Abstract
Purpose
Bladder pain is a debilitating symptom of many urologic conditions, and there is no generally effective treatment. Abnormal urothelial turnover is common to multiple disease states, but the specific components of urothelial injury and the resulting molecular signals that lead to bladder pain are unknown. We examined mouse models of bladder injury induced by uropathogenic E. coli (UPEC), protamine sulfate (PS), and bacterial lipopolysaccharide (LPS) to identify cellular and molecular correlates underlying pain sensitization in response to the stimuli.
Materials and Methods
C57BL/6 female mice were given intravesicular PS, LPS or UPEC, and the impact of each on nociception was determined by measuring the evoked visceromotor response to bladder distention at 24 hours post inoculation. Levels of pyuria and tissue inflammation were examined by urinary cytology and tissue histology. Quantitative PCR and gene expression analysis were used to identify injury profiles associated with nociception.
Results
PS treatment was significantly analgesic upon bladder distention. PS-treated bladders did not exhibit pyuria or extensive tissue damage. PS injury was associated with a global decrease in expression of inflammation-associated genes. In contrast, UPEC injury significantly increased the nociceptive response to bladder distention. LPS treatment did not affect nociception. Finally, injury-induced expression of inflammation-associated genes correlated with nociceptive responses.
Conclusion
PS treatment of the bladder is analgesic, tissue protective, and suppresses inflammatory cytokine expression normally associated with nociception. Additionally, the injury modalities that result in differential tissue response patterns provide an innovative method for identification of mediators of visceral pain.
Keywords: protamine sulfate, urothelium, nociception, visceromotor response, urinary tract infection
INTRODUCTION
The bladder is the site of several urologic conditions, and abnormal urothelial turnover is common to multiple painful disease states including interstitial cystitis/bladder pain syndrome (IC/BPS) and urinary tract infections (UTIs). IC/BPS and UTIs share the common clinical feature of increasing pelvic pain upon bladder filling (distention) leading to urinary frequency and urgency, with bladder pain being the most frequent reason for physician visits.1 IC/BPS affects 3–6% of women in the US.2 UTIs, caused primarily by uropathogenic E. coli (UPEC), affect 13 million women each year.3
Urothelial cell sloughing and defective urothelial barriers characterize patients with recurrent/chronic UTIs and IC/BPS. Disruption of the normally impermeable urothelial barrier leads to tissue injury and pain sensitization.4 Although the urothelium is recognized as a nociceptive (pain)-sensing structure that modulates and transmits noxious stimuli through mediators such as cytokines and ATP,5, 6 it is unclear how damage to each tissue layer of the bladder affects pain, and the specific molecular components generated by urothelial tissue injury that lead to bladder pain are unknown.
The urothelium exhibits a remarkable ability to renew in response to environmental insults (e.g., pathogens and cytoinjurious factors). Intriguingly, the regenerative responses to injury modalities are distinct.7 A murine model of bladder injury resulting from UPEC infection leads to urothelial barrier damage and inflammation within 24h.7 Urothelial regeneration following infection is fueled by a rapid activation of stem and early progenitor cells.7 In contrast, intravesicular treatment with protamine sulfate (PS), a highly cationic peptide that causes increased ionic permeability of the urothelium and chemically exfoliates urothelial barrier cells within 12h of instillation, does not induce inflammation.7, 8 Rather than activating urothelial stem cells, PS-induced injury appears to activate epithelial repair via transiently amplifying cells.7
We used these two well-characterized models of urothelial damage to dissect the cellular and molecular mechanisms underlying pain sensitization in response to injury and inflammation. We report a striking protection from distention-induced bladder pain upon PS injury, which is in contrast to the nociceptive response triggered by UPEC infection. Urine cytology and tissue histology show that PS treatment protects against pyuria and distention-induced tissue damage. Finally, we identified a molecular profile of globally down-regulated inflammatory cytokines with an increase in transient amplifying cell signatures, thus providing novel insights into the mechanisms of nociception and PS-induced analgesia.
MATERIALS AND METHODS
Mice
All animal experimental protocols were approved by the Washington University Institutional Animal Care and Use Committee. Female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), 9–13 weeks old were used for all experiments. Animals were housed on a 12-hour light/dark cycle and allowed ad libitum access to food and water.
Intravesicular inoculations
UTI89, a pathogenic Escherichia coli strain, was grown statically in Luria-Bertani (LB) broth for 17 h at 37 °C. 50 µl of PS (10 mg/ml in water; Sigma, St. Louis, MO) or 107 colony-forming units (CFU)/mL bacterial suspensions in saline were intravesicularly administered.7, 9 Littermate controls received 50 µl of sterile 1x saline (Fisher, Waltham, MA). Inflammatory damage was induced with lipopolysaccharide (LPS) (50 µl, 100 µg/ml LPS from E. coli strain 055:B5, Sigma) by intravesicular inoculation once a day for four days.10 Littermate controls received a four-day administration of 50 µl of 1x sterile saline. After intravesicular administration, the catheter was removed, and all animals were maintained under isoflurane anesthesia for 10 minutes before spontaneous waking.
Visceromotor Response (VMR) analysis
At 24 hours post last inoculation (hpi), animals were lightly anesthetized under isoflurane and the visceromotor response (VMR) of each animal was recorded. Visceral nociception was quantified by an electromyographic recording of the abdominal muscle response to bladder distention as described previously.11–13 For each distention, the VMR signals were subtracted from the baseline, rectified, and integrated over 20 seconds to quantify the area under the curve.
Tissue preparation and inflammation scoring
Immediately following completion of VMR analysis, mice were sacrificed, and bladder and kidney tissues were aseptically removed. Tissues were fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid), embedded in paraffin, sectioned, and stained with hemotoxylin and eosin. Photomicrographs were taken using a Hamamatsu NanoZoomer HT (Hamamatsu Corporation) and observed in a blinded fashion to score the level of tissue damage and inflammation using a modified semi-quantitative scoring system14: 0, normal; 1A, subepithelial edema without cellular infiltrate; 1B, subepithelial inflammatory infiltration (focal and multifocal); 2, edema and subepithelial inflammatory infiltration (diffuse); 3, marked subepithelial inflammatory cells with necrosis and polymorphonuclear neutrophils (PMNs) in and on bladder mucosal epithelium; 4, grade 3 criteria plus inflammatory infiltrate extends into muscle; 5, loss of surface epithelium (necrosis with full thickness inflammatory infiltration).
Urine collection, urine sediment, and bacterial titer analysis
Urines were collected prior to intravesicular treatment, at 6 hpi, 24 hpi, and twice during the VMR analysis. Urines collected prior to VMR were obtained as previously published.14 During VMR rest periods, catheterized animals had gentle pressure applied to the skin just below the occiput, and voided urine was gathered by pipet and transferred to sterile tubes. “Non-noxious pressure” (≤30 mmHg distention) and “noxious pressure” (≥40 mmHg) urines were collected per mouse and pooled. Urine sediments were obtained as previously detailed,14 fixed for 15 minutes in acetic acid/alcohol, and Papanicolaou stained following manufacturer’s instructions (PROTOCOL brand, Fisher). Photomicrographs were taken using a Nikon Eclipse E800 microscope (Japan) and analyzed using Image J (NIH). Stained urine sediments were examined and scored in a blinded manner by light microscopy on a 0–4 scale, where 0 indicates <1 and 4 indicates >20 PMNs/high-powered field as previously described.14 Pre-treatment urines exhibited no evidence of superficial cell sloughing or pyuria prior to intravesicular instillations. Bladder infection was confirmed by spotting serial dilutions of urine on LB agar plates and quantifying CFUs after overnight growth at 37 °C.
DNA Microarray inflammatory gene profiling
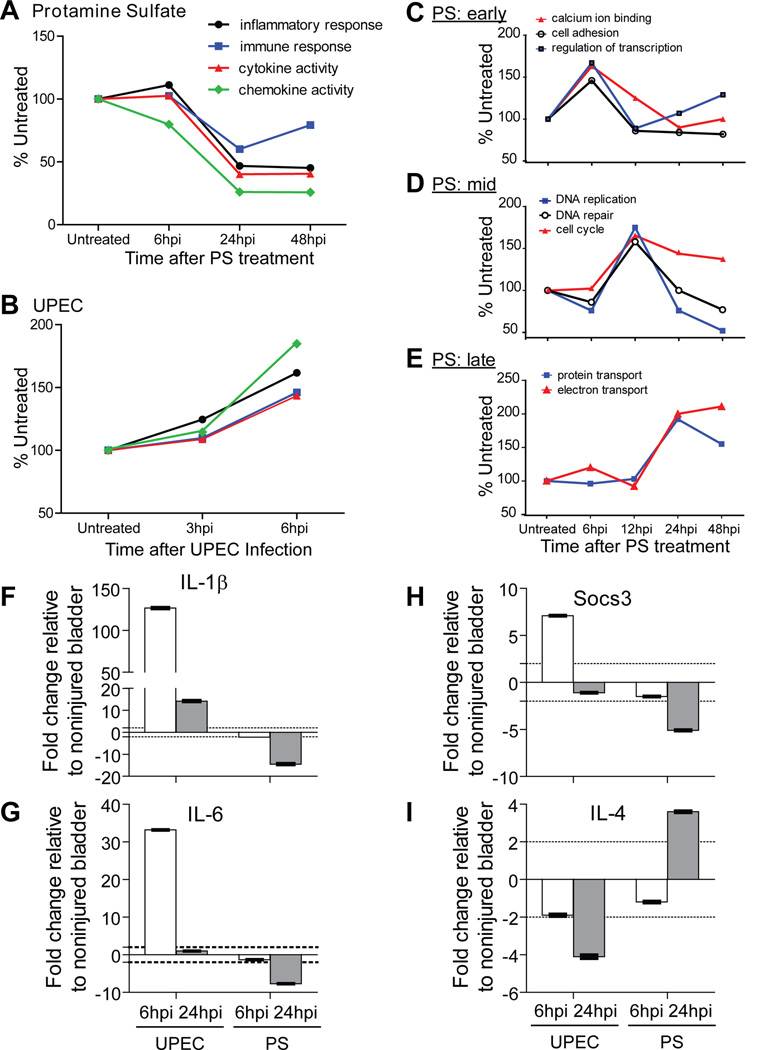
Total cellular RNA was isolated (RNeasy kit, Qiagen) from whole mouse bladders at 3 hpi, 6 hpi (for UPEC), and 3 hpi, 6 hpi, 24 hpi, and 48 hpi (for PS); (8–12 week old female mice, 10 animals/injury/time point, n=2 independent experiments). cRNAs were generated from pooled RNAs and used to interrogate U74 (UPEC) and 430 2.0 whole mouse genome GeneChips (Affymetrix) as previously published.7 Genes and Gene Ontology (GO) terms that were enriched in each treatment were determined using dChip and GOurmet software.15 To identify GO terms increased following PS treatment, enriched genes were associated with GO terms. GO terms that increased by ≥25%, and represented ≥3% of the genes expressed at one or more time points were determined. All such GO terms were plotted in Fig. 5C-E.
Figure 5. Global gene expression profiles after UPEC or PS injury reflect nociception trends.
A. UPEC infection lead to a rapid global increase in gene expression associated with an inflammatory state by 6 hpi. B. PS injury resulted in a global decrease in pro-inflammatory gene expression by 24 hpi maintained through 48 hpi. C-E. PS treatment induced increases in various GO terms that peaked at 6 h (C, early), 12 h (D, mid), or 24 h (E, late). The genes peaking at 6 h were associated with cell cycling, whereas those at 12 h had to do with transiently amplifying cell populations. F-I. qPCR analysis confirms inflammatory profiles of UPEC and PS injuries, where the pro-inflammatory, pro-nociceptive cytokines IL-1β (F) and IL-6 (G) were up-regulated by UPEC and down regulated by PS injury relative to untreated bladder. Socs3 was also up-regulated by UPEC and down-regulated by PS injury (H). Infection decreased mRNA expression levels of the anti-inflammatory cytokine, IL-4 (I) and PS injury up-regulated it by 24 hpi. Significant fold changes indicated by dotted line at ±2 fold.
Real time quantitative RT-PCR (qPCR)
Total cellular bladder RNA (TRIzol isolated following manufacturer’s protocols) was pooled (5–7 animals, 6–11 week old females) from mice at 3.5 hpi, 6 hpi and 24 hpi. cDNAs were reverse transcribed, assayed in triplicate, and gene expression changes were determined using the ΔΔCt method (normalized to 18s rRNA, and then to saline treated mice) as described.7, 16 Primer sequences (listed in Table 1) were obtained from the qPCR PrimerBank public database.17
Table 1.
Inflammatory genes differentially regulated by PS- or UPEC-mediated injuries.
| Gene | Forward Primer (5'-3') | Reverse Primer (5'-3') | Amplicon length (bp) |
Fold change in UPEC injury realtive to non- injured bladder |
Fold change in PS injury relative to non-injured bladder |
|---|---|---|---|---|---|
| IL-1β | 5' - GCAACTGTTCCTGAACTCAACT | 5' - ATCTTTTGGGGTCCGTCAACT | 89 | 126.5 (6hpi) | −14.5 (24hpi) |
| IL-6 | 5' - CCAGAAACCGCTATGAAGTTCCT | 5' - CACCAGCATCAGTCCCAAGA | 72 | 33.2 (6hpi) | −7.7 (24hpi) |
| Socs3 | 5' - CTTCCCATGCCGCTCACA | 5' - CCCAGCCCCATACCTGACTT | 110 | 7.1 (6hpi) | −5.1 (24hpi) |
| IL-4 | 5' - GGTCTCAACCCCCAGCTAGT | 5' - GCCGATGATCTCTCTCAAGTGAT | 102 | −4.1 (24hpi) | 3.6 (24hpi) |
| KC | 5' - ACCCAAACCGAAGTCATAGCC | 5' - TTCAGGGTCAAGGCAAGCC | 60 | 44.7 (6hpi) | N.S. |
| Cinc-1 | 5' - CTGGGATTCACCTCAAGAACATC | 5' - CAGGGTCAAGGCAAGCCTC | 117 | 26.4 (6hpi) | N.S. |
| Ccxl-10 | 5' - CCAAGTGCTGCCGTCATTTTC | 5' - GGCTCGCAGGGATGATTTCAA | 157 | 24.5 (6hpi) | N.S. |
| Tnf-α | 5' - CCCTCACACTCAGATCATCTTCT | 5' - GCTAC GACGTGGGCTACAG | 61 | 7.9 (6hpi) | N.S. |
| Stat5b | 5' - CACCCGCAATGATTACAGCG | 5' - CTCTTGATTCGTTTCAGGGACA | 117 | −5.6 (6hpi) | N.S. |
| IL-10 | 5' - GCTCTTACTGACTGGCATGAG | 5' - CGCAGCTCTAGGAGCATGTG | 105 | −5.3 (24hpi) | N.S. |
| IL-7 | 5' - TTCCTCCACTGATCCTTGTTCT | 5' - AGCAGCTTCCTTTGTATCATCAC | 200 | −4.4 (6hpi) | N.S. |
| SOCS7 | 5' - TCAGTCGCCTGTTTCGCAC | 5' - GTTTCCTCCCCGTATCCAGC | 153 | −3.6 (6hpi) | N.S. |
| Tgf-β 1 | 5' - CTCCCGTGGCTTCTAGTGC | 5' - GCCTTAGTTTGGACAGGATCTG | 133 | N.S. | 5.4 (24hpi) |
| IL-1α | 5' - GCACCTTACACCTACCAGAGT | 5' - AAACTTCTGCCTGACGAGCTT | 126 | 30.7 (6hpi) | 2.6 (6hpi) |
| IL-1Ra | 5' - GCTCATTGCTGGGTACTTACAA | 5' - CCAGACTTGGCACAAGACAGG | 132 | 5.7 (6hpi) | 9.4 (24hpi) |
| 18S | 5' - CGGCTACCACATCCAAGGAA | 5' - GCTGGAATTACCGCGGCT | 187 | - | - |
Statistical analysis
VMR measurements (mean ±SEM) were analyzed by 2-way ANOVA with Bonferroni post-hoc analysis. Inflammation scores were analyzed using two-tailed Mann-Whitney U-tests (where appropriate) comparing injuries to their respective controls.
RESULTS
PS is analgesic in the distention VMR assay
We hypothesized that various injuries to the bladder architecture and the respective renewal responses may have differential effects on the pain-like response to bladder distention. We have previously used the visceromotor response (VMR) to reliably measure hypersensitivity in a chemically-induced bladder inflammation model, demonstrating that the VMR is potentiated by inflammation (mustard oil, cyclophosphamide, and zymosan) and inhibited by analgesics (morphine and intravesicular lidocaine).11–13 The abdominal VMR is measured as the electromyographic signals of the external oblique muscle. Behaviorally, it corresponds to abdominal withdrawal and nocifensive guarding when an animal experiences pain while its bladder is distended. Therefore, the VMR is a surrogate measure of distension-evoked visceral nociception and represents a useful model for examining how different injuries to the bladder affect distention-induced bladder pain.
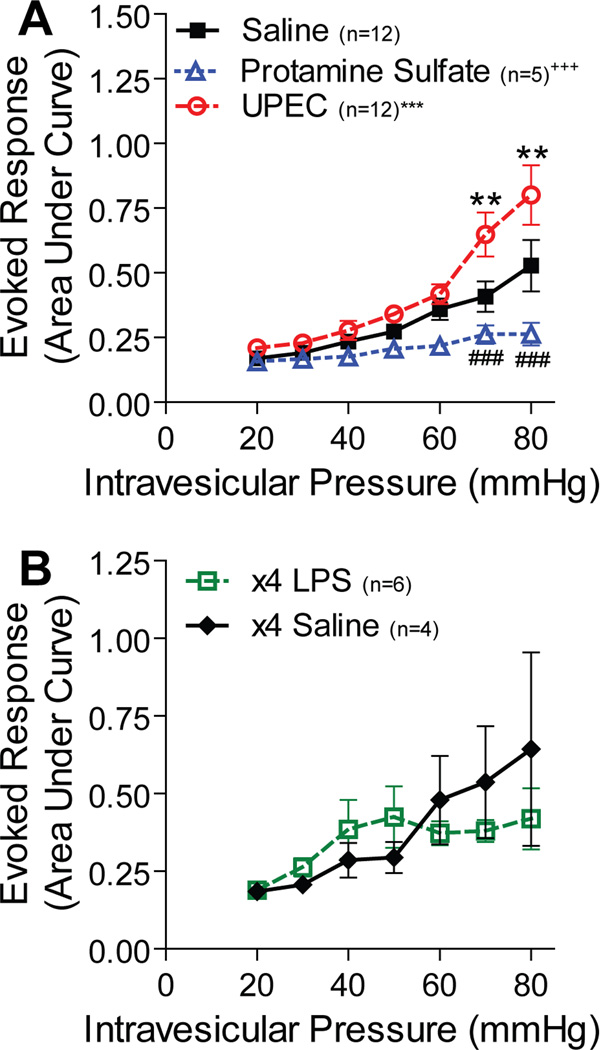
24 hours prior to VMR testing, mice were given intravesicular UPEC, PS or saline (control). As expected, UPEC infection significantly increased the evoked response to bladder distention when compared to controls (fig. 1A). In contrast, PS treatment resulted in a significantly decreased VMR when compared to controls (fig. 1A). To determine whether the differential effect was due to the absence of an inflammatory response in PS injury, we treated a separate cohort of mice with intravesicular LPS every 24 hours for 4 days, a regimen previously shown to elicit an inflammatory response.10 Multi-dose LPS injury did not significantly alter the VMR when compared to mice similarly treated with saline (fig. 1B).
Figure 1. PS injury is analgesic to bladder distention.
A. Female mice were administered saline, PS, or UPEC intravesicularly prior to VMR. PS-treated animals showed a significantly blunted VMR curve (+++p<0.0001) compared to saline controls with 70 and 80mmHg pressures (###p<0.0001) eliciting statistically significantly decreased VMRs. UPEC-infected animals had a significantly higher VMR curve (***p<0.0001) compared to saline controls, and sensitization was seen at the highest pressures of distention (70 and 80mmHg, **p<0.001). B. Female mice administered LPS or saline four times over four days had no significantly different VMR. The data is presented in arbitrary units as mean ±SEM, and p-values were determined by 2-way ANOVA with Bonferroni’s post-hoc test.
PS treatment does not induce pyuria
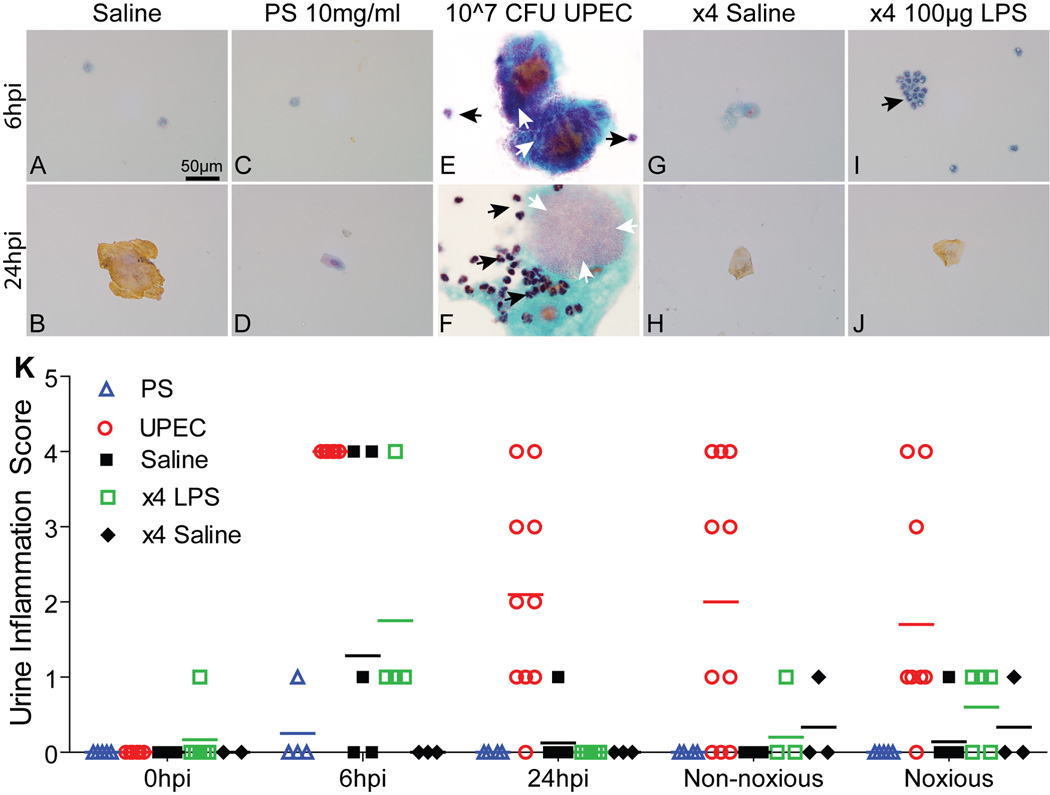
To track urothelial injury and the impact of distention on injury profiles, urine was collected for sediment analysis. Saline treatment did not induce injury as measured by cytology (fig. 2A-B, GH). UPEC injury has been shown to result in formation of intracellular bacterial communities (IBCs) by 6 hpi. These are shed into the urine along with neutrophils (PMNs) as part of the host response.14 Accordingly, we identified IBCs and PMNs in urines from UPEC-infected mice (fig. 2E-F). Analysis of urines from PS-treated mice showed minor cell sloughing prior to distention (fig. 2C-D). LPS injury resulted in PMN recruitment by 6 hpi without associated loss of superficial cells (fig. 2I-J). Urine sediments scored for inflammation at time points post injury revealed that UPEC injury lead to sustained pyuria (urinary inflammation), whereas PS treatment did not (fig. 2K). LPS injury resulted in acute pyuria, which was resolved prior to VMR (fig. 2K).
Figure 2. Pyuria is not induced by PS treatment.
Representative urine cytology images of PAP-stained urine sediments from control and injured (PS, UPEC, LPS) animals. A-B & G-H. Saline-treated controls showed minimal urothelial cell sloughing (highly kertanized vaginal cells are brown). By 6 hpi, both UPEC (E) and LPS (I) injuries displayed pyuria whereas PS (C) did not. IBCs were found throughout UPEC urines (E&F). Black arrows depict PMNs and white arrows demark IBCs. K. Urinary inflammation scores from PS- (n=6), UPEC- (n=10), saline-(n=8), x4 LPS- (n=6), and x4 saline- (n=4) treated animals. Bars represent median score values.
PS treatment induces hematuria at noxious pressures
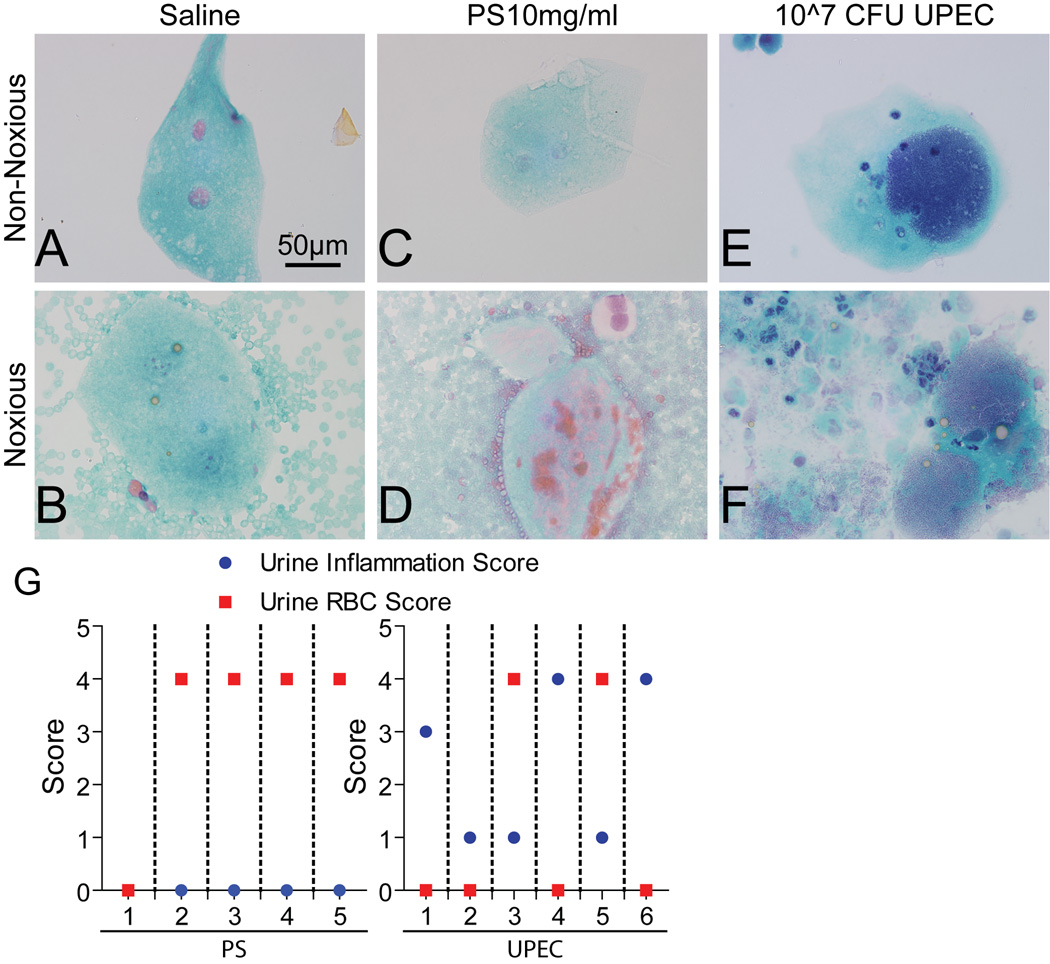
Hemorrhagic cystitis is frequently induced upon toxic chemical or infectious instillation into the bladder.18 We observed gross and microhematuria in urines and sediments from the majority of PS-injured animals (fig. 3D, 4/5, 80%), which was not evident in urines prior to pathologic bladder distention (fig. 3C). The incidence of hematuria from infectious injury was far less prevalent (fig. 3E-F, 5/10, 50%). Saline treated animals exhibited hallmarks of distentioninduced microhematuria (fig. 3A-B, 4/7, 57%). Moreover, we found that levels of hematuria and pyuria were inversely correlated in PS and UPEC injuries (fig. 3G).
Figure 3. Noxious distention induces hematuria in PS-injured animals.
Representative urine cytology images of PAP-stained urine sediments from control and injured animals. A, C, E. Distention-induced sloughing of superficial cells from all animals. At noxious distention pressures (>40 mmHg), hematuria was induced in control (B) and PS (D) treated animals. G. Individual PS- (left) or UPEC- (right) treated animals showed an inverse correlation between the levels of pyuria (urine inflammation score) and hematuria (urine RBC score). Dotted lines partition individual animals’ scores from one another.
PS treatment protects against distention-induced exacerbation of injury
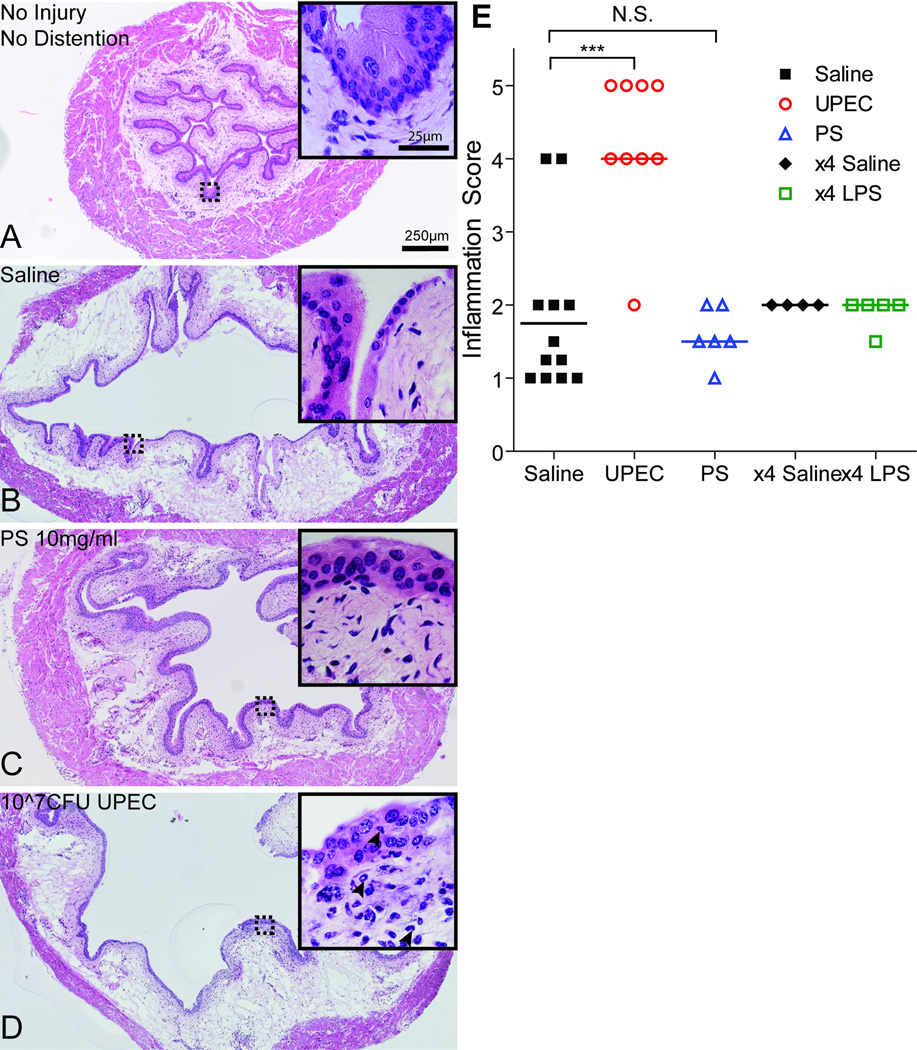
To track architectural changes as a result of both distention and injury, bladders were collected immediately following completion of the VMR recordings. We found that control animals displayed superficial cell loss and edema of the lamina propria upon distention (fig. 4A-B). Interestingly, PS injury was not associated with extensive urothelial or stromal damage (fig. 4C) and displayed a relative lack of tissue inflammation (fig. 4E). In contrast, histological characterization of UPEC-injured bladders after distention revealed stromal edema, urothelial damage, and PMN influx (fig. 4D, inset) associated with significantly higher tissue inflammation scores (fig. 4E) when compared to saline-treated controls.
Figure 4. Histologic analysis shows PS injury protects against distention-induced damage.
A. H&E of normal bladder histology without injury or distention. B. Saline treated bladder after distention showed edema and superficial cell disruption (inset). C. PS treatment prevented exacerbated superficial cell loss or edema due to distention. The urothelium and stromal compartment appeared almost normal, with no infiltration of immune cells (inset). D. Edema and major urothelial barrier disruption occurred in UPEC-infected bladders after distention. Immune cells were evident in the stromal and urothelial layers (black arrowheads, inset). Dotted boxes denote area depicted in insets. E. Tissue inflammation scores from control (saline n=12; x4 saline n=4) and injured (PS n=6; UPEC n=9; x4 LPS n=5) animals after distention. Bars represent median score values. ***p<0.0001. Two-tailed Mann-Whitney U-test comparing to saline controls. N.S. = Not Significant.
PS treatment suppresses pro-inflammatory responses
The bladder and urothelium produce distinct pro-inflammatory cytokines and chemokines in response to inflammatory (e.g., CPX, LPS, substance P) and infectious injury.10, 14, 19, 20 Pro-inflammatory cytokines (e.g., IL-6, IL-1β) can also lead to pain hypersensitivity.21, 22 To determine how PS treatment regulates inflammation, we compared gene expression in bladders from mice treated with PS to mice infected with UPEC. UPEC infection lead to increased expression of genes with ‘inflammation/immune response’ GO terms, whereas PS down-regulated the expression of those genes (fig. 5A-B).15 Nearly 10 % of all genes enriched by UPEC at 6 hpi were characterized by the GO term ‘inflammatory response’, whereas only about 0.1 % of those enriched in the PS at 48 hpi were characterized as having ‘chemokine activity’, indicating that the molecular signatures of bladder responses to PS were distinct from those induced by UPEC infection.
Microarray analysis following PS injury revealed an increase in expression of genes that function in tissue renewal mechanisms (fig. 5C-E). At early time points (6 h and before, fig. 5C), genes related to signaling and cell adhesion were elevated, and by 12 h, GO terms like ‘DNA replication’ and ‘cell cycle’, which characterize transient amplifying cells but not stem cells, were induced (fig. 5D).
We further confirmed these results by identifying multiple gene expression profiles that correlated between injury and its respective nociception response by qPCR (fig. 5F-I and Table 1). UPEC injury triggered an up-regulation of the early pro-inflammatory cytokines, IL-1β and IL- 6, and of Socs3, a negative regulator of IL-6. Furthermore, infection with UPEC resulted in down-regulation of the anti-inflammatory cytokine, IL-4, but PS treatment correlated with an upregulation of this cytokine. In contrast, PS injury resulted in down-regulation of IL-1β, IL-6, and Socs3 (fig. 5F-I). We have also identified molecular markers that were up-regulated by both UPEC and PS treatments or only regulated by UPEC or PS injury (Table 1).
DISCUSSION
Here we report that different bladder injuries result in distinct alterations in nociception and provide several lines of evidence that this is due to differential effects on inflammation. We find that although UPEC infection causes increases in distension-induced pain responses, PS treatment reduces these responses. Our urine and tissue inflammation assays and gene expression analyses all indicate that the pain modulation correlates with inflammation as a result of UPEC infection and suppression of inflammation by PS treatment.
Chronic bladder pain is a debilitating condition that is often unresponsive to conventional pain medications. Injury-induced sensitization can result in acute pain that resolves, but in some instances can transition to chronic pain that persists even in the absence of ongoing injury.21 Renewal after injury typically requires barrier restoration and resolution of inflammation.7 The pathophysiology of conditions such as IC/BPS remains poorly understood. Underlying mechanisms include occult UTI, non-infectious idiopathic inflammatory changes of the bladder, or urothelial cell sloughing and regeneration. Patients can be divided into subsets based on these underlying mechanisms. IC/BPS symptom exacerbation (“flare up”) bears remarkable resemblance to recurrent UTI, with increasing bladder pain and urinary urgency, and in some cases, may be associated with subclinical UTI.23 IC urine contains an antiproliferative factor that inhibits the regeneration of bladder epithelial cells in culture.6 In IC/BPS and UTIs, disruption of the normally impermeable urothelial barrier by continued cell sloughing may further impede renewal and lead to exacerbated tissue injury and pain sensitization.4, 6
The differential pain response to UPEC, LPS and PS stimuli could in part be explained by type of urothelial damage. Consistent with previous findings,11–13, 24 we find that UPEC infection results in visceral hyperalgesia and sensitization of nociceptive responses. UPEC infection appears to activate the nociceptive signals transmitted to the central nervous system; inhibition of a key receptor (mGluR5) in this pathway results in a blunted VMR to UPEC infection.13 One explanation for this activation could be the potent inflammatory response induced upon infection.
To better study the effect of inflammation on VMR activation, we simplified the complex host-pathogen interaction in infection by focusing on inflammation induced by the surface antigen LPS. Although adjuvant pre-treatment has been used to increase levels of inflammation,10, 20 we treated with LPS alone to mimic the naïve bladder’s response to bacterial products. In contrast to adjuvant administration, we found that LPS treatment, although displaying acute pyuria, is insufficient to induce changes in VMR. Our findings suggest that acute inflammation alone is not critical to evoke bladder pain. Future work should focus on identifying how urothelial status affects the integration of signals important for nociception sensitization.
Although the presence of a foreign body within the bladder and other PS injury models have been shown to result in cystitis-like inflammation,25, 26 our PS injury model uses short catheterization and incubation periods and does not result in inflammatory cystitis.7 We found that PS injury was analgesic upon distention and had improved bladder histology when compared to distended infectious injury and controls. This is the first report of urothelial injury eliciting analgesia, but the mechanism by which PS treatment is protective is unknown.
Our microarray analysis revealed contrasting expression profiles in UPEC- and PStreated bladders, consistent with the differential effects of UPEC and PS injury on VMR. UPEC infection resulted in increased pro-inflammatory gene expression changes within 3–6 hours, consistent with molecular changes preceding urothelial stem cell (USC) activation.7, 16 In contrast, we detected decreased expression of inflammatory-related genes 24 hours after PS injury. We confirmed the suppressed immune response after PS injury by qPCR, focusing on multiple pro-inflammatory and pro-nociceptive cytokines. Expression of IL-1β, a pro-inflammatory, pro-nociceptive cytokine that regulates the expression of other inflammatory cytokines, and is produced by the bladder in response to inflammatory injury,19, 22 was decreased in PS injury, but increased following UPEC infection. Expression of the pro-inflammatory cytokine IL-6 was similarly affected.14, 21, 27 Conversely, we observed decreased expression of IL-4, a prototypical anti-inflammatory cytokine, in UPEC-infected mice but increased expression following PS injury.
PS injury revealed an increase in renewal mechanisms initiating from transient amplifying regulation rather than USC activation.7 It is possible that injuries, such as UPEC infection, that activate stem cell-mediated renewal might amplify a feed-forward loop that leads to enhanced nociception, whereas injuries like PS that do not activate stem cell-mediated renewal do not contribute to increased nociception. Therefore, this combination of suppressed immune response and transient amplifying renewal could be contributing to the mode of PS injury-induced analgesic action.
CONCLUSIONS
We report that various injuries to the bladder lead to differential effects on distention-induced nociception. PS injury leads to a decrease in global inflammatory profile expression, is analgesic in a model of bladder distention, and decreases the level of injury from distention as compared to controls. Our PS injury model provides a new avenue to understand analgesia and how pain is sensed in the bladder.
ACKNOWLEDGMENTS
We thank Sherri K. Vogt for technical assistance.
GRANTS: This work was supported in part by NIH grants DK089969 (LWC), T32-A1007172 (KMS), MAPP Network grant DK82315 (RWG, HHL), NS48602 (RWG), DK080643 (IUM), NIHDK079798, NIH2P30 DK052574-12 (JCM); Multiplex Gene Analysis Core of the Siteman Cancer Center (supported in part by National Cancer Institute Grant P30 CA91842); the Alafi Neuroimaging Laboratory, the Hope Center for Neurological Disorders, and NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University.
Abbreviations and Acronyms
- UTI
urinary tract infection
- UPEC
uropathogenic E. coli
- PS
protamine sulfate
- IC/BPS
interstitial cystitis/bladder pain syndrome
- VMR
visceromotor response
- IBC
intracellular bacterial community
- PMN
polymorphonuclear leukocyte
- LPS
lipopolysaccharide
- hpi
hours post inoculation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no competing financial or non-financial interests to disclose.
AUTHORS’ CONTRIBUTIONS
K.M.S and L.W.C. contributed equally to this manuscript. K.M.S. and L.W.C. performed experiments and analyzed data. H.H.L. provided expertise on VMR analysis. J.C.M. conducted the bioinformatic analysis. K.M.S., L.W.C., R.W.G., and I.U.M. conceived the study. K.M.S., L.W.C., H.H.L., J.C.M., R.W.G., and I.U.M. contributed to writing the manuscript. All authors read and agreed on the final version of the manuscript.
REFERENCES
- 1.Warren JW, Brown J, Tracy JK, et al. Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology. 2008;71:444. doi: 10.1016/j.urology.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 5.Birder L, Ruggieri M, Takeda M, et al. How does the urothelium affect bladder function in health and disease?: ICI-RS 2011. Neurourol Urodyn. 2012;31:293. doi: 10.1002/nau.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008;20:2174. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Mysorekar IU, Isaacson-Schmid M, Walker JN, et al. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzan CJ, Berg J, Lewis SA. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J Membr Biol. 1993;133:227. doi: 10.1007/BF00232022. [DOI] [PubMed] [Google Scholar]
- 9.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saban MR, Nguyen NB, Hammond TG, et al. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am J Pathol. 2002;160:2095. doi: 10.1016/S0002-9440(10)61159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ness TJ, Elhefni H. Reliable visceromotor responses are evoked by noxious bladder distention in mice. J Urol. 2004;171:1704. doi: 10.1097/01.ju.0000116430.67100.8f. [DOI] [PubMed] [Google Scholar]
- 12.Lai H, Qiu CS, Crock LW, et al. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011 doi: 10.1016/j.pain.2011.05.017. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crock LW, Stemler KM, Song DG, et al. Metabotropic glutamate receptor 5 (mGluR5) regulates bladder nociception. Molecular Pain. 2012;8 doi: 10.1186/1744-8069-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty JM, Carmichael LK, Mills JC. GOurmet: a tool for quantitative comparison and visualization of gene expression profiles based on gene ontology (GO) distributions. BMC Bioinformatics. 2006;7:151. doi: 10.1186/1471-2105-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mysorekar IU, Mulvey MA, Hultgren SJ, et al. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002;277:7412. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 17.Spandidos A, Wang X, Wang H, et al. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traxer O, Desgrandchamps F, Sebe P, et al. Hemorrhagic cystitis: etiology and treatment. Prog Urol. 2001;11:591. [PubMed] [Google Scholar]
- 19.Wood MW, Breitschwerdt EB, Gookin JL. Autocrine effects of interleukin-6 mediate acute-phase proinflammatory and tissue-reparative transcriptional responses of canine bladder mucosa. Infect Immun. 2011;79:708. doi: 10.1128/IAI.01102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saban MR, Hellmich H, Nguyen NB, et al. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics. 2001;5:147. doi: 10.1152/physiolgenomics.2001.5.3.147. [DOI] [PubMed] [Google Scholar]
- 21.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binshtok AM, Wang H, Zimmermann K, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porru D, Politano R, Gerardini M, et al. Different clinical presentation of interstitial cystitis syndrome. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:198. doi: 10.1007/s00192-004-1129-9. [DOI] [PubMed] [Google Scholar]
- 24.Rudick CN, Billips BK, Pavlov VI, et al. Host-pathogen interactions mediating pain of urinary tract infection. J Infect Dis. 2010;201:1240. doi: 10.1086/651275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guiton PS, Hung CS, Hancock LE, et al. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect Immun. 78:4166. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soler R, Bruschini H, Freire MP, et al. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J Urol. 2008;180:1527. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Blalock EM, Korrect GS, Stromberg AJ, et al. Gene expression analysis of urine sediment: evaluation for potential noninvasive markers of interstitial cystitis/bladder pain syndrome. J Urol. 2012;187:725. doi: 10.1016/j.juro.2011.09.142. [DOI] [PubMed] [Google Scholar]