Abstract
Connexin mimetic peptides (CxMPs), such as Gap26 and Gap27, are known as inhibitors of gap junction channels but evidence is accruing that these peptides also inhibit unapposed/non-junctional hemichannels (HCs) residing in the plasma membrane. We used voltage clamp studies to investigate the effect of Gap26/27 at the single channel level. Such an approach allows unequivocal identification of HC currents by their single channel conductance that is typically ~220 pS for Cx43. In HeLa cells stably transfected with Cx43 (HeLa-Cx43), Gap26/27 peptides inhibited Cx43 HC unitary currents over minutes and increased the voltage threshold for HC opening. By contrast, an elevation of intracellular calcium ([Ca2+]i) to 200–500 nM potentiated the unitary HC current activity and lowered the voltage threshold for HC opening. Interestingly, Gap26/27 inhibited the Ca2+-potentiated HC currents and prevented lowering of the voltage threshold for HC opening. Experiments on isolated pig ventricular cardiomyocytes, which display strong endogenous Cx43 expression, demonstrated voltage-activated unitary currents with biophysical properties of Cx43 HCs that were inhibited by small interfering RNA targeting Cx43. As observed in HeLa-Cx43 cells, HC current activity in ventricular cardiomyocytes was potentiated by [Ca2+]i elevation to 500 nM and was inhibited by Gap26/27. Our results indicate that under pathological conditions, when [Ca2+]i is elevated, Cx43 HC opening is promoted in cardiomyocytes and CxMPs counteract this effect.
Keywords: Connexin 43, Connexin hemichannel, Mimetic peptides, Cardiomyocytes, Single channel
Introduction
In vertebrates, connexin (Cx)-based hemichannels (HCs) embedded in the plasma membrane interact with their counterparts on neighboring cells in a head-to-head arrangement, forming gap junction channels that coalesce into junctional plaques that allow the direct exchange of metabolic and signaling molecules with molecular weight below 1.5 kDa between cells [22, 37, 71]. On the other hand, unapposed/undocked HCs present in non-junctional plasma membrane can open and form a conduit between the cell’s interior and the extracellular milieu, allowing Na+ and Ca2+ to enter the cell and K+ and messengers such as ATP, glutamate and others to leave the cell [27, 31,34, 58, 73]. Cx HCs are typically closed under resting conditions, but may open under ischemic conditions [10,26, 39, 61] by alterations in the redox status [50], the connexin phosphorylation status [2, 30] or by an increased presence of these channels in the plasma membrane [49,56].
Cx mimetic peptides (CxMPs) are peptides identical to sequences located on one of the two extracellular loops of the Cx protein [21]. They were introduced in the 1990s based on the hypothesis that such peptides could prevent the docking of two HCs, thereby preventing the formation of GJCs [20, 70]. Among them, Gap26 and Gap27, mimicking sequences on the first and second extracellular loop regions of Cx43, respectively (see Online Resource Fig. S1), have been most extensively investigated and used as gap junction blockers [4, 9, 55, 72]. Recent evidence raised the possibility that CxMPs may also affect the function of HCs [5,20], and to date, they have become a frequently applied pharmacological tool to explore new functions of the Cx HC signaling pathway. CxMPs such as Gap26 and Gap27 have been applied to establish the contribution of HCs in cell death propagation [17]. In embryonic neural retina, these peptides have been used to demonstrate an essential role of HCs as an ATP release pathway in the development of neural retina progenitor cells [43]. In the context of cardiac ischemia, targeting of Cx43 HC at the cell surface by Gap26/27 showed cardioprotective effect in both primary myocytes culture and intact heart [24, 26, 61]. Further work with CxMPs has suggested a role of HCs in a variety of pathological insults ranging from amyloid β-mediated neuronal death to hypoxia-induced inflammation [19, 28,40]. Intriguingly, despite the growing interest in CxMPs-based HC studies, no conclusive arguments are available to support a direct action of the peptides on HCs. Short exposure to CxMPs has been reported to inhibit indirect measures of HC function based on ATP release or dye uptake studies. Electrophysiological studies further indicated that Gap26 rapidly diminished macroscopic currents in Cx43 expressing cells [24, 25, 53]. Studies performed in the Xenopus oocyte expression system, however, failed to demonstrate clear inhibition of macroscopic currents; limited inhibition was only observed with long exposures (3 h) and appeared to depend on peptide size rather than sequence [69]. Due to the lack of studies at single channel level, it remains uncertain whether CxMPs block HC openings and if so, whether this effect is sequence-specific or merely the consequence of steric occlusion.
Here, we investigated the effect of Gap26 and Gap27 at the level of unitary currents through Cx43 HCs, allowing detailed analysis at the highest resolution. In HeLa cells expressing exogenous Cx43, unitary events associated with Cx43 HC opening can be unambiguously identified by the single channel conductance that is typically ~220 pS. Our data show that HC-based unitary events are inhibited by Gap26/27 within several minutes, by shifting the voltage-dependence of Cx43 HC opening to more positive trans-membrane potentials (Vm). In addition, moderate elevation of the intracellular calcium concentration ([Ca2+]i) to 200–500 nM shifted the Vm dependence of HC opening to lower Vm values (shift to the left), while Gap26/27 fully prevented this shift. Interestingly, low pH-induced HC inhibition was also found to depend on [Ca2+]i changes, with HC closure being mediated by (strong) [Ca2+]i elevation to micromolar concentrations. In isolated left ventricular cardiomyocytes of adult pig, single channel openings triggered by stepping Vm to positive potentials were abolished by short interfering RNA (siRNA) against Cx43. These unitary current activities were promoted by 500 nM [Ca2+]i and inhibited by Gap26/27. We conclude that (a) functional Cx43 HCs are present in ventricular cardiomyocytes, (b) when [Ca2+]i is moderately elevated, these channels open at Vm = + 40 mV, and (c) CxMPs prevent Ca2+ and voltage-dependent HC activation. These effects are likely to play a prominent role in the protective effect of CxMPs on cardiac ischemia.
Materials and methods
Chemical reagents
Gap26 (VCYDKSFPISHVR) and Gap27 (SRPTEKTIFII), two connexin mimetic peptides corresponding to a sequence in the first and second extracellular loop regions of Cx43, respectively, were used in the study. Two associated scrambled peptides SGap26 (PSFDSRHCIVKYV) [43] and SGap27 (TFEPIRISITK) [72] were applied in some experiments as inactive controls (overviewed in Online Resource Table S1). All peptides were synthesized to a purity >90 %.
Cell culture
HeLa-wild type (HeLa-WT) cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Gent, Belgium) supplemented with 20 % fetal bovine serum (FBS), 2 mM glutamine, 10 U/mL penicillin, 10 μg/mL streptomycin, and 0.25 μg/mL fungizone (Invitrogen, Gent, Belgium). Cells stably transfected with Cx43 [18] were grown in the medium with additional 1 μg/mL puromycin (Sigma-Aldrich, Bornem, Belgium). Mouse Cx43 gene was cloned into the EcoRI/BamHI restricted cloning site of the expression vector pMJgreen. CytoMegalovirus (CMV) promoter was used. The vector also contains a puromycin N-acetyl-transferase (Pac) gene encoding region.
Calcein efflux
Subconfluent cultures of HeLa-Cx43 seeded on glass coverslips (Knittel, Novolab, Geraardsbergen, Belgium) were loaded with 10 μM calcein-acetoxymethyl ester (calcein-AM) for 1 h at room temperature. Thereafter, cells were washed and left for another 30 min to allow for deesterification. Cultures were then transferred to an inverted epifluorescence microscope (Eclipse TE300, Nikon Belux, Brussels, Belgium) equipped with a ×40 oil-immersion objective and an electron-multiplying CCD camera (Quantem 512SC, Photometrics, Tuscon, AZ, USA). Calcein was excited at 482 nm by a Lambda DG-4 filterswitch (Sutter Instrument, Novato, CA, USA). A 505 nm long-pass dichroic mirror and a 535 nm bandpass filter (35 nm bandwidth) were used to capture calcein emission light. Fluorescent images were recorded every second and the fluorescent decay as a function of time was quantified as described in [14].
Cardiomyocyte isolation
Left ventricular cardiomyocytes from adult domestic pigs were enzymatically isolated as previously described [63]. Briefly, the left anterior descending coronary artery was cannulated, and the cells were dissociated by enzymatic tissue digestion through Langendorff perfusion at 37 °C. The cells were then filtered and resuspended in a low Ca2+ Tyrode solution containing (in mM): 130 NaCl, 5.4 KCl, 1.2 KHPO4, 1.2 MgSO4, 0.18 CaCl2, 6 HEPES, 10 glucose, pH 7.2. Ca2+-tolerant cells were stored at room temperature and used within 8–10 h after isolation.
RNA interference
Acutely isolated cardiomyocyte suspension was centrifuged at 300 rpm for 5 min. The cell pellets containing both cardiomyocytes and fibroblasts were resuspended in Medium 199 (Sigma-Aldrich) supplemented with 10 % FBS, 10 U/mL penicillin and 10 μg/mL streptomycin. After 2-h incubation at 37 °C in 5 % CO2, cardiomyocytes remained suspended in the medium while fibroblasts adhered to the bottom of the polystyrene flask. The fibroblast-depleted suspension was again collected and cultured at a density of 2 × 104 cells/cm2 in a Petri dish (9.2 cm2; TPP Techno Plastic Products AG, Trasadingen, Switzerland) or glass coverslip (2.5 cm2) coated in advance with natural mouse laminin (Invitrogen).
Cultured cardiomyocytes were transfected the following day with siRNA using DharmaFECT lipid reagent (Dharmacon, Thermo Fisher Scientific, Aalst, Belgium). siRNA targeting the porcine Cx43 gene gja1 (5′-GAAAGAGGAGGAACUCAAA-dTdT-3′) was synthesized and annealed by Eurogentec (Luik, Belgium). Cultures transfected with scrambled sequence (siCx43scr: 5′-AGAGAUACGAACAAGAGAG-3′) or lipid reagent alone (MOCK) were used as negative controls. The transfection mixture was removed from the cultures after 24-h treatment.
Western blot analysis
Whole cell lysates were prepared by treating confluent cell culture with RIPA buffer [25 mM Tris, 50 mM NaCl, 0.5 % NP-40, 0.5 % deoxycholate, 0.1 % SDS, 5.5 % β-glycerophosphate, 1 mM dithiothreitol, 10 μL/mL protease inhibitor cocktail (Sigma-Aldrich), 30 μL/mL phosphatase inhibitor cocktail (Sigma-Aldrich), and 20 μL/mL mini EDTA-free protease inhibitor cocktail]. Proteins were resolved in 10 % SDS-poly-acrylamide (SDS-PAGE) gel and transferred to a nitrocellulose membrane (Amersham, Buckinghamshire, UK). Blots were probed with a rabbit polyclonal anti-Cx43 antibody (Sigma-Aldrich) or a rabbit anti-Panx1 (a gift kindly provided by Dr. Dale W. Laird, University of Western Ontario, Canada) followed by alkaline phosphatase-conjugated goat anti-rabbit IgG anti-body (Sigma-Aldrich). The blots were then developed with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate reagent (Zymed, Invitrogen). Total protein stains by SYPRO® Ruby (Invitrogen) prior to antibody staining or detection with a rabbit anti-β-tubulin antibody (Abcam, Cambridge, UK) was used as a loading control.
Triton X-100 (Tx-100) insoluble fraction was obtained by harvesting cells in 1 % Trixton X-100 supplemented with 50 mM NaF, 1 mM Na3VO4, 10 μL/mL protease inhibitor cocktail, 30 μL/mL phosphatase inhibitor cocktail. Samples were centrifuged at 16,000g for 10 min and the Tx-100 insoluble pellets were resuspended in 1×Laemmli buffer (10 % glycerol, 2.3 % SDS and 125 mM Tris pH 6.8).
Detection of the fraction of unapposed Cx43 in the plasma membrane was achieved by biotin-labeling of surface protein with the Pierce cell surface protein isolation kit (Thermo Scientific, Erembodegem, Belgium). Confluent cultures were incubated with 0.25 mg/mL sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (Sulfo-NHS-SS-Biotin) phosphate-buffered saline for 2 h at 4 °C. This membrane-impermeable, thiol-cleavable and amine-reactive reagent labels free surface membrane proteins including unapposed Cx43 HCs that have their extracellular domains unoccupied. By contrast, HCs incorporated into gap junctions have no accessible extracellular domains and the amine-reactive sites are effectively sealed. Unreacted biotin was quenched by glycine-based solution and the cells were lysed immediately. Cell extracts were then mixed with Neutravidine™ agarose for purification of biotin-labeled proteins. Biotinylated proteins containing the pool of unapposed HCs were subsequently eluted from the Neutravidine™ agarose and separated by a 10 % SDS-PAGE gel for further immunoblotting with Cx43 antibody (Sigma-Aldrich).
Electrophysiological recording
Hela-Cx43cells were bathed in a recordingchamber filled with a modified Krebs–Ringer solution consisting of (in mM): 150 NaCl, 6 CsCl, 2 CaCl2, 2 MgCl2, 1 BaCl2, 2 pyruvate, 5 glucose, 5 HEPES and pH adjusted to 7.4. The standard whole-cell recording pipette solution was composed of (in mM): 130 CsCl, 10 Na-aspartate, 0.26 CaCl2, 1 MgCl2, 2 EGTA, 5 tetraethylammonium (TEA)-Cl and 5 HEPES; pH was adjusted to 7.2 and [Ca2+] was 50 nM as calculated with Webmax Standard software application (http://www.stanford.edu/~cpatton/webmaxcS.htm). Estimations of attained [Ca2+]i mentioned in the text were also calculated using this software. Intracellular acidification was applied via the patch pipette; to this purpose, HEPES was replaced by MES (5 mM) and pH was adjusted to 6.4 or 6.0 [33]. Pipette resistance was 1–3 MΩ. A 3-M KCl agar bridge connected the recording chamber to a grounding compartment.
For the experiments on cardiomyocytes, cells were initially perfused with the standard Tyrode solution (in mM): 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 10 glucose, 11.8 HEPES and pH adjusted to 7.4. When recording unitary HC currents, the solution was switched to a solution with the same composition but with all K+ replaced by Cs+. In addition, 1 mM BaCl2 was added to the external solution to further inhibit K+ currents. The pipette solution was composed of (in mM): 120 CsCl, 5 NaCl, 10 TEACl, 1 CaCl2, 1 MgCl2, 2 MgATP, 10 EGTA, 10 HEPES and pH adjusted to 7.2.
Single channel recordings were performed by making use of an EPC 7 PLUS patch-clamp amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). Data were acquired at 4 kHz using a NI USB-6221 data acquisition device from National Instruments (Austin, TX, USA) and Clampex 10.2 acquisition software (Axon instruments). All currents in whole-cell configuration were filtered at 1 kHz (7-pole Besselfilter). Liquid junction potentials were measured in current mode by placing pipette solution or bath solution in the recording pipette and the other in the bath. Membrane potentials were corrected for the liquid junction potentials.
For single channel analysis, holding currents were subtracted from the recorded current traces, giving traces that only contained unitary current events. Unitary conductances were calculated from the elementary current transitions Δi as: γ = Δi/Vm. From these data, we constructed all-point conductance histograms that displayed one or more Gaussian distributions. These were fit by a probability density function assuming independent channel opening [48, 68]. Channel activity was quantified from the charge transfer Qm associated with unitary currents; this was done by integrating the unitary current traces (i.e., a function of time) over the duration of the voltage step as: Qm = ∫idt.
Statistical analysis
Data are expressed as mean ± SEM with n denoting the number of independent experiments. Multiple groups were compared by one-way ANOVA with Bonferroni post-test. Two groups were compared with an unpaired Student’s t test and two-tail P value.
Results
Single channel unitary currents in HeLa cells stably transfected with Cx43
Previous studies revealed that unitary Cx43 HCs events appeared upon depolarizing the membrane potential (Vm) to >+50 mV [11]. Figure 1a illustrates single channel openings observed with whole-cell patch-clamp recording from a solitary Hela-Cx43 cell upon stepping Vm from −30 to +70 mV. Recordings were performed in the presence of extracellular Ca2+ and Mg2+ and under conditions of K+-channel blockade with Cs+, Ba2+ and TEA+ (see “Materials and methods”). Transitions between the closed and fully open state were characterized by a unitary conductance (γo) of ~220 pS (Fig. 1a). Transitions from the open state occasionally occurred to a sub-conductance state characterized by a unitary conductance (γsub) of ~58 pS (Fig. 1b), as reported by Contreras et al. [11]. Such transitions were not frequent and event histograms obtained from recordings of 30 s typically did not show a resolvable subconductance peak under control conditions. The typical 220 pS opening activity was not observed in single HeLa-WT cells (Online Resource Fig. S2a). In HeLa-Cx43 cells, unitary current activity induced by Vm = +70 mV rapidly disappeared following application of carbenoxolone (100 μM), a gap junction and HC blocker (Online Resource Fig. S3).
Fig. 1.
Unitary currents recorded in HeLa-Cx43 cells. a Single channel events were activated by stepping Vm from −30 to +70 mV. The vertical graph at the left represents an all-point histogram illustrating the ~220 pS unitary conductance of a fully open channel. b Illustration of two fully open channels closing to a substate of ~58 pS as shown in the expanded traces
Gap26 and Gap27 inhibit Cx43 hemichannel unitary currents
We tested whether Gap26 and Gap27 inhibited unitary current events recorded in HeLa-Cx43 cells at concentrations commonly used to inhibit gap junctions. Pre-incubation of cells for 30 min with Gap26 (160 μM) or Gap27 (200 μM) reduced unitary events typically observed at Vm = +70 mV (Fig. 2a, b). The conductance of the remaining events showed a peak at ~220 pS, indicating that Gap26/27 did not influence the γo. Experiments with scrambled versions of Gap26 and Gap27 (SGap26 and SGap27, respectively) did not reveal any inhibition of HC-mediated unitary events (Fig. 2a, c). Unitary current activities were quantified by integrating the current traces (holding current subtracted) over the time of Vm step, giving the charge transfer (Qm) via open HCs. Figure 2c shows a more than threefold decrease in Qm by Gap26/27, whereas Qm recorded in cells exposed to SGap26/27 was not different from control. Western blot analysis of Cx43 expression demonstrated that the density of membrane-located biotin-labeled Cx43 HCs relative to the total membrane Cx43 pool (Tx-100 insoluble fraction) was not influenced by Gap26 treatment (Fig. 2d), in line with the findings reported by others [36].
Fig. 2.
Cx43 HC unitary current events are inhibited by Gap26 and Gap27 in HeLa-Cx43 cells. a Example traces depicting unitary current activities induced by stepping Vm to +70 mV (30 s) in control and after addition of Gap26/27 or scrambled sequences. b All-point histograms determined from the corresponding traces in a. Dashed vertical lines through the peaks are each separated by ~220 pS. c Summary data of Qm, illustrating significant inhibition by Gap26/27 but not by the scrambled sequences. **P < 0.01 versus control; ##P < 0.01 versus scrambled peptides. The number of experiments are given in the bars. d Biotin-labeling studies illustrating that Gap26 has no effect on the fraction of unapposed HCs. For assessment of unapposed HC fraction present in the plasma membrane, the biotin-labeled fraction was normalized to the total protein stained by SYPRO® Ruby. Membrane-associated Cx43 was estimated by the Tx-100 insoluble fraction proportional to the total lysate. Treatment with Gap26 (160 μM, 30 min) did not influence the biotin-labeled Cx43 protein fraction, indicating that the number of HCs in the plasma membrane is not altered (n = 4)
Gap26/27 inhibition of Cx43 HC unitary currents is concentration-dependent and occurs within minutes
We examined the kinetics of Gap26/27 inhibition of unitary current activity. In this experiment, we applied repeated (every 40 s) Vm steps from −30 to +70 mV (30 s) over a time period of 10 min and calculated the Qm at different time points. Figure 3a illustrates an example recording, showing a decrease of Qm with time after addition of Gap26 (250 μM). There was some inherent variability in these recordings, related to shot noise associated with low open probability events [27]. Figure 3b represents changes of current under control conditions and 240 s after exposure to Gap26. Figure 3c shows averaged Qm changes in experiments similar to the one illustrated in Fig. 3a. After the addition of Gap26, Qm progressively decreased with a time constant (τ) of 148 s (determined by fitting the data points to a mono-exponentially decaying function). At steady-state conditions, Qm was reduced to ~35 %. In contrast, SGap26 did not cause any Qm decay. We further examined the concentration dependence of Gap26 inhibition (148 s exposure time) and found an IC50 of ~81 μM and a Hill coefficient (nH) = 2 (Fig. 3d). SGap26 did not exhibit inhibitory effects over the concentration range at which Gap26 displayed inhibition. Only when the concentration of SGap26 was increased to 1 mM, strong inhibition was observed (Fig. 3e). Figure 4 illustrates a similar set of experiments performed with Gap27 (300 μM). Inhibition was somewhat slower (τ = 223 s) while the steady-state reduction of Qm was stronger (reduction to ~15 %) compared to Gap26 (Fig. 4c). Concentration-dependent inhibition of Qm (223 s exposure time) was characterized by an IC50 of ~161 μM and nH = 2 (Fig. 4d). As expected, SGap27 did not affect Qm unless it was applied at 1 mM concentration (Fig. 4e). The abrupt kink in the Qm curve at 1 mM with both scrambled peptides (SGap26 and SGap27) indicates the appearance of steric occlusion of the channel pore.
Fig. 3.
Gap26 inhibition occurs within minutes and is concentration-dependent. a Qm for repeated Vm steps to +70 mV before and after application of Gap 26 (250 μM) in HeLa-Cx43 cells. b Example traces and all-point histograms before and after addition of Gap26 (points indicated as 1 and 2, respectively, in a). c Average data illustrating the kinetics of Gap26 inhibition (n = 5). For SGap26, the curve remained fluctuating around 100 % (n = 3). Qm was expressed as a percentage normalized to the averaged measurement before Gap26 addition. *P < 0.05 Gap26 versus SGap26. d Concentration-dependence of Gap26 inhibition; Qm was normalized to data values obtained without peptide. e Experiment as in d but with the scrambled sequence
Fig. 4.
Gap27 inhibition occurs within minutes and is concentration-dependent. a Qm for repeated Vm steps to +70 mV before and after application of Gap 27 (300 μM). b Example traces and all-point histograms before and after addition of Gap27 (points indicated as 1 and 2, respectively, in a). c Average data illustrating the kinetics of Gap27 inhibition (n = 5). For SGap27, the curve remained fluctuating around 100 % (n = 3). Qm was expressed as a percentage normalized to the averaged measurement before Gap27 addition. **P < 0.01 Gap27 versus SGap27. d Concentration dependence of Gap27 inhibition; Qm was normalized to data values obtained without the peptide. e Experiment as in d but with the scrambled sequence. f Gap27 inhibits dye transfer triggered by exposure to nominally divalent-free (NDF) solution. NDF triggered the efflux of calcein preloaded in HeLa-Cx43 cells and this was inhibited by Gap27 (300 μM; n = 31 cells from 5 experiments). ***P < 0.001, NDF versus baseline. g Gap27 inhibits NDF-promoted HC currents in HeLa-Cx43 cells. Traces show unitary current activity upon stepping from −30 to +60 mV (40 s) that was strongly promoted by NDF conditions. Gap27 (300 μM, added just after the 225 s trace) inhibited NDF-potentiated currents (300 μM)
We tested whether Gap27 could also inhibit the passage of larger molecules through Cx43 HCs. To that purpose, we used calcein (MW 623 Da) as a HC-permeable fluorescent reporter dye [1] and quantified the loss of fluorescence from preloaded HeLa-Cx43 cells, as previously done [14]. The calcein efflux rate increased upon exposure to nominal divalent-free (NDF, no Ca2+ or Mg2+ added) solution and the efflux rate-increase was inhibited by Gap27 (300 μM) (Fig. 4e). Figure 4f illustrates the effect of NDF and NDF + Gap27 on unitary HC currents, demonstrating comparable effects as observed in the calcein experiments. Thus, Gap27 has comparable effects on calcein passage and current flow through Cx43 HCs.
Gap26 and Gap27 increase the Vm necessary to open HCs
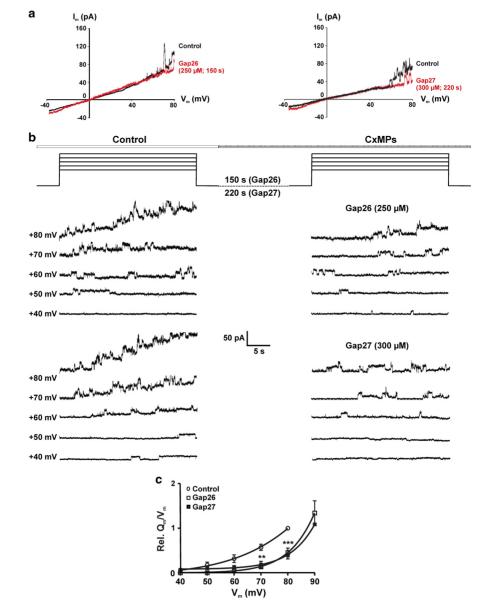
Gap26 and Gap27 had no effect on γo, therefore, we examined whether they exerted their effect through a change in the voltage gating. Figure 5a shows an I–V plot obtained by applying voltage ramps from −40 to +80 mV (1.8 mV/s). Unitary current events started to appear from Vm = +50 mV while after addition of Gap26/27, unitary events appeared at more positive potentials. A similar influence was observed on I–V plots derived from slower voltage ramps (0.5 mV/s; Online Resource Fig. S4). By applying Vm steps from −30 mV to voltages in the range of +40 to +80 mV, we observed that Gap26 and Gap27 (for concentrations and exposure times see Fig. 5b) significantly shifted the Vm dependence of HC opening to the right over ~15 mV, i.e. they increased the Vm for HC activation (Fig. 5c).
Fig. 5.
Gap26/27 increases the Vm required for hemichannel activation in HeLa-Cx43 cells. a Voltage ramp I–V plot illustrating that unitary current activation occurs at more positive Vm after exposure to Gap26/27. b Voltage-dependent activation studies performed by Vm steps in the range of +40 to +80 mV in 10 mV increments (30 s). c Voltage activation curve, obtained by normalizing the Qm/Vm data to the control value at +80 mV as a relative measure of HC openings (n = 5). **P < 0.01 and ***P < 0.001, Gap26 and Gap27 versus control
[Ca2+]i-dependent modulation of Cx43 HC unitary currents under control/normal conditions and in the presence of Gap26/27
Unitary Cx43 HC currents appear only at strong positive Vm as reported earlier [11, 27] and confirmed here. Previous studies demonstrated that an elevation of [Ca2+]i stimulates HC-mediated ATP release and dye uptake [15,16, 47, 57]. We investigated whether [Ca2+]i elevation alters the properties of unitary HC currents in HeLa-Cx43 cells. For this purpose, the pipette solutions used for whole-cell recordings were buffered to different [Ca2+] of 50 (standard pipette solution), 200, 500, and 1,000 nM. Figure 6a demonstrates that unitary current activity measured at Vm = + 60 mV was clearly potentiated at moderately elevated [Ca2+]i levels of 200 and 500 nM, while the effect disappeared with further elevation to 1 μM. Figure 6b shows the corresponding all-point histograms, demonstrating an increased frequency of ~220 pS openings and less activity in the closed state. Average data of such experiments are shown in Fig. 6c, illustrating a significant increase of Qm at 200 and 500 nM (compared to 50 nM) while this potentiation disappeared at 1 μM. HeLa-WT cells exhibited no unitary current activities upon [Ca2+]i elevation to 500 nM (Online Resources Fig. S2b). The bell-shaped profile of these responses in HeLa-Cx43 cells is very similar to previously reported results that were based on indirect measurements of HC activity [15, 16]. Moreover, we found that [Ca2+]i elevation from 50 to 200 and 500 nM significantly altered the Vm dependence of HC opening by shifting it ~15 mV leftwards, i.e. 200–500 nM [Ca2+]i decreased the Vm for HC opening (Fig. 6d). The effect of [Ca2+]i elevation to 200 nM was stronger than for 500 nM. [Ca2+]i elevation had no effect on the reversal potential of the unitary current recordings (0 mV; Online Resource Fig. S5). Interestingly, Gap26 (250 μM) and Gap27 (300 μM) inhibited the Qm increase mediated by [Ca2+]i elevation to 200/500 nM (Fig. 6c) and completely prevented changes of Vm dependence induced by [Ca2+]i increase (Fig. 6d).
Fig. 6.
Effect of [Ca2+]i elevation on Cx43 HC unitary currents recorded in HeLa-Cx43 cells. a Representative current traces obtained by Vm steps to +60 mV (30 s). Ten consecutive runs were recorded and the beginning of each 30-s example trace was separated by 40 s. The various [Ca2+]i depicted were applied via the whole-cell recording pipette. b All-point histograms of each set of recordings depicted in a. [Ca2+]i elevation to 200 and 500 nM increased the number of peaks and reduced the frequency of the closed state while elevation to 1 μM had no effect. c Summary data illustrating the effect of various [Ca2+]i levels on Qm (n = 8). Gap26 (250 μM) significantly attenuated the Ca2+-promoted Qm (n = 5); Gap27 had similar effects (data not shown). **P < 0.01 versus 50 nM [Ca2+]i. #P < 0.05 versus 200 or 500 nM [Ca2+]i. d Effect of [Ca2+]i elevation to 200 and 500 nM on the Vm dependence of HC activation (relative Qm/Vm normalized to control value at +90 mV). The curve was left-shifted and this was completely prevented by Gap26 (250 μM, 150 s) and Gap27 (300 μM, 220 s) (n = 5). *P < 0.05, **P < 0.01 and ***P < 0.001 for 200 or 500 nM [Ca2+]i versus 50 nM [Ca2+]i
[Ca2+]i-dependent modulation is involved in acidification-induced Cx43 HC closure
Acidification is known to inhibit gap junction channels [8, 42] and it has been suggested that this effect depends on a [Ca2+]i elevation [13, 32, 45]. We further tested whether HCs close with pH lowering and whether a [Ca2+]i increase is involved in this. Intracellular acidification from pH 7.2 to 6.4 induced by 2 % CO2 [42] rapidly inhibited Cx43 HC unitary currents (Fig. 7a, b). Because pHi lowering decreases the affinity of EGTA present in the pipette and cell, it is possible that inhibition is partly caused by an associated and prominent (>500 nM) [Ca2+]i elevation. Calculation of the expected [Ca2+]i increase (making use of the Webmax program; see, “Materials and methods”) indeed indicated a micromolar increase of [Ca2+]i in response to CO2-induced pH lowering to 6.4. We therefore performed experiments with MES-buffered acidic pipette solutions with defined [Ca2+]. When [Ca2+]i was 50 nM, pHi lowering to 6.4 or 6.0 did not inhibit HC currents (traces shown in Fig. 7c; summary data given in Fig. 7d). However, when [Ca2+]i was set to 6 μM, HC currents were strongly inhibited by the pH 6.0 condition as they were at pH 7.2 (Fig. 7d). Thus, large [Ca2+]i elevations can be involved in the low pH closure of Cx43 HCs. Of notice, Fig. 6c indicates that HC activity at 1 μM [Ca2+]i is slightly lower than at 50 nM while Fig. 7d (first two bars) shows that HC activity is significantly depressed by further elevating [Ca2+]i to 6 μM.
Fig. 7.
Role of [Ca2+]i in acidification-induced Cx43 HC closure. a Intracellular acidification triggered by 2 % CO2 abolished HC unitary current activities in HeLa-Cx43. b Corresponding time evolution of single channel activities of the experiment demonstrated in a. c Example unitary current traces recorded in HeLa-Cx43 cells under various pH and [Ca2+]i conditions as indicated. Voltage steps to +70 mV (30 s) were applied. Top-down current traces consist of ten consecutive runs, each separated by 40 s. d Summary data of experiments as in c. At pH 7.2, 6 μM [Ca2+]i strongly inhibited HC unitary activity compared to 50 nM [Ca2+]i. Acidosis (pH 6.4 and 6.0) did not significantly influence HC currents when [Ca2+]i was 50 nM. However, pH 6.0 combined with 6 μM 50 nM strongly suppressed the currents. ***P < 0.01 [Ca2+]i = 6 μM (pH 6.0) versus [Ca2+]i = 50 nM (pH 6.0). ##P < 0.01 [Ca2+]i = 6 <M (pH 7.2 and 6.0) versus [Ca2+]i = 50 nM (pH 7.2). The number of experiments are given in the bars
Gap26/27 inhibits [Ca2+]i-dependent modulation of Cx43 HC unitary currents in ventricular cardiomyocytes
In a next step, we examined whether we could reproduce the modulation of Cx43 HC opening by [Ca2+]i and Gap26/27 measured in HeLa transfectants with those obtained from primary cardiomyocytes, acutely isolated from the left ventricle of pigs. In these experiments, perfusion conditions were similar to those used for HeLa-Cx43 cells. Stepping the Vm from −70 to +80 mV demonstrated the appearance of unitary current events characterized by a γo of ~200 pS (Fig. 8a, left panel). These unitary current events were not influenced by a low concentration of carbenoxolone (20 μM) that is known to block Panx1 HCs but not Cx43 HCs [6] (Fig. 8a, middle panel). Carbenoxolone at higher concentration (100 μM) acted inhibitory, as expected for Cx43 HCs (Fig. 8a, right panel). Western blot analysis (Online Resource Fig. S6) showed no Panx1 signal in ventricular cardiomyocytes, in line with a recent report by Penuela et al. [44]. Thus, the ~200 pS opening events are likely to be mediated by Cx43 HCs. To further confirm this, we used an siRNA approach to silence Cx43 expression: this led to ~50 % knockdown of Cx43 expression in cultured pig ventricular cardiomyocytes (Fig. 8b). Unitary current activities were present in MOCK-treated cultured cardiomyocytes (Fig. 8c), although the activities were less frequent compared to those in acutely isolated cells. Histogram analysis indicated a γo of ~220 pS, which suggests that culturing of cardiomyocytes slightly increases the γo to the levels recorded in cultured HeLa-Cx43 cells. The bar chart in Fig. 8c summarizes average Qm data, demonstrating that current activity was significantly inhibited by siRNA directed against Cx43 while siCx43scr had no effect. We next tested the effect of [Ca2+]i elevation and Gap26/27 on unitary HC current activity in freshly isolated cardiomyocytes. Figure 8d shows that a [Ca2+]i increase from 50 to 500 nM reduced the voltage threshold for HC opening from +60 to +40 mV. Furthermore, we found that Gap26 (160 μM, 5 min) reduced the Ca2+-mediated enhancement of unitary current activity in a similar fashion as it was observed in HeLa-Cx43 cells (Fig. 8e). Average Qm data (Fig. 8f) obtained from such cardiomyocyte recordings demonstrated strong inhibition of Ca2+-potentiated Cx43 HC activity by Gap26 (160 μM, 5 min) and Gap27 (200 μM, 5 min).
Fig. 8.
Effect of [Ca2+]i elevation and Gap26/27 on unitary current activities in isolated pig ventricular cardiomyocytes. a Representative traces illustrating single channel events triggered by stepping Vm to +80 mV. The all-point histograms at the right illustrate that the distribution peaks are separated by a γo of ~200 pS. Carbenoxolone had no effect when applied at 20 μM but clearly inhibited the currents at 100 μM. b Treatment of cultured ventricular cardiomyocytes with siCx43 brought down Cx43 expression to 50 % of control (MOCK) while siCx43scr had no effect. *P < 0.05 siCx43 versus siCx43scr and MOCK. c Example traces depicting unitary currents recorded in MOCK-, siCx43- and siCx43scr-treated cells. The bar chart summarizes average data for Cx43 HC currents activated at +70 mV (MOCK: n = 3, siCx43: n = 9, siCx43scr: n = 6). All-point histogram depicting the γo of the unitary current activities recorded in cultured cardiomyocytes treated with siCx43scr and MOCK, ***P < 0.001 siCx43 versus MOCK and siCx43scr. d Elevating [Ca2+]i to 500 nM resulted in increased activity and a lowered Vm for channel activation. e Unitary current events triggered by Vm steps to +60 mV (30 s) were potentiated by 500 nM [Ca2+]i and the increased activity was inhibited by Gap26 (160 μM, 5 min). The beginning of each trace is separated by 35 s. The all-point histograms at the right illustrate the effect on conductance distributions. f Average data of [Ca2+]i and Gap26/27 effects on Qm. [Ca2+]i elevation to 500 nM strongly and significantly increased Qm and this effect was completely prevented by Gap26 (160 μM, 5 min) and Gap27 (200 μM, 5 min). ***P < 0.001 versus 50 nM [Ca2+]i; ###P < 0.001 versus 500 nM [Ca2+]i without peptides. The number of experiments are given in the bars
Discussion
Gap26/27 and related peptides have been reported to inhibit HC-related ATP and dye uptake [5, 46, 52, 61] and have been frequently applied to interfere with HC function under various conditions [14, 19, 38, 41, 43,66]. Macroscopic current studies have been inconclusive, with some reports showing inhibition by Gap26/27 [24,53] and others claiming that inhibition is related to non-specific pore block [69]. Here, we demonstrate at single channel level that these peptides inhibit function of Cx43 HCs and that this effect is sequence specific. Moreover, we show that the peptides also inhibit Cx43 HC openings in acutely isolated ventricular cardiomyocytes, including HC currents promoted by moderate (≤500 nM) [Ca2+]i elevation. Interestingly, the Ca2+-mediated potentiation of HC function indicates that Cx43 HCs can be opened at physiologically relevant Vm (+40 mV) and Gap26/27 antagonizes this Ca2+-promoted HC opening.
Inhibition of HC currents by Gap26/27 occurring over a time span of minutes was incomplete and not associated with alterations in single channel conductance or number of unapposed HCs in the plasma membrane. Currently, the mechanisms as to how CxMPs inhibit the function of HCs are not known. We assume that Gap26/27 peptides bind to as yet undefined domains in the extracellular loops of Cx43 resulting in HC inhibition [3]. For Gap26, evidence is available that this peptide indeed interacts with the extracellular Cx43 domains [35]. If CxMPs inhibit HCs by interacting with freely accessible, unoccupied extracellular domains of Cx43, then the inhibition kinetics in duration of minutes appears to be slow. In fact, this suggests that the interaction site is less accessible than hypothesized, either as a consequence of being located deeper in the channel pore or being only accessible when the channel is open. We demonstrate that Gap26/27 reduces HC opening by shifting the Vm dependence of opening to more positive voltages (right-shift). This may be mediated by the shielding of a charged amino acid residue, as a consequence of the interaction, that changes the local electrical field sensed by the voltage sensor and alters the activation threshold, as has been suggested for the shift in voltage-dependence of Na+ and K+ channels by a variety of stimuli [64, 74].
Millimolar extracellular Ca2+ concentrations normally keep Cx43 HCs closed but when extracellular Ca2+ decreases, for example during ischemia [62], HCs open and HC currents at positive Vm are promoted [11]. It has been proposed that divalent cations such as Ca2+ and Mg2+ influence the intrinsic voltage-gating and lock the channel in the closed state by interacting with the extracellular side of the channel [67]. We found that Gap27 inhibited the passage of the fluorescent dye calcein triggered by exposure to nominally Ca2+- and Mg2+-free extracellular conditions. Thus, CxMPs inhibit currents equally well as the passage of larger molecules through Cx43 HCs.
In addition to extracellular Ca2+ also intracellular Ca2+ modulates Cx43 HC function. Here, we show that a moderate increase of [Ca2+]i promotes HC unitary current activity while this effect disappears upon further elevation to 1 μM (Fig. 6c), as reported in previous studies based on dye transfer or ATP release [15, 47]. These indirect measurements of HC opening have suggested that increased HC opening with moderate [Ca2+]i elevation is dependent on Ca2+-calmodulin signaling [16]; Cx43 contains several putative calmodulin binding sites located on the amino terminus and cytoplasmic loop [65, 75]. We further demonstrate here that increasing [Ca2+]i to micromolar concentrations not only removes the [Ca2+]i-promotion effect (1 μM) but also actively inhibits HC opening at 6 μM (Fig. 7d). Apparently, this inhibitory effect also plays a role in the low pH-induced closure of HCs. It is very much likely that the transient nature of hemichannel opening reported in in vitro simulated ischemia is caused by the dual effects (stimulation vs. inhibition) of [Ca2+]i on Cx43 HC opening [61]. Interestingly, the HC-stimulation by moderate [Ca2+]i elevation was related to a left-shift of the Vm dependence and this shift along the voltage axis was completely reversed by Gap26/27. Thus, Gap26/27 peptides inhibit HC function by interacting with extracellular domains of Cx43, while [Ca2+]i potentiates HC function by interacting with intracellular Cx43 domains, both possibly acting on a common target that is the voltage sensor [7, 23].
While HCs are activated from +40 mV on in the presence of a moderate [Ca2+]i increase, Online Resource Fig. S7 clearly demonstrates that unitary currents appear at +30 mV upon exposure to chemical ischemia conditions applied by metabolic inhibition. These potentials can realistically be attained during the plateau phase of the cardiac action potential.
In the heart, Cx43, predominantly expressed in ventricular cardiomyocytes, is an important determinant of myocardial ischemic injury [54]. Cx43 proteins form gap junction channels that are clustered in the nexus, which is one of the major components of the intercalated disks. Non-junctional (unapposed) Cx43 HCs reside in the zone surrounding the junctional nexus area, called the perinexus [51]. As discussed above, Cx43 HC opening may be triggered under ischemic conditions [12, 60, 61], and this has been associated with cardiomyocyte cell swelling in response to simulated ischemia [59]. When exposed to metabolic inhibition, rabbit ventricular myocytes have also been reported to display a [Ca2+]i-dependent non-selective current which may be attributed to Cx43 HC opening [31]. Here, we demonstrate, for the first time, unitary currents in acutely isolated left ventricular cardiomyocytes from pig that are characterized by an activation threshold ≥+50 mV and a single channel conductance of ~200 pS. These biophysical properties are similar to those of mouse Cx43 HCs observed in HeLa transfectants (≥+50 mV, ~220 pS). Moreover, the unitary current activity was also promoted by moderate [Ca2+]i elevation and was inhibited by Gap26 and Gap27. A recent study has proposed Panx1 HCs as the conductance pathway exhibiting large unitary conductance and [Ca2+]i-dependent currents in cultured atrial cardiomyocytes from rat [29]. Panx1 HCs can, however, be ruled out based on the following arguments: (a) Panx1 proteins could not be detected in acutely isolated pig left ventricular cardiomyocytes, (b) the recorded unitary currents were not affected by 20 μM carbenoxolone which is known to inhibit Panx1 HCs and (c) a specific siRNA targeting Cx43 abolished the single channel activities. An absence of Panx1 expression and therefore of Panx1 HC function in cardiomyocytes used in our studies may be related to differences in cell preparation (acutely isolated cells vs. cultured cells), differences in tissue types (ventricles vs. atrium) and species (pig vs. rat).
In summary, our data show that Cx43 HCs in ventricular cardiomyocytes can be activated by positive Vm (≥+30 mV) and [Ca2+]i elevation (~500 nM). As a consequence, this may cause a leakage of ions and metabolites that may reduce excitability, safety of signal transfer between the cardiomyocytes and contribute to arrhythmogenesis. Gap26 and Gap27 inhibit these Vm/Ca2+-mediated HC currents and thus open a new pathway for novel therapeutic approaches in treating cardiac arrhythmia.
Supplementary Material
Acknowledgments
This work was supported by the Interuniversity Attraction Poles Program (Belgian Science Policy Project P6/31 and P7/10 to K.R. Sipido and L. Leybaert), the Fund for Scientific Research Flanders (FWO Grant Numbers G.0140.08, 3G.0134.09 and G.0298.11 to L. Leybaert) and supported by NIH Grants (R01NS072238 and RO1HL084464 to F.F. Bukauskas).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-012-0304-2) contains supplementary material, which is available to authorized users.
Contributor Information
Nan Wang, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium.
Marijke De Bock, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium.
Gudrun Antoons, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Ashish K. Gadicherla, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium
Mélissa Bol, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium.
Elke Decrock, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium.
William Howard Evans, Department of Medical Biochemistry and Immunology, Cardiff University School of Medicine, Heath Park, Cardiff CF14 4XN, Wales, UK.
Karin R. Sipido, Department for Experimental Cardiology, K.U.Leuven, Herestraat 49, 3000 Leuven, Belgium
Feliksas F. Bukauskas, Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461, USA
Luc Leybaert, Physiology Group, Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan 185 (Block B-Rm 031), 9000 Ghent, Belgium.
References
- 1.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. doi:10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. doi:10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud VM, Beyer EC, Seul KH. Peptide inhibitors of intercellular communication. Am J Physiol Lung Cell Mol Physiol. 2000;279:L619–L622. doi: 10.1152/ajplung.2000.279.4.L619. [DOI] [PubMed] [Google Scholar]
- 4.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 5.Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 6.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. doi:10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 7.Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol. 2002;119:171–185. doi: 10.1085/jgp.119.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. doi:10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol. 1997;503(Pt 1):99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke TC, Williams OJ, Martin PE, Evans WH. ATP release by cardiac myocytes in a simulated ischaemia model: inhibition by a connexin mimetic and enhancement by an anti-arrhythmic peptide. Eur J Pharmacol. 2009;605:9–14. doi: 10.1016/j.ejphar.2008.12.005. doi:10.1016/j.ejphar.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. doi:10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. doi:10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, Dauwe I, Vinken M, Simon AM, Rogiers V, De LG, Evans WH, Bultynck G, Dupont G, Cecchelli R, Leybaert L. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J Cereb Blood Flow Metab. 2011;31:1942–1957. doi: 10.1038/jcbfm.2011.86. doi:10.1038/jcbfm.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. doi:10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. doi:10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Decrock E, DeVuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D’Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009;16:151–163. doi: 10.1038/cdd.2008.138. doi:10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 18.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. doi:10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 20.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 21.Evans WH, Carlile G, Rahman S, Torok K. Gap junction communication channel: peptides and anti-peptide antibodies as structural probes. Biochem Soc Trans. 1992;20:856–861. doi: 10.1042/bst0200856. [DOI] [PubMed] [Google Scholar]
- 22.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. doi:10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 23.Harris AL. Voltage-sensing and substate rectification: moving parts of connexin channels. J Gen Physiol. 2002;119:165–169. doi: 10.1085/jgp.119.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawat G, Benderdour M, Rousseau G, Baroudi G. Connexin 43 mimetic peptide Gap26 confers protection to intact heart against myocardial ischemia injury. Pflugers Arch. 2010;460:583–592. doi: 10.1007/s00424-010-0849-6. doi:10.1007/s00424-010-0849-6. [DOI] [PubMed] [Google Scholar]
- 25.Hawat G, Helie P, Baroudi G. Single intravenous low-dose injections of connexin 43 mimetic peptides protect ischemic heart in vivo against myocardial infarction. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.07.008. doi: 10.1016/j.yjmcc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Johansen D, Cruciani V, Sundset R, Ytrehus K, Mikalsen SO. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell Physiol Biochem. 2011;28:103–114. doi: 10.1159/000331719. doi:10.1159/000331719. [DOI] [PubMed] [Google Scholar]
- 27.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. doi:10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. doi:10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem. 2011;286:290–298. doi: 10.1074/jbc.M110.163477. doi:10.1074/jbc.M110.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J Biol Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- 31.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. doi:10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 32.Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J. 1993;65:2002–2012. doi: 10.1016/S0006-3495(93)81242-6. doi:0.1016/S0006-3495(93)81242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewandowski R, Procida K, Vaidyanathan R, Coombs W, Jalife J, Nielsen MS, Taffet SM, Delmar M. RXP-E: a connexin43-binding peptide that prevents action potential propagation block. Circ Res. 2008;103:519–526. doi: 10.1161/CIRCRESAHA.108.179069. doi:10.1161/CIRCRESAHA.108. 179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Sugishita K, Su Z, Ueda I, Barry WH. Activation of connexin-43 hemichannels can elevate [Ca(2+)]i and [Na(+)]i in rabbit ventricular myocytes during metabolic inhibition. J Mol Cell Cardiol. 2001;33:2145–2155. doi: 10.1006/jmcc.2001.1477. doi:10.1006/jmcc.2001.1477. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Arce FT, Ramachandran S, Lal R. Nanomechanics of hemichannel conformations: connexin flexibility underlying channel opening and closing. J Biol Chem. 2006;281:23207–23217. doi: 10.1074/jbc.M605048200. doi:10.1074/jbc.M605048200. [DOI] [PubMed] [Google Scholar]
- 36.Martin PE, Wall C, Griffith TM. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br J Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. doi:10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuyama D, Kawahara K. Proliferation of neonatal cardiomyocytes by connexin43 knockdown via synergistic inactivation of p38 MAPK and increased expression of FGF1. Basic Res Cardiol. 2009;104:631–642. doi: 10.1007/s00395-009-0029-z. doi:10.1007/s00395-009-0029-z. [DOI] [PubMed] [Google Scholar]
- 38.O’Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes. 2008;15:27–42. doi: 10.1080/15419060802014164. doi:10.1080/15419060802014164. [DOI] [PubMed] [Google Scholar]
- 39.Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. doi:10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011;31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. doi:10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oviedo-Orta E, Perreau M, Evans WH, Potolicchio I. Control of the proliferation of activated CD4+ T cells by connexins. J Leukoc Biol. 2010;88:79–86. doi: 10.1189/jlb.0909613. doi:10.1189/jlb.0909613. [DOI] [PubMed] [Google Scholar]
- 42.Palacios-Prado N, Briggs SW, Skeberdis VA, Pranevicius M, Bennett MV, Bukauskas FF. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc Natl Acad Sci USA. 2010;107:9897–9902. doi: 10.1073/pnas.1004552107. doi:10.1073/pnas.1004552107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. doi:10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. doi:10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. doi:10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, Brandner JM. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med. 2011;15:861–873. doi: 10.1111/j.1582-4934.2010.01057.x. doi:10.1111/j.1582-4934.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponsaerts R, De Vuyst E, Retamal M, D’hondt C, Vermeire D, Wang N, De Smedt H, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. doi:10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 48.Ramanan SV, Brink PR. Multichannel recordings from membranes which contain gap junctions. II. Substates and conductance shifts. Biophys J. 1993;65:1387–1395. doi: 10.1016/S0006-3495(93)81193-7. doi:10.1016/S0006-3495(93)81193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Saez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. doi:10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. doi:10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. doi:10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 53.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. doi:10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia-Dorado D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia–reperfusion injury. Cardiovasc Res. 2008;77:325–333. doi: 10.1093/cvr/cvm062. doi:10.1093/cvr/cvm062. [DOI] [PubMed] [Google Scholar]
- 55.Samoilova M, Wentlandt K, Adamchik Y, Velumian AA, Carlen PL. Connexin 43 mimetic peptides inhibit spontaneous epileptiform activity in organotypic hippocampal slice cultures. Exp Neurol. 2008;210:762–775. doi: 10.1016/j.expneurol.2008.01.005. doi:10.1016/j.expneurol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez HA, Orellana JA, Verselis VK, Saez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol. 2009;297:C665–C678. doi: 10.1152/ajpcell.00200.2009. doi:10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martinez AD, Saez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell. 2008;19:3501–3513. doi: 10.1091/mbc.E07-12-1240. doi:10.1091/mbc.E07-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schalper KA, Sanchez HA, Lee SC, Altenberg GA, Nathanson MH, Saez JC. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am J Physiol Cell Physiol. 2010;299:C1504–C1515. doi: 10.1152/ajpcell.00015.2010. doi:10.1152/ajpcell.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. doi:10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 60.Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. doi:10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 61.Shintani-Ishida K, Uemura K, Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714–H1720. doi: 10.1152/ajpheart.00022.2007. doi:10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- 62.Silver IA, Erecinska M. Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol. 1990;95:837–866. doi: 10.1085/jgp.95.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stankovicova T, Szilard M, De Scheerder I, Sipido KR. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res. 2000;45:952–960. doi: 10.1016/s0008-6363(99)00418-6. [DOI] [PubMed] [Google Scholar]
- 64.Thibault O, Porter NM, Landfield PW. Low Ba2+ and Ca2+ induce a sustained high probability of repolarization openings of L-type Ca2+ channels in hippocampal neurons: physiological implications. Proc Natl Acad Sci USA. 1993;90:11792–11796. doi: 10.1073/pnas.90.24.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torok K, Stauffer K, Evans WH. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem J. 1997;326(Pt 2):479–483. doi: 10.1042/bj3260479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verma V, Hallett MB, Leybaert L, Martin PE, Evans WH. Perturbing plasma membrane hemichannels attenuates calcium signalling in cardiac cells and HeLa cells expressing connexins. Eur J Cell Biol. 2009;88:79–90. doi: 10.1016/j.ejcb.2008.08.005. doi:10.1016/j.ejcb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008 doi: 10.1085/jgp.200810029. doi:10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang HZ, Day N, Valcic M, Hsieh K, Serels S, Brink PR, Christ GJ. Intercellular communication in cultured human vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C75–C88. doi: 10.1152/ajpcell.2001.281.1.C75. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. doi:10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 70.Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol. 1995;488(Pt 3):721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. doi:10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 72.Wright CS, van Steensel MA, Hodgins MB, Martin PE. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen. 2009;17:240–249. doi: 10.1111/j.1524-475X.2009.00471.x. doi:10.1111/j.1524-475X.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- 73.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Hartmann HA, Satin J. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol. 1999;171:195–207. doi: 10.1007/s002329900571. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y, Yang W, Lurtz MM, Ye Y, Huang Y, Lee HW, Chen Y, Louis CF, Yang JJ. Identification of the calmodulin binding domain of connexin 43. J Biol Chem. 2007;282:35005–35017. doi: 10.1074/jbc.M707728200. doi:10.1074/jbc.M707728200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.