Abstract
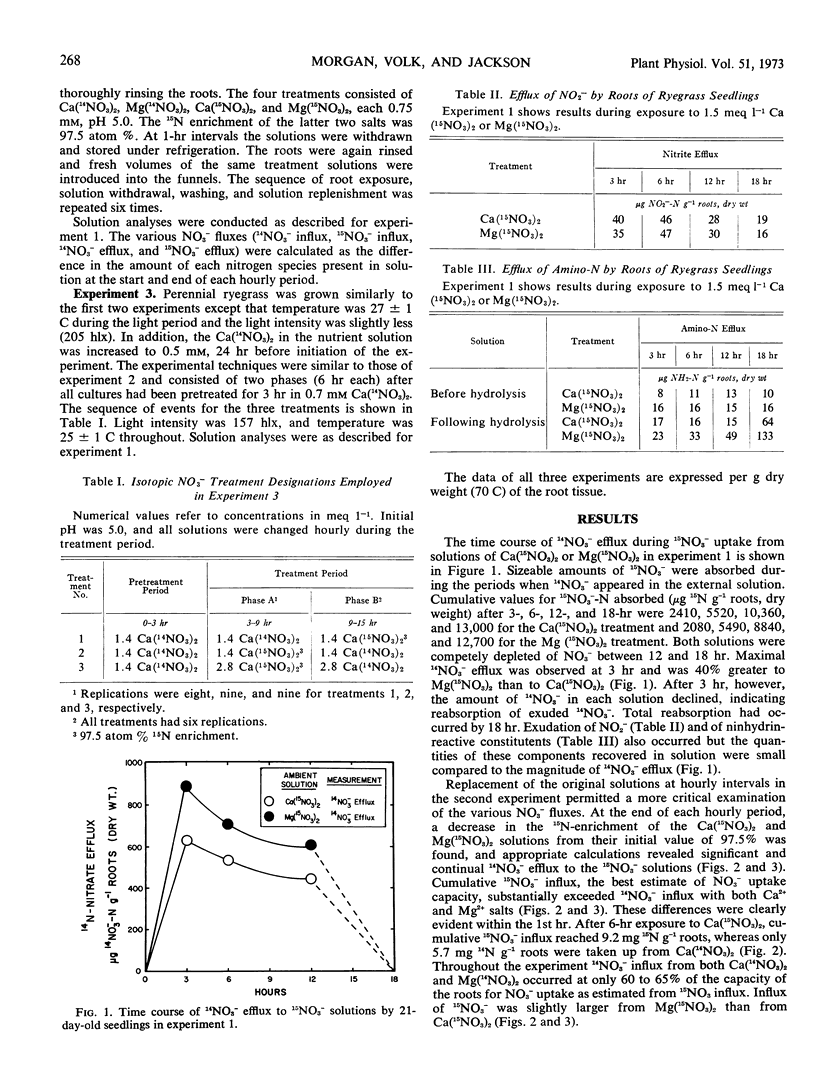
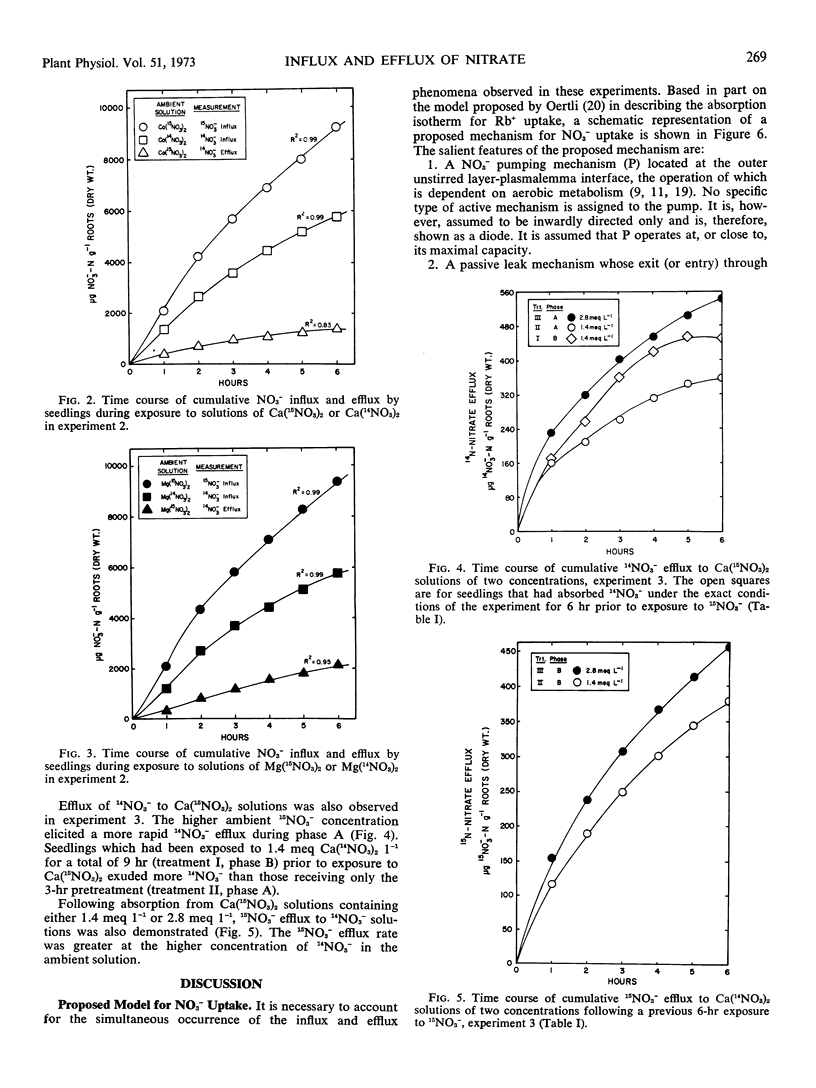
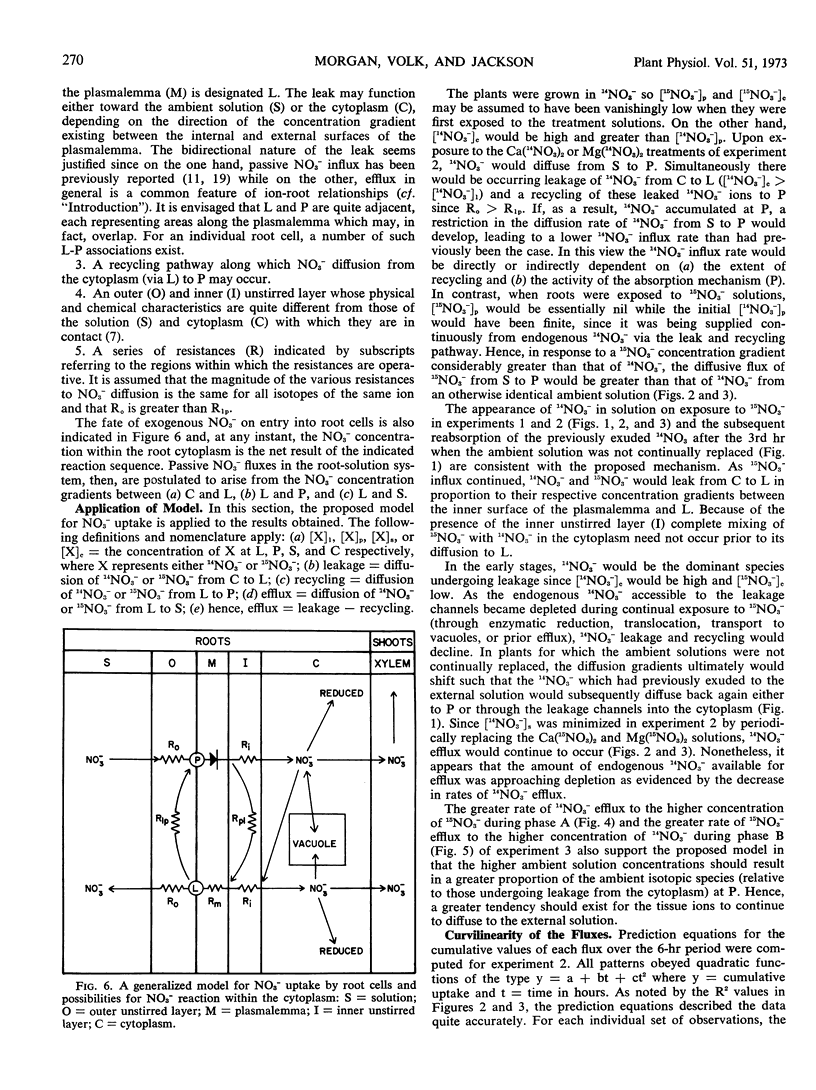
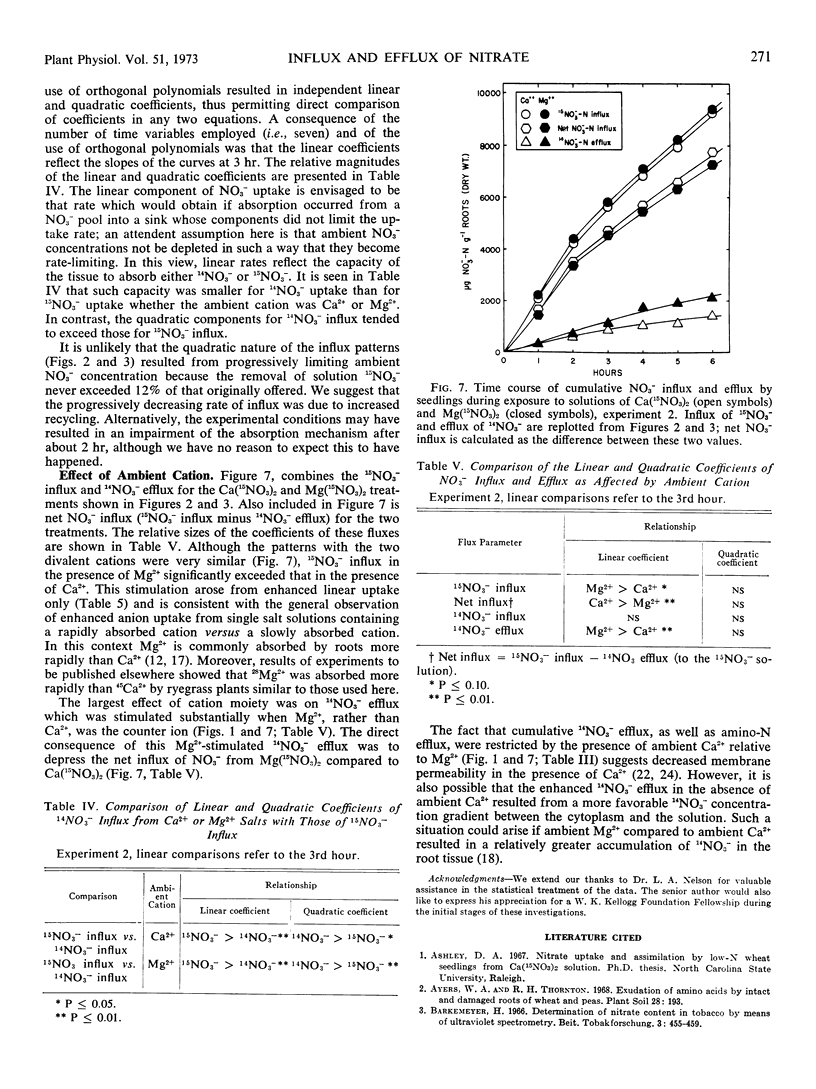
Experiments with intact plants of Lolium perenne previously grown with 14NO3− revealed significant efflux of this isotopic species when the plants were transferred to solutions of highly enriched 15NO3−. The exuded 14NO3− was subsequently reabsorbed when the ambient solutions were not replaced. When they were frequently replaced, continual efflux of the 14NO3− was observed. Influx of 15NO3− was significantly greater than influx of 14NO3− from solutions of identical NO3− concentration. Transferring plants to 14NO3− solutions after a six-hour period in 15NO3− resulted in efflux of the latter. Presence of Mg2+, rather than Ca2+, in the ambient 15NO3− solution resulted in a decidedly increased rate of 14NO3− efflux and a slight but significant increase in 15NO3− influx. Accordingly, net NO3− influx was slightly depressed. A model in accordance with these observations is presented; its essential features include a passive bidirectional pathway, an active uptake mechanism, and a pathway for recycling of endogenous NO3− within unstirred layers from the passive pathway to the active uptake site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cram W. J. Compartmentation and exchange of chloride in carrot root tissue. Biochim Biophys Acta. 1968 Nov 5;163(3):339–353. doi: 10.1016/0005-2736(68)90119-3. [DOI] [PubMed] [Google Scholar]
- Hiatt A. J., Lowe R. H. Loss of organic acids, amino acids, k, and cl from barley roots treated anaerobically and with metabolic inhibitors. Plant Physiol. 1967 Dec;42(12):1731–1736. doi: 10.1104/pp.42.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J. E., Gilbert W. A. Localization of the ca-mediated apparent ion selectivity in the cross sectional volume of soybean roots. Plant Physiol. 1967 Dec;42(12):1658–1664. doi: 10.1104/pp.42.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H., Handley R., Overstreet R. Potassium Loss and Changes in the Fine Structure of Corn Root Tips Induced by H-ion. Plant Physiol. 1966 Dec;41(10):1725–1735. doi: 10.1104/pp.41.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. P., Overstreet R., Jacobson L. Uptake of magnesium & its interaction with calcium in excised barley roots. Plant Physiol. 1961 May;36(3):290–295. doi: 10.1104/pp.36.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Jackson W. A., Volk R. J. Nitrate absorption and assimilation in ryegrass as influenced by calcium and magnesium. Plant Physiol. 1972 Oct;50(4):485–490. doi: 10.1104/pp.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J. Carrier-mediated Potassium Efflux Across the Cell Membrane of Red Beet. Plant Physiol. 1969 Apr;44(4):485–490. doi: 10.1104/pp.44.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Schmid W. E., Epstein E. Absorption of Cations by Roots. Effects of Hydrogen Ions and Essential Role of Calcium. Plant Physiol. 1964 Mar;39(2):274–278. doi: 10.1104/pp.39.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]



