Abstract
Carbohydrate metabolism in humans is regulated by insulin secretion from pancreatic β-cells and glucose disposal by insulin-sensitive tissues. Insulin facilitates glucose utilization in peripheral tissues and suppresses hepatic glucose production. Any defects in insulin action predispose an individual to glucose intolerance and Type 2 diabetes mellitus. Early detection of defects in insulin action could provide opportunities to prevent or delay progression of the disease state. There are different approaches to assess insulin action. Initial methods, such as peripheral insulin concentration and simple indices, have several limitations. Subsequently, researchers developed methodologies using intravenous glucose infusion to determine glucose fluxes. However, these methodologies are limited by being non-physiological. Newer, innovative techniques that have been developed are more sophisticated and physiological. By modelling glucose kinetics using isotope dilution techniques, several robust parameters can be obtained that are physiologically relevant and sound. This brief review summarizes most of the non-physiological and physiological methodologies used to measure the variables of insulin action.
Introduction
Insulin, secreted by β-cells of the pancreatic islets, is fundamentally involved in maintaining glucose homeostasis both in post-absorptive and postprandial states. It suppresses hepatic glucose production and stimulates whole body glucose uptake in insulin-sensitive tissues, i.e. muscle, fat and, to an extent, liver. Hence, impaired insulin action signifies a state of reduced ability of insulin-sensitive tissues to respond to the biological action of insulin on carbohydrate metabolism. In addition, insulin has multiple vital influences on protein and fat metabolism as well as mitogenic effects.
Impaired insulin action is the hallmark of metabolic abnormalities that include pre-diabetes (impaired fasting glucose and/or impaired glucose tolerance), Type 2 diabetes, obesity, dyslipidaemia and the metabolic syndrome (insulin resistance syndrome). It is estimated that diabetes affects about 285 million people worldwide, and the prevalence could double by the year 2030 in parallel with the worldwide rise in obesity, with two-thirds of all diabetes cases occurring in low- to middle-income countries [1]. This exponentially emerging global public health crisis is fuelled by rapid urbanization, change in eating habits and increasingly sedentary lifestyles.
Rational therapeutic approaches need to be fashioned to contain and prevent this global pandemic. However, such interventions need to be grounded on accurate estimation of the pathophysiological abnormalities that predispose and lead to these metabolic conditions that in turn translate to increased burden of cardiovascular morbidity and mortality. As insulin resistance is the fundamental problem in these individuals, it is critical to have physiologically pertinent and accurate methods to measure this variable in free living humans.
Various methods have been developed over the years to assess insulin action. These can be further classified as ‘hot’ or ‘cold’ techniques, depending on whether glucose isotopes are utilized or not:
Cold methods
These techniques do not involve use of glucose isotopes.
Assessment of basal insulin action
Fasting plasma insulin concentrations
This has been suggested as a simple way to assess insulin action because of its ease of measurement. However, limitations include extreme variability of insulin assays (intra-day, inter-laboratories, etc.); variability in, and dependence on, insulin secretion and insulin clearance; dependence on fasting glucose levels, etc., which makes this a poor indicator of insulin action [2,3]. This measure has been applied to provide an indication of insulin action, with all the caveats, in large epidemiological studies.
Homeostasis model assessment (HOMA)
In population-based studies, the proponents of this method have used the product of fasting plasma glucose (mM) and fasting plasma insulin (mU/l) divided by 22.5 as an index of insulin action [4].This model is based on various assumptions that include: (1) the degree to which fasting plasma glucose is increased in individuals with impaired β-cell function, reflects the shape of the normal insulin secretory response to a glucose challenge. This premise is likely non-physiological except in a most general sense; (2) fasting plasma insulin concentration is directly related to the severity of insulin resistance and assumes that hepatic and peripheral insulin resistance is equivalent in a given individual. Numerous investigations in humans with and without Type 2 diabetes have suggested that this assumption is an oversimplification of physiology; (3) the relationship between insulin secretion and insulin action is independent of age and sex. Again, elegantly performed investigations have demonstrated the considerable influence of age and sex on these physiological factors [5,6,7]; (4) finally, the dynamic relationship between glucose and insulin concentrations in any given individual, reduced to a mathematical factor that is valid and relevant to people of all ages and ethnicities, is likely an oversimplification of physiological reality. The main advantage of this model is its usability in population-based studies. However, extreme care needs to be exercised in the interpretation of this index, especially as a measure of individual insulin action or in investigations that employ limited sample sizes.
Quantitative insulin sensitivity check index (QUICKI)
Katz and colleagues [8] have proposed this index derived from the logarithmic transformation of fasting glucose and insulin levels as a measure of estimating insulin sensitivity. They reported that the correlation of this measure to the gold standard euglycaemic clamp is high (r = 0.78). However, the coefficient of determination (r2) is perhaps a more robust and useful measure of correlation and represents the per cent of the data closest to the line of best fit. Hence, r2 of 0.6 denotes that only 60% of the relationships between QUICKI and clamp assessments of insulin action are reliable and true.
Even although, QUICKI and HOMA were derived in a completely different conceptual fashion, these two surrogate indexes are mathematically related; i.e. QUICKI is proportional to 1/log (HOMA) [9].
Assessment of post-glucose challenge insulin action
Frequently sampled intravenous glucose tolerance test (FSIVGTT)
A mathematical minimal model devised by Bergman, Cobelli and colleagues [10], allows measurement of insulin sensitivity index (SI) from insulin and glucose concentrations obtained from a frequently sampled intravenous glucose tolerance test. The development of this model is based on several assumptions and premises: (1) the dynamics of glucose kinetics following an intravenous bolus is described by a single compartment; (2) there is a lag effect of insulin on glucose concentration, which represents the time necessary for insulin to traverse the capillary endothelium and elevate interstitial fluid insulin levels; (3) glucose inhibition of its production and stimulation of its utilization is proportional to its plasma concentration (so-called glucose effectiveness); and (4) insulin inhibition of glucose production and stimulation of glucose utilization is proportional to insulin concentration in a compartment remote from plasma. Based on the above principles, the minimal model began to be widely used for estimations of the insulin sensitivity index. The protocol used for the frequently sampled intravenous glucose tolerance test entailed the administration of an intravenous glucose bolus of 300 mg/kg, followed 20 min later by a bolus of regular insulin (0.03 U/kg) or intravenous tolbutamide. Fitting the resultant glucose and insulin values to the model provided estimates of the sensitivity index. Saad and colleagues directly compared with each other and with the glucose clamp, estimates of insulin sensitivity obtained from the tolbutamide-boosted and insulin-boosted protocols [11]. They found that, although these indices derived from each of the protocols correlated with each other (r2 = 0.5), they were quantitatively different. Subsequently, modifications of this method that included use of glucose tracers enabled investigators to distinguish between the effects of insulin on peripheral and hepatic insulin action [12–14]. The advantages of this technique include the relative simplicity of execution and the reasonable reliability in the assessment of insulin sensitivity in moderately sized clinical research studies. However, the clear limitation of this method has been the non-physiological intravenous glucose challenge and the relevance of the sensitivity index estimate in real-life situations.
Oral glucose tolerance test (OGTT)
The standard 75-g oral glucose tolerance test with unlabelled glucose provides an estimate of overall glucose tolerance and has been used to diagnose diabetes mellitus or impaired glucose tolerance. Prior to the advent of more sophisticated and reliable techniques to measure insulin sensitivity, the glucose:insulin ratio obtained from the oral glucose tolerance test had been used as an estimate of insulin action. Although more physiological than the intravenous glucose tolerance test, this method suffers from confounders that include limited reproducibility, variability of insulin secretion and assay precision, etc., that detracts it as a reliable method to measure insulin action. However, Cobelli et al., using sophisticated and robust modelling techniques (oral minimal model; OMM), have demonstrated the efficacy of this simple, yet physiological tool to estimate both whole body insulin action (sensitivity index oral glucose tolerance test) and insulin secretion [15].
Matsuda index
From information derived from the oral glucose tolerance test, a surrogate index was proposed by Matsuda and DeFronzo [16]. The Matsuda index is a simple measure of whole-body insulin sensitivity. It is defined as 10 000/square root of (fasting glucose × fasting insulin) × (mean glucose × mean insulin during oral glucose tolerance test). Its correlation with the rate of whole-body glucose disposal during the euglycaemic insulin clamp was reported by the authors to be r = 0.73 (r2 = 0.5).
Surrogates indices based on dynamic tests
Data obtained from dynamic tests such as the oral glucose tolerance test, intravenous glucose tolerance test and meal tolerance tests were used to develop several other insulin sensitivity indices that include the Stumvoll index, Avignon index, oral glucose insulin sensitivity index, Gutt index and Belfiore index. These indices use specific sampling protocols for glucose and insulin during the oral glucose tolerance test, incorporate both hepatic and peripheral insulin sensitivities and are reasonable choices to predict the development of Type 2 diabetes in epidemiologic studies involving large sample sizes [17].
Other insulin modified tests
Insulin tolerance test
In an overnight fasted individual, the glucose response over 80 min to a fixed intravenous bolus dose of regular insulin has been proposed as a measure of insulin sensitivity. The K index of the insulin tolerance test (KITT) represents the per cent decline in plasma glucose levels per min and is determined by the ratio of 0.693/t1/2, where the denominator is the half-life of plasma glucose decay [18]. Several practical issues limit its use. This includes the likelihood of symptomatic unpleasant hypoglycaemia in those tested and consequent rise in counter-regulatory hormones that tend to offset insulin action, hence its measurement.
Insulin suppression test
This test is now rarely used. In an overnight fasted individual, 5 mg of propranolol is given as an intravenous bolus. Thereafter, infusions of glucose (6 mg kg−1 min−1), epinephrine (6 µg/min), propranolol (0.08 mg/min) and regular insulin (80 mU/min) are started and maintained for 180 min. Steady-state plasma glucose and steady-state plasma insulin are obtained in the last hour of the test. The higher the steady-state plasma glucose, the lesser is the insulin sensitivity [19]. This test had multiple safety problems, especially with use of epinephrine and propranolol on insulin action and the cardiovascular system. The method was also subsequently modified to include use of somatostatin and later on led to the development of the insulin clamp technique described below.
Glucose clamp technique
This technique, first described by DeFronzo [20], consists of the administration of a fixed insulin infusion (commonly high-dose insulin) and of a variable rate of glucose infusion in order to ‘clamp’ the plasma glucose concentration at a required concentration (euglycaemic or hyperglycaemic). This method is based on the assumption that under steady-state plasma glucose conditions, the glucose infused must equal the glucose being translocated out of the glucose space (i.e. glucose metabolized) provided that endogenous glucose production is completely suppressed. This technique is purported to be the gold standard for assessment of insulin action [21]. This method, although non-physiological, labour-intensive and operator dependent, provides assessment of insulin sensitivity by analysing the rate of glucose infusion required to maintain the targeted glucose concentration.
Hot techniques
These techniques use glucose isotopes to measure glucose fluxes and to partition hepatic and peripheral insulin sensitivity measures.
Labelled intravenous glucose tolerance test (IVGTT)
An unlabelled intravenous glucose tolerance test provides estimates of insulin sensitivity and glucose effectiveness using a mathematical model of glucose disappearance. However, this method does not allow the segregation of glucose production from its disposal. Addition of a glucose tracer to the intravenous glucose tolerance test glucose bolus resolves this limitation. The tracer allows measurement of glucose disposal and endogenous glucose production using the single compartment minimal model of hot glucose kinetics [22]. Subsequently, a two-compartment minimal model has been developed [23], which allows estimation of a plausible profile of glucose production. Thus, the glucose metabolism assessment using the labelled intravenous glucose tolerance test data is more precise and accurate than the unlabelled frequently sampled intravenous glucose tolerance test data.
Labelled oral glucose tolerance test (OGTT)
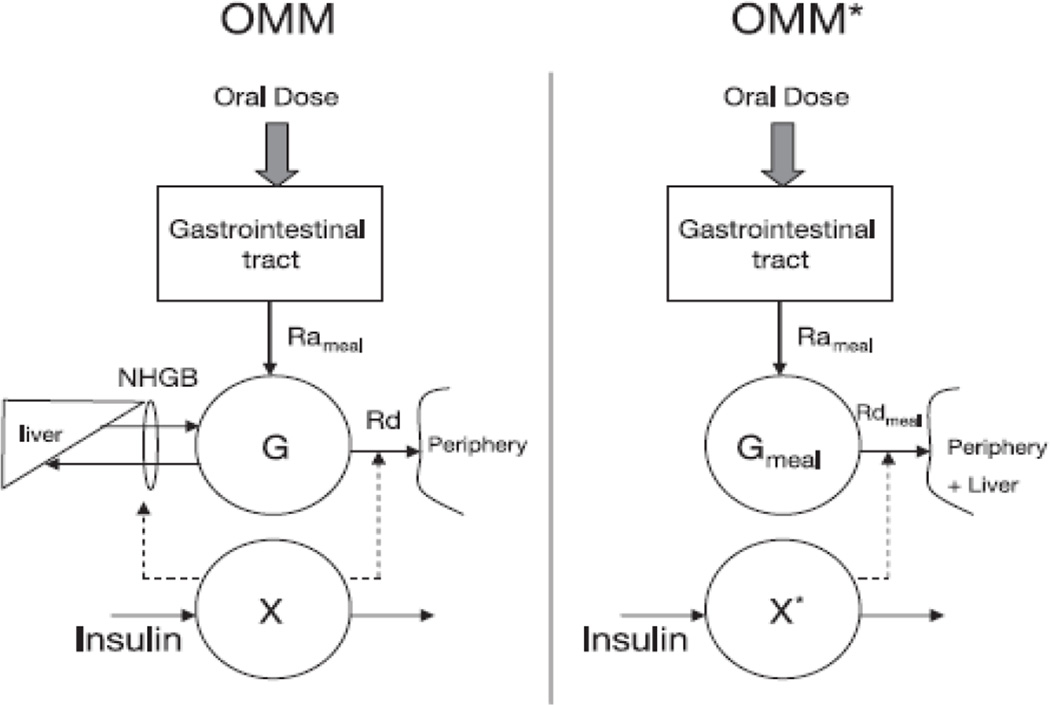
An oral glucose model is closer to normal life conditions and it can be more easily implemented for epidemiological studies. However, there are several limitations to the oral glucose tolerance test. One such limitation is that the rate of appearance (Ra) of orally administered glucose is unknown. This limitation was overcome by administering an oral glucose tracer and measuring plasma concentration of total and labelled glucose. The oral tracer permits us to derive the exogenous glucose by means of tracer:tracee ratio of the meal or glucose [24,25]. This novel approach, which was named ‘hot’ or labelled oral minimal model (OMM*), can simultaneously measure insulin action and the rate of glucose appearance after a meal or glucose ingestion (Fig 1). The ‘hot’ or labelled oral minimal model has been validated by means of a reference-labelled model in which two additional tracers were infused intravenously. This allowed reliable and virtually model-independent estimates of rate of appearance of ingested glucose (Ra meal), which was in turn used to obtain reference values for the ‘hot’ or labelled oral minimal model variables, in particular the ‘hot’ or labelled insulin sensitivity index (SI*) [24].
Figure 1.
(a) Oral minimal model (OMM). (b) ‘Hot’ or labelled oral minimal model (OMM*). G, total plasma glucose concentration; Gmeal, glucose coming from the meal; Rameal, rate of appearance of Gmeal; Rd, rate of glucose disappearance; Rdmeal, rate of disappearance of Gmeal; X and X*, insulin action on glucose disposal and production; NHGB, net hepatic glucose balance [24].
Glucose clamps with glucose tracer and somatostatin
In the present and most advanced stage, the clamp technique involves the following: in the presence of somatostatin infusion to inhibit endogenous insulin secretion, an intravenous infusion of insulin is administered at predetermined rates to ensure constant and equal portal and peripheral insulin concentrations. The level of insulin desired is dependent on the research question and can thus vary from the physiological to the pharmacological (hyperinsulinaemic) concentrations. Concomitant basal infusions of glucagon and growth hormone ensures a controlled hormonal milieu. These methods utilize isotope dilution technique in order to assess the rate of appearance of total glucose, endogenous glucose production and glucose disposal (Rd) using Steele’s equation [26]. Insulin sensitivity indices are also derived using mathematical models [27,28].
Dual tracer mixed meal test
The above-listed methods, except for the oral glucose tolerance test, assess insulin action by creating a non-physiological milieu. Therefore, methods that are able to assess insulin sensitivity and actions in a physiological milieu are required, especially after a meal. After carbohydrate ingestion as part of a mixed meal, plasma glucose levels depend on the net balance of rate of appearance of ingested glucose, endogenous glucose production and glucose disposal. Steele et al., in order to assess these glucose fluxes, developed a dual-isotope method [26]. This approach utilizes two glucose tracers: one ingested and one infused intravenously. The intravenously infused tracer measures the rate of appearance of the ingested tracer and of total glucose (i.e. labelled and unlabelled, respectively). Appearance of the ingested glucose is calculated by multiplying the rate of appearance of the ingested tracer by the specific activity (or tracer:tracee ratio if a non-radioactive tracer is used) of the meal. Initial splanchnic glucose uptake is calculated by subtracting the portion of the ingested glucose that reaches the systemic circulation from the total amount of glucose ingested. Endogenous glucose production is calculated by subtracting the rate of appearance of the ingested glucose from the total glucose appearance. Glucose disposal is calculated by subtracting the change in glucose mass from the total rate of glucose appearance [29]. As extensively discussed in a number of reports [29–32], the marked non-steady-state condition of the tracer:tracee ratio that occurs with the dual-tracer method introduces errors and renders the calculation of rate of appearance of ingested glucose, endogenous glucose production and glucose disposal dependent on both the model used in the calculation (e.g. one or two compartments) and its parameters (e.g. the volume of distribution) [33].
Triple tracer mixed meal test
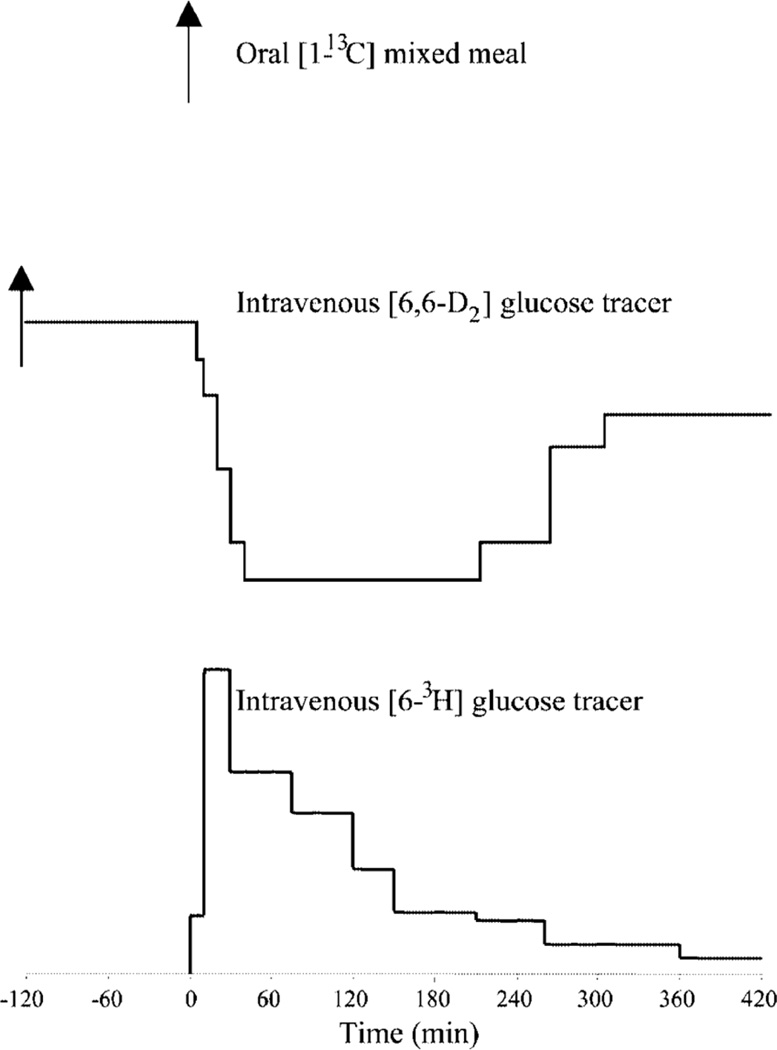
In an effort to overcome these problems, Basu et al. proposed a novel triple tracer method that could be used to minimize changes in both meal and endogenous tracer:tracee ratios. This method uses an oral glucose tracer [e.g. (1-13C) glucose], mixed within the meal and two intravenous tracers [e.g. (6-3H) glucose and (6,6-2H2) glucose]. (6-3H) glucose traces the systemic rate of appearance of the (1-13C) glucose contained in the meal, whereas (6,6-2H2) glucose traces the rate of appearance of endogenously produced glucose (Fig. 2). The ratio of the plasma concentration of (6-3H) glucose to (1-13C) glucose is used to calculate the rate of appearance (1-13C) glucose, and the ratio of the plasma concentration of (6,6-2H2) glucose to the plasma concentration of endogenously derived glucose is used to calculate endogenous glucose production. (6,6-2H2) glucose is infused to mimic the anticipated changes to endogenous glucose production, whereas (6-3H) glucose is infused at variable rates to match the systemic rate of appearance of the ingested (1-13C) glucose. Even although it is impossible to predict in every individual, as the rate of appearance of (1-13C) glucose is influenced by multiple factors, including the rate of gastric emptying, the rate of glucose absorption and the rate of hepatic glucose uptake, by minimizing non-steady-state errors, this approach is essentially model independent, thereby enabling more accurate measurement of postprandial fluxes, rate of appearance of ingested glucose, endogenous glucose production and glucose disposal [29,33].
Figure 2.
A mixed meal containing [1-13C] glucose was ingested at time 0 (a). A primed-continuous infusion of [6,6 2H2] glucose was started at time −120 min and then varied from time 0 onward to mimic the anticipated pattern of change of endogenous glucose production (b). An intravenous infusion of [6-3H] glucose was started at time 0, and the rate varied to mimic the anticipated pattern of appearance of the ingested glucose (c) [29].
Although the triple-tracer method is undoubtedly more accurate, it is experimentally more complex and expensive than the conventional dual-tracer method. Haidar et al. compared both techniques and concluded that the triple-tracer approach renders highly accurate measurements of early postprandial period [34]. Hence, the triple-tracer approach, although not absolutely necessary, is preferred to accurately assess postprandial glucose metabolism. In practical terms, if the purpose of the experiment is to assess meal appearance alone or endogenous glucose production alone, then only two tracers are required. In the first instance, if changes in the plasma tracer:tracee ratio are to be minimized, the profile of the intravenously infused tracer needs to be varied to mimic the anticipated rate of appearance of the ingested glucose. In the second instance, the profile of the intravenously infused tracer needs to be varied to mimic the anticipated pattern of change of endogenous glucose production. In contrast, if the purpose of the experiment is to simultaneously assess both rate of appearance meal and endogenous glucose production, then three tracers are preferred [29].
A limitation of the triple-tracer approach is that there may still be an error in the calculation of glucose disposal. Because the change in glucose mass is calculated by multiplying the change in plasma glucose concentration by the volume of distribution (V) of glucose (pV in Steele’s equation) and glucose disposal is calculated by subtracting the change in glucose mass from the total rate of glucose appearance, an error in the value (generally assumed) of the volume will introduce error in the calculation of glucose disposal [29].
A word on the disposition index
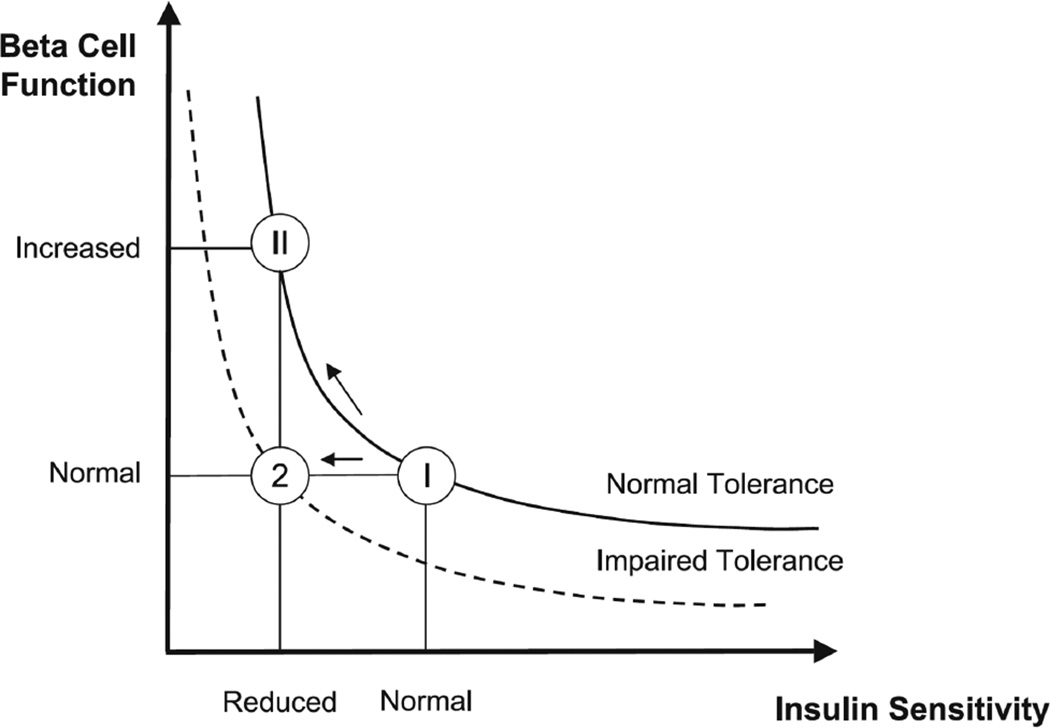
This index is an extremely useful index and represents a mathematical function defined as the product of insulin sensitivity and β-cell responsivity. It is a measure of β-cell function appropriate for the prevailing level of insulin action [35,36]. In other words, the disposition index evaluates the ability of β-cells to regulate insulin secretion appropriate to the level of insulin resistance [37]. The concept of the disposition index is evident in Fig. 3 and usually follows a hyperbolic pattern. As observed in the figure, if an individual’s β-cells respond to a decrease in insulin sensitivity by adequately increasing insulin secretion (state II), the product of β-cell function and insulin sensitivity (the disposition index) is unchanged and normal glucose tolerance is retained. In contrast, if there is not an adequate compensatory increase in β-cell function to the decreased insulin sensitivity (state 2), the individual develops glucose intolerance [38].
Figure 3.
Different hyperbolas representing the disposition index: a normal subject reacts to impaired insulin sensitivity by increasing β-cell responsivity (state II), whereas a subject with impaired tolerance does not (state 2). In state II, β-cell responsivity is increased, but the disposition index β-cell metric is normal; whereas in state 2, β-cell responsivity is normal, but the disposition index is impaired [38].
Conclusion
With the worldwide rise in the prevalence of obesity and Type 2 diabetes, it is important to understand the abnormalities in carbohydrate metabolism and insulin action.
Single indices, although simpler, are not precise methods to assess insulin action. Methods such as the intravenous glucose tolerance test and clamps are more precise, but are complex, non-physiological and require sophisticated laboratory facilities. Hence, they cannot be used for epidemiological studies. Moreover, insulin action and glucose fluxes following an oral meal cannot be completely evaluated unless the isotope dilution technique is applied with the use of glucose tracers.
In order to assess insulin action in a more physiologically pertinent manner, approaches such as the labelled oral glucose tolerance test or mixed meal studies have been proposed that are more accurate and experimentally feasible. The dual and triple tracer methods comprehensively assess basal and postprandial glucose fluxes.
Use of mathematical models of glucose metabolism, such as oral minimal model and the ‘hot’ or labelled oral minimal model, derives insulin sensitivity indices along with rate of appearance and glucose disposal.
Acknowledgments
Funding sources
This review has been supported by National Institutes of Health Grants DK 29953 to RB, CC and AB, DK 85516 to AB, RB and CC, DK 94331 to AB and CC and UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH).
Footnotes
Competing interests
None declared.
References
- 1.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenbeck CB, Chen N, Chen Y-DI, Reaven GM. Relationship between the plasma insulin response to oral glucose and insulin-stimulated glucose utilization in normal subjects. Diabetes. 1984;33:460–463. doi: 10.2337/diab.33.5.460. [DOI] [PubMed] [Google Scholar]
- 3.Olefsky JM, Farquhar JW, Reaven GM. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Diabetes. 1973;22:507–513. doi: 10.2337/diab.22.7.507. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55:2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 6.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, et al. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56:753–766. doi: 10.2337/db06-1504. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30:1972–1978. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- 8.Katz A, Nambu S, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 9.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 11.Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Chen Y-DI, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Diabetes. 1994;43:1114–1121. doi: 10.2337/diab.43.9.1114. [DOI] [PubMed] [Google Scholar]
- 12.Caumo A, Vicini P, Valerio A, Avogaro A, Cobelli C. Insulin sensitivity in NIDDM subjects: precise assessment by the labeled IVGTT minimal model. Diabetes J. 1995;44:154A. doi: 10.1152/ajpendo.1996.270.3.E532. [DOI] [PubMed] [Google Scholar]
- 13.Avogaro A, Bristow JD, Bier DM, Cobelli C, Toffolo G. Stable-label intravenous glucose tolerance test minimal model. Diabetes. 1989;38:1048–1055. doi: 10.2337/diab.38.8.1048. [DOI] [PubMed] [Google Scholar]
- 14.Cobelli C, Pacini G, Toffolo G, Sacca L. Estimation of insulin sensitivity and glucose clearance from minimal model: new insights from labeled IVGTT. Am J Physiol. 1986;250:E591–E598. doi: 10.1152/ajpendo.1986.250.5.E591. [DOI] [PubMed] [Google Scholar]
- 15.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol. Minimal model assessment of β-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 17.Ranganath M, Sihoon L, Hui Chen, Michael JQ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 18.Lundbaek K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br Med J. 1962;1:1507–1513. doi: 10.1136/bmj.1.5291.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen S-W, Reaven GM, Farquhar JW, Nakanishi RH. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–2160. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 21.Tripathy D, Wessman Y, Gullström M, Tuomi T, Groop L. Importance of obtaining independent measures of insulin secretion and insulin sensitivity during the same test: results with the Botnia clamp. Diabetes Care. 2003;26:1395–1401. doi: 10.2337/diacare.26.5.1395. [DOI] [PubMed] [Google Scholar]
- 22.Cobelli C, Pacini G, Toffolo G, Saccà L. Estimation of insulin sensitivity and glucose clearance from minimal model: new insights from labeled IVGTT. Am J Physiol. 1986;250:E591–598. doi: 10.1152/ajpendo.1986.250.5.E591. [DOI] [PubMed] [Google Scholar]
- 23.Toffolo G, Cobelli C. The hot IVGTT two-compartment minimal model: an improved version. Am J Physiol Endocrinol Metab. 2003;284:E317–E321. doi: 10.1152/ajpendo.00499.2001. [DOI] [PubMed] [Google Scholar]
- 24.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Measurement of selective effect of insulin on glucose disposal from labeled glucose oral test minimal model. Am J Physiol Endocrinol Metab. 2005;289:E909–E914. doi: 10.1152/ajpendo.00299.2004. [DOI] [PubMed] [Google Scholar]
- 25.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–E643. doi: 10.1152/ajpendo.00319.2003. [DOI] [PubMed] [Google Scholar]
- 26.Steele R, Wall J, DeBodo R, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, et al. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–283. doi: 10.2337/diabetes.49.2.272. [DOI] [PubMed] [Google Scholar]
- 28.Basu A, Caumo A, Bettini F, Gelisio A, Alzaid A, Cobelli C. Impaired basal glucose effectiveness in NIDDM. Contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes. 1997;46:421–432. doi: 10.2337/diab.46.3.421. [DOI] [PubMed] [Google Scholar]
- 29.Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E55–E69. doi: 10.1152/ajpendo.00190.2001. [DOI] [PubMed] [Google Scholar]
- 30.Allsop JR, Wolfe RR, Burke JF. The reliability of rates of glucose appearance in vivo calcuated from constant tracer infusions. Biochem J. 1978;172:407–416. doi: 10.1042/bj1720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livesey JH, Wilson PDG, Dainty JR, Brown JC, Faulks RM, Roe MA, et al. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol Endocrinol Metab. 1998;275:E717–E728. doi: 10.1152/ajpendo.1998.275.4.E717. [DOI] [PubMed] [Google Scholar]
- 32.Mari A, Wahren J, DeFronzo R, Ferrannini E. Glucose absorption and production following oral glucose: comparison of compartmental and arteriovenous-difference methods. Metabolism. 1994;43:1419–1425. doi: 10.1016/0026-0495(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 33.Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab. 2006;291:E800–E806. doi: 10.1152/ajpendo.00461.2005. [DOI] [PubMed] [Google Scholar]
- 34.Haidar A, Elleri D, Allen JM, Harris J, Kumareswaran K, Nodale M, et al. Validity of triple- and dual-tracer techniques to estimate glucose appearance. Am J Physiol Endocrinol Metab. 2012;302:E1493–E1501. doi: 10.1152/ajpendo.00581.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 37.Denti P, Toffolo GM, Cobelli C. The disposition index: from individual to population approach. Am J Physiol Endocrinol Metab. 2012;303:E576–E586. doi: 10.1152/ajpendo.00139.2011. [DOI] [PubMed] [Google Scholar]
- 38.Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, et al. Assessment of beta cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293:E1–E14. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]