Abstract
The purpose of this study was to describe the hemolytic effects of both negative pressure and an air-blood interface independently and in combination in an in-vitro static blood model. Samples of fresh ovine or human blood (5 mL) were subjected to a bubbling air interface (0–100 mL/min) or negative pressure (0–600 mmHg) separately, or in combination, for controlled periods of time, and analyzed for hemolysis. Neither negative pressure nor an air interface alone increased hemolysis. However, when air and negative pressure were combined, hemolysis increased as a function of negative pressure, the air interface, and time. Moreover, when blood samples were exposed to air prior to initiating the test, hemolysis was 4–5 times greater than samples not pre-exposed to air. When these experiments were repeated using freshly drawn human blood the same phenomena were observed, but the hemolysis was significantly higher than that observed in sheep blood. In this model, hemolysis is caused by combined air and negative pressure and is unrelated to either factor alone.
Keywords: Hemolysis, Negative Pressure, Air Interface, Cardiopulmonary Bypass, Cardiotomy Suction
Introduction
It is considered “common knowledge” that air exposure and negative pressure cause hemolysis. In cardiac surgery, it has been observed for over 50 years that hemolysis always occurs with cardiopulmonary bypass (CPB) and its extent is related in part to the air-blood interface and negative pressure that occurs with cardiotomy suction. An air-blood interface not only occurs as a result of using an open venous reservoir, but results from the mixing of air and blood from venting and cardiotomy suction, where bubbles of all sizes can be generated as a result of mixing blood and air together, then re-introducing the blood into the circulation after the air-blood mix has been defoamed. Is there a critical amount of air and negative pressure that causes hemolysis, and how do these two variables interrelate? Surprisingly, no definitive experiments have been reported that address these questions. Standard textbooks merely offer advice to keep the air flow and suction pressure to a minimum, but these recommendations are not supported by quantitative studies. If the specific effects of air and suction, both alone and in combination, on hemolysis were quantitated, the findings could be used to diminish or eliminate hemolysis associated with CPB, especially with cardiotomy suction. In order to study the relationship of negative pressure and the air-blood interface on hemolysis, a simple in-vitro model was developed. The model controls the degree of negative pressure and air-blood interface independently, allowing the hemolytic effects of each factor to be measured separately as well as in combination in static blood samples.
METHODS
In-vitro blood collection
Fresh blood was drawn slowly from the jugular vein of healthy adult sheep into a syringe containing 6 mL of citrate phosphate dextrose anticoagulant solution to a total volume of 60 mL. The collection syringe was de-aired and capped to prevent exposure to air. Fresh human blood was acquired through venipuncture from normal, healthy volunteers after written consent was obtained. The human study was approved by the University of Michigan Internal Review Board.
Experimental Model
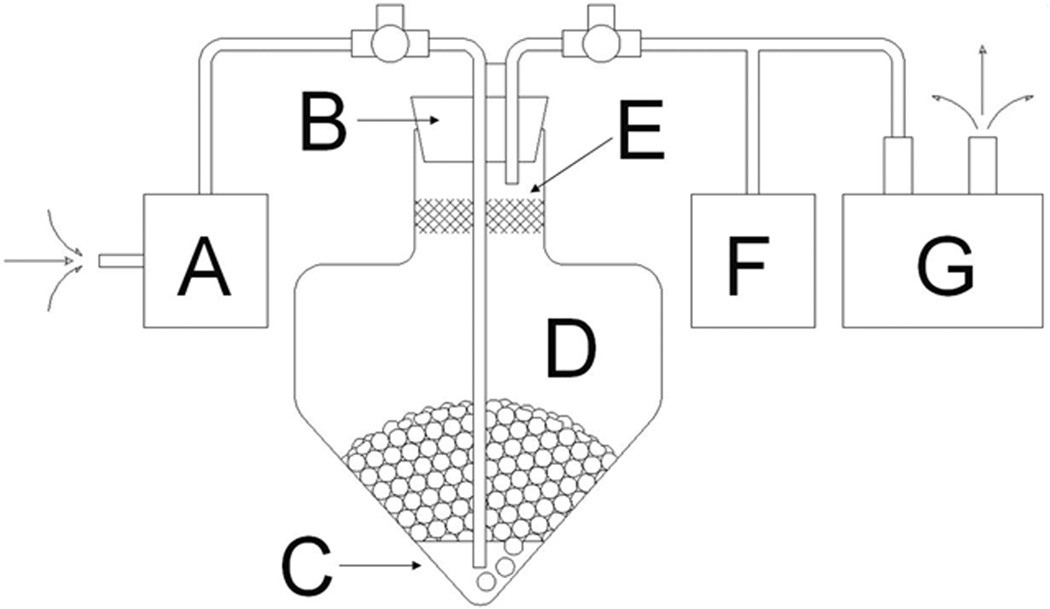
The test model used is diagramed in Figure 1. The apparatus consisted of a test chamber made from a 500 mL polyethylene centrifuge tube (Corning Inc., Acton, MA), a vacuum pump used to generate subatmospheric pressures (Model ME4, Vacuubrand Inc., Essex, CT), and a Matheson 601 and 602 series precision gas flow meter (Matheson Trigas, Montgomeryville, PA) to vary the rate of room air flow through the static blood sample and control the creation of an air-blood interface. The gas flow meter was connected to an air line with the tip positioned just above the bottom of the blood in the test chamber. The air-blood interface was generated using room air to bubble through the sample at a controlled rate. At atmospheric pressure the air flow was generated by positive pressure through the flow meter to the test chamber. For subatmospheric test conditions, the air flow was generated by negative pressure and controlled by the flowmeter. The room air was filtered to prevent any particulate matter from contaminating the sample. As air bubbles travelled through the blood sample, an air-blood interface was generated and a blood-air bubble column expanded from the base of the test chamber. The air-blood column was contained within the test chamber by a polyurethane sponge plug coated with anti-foam A and a rubber stopper. The pressure within the circuit was monitored continuously with a digital pressure indicator (Model DPI 705, Omega Engineering Inc., Stamford, CT). All experiments were conducted at room temperature.
Figure 1. Test Model.
Blood sample (C) is administered into test chamber (D) and sealed with antifoam sponge (E) and rubber stopper (B). Negative pressure is generated and regulated by vacuum pump (G) and measured by pressure monitor (F). Bubble rate through sample is driven by vacuum pump and regulated by gas flow meter (A) to generate the air-blood sample illustrated by the round circles in the test chamber.
Experimental Protocols
Series 1: The Effect of Air and Negative Pressure in Sheep Blood
A 5.0 mL sample of whole blood was administered from the collection syringe into the test chamber. The chamber was immediately sealed and the vacuum initiated, exposing the sample to a preset negative pressure and air flow rate that was maintained for a 15 minute test period. Five unique air flow rates were used (0, 2, 10, 50, 100 mL/min) to vary the air-blood interfacial surface area at four arbitrary pressures (0, −200, −400, or −600 mmHg). Once the test period was concluded, the air flow was stopped and atmospheric pressure was gently restored over 2–5 seconds by venting the system to atmosphere through the air line while orienting the chamber to avoid passing additional gas through the sample. The sample was then assayed in triplicate for hemolysis using Cripp’s method for measuring plasma free hemoglobin (PFHb).1 Each pressure and flow combination was performed 10 times with individual blood samples. Before initiating each test, an extra 2.5 mL sample was taken to measure baseline hemolysis related to blood handling. Maximal hemolysis occurred at an air flow of 50mL/min and −600 mmHg pressure. These settings were used for subsequent experiments and referred to as standard test settings.
Series 2: Hemolysis as a Function of Time
Experiments were conducted to describe the rate of hemolysis in the test chamber as a function of time. Sheep blood samples were exposed to the standard test settings (50 mL/min and −600 mmHg) for 1, 3, 5, 10, 15 or 30 minutes. The experiment was performed 10 times at each time setting, each with a separate 2.5 mL control sample used to assess baseline PFHb.
Series 3: Hemolysis and the pH Effect
The large room air-blood interface associated with this model could result in removal of the majority of CO2 and create alkalosis. To determine whether or not a severely elevated pH was contributing to the hemolysis observed in this model, a set of experiments were conducted that exposed sheep blood to the standard test settings for a 10 minute test period using either room air (pCO2 ≈ 0 mmHg) or air with 25% CO2 (pCO2 ≈ 30 mmHg at −600 mmHg), followed by analysis of PFHb, pCO2, and pH. Paired control samples were taken to measure baseline values. Each test condition was repeated 10 times.
Series 4: Effect of Static Exposure to Air Prior to Standard Test Settings
During cardiac surgery, blood may be exposed to air in the venous reservoir as well as the surgical field which may sensitize blood to damage created by negative pressure. In order to simulate this scenario, static blood samples were exposed to air in the test chamber for 1, 5, 10, 15, 30, 45, or 60 minutes, followed by exposure to the standard test settings for 15 minutes. The experiment was performed 5 times for each time period.
Series 5: The Effect of Air and Negative Pressure in Human Blood
Series 1–4 were all performed using sheep blood due to ease of collection and its availability. To understand whether human blood would yield a response similar to sheep blood in this model, a subset of Series 1 experimental conditions were conducted using fresh human blood. The air flows (10 and 50 mL/min) and negative pressures (0, −200, or −400 mmHg) were evaluated for 15 minutes separately or in combination. Each air flow and pressure combination was performed 10 times with human blood and compared and matched to results in sheep blood from Series 1.
Statistical Methods
Summary statistics were used for presentation of the raw data. The data in each group was presented as the mean and standard error of the mean for purposes of graphical presentation. Single or multivariable mixed model ANOVA for fixed and random variables was used for comparison of groups (SAS® 9.1). Students’ Standard two-tailed T-test was used for univariate analysis of continuous variables.
Results
The test apparatus was effective at containing the blood mixture while consistently maintaining the applied gas flows and negative pressures. An air-blood bubble column would form with the gas exposure, expand and then break. The blood would then be re-exposed to the stimulus as it drained back to the bottom of the chamber. Although the study did not focus on bubble size or shear forces, the bubbles appeared consistent in size (2–3mm) under the various test conditions upon initial exposure. The blood mixture did expand and the rate was larger as a function of higher gas flows. Any bubbles that contacted the defoaming media would break and drain back to the bottom of the chamber for re-exposure to the stimulus.
Series 1: The Effect of Air and Negative Pressure in Sheep Blood
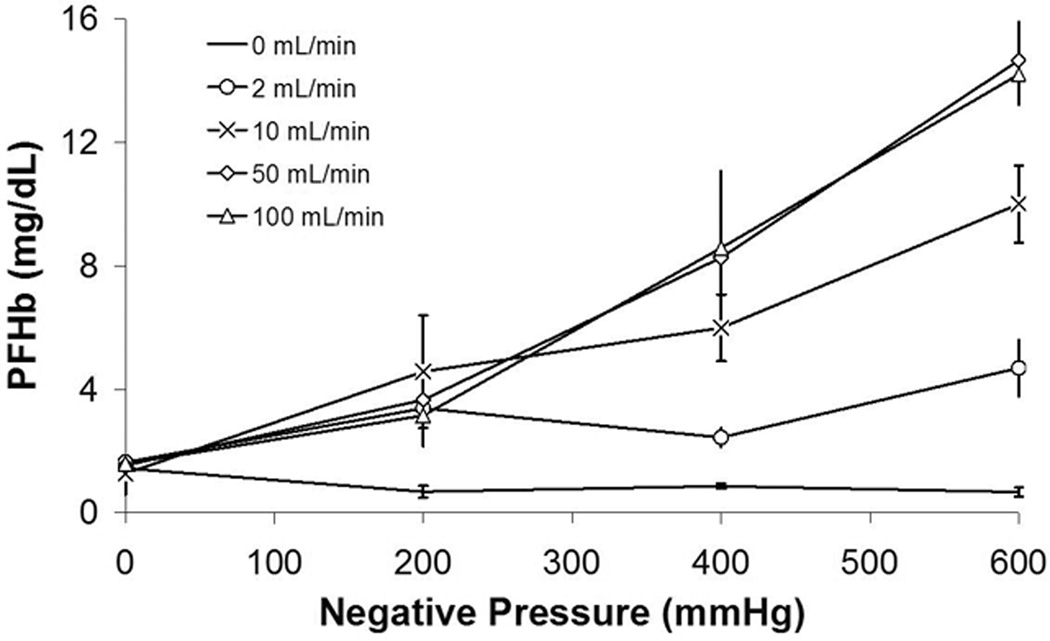
The PFHb data for Series 1 are shown in Figure 2.The average baseline PFHb of all test samples was 1.44 ± 0.18 mg/dL, which is shown at 0 mmHg in the 0 mL/min group. In both the isolated negative pressure and air-blood interface groups, the PFHb were similar to the control samples. Specifically, neither air exposure up to 100 ml/min nor negative pressure up to −600 mmHg caused hemolysis.
Figure 2. Series 1.
PFHb after exposing sheep blood to negative pressure alone, an air-interface alone, or combined air and negative pressure.
The PFHb did increase as a function of the combined air/blood interface and negative pressure. At an air flow of 2mL/min, the addition of negative pressure did not cause hemolysis (p=0.8355). There was a significant rise in PFHb at all subatmospheric pressures for air flow of 10mL/min (p < 0.0002), 50mL/min (p <0.0001) and 100mL/min (p <0.0001) groups. The amount of hemolysis was directly related to the amount of negative pressure.
Series 2: Hemolysis as a Function of Time
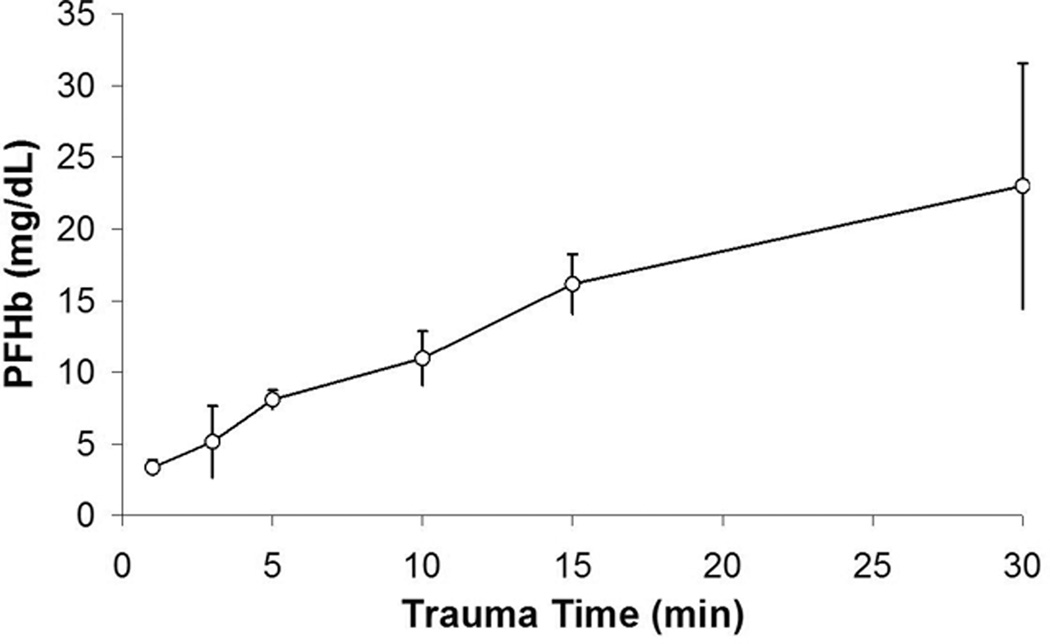
The PFHb data for Series 2 are shown in Figure 3. The average PFHb of all baseline measurements was 1.48 ± 0.51 mg/dL. The mean PFHb increased as a function of time at standard test settings. When compared to starting conditions, simple linear regression modeled changes in PFHb that approached significance at 5min (p= 0.0617) and became significant thereafter at all later time points (p <0.0001).
Figure 3. Series 2.
PFHb after exposing sheep blood to air and negative pressure for 1–30 minutes.
Series 3: Hemolysis and the pH effect
Baseline measurements were PFHb of 1.84 ± 0.40 mg/dL, pCO2 of 45.4 ± 5.4 mmHg, and pH of 7.243 ± 0.028. After 10 minutes in the test chamber, the pCO2 of samples ventilated with room air and 25% CO2 was 0.37 ± 0.03 mmHg and 45.4 ± 5.4 mmHg respectively, resulting in an average pH of 8.283 ± 0.027 and 7.242 ± 0.028, respectively. While PFHb was significantly higher than baseline in both groups (p < 0.0001), there was no significant difference in PFHb between groups (p = 0.760) when 25% CO2 was used in place of room air to prevent severe alkalosis and maintain samples within a more normal physiologic range.
Series 4: Effect of Static Exposure to Air Prior to Standard test Conditions
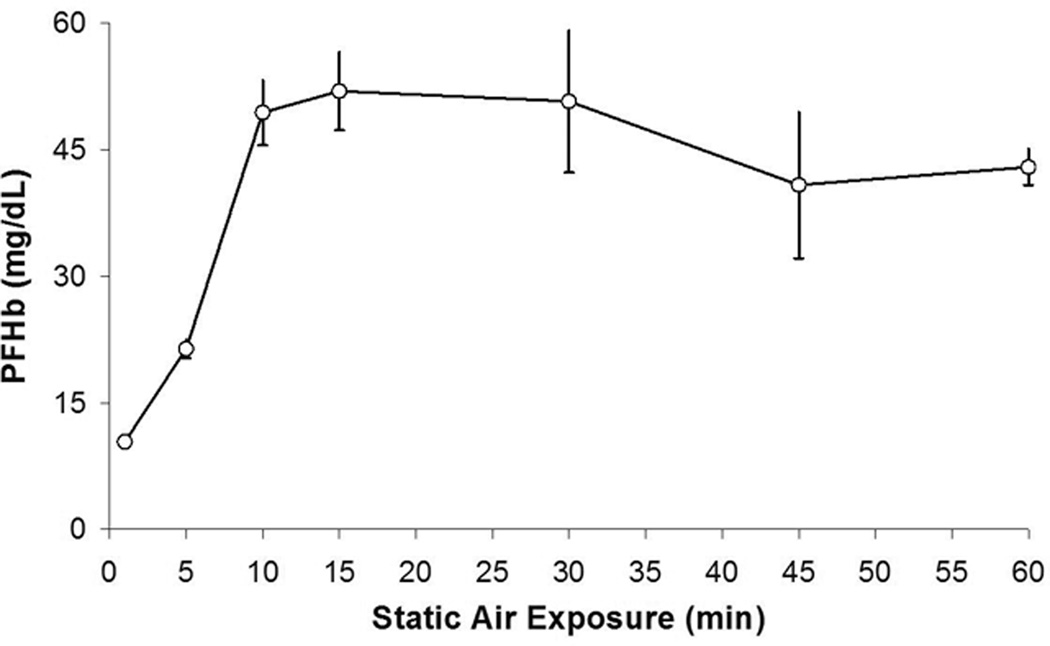
The PFHb data for Series 4 are shown in Figure 4. There was a step-wise increase in PFHb as the static air exposure time increased from 1 minute to 10 minutes. After 10 minutes, the levels of PFHb reached a plateau. Specifically, there was no significant change in PFHb at standard test settings after 1 minute of static air exposure (p=0.1311), but a significance increase in PFHb was observed at 5 minutes (p=0.0014) and beyond (p < 0.0001).
Figure 4. Series 4.
PFHb after sheep blood was allowed to sit exposed to air for 1–60 minutes, then tested with combined air and negative pressure.
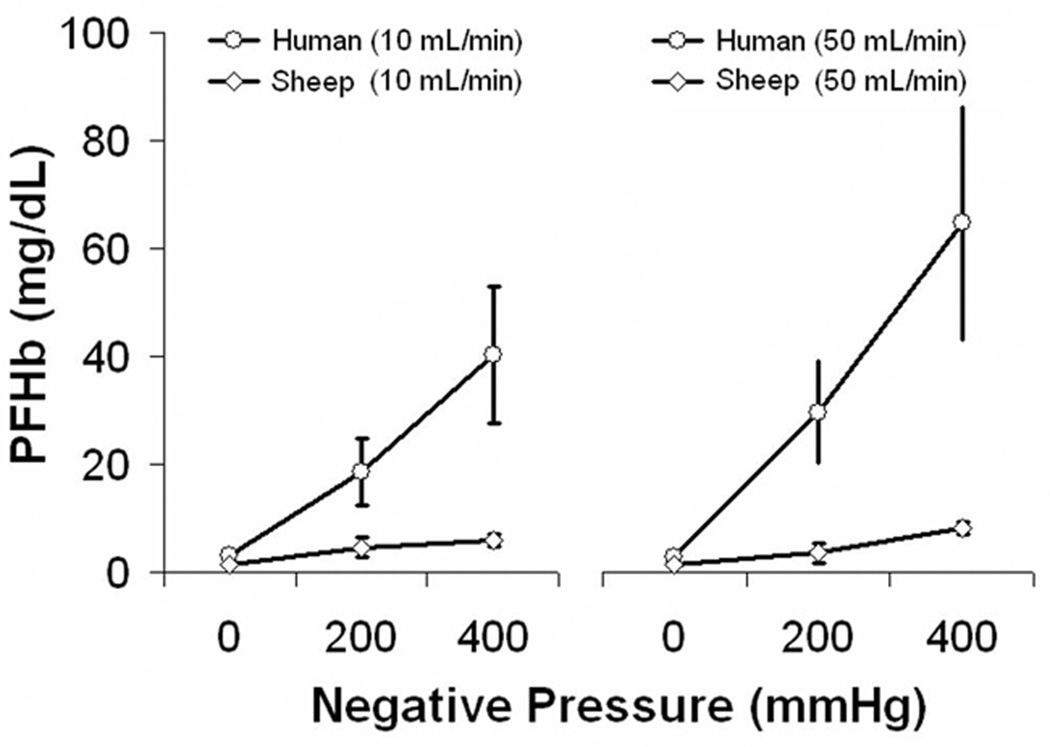
Series 5: The Effect of Air and Negative Pressure in Human Blood
The PFHb data for human blood are shown in Figure 5. The average baseline PFHb of all human samples was 4.54 ± 0.83 mg/dL which was higher compared to 1.44 ± 0.18 mg/dL in sheep blood from Series 1. As with sheep blood there was no hemolysis created by air exposure or negative pressure alone. When air flow and negative pressure were combined, the PFHb of human blood was significantly higher compared to sheep blood under identical test conditions (p < 0.0001).
Figure 5. Series 5.
PFHb after exposing human blood to air and negative pressure, or air alone. Data from series 1 in sheep blood is shown for comparison.
Discussion
There are many sources of hemolysis that relate to the operation of the extracorporeal circuit components. Roller pump over occlusion2 and improper use of pulsatile flow or a centrifugal pump3 can all contribute to hemolysis. Many circuit components (blood pumps, oxygenators) and physical factors (shear forces, air-blood interface, artificial surfaces) have also been investigated for their impact on blood damage.4 It is generally thought that hemolysis associated with the isolated high positive pressure generated by roller or centrifugal pumps is related more to shear forces than positive pressure.5 Highly oxygenated blood from the heart-lung machine may release gas filled bubbles when blood flows at a high velocity through the aortic cannula.6 This is caused by negative hydrostatic pressure generated at the tip of an aortic cannula when blood emerges into the aorta by either laminar or turbulent flow.7
The combination of air in conjunction with excessive negative pressure has been linked to hemolysis.8,9 This occurs during cardiotomy suction and the use of cardiotomy suction is the principle source of hemolysis during CPB.4,10,11 Based on these observations, it has been advocated in standard textbooks and practice guidelines to minimize or eliminate cardiotomy suction, but that recommendation is not based on definitive measurements. As early as 1958, McCaughan et al showed the suctioning of an air-blood mixture contributed to a much higher level of hemolysis when compared to intermittent suctioning of a pool of blood followed by air or continuous collection of blood without an air interface. Although neither the amount of gas relative to the blood flow nor the negative pressure was measured, the major cause of hemolysis was the mixture of air and blood with an applied vacuum.12 It was also shown that hemolysis was slightly higher when reinfusing suction blood from an air-blood mixture with a bubble oxygenator compared to the use of the bubble oxygenator alone, in-vitro.13 Pearson et al attributed hemolysis in cardiotomy suction to turbulence and high shear to the air-blood interface, but did not control or measure the critical variables.14
Several investigators have studied the effect of negative pressure on hemolysis but used models that pre-exposed the blood to air prior to any test stimuli. Thus, the red cells were pre-conditioned and the effect of the air-blood interface was not considered or controlled. Even the classic studies have this flaw. In a widely cited study, Blackshear et al demonstrated that blood sealed in a ventricle and “subjected to 1/3 atmosphere or −200 mmHg gave mixed results with an occasional very high hemolysis rate over the test period”.15 It is important to note that, although the ventricle was sealed, it did contain an air-blood interface. Bernstein and associates also reported that blood begins to hemolyze as the pressure falls more than 300 mmHg below atmospheric, but once again an air-blood interface was present.16 Yarborough and associates attempted to quantify elements of Bernstein’s model and found that blood that was exposed to air hemolyzed as a function of negative pressure over the range from zero to −700 mmHg.17 None of these investigations controlled for the effects of the air-blood interface.
Discounting those studies as uninterpretable or inaccurate, other investigators have studied the effect of air and air exposure with negative pressure as independent variables. Chambers et al showed that high positive pressure applied to in-vitro blood samples did not cause hemolysis, even if the blood was exposed to air. When negative pressure was applied, hemolysis did not occur if the blood was isolated from the air interface by a layer of mineral oil, until an extreme negative pressure (−680mmHg) was applied which caused the blood to cavitate. This was observed in a static model and in flowing blood.18–20 Gas bubbles were generated by cavitation in and around the red blood cells that caused those cells to rupture. This corroborated the observation by Weilogorski and colleagues who concluded that “cavitation may be the main cause of hemolysis”.10
The current study demonstrates that hemolysis does not occur with air exposure or negative pressure (up to −600mmHg) alone. However hemolysis does occur with simultaneous air and negative pressure. Hemolysis was not related to an alkalotic blood pH. Blood pre-exposed to a static air interface for as little as one minute had more hemolysis when exposed to standard test settings. This suggests that while an air-blood interface may not directly cause hemolysis, it may render blood more susceptible to hemolysis, when the red cells are exposed to negative pressure.
There are several limitations to this study and model. Sheep blood was tested and may not be an accurate reflection of human blood. When human blood was tested under similar conditions, hemolysis was increased compared to sheep. Shear forces, bubble sizes and collisions were not measured, but considering there was no hemolysis despite vigorous bubbling of air through the blood, these variables may not be important at normal atmospheric pressures. These studies were also conducted on static blood and the results in flowing blood may be different. Although our findings focus on the air-pressure aspects that have similarities to cardiotomy suction, there are other factors which have been implicated with using cardiotomy suction that cause blood damage that were not evaluated in this study. These include pericardial fluid contents, lipids and filtration media associated with cardiotomy suction. It has been shown that blood from the surgical field also contains lipid emboli which contribute to inflammation and post-operative morbidity.21–23 Blood contact with extravascular surfaces in the surgical field can induce hemolysis and release inflammatory mediators including thrombin, IL-6, and TNF-α.24,25 In addition, it is difficult or impossible to control or isolate these factors responsible for hemolysis.26, 27 Therefore, with all the factors that cause blood damage during cardiotomy suction, it has been suggested that the suction contents from the surgical field be either discarded28 or at least centrifuged and washed to salvage red blood cells. However, these red cells are preconditioned to hemolyze when exposed to negative pressure. The routine use of cardiotomy suction could be either curtailed or abandoned in routine operations in which blood loss is minimal. In more complicated procedures, the volume and rate of shed blood requires the use of cardiotomy suction to quickly salvage and reinfuse shed blood back into the extracorporeal circuit.
This study implies that red blood cells exposed to air in an open venous reservoir are preconditioned to hemolysis when later subjected to negative pressure in the venous drainage line or cardiotomy suction line. During cardiotomy suction the aspirated blood is mixed with a large quantity of air under different and often variable negative pressures as a function of the pump speed. When the suction catheter becomes occluded in the operative field, the pressure in the suction line can reach cavitation pressures within seconds.29, 30 Blood will cavitate at approximately −700mmHg, so it will hemolyze quickly.10,19,20
The current study identified that neither air exposure nor negative pressure alone causes hemolysis, but rather the combination of the amount of suction pressure applied to blood exposed to air. If cardiotomy suction is required due to the complexity of the operation, the amount of air aspiration and suction pressure should be minimized to limit the degree of hemolysis. Our future studies will investigate the mechanism of hemolysis caused by the air-pressure interaction while studying these relationships in flowing blood and the evaluation of cardiotomy suction systems designed to limit air aspiration and negative pressure.
CONCLUSION
In this in-vitro model, hemolysis is not caused by air exposure or negative pressure alone, but by the combination of these factors. The amount of hemolysis is directly related to the amount of negative pressure applied to the air-blood interface. This suggests that hemolysis during CPB could be minimized by eliminating any air exposure and cardiotomy suction. When cardiotomy suction is needed, hemolysis could be minimized by reducing the suction pressure.
Acknowledgments
Supported in part by NIH Grant: R01 HD015434-25A1
REFERENCES
- 1.Cripps CM. Rapid method for the estimation of plasma haemoglobin levels. J Clin Pathol. 1968;21:110–112. doi: 10.1136/jcp.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessel EA. Cardiopulmonary bypass circuitry and cannulation techniques. In: Gravlee GP, Davis RF, Utley Junior, editors. Cardiopulmonary bypass, principles and practice. Baltimore: Williams and Wilkins; 1993. p. 68. [Google Scholar]
- 3.Wheeldon DR, Bethune DW, Gill RD. Vortex pumping for routine cardiac surgery: a comparative study. Perfusion. 1990;5:135–143. doi: 10.1177/026765919000500207. [DOI] [PubMed] [Google Scholar]
- 4.Hirose T, Burman SO, O’Connor RA. Reduction of perfusion hemolysis by the use of atraumatic low-pressure suction. J Thorac Cardiovasc Surg. 1964;47:242–247. [PubMed] [Google Scholar]
- 5.High KM, Snider MT, Bashein G. Principles of oxygenator function. In: Gravlee GP, Davis RF, Utley Junior, editors. Cardiopulmonary bypass, principles and practice. Baltimore: Williams and Wilkins; 1993. p. 43. [Google Scholar]
- 6.Ross J., Jr Factors influencing the formation of bubbles in the blood. ASAIO Trans. 1959;5:140–144. [Google Scholar]
- 7.Galletti PM, Brecher GA. Connection of the vascular system with an extracorporeal circuit. In: Galletti PM, Brecher GA, editors. Heart-Lung Bypass, principles and techniques of extracorporeal circulation. New York: Grune and Stratton; 1962. p. 185. [Google Scholar]
- 8.Wright G, Sanderson JM. Cellular aggregation and trauma in cardiotomy suction systems. Thorax. 1979;34:352–358. doi: 10.1136/thx.34.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong JCF, ten Duis HJ, Sibinga CT, et al. Hematologic aspects of cardiotomy suction in cardiac operations. J Thorac Cardiovasc Surg. 1980;79:227–236. [PubMed] [Google Scholar]
- 10.Wielogorski JW, Cross DE, Nwadike EV. The effects of subatmospheric pressure on the haemolysis of blood. J Biomech. 1975;8:321–325. doi: 10.1016/0021-9290(75)90084-6. [DOI] [PubMed] [Google Scholar]
- 11.Hessel EA, Hill AG. Cardiotomy suction and venting. In: Gravlee GP, Davis RF, Kurusz M, Utley JR, editors. Cardiopulmonary bypass, principles and practice. 2nd edition. Philadelphia: Lippincott, Williams and Wilkins; 2000. p. 99. [Google Scholar]
- 12.McCaughan JS, McMichael H, Schuder JC, Kirby CK. An evaluation of various devices fore intracardiac suction. ASAIO Trans. 1958;4:130–142. [Google Scholar]
- 13.McCaughan JS, McMichael H, Schuder JC, Kirby CK. The use of a totally occlusive pump as a flowmeter with observations of hemolysis caused by occlusive and nonocclusive pumps and other pump-oxygenator components. Surgery. 1958;44:210–219. [PubMed] [Google Scholar]
- 14.Pearson DT, Watson BG, Waterhouse PS. Ultrasonic analysis of the comparative efficiency of various cardiotomy reservoirs and micropore blood filters. Thorax. 1978;33:352–358. doi: 10.1136/thx.33.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackshear PL, Jr, Dorman FD, Steinbach JH. Some mechanical effects that influence hemolysis. ASAIO Trans. 1965;11:112–117. doi: 10.1097/00002480-196504000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein EF, Castenada AR, Blackshear PL, et al. Prolonged mechanical circulatory support: Analysis of certain physical and physiological considerations. Surgery. 1965;57:103–122. [PubMed] [Google Scholar]
- 17.Yarborough KA, Mockros LF, Lewis FJ. Hydrodynamic hemolysis in extracorporeal machines. J Thorac Cardiovasc Surg. 1966;52:550–557. [PubMed] [Google Scholar]
- 18.Chambers SD, Bartlett RH, Ceccio SL. Hemolytic potential of hydrodynamic cavitation. J Biomech Eng. 2000;122:321–326. doi: 10.1115/1.1286560. [DOI] [PubMed] [Google Scholar]
- 19.Chambers SD, Laberteaux KR, Merz SI, et al. Effects of static pressure on red blood cells on removal of the air interface. ASAIO J. 1996;42:947–950. doi: 10.1097/00002480-199642060-00005. [DOI] [PubMed] [Google Scholar]
- 20.Chambers SD, Ceccio SL, Annich GA, et al. Extreme negative pressure does not cause erythrocyte damage in flowing blood. ASAIO J. 1999;45:431–435. doi: 10.1097/00002480-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Morris KN, Kinross FM, Stirling GR. Hemolysis of blood in the pericardium: The major source of plasma hemoglobin during total body perfusion. J Thorac Cardiovasc Surg. 1965;49:250–258. [PubMed] [Google Scholar]
- 22.Fabre O, Vincentelli A, Corseaux D, et al. Comparison of blood activation in the wound, active vent, and cardiopulmonary bypass circuit. Ann Thorac Surg. 2008;86:537–542. doi: 10.1016/j.athoracsur.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 23.Svenmarker S, Engstrom KG. The inflammatory response to recycled pericardial suction blood and the influence of cell-saving. Scand Cardiovasc J. 2003;37:158–164. doi: 10.1080/14017430310001465. [DOI] [PubMed] [Google Scholar]
- 24.Skrabal CA, Khosravi A, Choi YH, et al. Pericardial suction blood separation attenuates inflammatory response and hemolysis after cardiopulmonary bypass. Scand Cardiovasc J. 2006;40:219–223. doi: 10.1080/14017430600628201. [DOI] [PubMed] [Google Scholar]
- 25.Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg. 2004;78:54–59. doi: 10.1016/j.athoracsur.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Moody DM, Brown WR, Challa VR, et al. Brain microemboli associated with cardiopulmonary bypass: a histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304–1307. doi: 10.1016/0003-4975(95)00057-r. [DOI] [PubMed] [Google Scholar]
- 27.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–1655. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 28.Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. doi: 10.1016/j.jtcvs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Mulholland JW, Massey W, Shelton JC. Investigation and quantification of the blood trauma caused by the combined dynamic forces experienced during cardiopulmonary bypass. Perfusion. 2000;15:485–494. doi: 10.1177/026765910001500603. [DOI] [PubMed] [Google Scholar]
- 30.Gregoretti S. Suction-induced hemolysis at various vacuum pressures: implications for intraoperative blood salvage. Transfusion. 1996;36:57–60. doi: 10.1046/j.1537-2995.1996.36196190516.x. [DOI] [PubMed] [Google Scholar]