Abstract
Enhancing the effects of endogenously-released cannabinoid ligands in the brain might provide therapeutic effects more safely and effectively than administering drugs that act directly at the cannabinoid receptor. Inhibitors of fatty acid amide hydrolase (FAAH) prevent the breakdown of endogenous ligands for cannabinoid receptors and peroxisome proliferator-activated receptors (PPAR), prolonging and enhancing the effects of these ligands when they are naturally released. This review considers recent research on the effects of FAAH inhibitors and PPAR activators in animal models of addiction and cognition (specifically learning and memory). These studies show that FAAH inhibitors can produce potentially therapeutic effects, some through cannabinoid receptors and some through PPAR. These effects include enhancing certain forms of learning, counteracting the rewarding effects of nicotine and alcohol, relieving symptoms of withdrawal from cannabis and other drugs, and protecting against relapse-like reinstatement of drug self-administration. Since FAAH inhibition might have a wide range of therapeutic actions but might also share some of the adverse effects of cannabis, it is noteworthy that at least one FAAH-inhibiting drug (URB597) has been found to have potentially beneficial effects but no indication of liability for abuse or dependence. Although these areas of research are new, the preliminary evidence indicates that they might lead to improved therapeutic interventions and a better understanding of the brain mechanisms underlying addiction and memory.
Keywords: Endocannabinoid system, CB1 receptors, Nuclear receptors, Behavior, Drug abuse, Animal models
1. Introduction
Cannabis has been used as a drug for millennia, but only recently have we begun to understand the brain systems that cannabis affects and to develop safer, more effective ways to manipulate these systems for therapeutic purposes. Like THC, which is the main psychoactive constituent of cannabis, endogenous cannabinoid ligands activate inhibitory presynaptic CB1 receptors that are richly expressed in the hippocampus, basal ganglia, cerebellum and cerebral cortex (see reviews by Freund et al., 2003; Piomelli, 2003; Lovinger, 2008; Pertwee et al., 2010). These endocannabinoids are synthesized and released on demand by the postsynaptic cell and limit the activity of glutamate, GABA, glycine, acetylcholine, norepinephrine and serotonin neurons (Schlicker & Kathmann, 2001; Szabo & Schlicker, 2005). Thus, an important role of CB1 receptors seems to be providing a damping function when these transmitter systems become overactive, such as during stress (Katona & Freund, 2008; Hill et al., 2010).
Endocannabinoids such as anandamide and arachidonoylglycerol (2-AG) are believed to have a number of beneficial effects, such as modulating anxiety, depression and pain (see reviews by Schlosburg et al., 2009b; Zanettini et al., 2011). However, simply administering these compounds exogenously as therapeutic agents is problematic because they are rapidly degraded in vivo. One way to deal with this problem is to develop longer-acting forms of these ligands, such as methanandamide (Abadji et al., 1994). An alternative and potentially more desirable way is to prolong the effects of endogenously-released cannabinoids, using a general strategy that has been successful with endogenously-released serotonin (Taylor et al., 2011) and dopamine (Godfrey, 2009). This can be achieved by inhibiting the enzymes responsible for the degradation of endocannabinoids. An advantage of this approach is that it might be specific with regard to site of action, enhancing endocannabinoid actions locally when and where they are endogenously released. In contrast, administration of directly-acting ligands affects receptors globally, relatively independent of endogenous activity. A potential disadvantage of inhibiting degradation is that it is less specific chemically; the degradation mechanisms are shared by families of ligands, and the ligands within a family do not all target the same type of receptor. [Allosteric modulators that enhance or diminish the actions of endogenously released cannabinoids also have the potential to provide therapeutic effects without the drawbacks of direct agonist drugs (Price et al., 2005); although this would presumably be more specific chemically than FAAH inhibition, the behavioral effects of these compounds have yet to be characterized.]
The main degradation mechanism for anandamide is enzymatic breakdown by fatty acid amide hydrolase (FAAH). A variant, FAAH-2, has been found to occur in humans and some other vertebrates, but not in rats or mice (Wei et al., 2006). There are a number of highly-selective FAAH inhibitors available, including URB597, OL-135, PF-3845 and AM3506, although the preponderance of studies have involved URB597 (Lichtman et al., 2004; Piomelli et al., 2006; Vandevoorde, 2008; Ahn et al., 2009; Godlewski et al., 2010; Petrosino & Di Marzo, 2010). The main degrading enzyme for the endocannabinoid 2-AG is monoacylglycerol lipase (MGL), and there are inhibitors available for this enzyme (e.g., JZL184), and also dual inhibitors that are equipotent for FAAH and MGL (e.g., AM6701). Research into the effects of endocannabinoids has also been facilitated by the development of knockout mice that lack either FAAH (Cravatt et al., 2001) or MGL (Pan et al., 2011). An additional means by which anandamide is deactivated is by a transport mechanism that removes it from the synapse; inhibitors of this recently discovered FAAH-like anandamide transporter (FLAT), such as AM404, VDM11, UCM707, OMDM-1 and ARN272, selectively inhibit anandamide deactivation (Fu et al., 2012). This finding raises the possibility that lipid transporters might be found in the future that selectively deactivate other FAAH substrates such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) or MGL substrates such as 2-AG.
Inhibition of FAAH increases endogenous levels of the fatty acid ethanolamides (FAEs) anandamide, oleamide, PEA and OEA. Among these, only anandamide and oleamide act at cannabinoid CB1 receptors, but all are considered members of the extended endocannabinoid family (O’Sullivan, 2007; Pistis & Melis, 2010). Anandamide also acts at peroxisome proliferator-activated receptors (PPARs), specifically the PPAR-α and PPAR-γ subtypes (Bouaboula et al., 2005; Sun et al., 2006; Sun et al., 2007). PEA and OEA act at PPAR-α, and OEA also acts at PPAR-β/δ (Fu et al., 2003; Lo Verme et al., 2005a). FAAH and FAAH-2 degrade the endogenous sleep-inducing factor oleamide (Wei et al., 2006), which is a CB1 agonist (Leggett et al., 2004) and weak PPAR-γ agonist (Dionisi et al., 2012) and may also act by other mechanisms (Fedorova et al., 2001). N-acyltaurines, which are agonists of transient receptor potential calcium channels (TRP), are also FAAH substrates (Saghatelian et al., 2006).
1.1. Overview
Since FAAH inhibition affects PPAR ligands, it is natural to consider the effects of FAAH inhibition and PPAR activation together. Since FAAH inhibition increases levels of the endocannabinoid anandamide, it is also natural to relate the findings obtained with FAAH manipulations to those obtained by administering cannabinoid-receptor agonists and antagonists. This review will focus on research into the effects of these manipulations in two relatively new but promising areas, cognition (specifically learning and memory) and addiction. PPARs have received much attention for their roles in lipid metabolism, energy balance, feeding, inflammation, and neuroprotection. Some of their effects on cognition and addiction may actually be extensions of these other roles. In some cases, activation of PPAR and CB1 systems may have opposing effects. Since CB1 receptors mediate the abuse-related effects of cannabis and possibly other drugs, it is important to evaluate the possibility that FAAH inhibitors and other potentially therapeutic drugs that affect the endocannabinoid system might themselves have abuse and addiction liability. By the same token, the ability of the endocannabinoid system to modulate the effects of drugs and drug-associated environmental cues makes this system a potential target for treating addiction.
2. Interactions between the endocannabinoid and peroxisome proliferator-activated receptor systems
Cannabinoid CB1 and CB2 receptors and all PPAR isoforms (α, β/δ and γ) are widely expressed in the central and peripheral nervous systems (Mackie, 2005; Pistis & Melis, 2010). The endocannabinoid system consists of cannabinoid receptors, the genes that encode them, their endogenous ligands (endocannabinoids like anandamide and 2-AG), and mechanisms for the synthesis, uptake and metabolism of these ligands (Pertwee, 2006). CB1 receptors are G protein-coupled receptors that are localized mainly at the terminals of central and peripheral neurons, where they typically mediate inhibition of ongoing neurotransmitter release (Szabo & Schlicker, 2005). CB1 receptors are implicated in the pathophysiology of many psychiatric and neurological disorders, including schizophrenia, depression, Alzheimer’s disease, Parkinson’s disease, eating disorders, addiction, chronic pain, and anxiety (Porter & Felder, 2001; Croxford, 2003; Iversen, 2003). CB2 receptors are located predominantly in immune cells and modulate immune processes like cell migration and cytokine release in the periphery and the brain (Pertwee, 2005). However, there is mounting evidence that CB2 receptors are also expressed by neuronal, glial and endothelial cells within the brain (reviewed by Onaivi et al., 2012). Recently, CB2 receptors were implicated in neurological disorders involving neuroinflammation and neuropathic pain (Ashton & Glass, 2007; Arevalo-Martin et al., 2008; Guindon & Hohmann, 2008; Cabral & Griffin-Thomas, 2009), and also depression and drug abuse (Onaivi et al., 2006; Ishiguro et al., 2007; Torres et al., 2010; Xi et al., 2011). Both types of cannabinoid receptor can inhibit adenylyl cyclase and activate mitogen-activated protein kinase by signaling through Gi/o proteins (Howlett, 2005), and CB1 receptors can also signal through Gs proteins (Glass & Felder, 1997).
PPARs are ligand-activated transcription factors that bind to sequence-specific promoter elements of target genes and directly regulate the genes’ expression (Glass, 2006). In addition to these genomic effects in the cell nucleus, PPARs have more recently been recognized to have rapid, non-genomic, extranuclear effects, such as affecting protein kinases (e.g., Melis et al., 2010); genomic and non-genomic effects probably occur in the same cells and mutually influence each other (Luconi et al., 2010). PPARs are expressed in tissues throughout the body, including many areas of the brain (Moreno et al., 2004). They are known to modulate inflammation and immune responses and lipid/glycidic homeostasis. Activators of PPAR-α are used to treat lipid disorders such as hypercholesterolemia, and activators of PPAR-γ are used to treat disorders of glucose metabolism such as type-2 diabetes. The functions of the β/δ subtype, are less well established, but it might be involved in regulation of metabolism and differentiation of keratinocytes, adipocytes and oligodendrocytes (Matsuura et al., 1999; Jehl-Pietri et al., 2000; Saluja et al., 2001; Barish et al., 2006). Since the pharmacology of the endocannabinoid and PPAR systems has been reviewed in detail elsewhere (Piomelli, 2003; O’Sullivan, 2007; Sun & Bennett, 2007; O’Sullivan & Kendall, 2010; Pertwee et al., 2010; Pistis & Melis, 2010), we will concentrate on interactions between these systems.
The unusually large ligand-binding domain of PPARs can be activated by natural and synthetic ligands of vastly different chemical structures, including endogenous FAEs, natural and synthetic cannabinoids, and members of the clinically-used fibrate (PPAR-α agonists) and thiazolidinedione (PPAR-γ agonists) drug classes. Many of these ligands also bind to cannabinoid receptors and/or are metabolized by FAAH (Lenman & Fowler, 2007). Anandamide has been found to directly activate PPAR-γ (Bouaboula et al., 2005) and PPAR-α (Sun et al., 2006). 2-AG can also directly activate PPAR-γ (Bouaboula et al., 2005). Other endocannabinoids, virodhamine and noladin ether, activate PPAR-α (Sun et al., 2006) but not PPAR-γ. The endocannabinoid N-arachidonoyl-dopamine (NADA) was shown to increase transcriptional activity of PPAR-γ (O’Sullivan et al., 2009). The FAEs and FAAH substrates OEA and PEA activate PPAR-α (Fu et al., 2003; Sun et al., 2006), but neither increases transcriptional activity of PPAR-γ (Fu et al., 2003; Lo Verme et al., 2006). The natural phytocannabinoids THC and cannabidiol activate transcriptional activity of PPAR-γ (O’Sullivan et al., 2005, 2009). Synthetic CB1 agonists that activate PPAR-γ include HU210, WIN 55,212-2 and CP 55,940 (Matias et al., 2006; O’Sullivan, 2007). WIN 55,212-2 also activates PPAR-α (Sun et al., 2006).
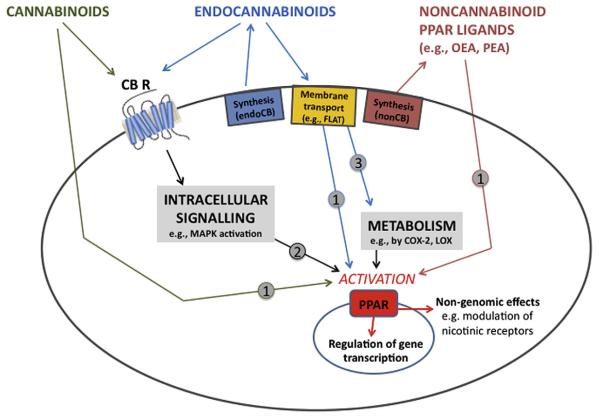
The mechanisms of cannabinoid-PPAR interactions are not completely clear, but there is evidence for several types of mechanism (Fig. 1; see reviews by O’Sullivan, 2007; Sun & Bennett, 2007; O’Sullivan & Kendall, 2010). Some studies have demonstrated direct binding of cannabinoid ligands (anandamide, OEA, WIN 55,212-2, virodhamine and noladin ether) to PPARs that cause changes in target gene expression (Fu et al., 2003; Bouaboula et al., 2005; Sun et al., 2006). Direct binding of these highly lipophilic cannabinoids to nuclear PPARs requires that the cannabinoids pass through plasma membranes and are transported into the nucleus. Transport through the membrane may depend on certain membrane and intracellular transporters such as the FAAH-like anandamide transporter (Fu et al., 2012) and the fatty acid binding proteins FABP5 or FABP7 (Kaczocha et al., 2009; Fu et al., 2012). Some endocannabinoids also have fatty acid, ethanolamine, COX-2 or LOX metabolites that bind to PPARs. For example, COX-2 metabolites of anandamide or LOX metabolites of 2-AG have been shown to activate PPARs (Kozak et al., 2002; Rockwell & Kaminski, 2004). Finally, activation of cannabinoid receptors at the cell surface may initiate intracellular signaling cascades leading to PPAR activation. Ligand-binding to cannabinoid receptors increases activity of mitogen-activated protein kinase (MAPK), which can regulate PPAR activation through direct phosphorylation (Gelman et al., 2005). Although much remains to be explained about cannabinoid-PPAR interactions, it is clear that PPAR activation provides a mechanism by which cannabinoids can regulate gene transcription, producing long-term exposure consequences and potentially therapeutic effects.
Fig. 1.
Schematic depiction of proposed mechanisms by which cannabinoid receptors and PPARs interact. There is evidence that cannabinoids (e.g., THC) and endocannabinoids (e.g., anandamide and 2-AG) can activate nuclear PPAR receptors by three mechanisms: 1) being transported into the cell (e.g., by FLAT) and binding directly to PPARs; 2) activating cannabinoid receptors at the cell surface, which initiates intracellular signaling cascades (e.g., MAPK pathways) that activate PPARs; and 3) being transported into the cell and enzymatically modified into metabolites that activate PPARs. This activation of PPARs by noncannabinoid PPAR ligands, cannabinoids, endocannabinoids, or endocannabinoid metabolites can have two general types of effect: regulating gene transcription and producing non-genomic effects. The relative contribution of each mechanism in different cells and tissues is dependent on the expression of various receptors and enzymes. CB R, cannabinoid receptor; COX-2, cyclooxygenase-2; endoCB, endocannabinoid; FLAT, FAAH-like anandamide transporter; LOX, lipoxygenase; MAPK, mitogen-activated protein kinase; nonCB, non-cannabinoid; PPAR, peroxisome proliferator-activated receptor. Modified from O’Sullivan (2007).
3. Fatty acid amide hydrolase inhibition and cognition
3.1. Learning and memory
Although marijuana and CB1 agonists are well known for their amnestic effects, and FAAH inhibition increases levels of the CB1 agonist anandamide, several studies have indicated that FAAH inhibition might enhance learning and memory. Varvel et al. (2007) studied effects of the FAAH inhibitor OL-135 and of genetic deletion of FAAH in mice. Both FAAH manipulations enhanced acquisition of spatial learning in a water maze, and this enhancement was blocked by treatment with the CB1 antagonist/inverse agonist rimonabant, suggesting the enhancement was mediated by CB1 receptors. FAAH deletion also enhanced acquisition of a spatial learning task designed to focus on working memory (Varvel et al., 2006); however, the FAAH inhibitor OL-135 and the PPAR agonists OEA, PEA had no effect on learning in this working memory procedure, either in FAAH(−/−) or FAAH(+/+) mice.
Mazzola et al. (2009) found that the FAAH inhibitor URB597 enhanced passive avoidance learning only when given before the learning trial (to test for effects on acquisition), not when given immediately after the learning trial (to test for effects on consolidation) or before the test trial (to test for effects on retrieval). While this enhancement of acquisition, like that observed by Varvel et al. (2007), was attenuated when the CB1 antagonist/inverse agonist rimonabant was given before the FAAH inhibitor, Mazzola et al. (2009) also found that it could be attenuated by the PPAR-α antagonist MK886. As described below (in the Peroxisome proliferator-activated receptors and cognition section), this led to further investigation of passive avoidance learning with selective PPAR-α ligands.
The water-maze and passive avoidance procedures of Varvel et al. (2006, 2007) and Mazzola et al. (2009) involved aversively-motivated learning (i.e., escape from deep water or avoidance of a footshock-associated area, respectively). The tests that have been performed thus far with appetitively-motivated tasks have not detected enhancing effects of FAAH inhibition. Wise et al. (2009) compared the effects of FAAH deletion in aversively and appetitively motivated versions of the Barnes maze that involved either reward with drinking water or escape from bright light and air turbulence. FAAH null mice showed accelerated acquisition in the aversively-motivated task, but did not differ from controls in the appetitively-motivated task. The enhancement of escape learning was blocked by treatment with rimonabant, suggesting that this effect of FAAH deletion was due to anandamide’s action at CB1 receptors.
In another appetitively-motivated task (water-reinforced delayed nonmatching to position), Goonawardena et al. (2011) found that working memory in rats was impaired by the FAAH inhibitor URB597, the anandamide uptake inhibitor AM404, and methanandamide. FAAH inhibition and methanandamide also suppressed hippocampal ensemble activity in a manner similar to that of the synthetic CB1 agonists THC and WIN 55,212-2. This disruption of baseline patterns of neural activity was seen during encoding, but not during recall. In another working memory task (T-maze with food reward), URB597 produced impairments (Seillier et al., 2010), but paradoxically it reversed impairments induced by subchronic treatment with phencyclidine.
The tentative conclusion that FAAH inhibition or deletion enhances memory most effectively in animal models that involve aversive situations suggests that this enhancement is due to modulation of the response to stressful situations. This would be consistent with findings that FAAH inhibitors have anxiolytic effects under moderately but not mildly stressful conditions in rats (Haller et al., 2009) and that a gene variant that decreases FAAH expression in humans is associated with low stress reactivity (Gunduz-Cinar et al., 2012). If FAAH inhibitors enhance learning by increasing the ability to cope with stress (as suggested by Haller et al., 2009), as opposed to increasing the salience of aversive stimuli, these compounds might improve memory deficits associated with anxiety disorders and depression.
Two recent studies indicate that, by regulating anandamide levels, FAAH has important effects on the basolateral amygdala related to stress, anxiety, and learning. Chronic stress in mice increased FAAH content of the amygdala, increased anxiety-like behavior, and induced FAAH-dependent changes in amygdalar structure and function (Hill et al., 2012). The FAAH inhibitor AM3506 facilitated extinction learning through a CB1-mediated mechanism in a fear-conditioning procedure with mice (Gunduz-Cinar et al., 2012; see Extinction learning section, below). In brain slices, AM3506 enhanced synaptic plasticity in the basolateral amygdala consistent with a lasting suppression of GABA release from amygdala interneurons (Gunduz-Cinar et al., 2012).
The effects of inhibiting MGL, the main deactivating enzyme of the endogenous CB1 agonist 2-AG, have received less research attention than those of inhibiting FAAH. However, Pan et al. (2011) have found that mice lacking MGL show enhanced learning in water-maze and object recognition procedures. MGL knockout mice also exhibited changes in the synaptic plasticity of hippocampal neurons indicating that MGL determines the time course of CB1-mediated depression of inhibitory postsynaptic potentials. The effect of genetic deletion of MGL on spatial learning in the water maze was reversed by the CB1 antagonist/inverse agonist AM251, suggesting that enhanced learning and memory in these mice was due to actions of 2-AG at CB1 receptors. Thus, sustained elevation of 2-AG had effects opposite to the amnestic effects that are typically produced by systemic administration of exogenous CB1 agonists such as THC (Zanettini et al., 2011), but similar to those of sustained elevations of anandamide and endogenous PPAR ligands in FAAH knockout mice (Varvel et al., 2006, 2007).
3.2. Paradoxical effects?
In light of the known amnestic effects of CB1 agonists and the fact that FAAH inhibition increases endogenous levels of the CB1 agonist anandamide, findings that FAAH inhibition enhances learning and that this enhancement can be blocked by a CB1 antagonist are puzzling. It might be that learning enhancements produced by FAAH inhibition are not truly CB1 mediated, but are blocked by rimonabant due to rimonabant’s inverse agonist effects at the CB1 receptor. It should also be noted that endogenous ligands of the extended endocannabinoid family tend to interact. For example, even though FAAH does not play a prominent role in degradation of the endogenous CB1 agonist 2-AG (Di Marzo & Maccarrone, 2008), FAAH inhibition or genetic inhibition of anandamide degradation has been observed to decrease 2-AG levels, possibly as a compensatory response to increased levels of anandamide (Maccarrone et al., 2008; Justinova et al., 2008a). Also, the wider spectrum of effects caused by inhibiting degradation of a family of neurochemicals (i.e., an “entourage” effect; Mechoulam et al., 1998) might be more powerful than the more selective effects caused by manipulating a single member of the family. Finally, it should be noted that systemic injection of CB1 agonists would be expected to affect all populations of CB1 receptors, and therefore could produce CB1-mediated effects that are quite different from those produced by FAAH inhibitors or FAAH deletion, which presumably have a more specific effect, magnifying the effects of anandamide when and where it is released (Kathuria et al., 2003; Fegley et al., 2005). For example, FAAH knockout mice and wild-type rats given URB597 have higher levels of anandamide (and other fatty acid amides) than controls, but their baseline behavioral profile does not resemble that of animals given exogenous anandamide or THC in the tetrad test of cannabinoid effects (Smith et al., 1994; Cravatt et al., 2001; Fegley et al., 2005).
3.3. Extinction learning
The endocannabinoid system has been found to play a role in extinction learning, a behavioral adaptation to situations where previously learned behavior is no longer reinforced. This adaptation is considered a form of inhibitory learning, as opposed to unlearning or forgetting of the original learning. In general, the effect of cannabinoid CB1 agonists on extinction learning has been opposite to those on other forms of learning, having an enhancing rather than impairing effect (Zanettini et al., 2011). Enhanced extinction learning could be useful for treating conditions, such as post-traumatic stress disorder, that involve lasting effects of stressful experiences. Murillo-Rodriguez et al. (2001) found that administration of oleamide facilitated extinction of passive avoidance in rats, presumably by acting as a CB1 agonist. Also consistent with a role of the endocannabinoid system in extinction learning, CB1 knockout mice and mice treated with rimonabant show impairments of extinction learning (Marsicano et al., 2002; Suzuki et al., 2004; Varvel et al., 2005). Varvel et al. (2007) studied extinction learning in FAAH knockout mice and in mice treated with the FAAH inhibitor OL-135 in a water maze task. They found that both FAAH manipulations enhanced extinction learning, and that the effect of OL-135 was blocked by rimonabant (although it should be noted that rimonabant by itself impaired extinction learning). They also showed that this was indeed an effect on extinction learning, as opposed to forgetting, since exposure to extinction trials (not just the passage of time) was necessary for the effect to occur. Gunduz-Cinar et al. (2012) found that the FAAH inhibitor AM3506 did not enhance acquisition or retrieval of fear conditioning in mice, but enhanced extinction of fear conditioning when given during the extinction trials; this effect was reversed by microinjection of rimonabant into the basolateral amygdala. In an addiction-related procedure, Manwell et al. (2009) found that the FAAH inhibitor URB597 facilitated extinction learning in a conditioned place-aversion procedure where a distinctive environment was paired with morphine withdrawal during conditioning.
3.4. Neuroprotection
Another important way in which FAAH-related treatments might affect memory is by protecting against insult from neurodegenerative disease, exposure to neurotoxic chemicals, and traumatic brain injury. There is much evidence that the endocannabinoid system can have a neuroprotective function, and there is some evidence that neuroprotective effects can be achieved by manipulating endocannabinoid levels. Karanian et al. (2005) found that a combined treatment that inhibited FAAH and blocked anandamide transport protected against the effects of intracranial injection of excitotoxic doses of the glutamate analog AMPA, protecting hippocampal cells and preventing memory impairment. Since FAAH inhibition might have neuroprotective effects through both CB1 and PPAR mechanisms, this is clearly an area that should be further investigated.
4. Peroxisome proliferator-activated receptors and cognition
4.1. Peroxisome proliferator-activated receptor-α
The effects of PPAR-α activators, per se, on learning and memory have received less attention than the effects of FAAH inhibition, which enhances endogenous levels of ligands for not only PPAR-α but also PPAR-γ, CB1 and TRP. As mentioned above, the PPAR ligands OEA and PEA were not found to affect spatial working memory in mice (Varvel et al., 2006). However, the results of two studies indicate that PPAR-α activation can enhance learning in other procedures. Mazzola et al. (2009), following up on the finding that the learning enhancement induced by FAAH inhibition in a passive-avoidance procedure could be blocked by the PPAR-α antagonist MK886, found that the synthetic PPAR-α agonist WY14643 produced enhancing effects similar to those of the FAAH inhibitor URB-597 in a passive-avoidance learning procedure; also like the effects of URB597, the effects of WY14643 were blocked by the PPAR-α antagonist MK886.
Campolongo et al. (2009) also found evidence that PPAR-α activation can enhance learning; administration of OEA enhanced learning in both a passive-avoidance procedure and a spatial-learning procedure (water maze) in rats. In contrast to Mazzola et al. (2009), who found that FAAH inhibition and WY14643 specifically facilitated acquisition, Campolongo et al. (2009) found that OEA had effects when given immediately post-training, indicating an effect on consolidation. This post-training effect also indicates that the effect of OEA was not due to sensorimotor or motivational effects of PPAR-α during the learning trial (e.g., antinociception, anxiolysis).
4.2. Peroxisome proliferator-activated receptor-γ
The area where a PPAR-based approach has been most studied with respect to cognitive effects is in the development of treatments for Alzheimer’s disease (AD). Due to its anti-inflammatory and neuroprotective properties, PPAR-γ may be useful for treating a number of neurological disorders, but it may also be well suited for AD because it affects synaptic function, energy balance, and lipid metabolism, all of which play a role in AD. Positive effects on learning and memory have been observed in animal models of AD and even in clinical trials. Since this area has been reviewed in detail recently (Mandrekar-Colucci & Landreth, 2011), it will only be briefly described here.
Much of this research has involved the thiazolidinedione compounds, pioglitazone and rosiglitazone, which have been used extensively to treat type-2 diabetes. In some preclinical studies, PPAR-γ activation ameliorated the development of AD-like pathophysiology. However, even though results were promising in early clinical trials, rosiglitazone did not have significant effects on memory in a much larger clinical trial (Gold et al., 2010). This might be due to these compounds passing the blood–brain barrier poorly (Mandrekar-Colucci & Landreth, 2011). In addition, the potential for side effects has recently led to a decrease in the clinical use of thiazolidinediones such as pioglitazone and encouraged a search for improved medications (Cariou et al., 2012). Ligands for retinoid X receptors, which form heterodimers with PPAR-γ and readily cross the blood brain barrier, show promise for this purpose (Mandrekar-Colucci & Landreth, 2011).
5. Fatty acid amide hydrolase inhibition and addiction
Inhibitors of FAAH were proposed as targets for the treatment of pain and neuropsychiatric disorders soon after FAAH was identified and characterized (Cravatt et al., 1996; Giang & Cravatt, 1997; Cravatt et al., 2001). Interest in FAAH inhibitors as potential treatments for drug addiction arose later, as it became clear that the endocannabinoid system can modulate the primary rewarding effects of many drugs of abuse (for reviews see Piomelli, 2003; Le Foll & Goldberg, 2005; Maldonado et al., 2006). It is well-established that addictive drugs (including alcohol, nicotine, opiates, THC, and psychostimulants) activate reward-related circuitry in the mesolimbic dopamine system, elevating extracellular dopamine levels in the nucleus accumbens (Koob, 1992a, 1992b; Wise, 2004). Cannabinoid CB1 receptors are abundantly expressed in this circuitry, where they modulate excitatory and inhibitory inputs that control dopaminergic neurons of the mesocorticolimbic pathway. Active cells in the nucleus accumbens and ventral tegmental area (VTA) release endocannabinoids that bind to presynaptic CB1 receptors and thereby act as retrograde messengers, performing a regulatory feedback function (Robbe et al., 2002; Melis et al., 2004; Riegel & Lupica, 2004).
There is plentiful evidence that noncannabinoid drugs of abuse can alter anandamide and 2-AG levels in reward-related areas of the brain, that these two endocannabinoids can be affected differentially by the same drug, and that these differential effects may be area-specific (for review see Serrano & Parsons, 2011). Moreover, administration of exogenous endocannabinoids can activate reward circuitry. For example, administration of exogenous anandamide increases extracellular dopamine levels in the nucleus accumbens shell, and this effect is blocked by the CB1 antagonist/inverse agonist rimonabant and magnified and prolonged by the FAAH inhibitor URB597 (Solinas et al., 2006). Rats will self-administer micro-injections of THC directly into the VTA or nucleus accumbens shell (Zangen et al., 2006). Thus it is clear that manipulation of endocannabinoid levels via FAAH inhibition can have profound effects on brain reward systems and might impact the rewarding effects of many drugs of abuse, as discussed further in this section.
5.1. Cannabinoids
Abuse-related effects of marijuana, including reward and dependence, are primarily mediated by CB1 receptors located on specific neuronal subpopulations in the central nervous system. CB1 knockout mice and CB1 antagonists are excellent tools for investigating the role of CB1 receptors in the effects of cannabinoids (Rinaldi-Carmona et al., 1994; Ledent et al., 1999). For example, Ledent et al. (1999) showed that CB1 knockout mice are unresponsive to cannabinoid agonists in a wide range of procedures, including precipitated withdrawal after long-term exposure to THC and intravenous self-administration of WIN 55,212-2.
Chronic administration of THC decreases levels of anandamide and 2-AG in the striatum, and it decreases levels of anandamide but not 2-AG in the midbrain and diencephalon (Di Marzo et al., 2000; Gonzalez et al., 2004a). CB1 receptor blockade by rimonabant increases anandamide release in the rat hypothalamus, and CB1 activation by WIN 55,212-2 decreases it; the same treatments induce opposite changes in 2-AG release (Bequet et al., 2007). This study – which employed a combination of online brain microdialysis and mass spectrometry to detect trace amounts of endocannabinoids in the extracellular fluid in real time – also showed that inhibition of FAAH by URB597 in rats increased levels of anandamide but not 2-AG in the hypothalamus. In non-human primates, URB597 increased anandamide levels throughout the brain, including the midbrain, nucleus accumbens, and prefrontal cortex (Justinova et al., 2008a). These increases in anandamide were accompanied by decreases in 2-AG levels that were presumably compensatory in nature.
5.1.1. Cannabinoid tolerance
Tolerance has been shown to develop to many cannabinoid effects, including antinociception, hypolocomotion, hypothermia, catalepsy, learning and memory impairment, subjective effects, changes in operant responding, and neuroendocrine effects (Wiley et al., 1993; Fan et al., 1994; Delatte et al., 2002; Martin, 2005; McKinney et al., 2008; McMahon, 2011; Singh et al., 2011). The development of cannabinoid tolerance, which is rapid and can sometimes be observed upon the second administration (Abood & Martin, 1992; Hutcheson et al., 1998), involves downregulation of CB1 receptors along with changes in second messenger systems (Rodriguez de Fonseca et al., 1994; Rubino et al., 2000a, 2000b; Rubino et al., 2004, 2005). Rats tolerant to THC display alterations in endocannabinoid content in various brain regions (Gonzalez et al., 2004a; Martin, 2005). However, repeated administration of anandamide itself does not appear to induce a robust downregulation of cannabinoid receptors, possibly because anandamide is rapidly metabolized by FAAH. But, even in FAAH knockout mice, subchronic treatment with THC produced greater rightward shifts (i.e., tolerance) in dose–response curves than did subchronic anandamide treatment when the mice were tested for antinociception, catalepsy and hypothermia (Falenski et al., 2010). THC produced levels of CB1 receptor desensitization and downregulation that were of similar magnitude in FAAH knockouts and their wild-type littermates, but anandamide produced only minimal CB1 receptor adaptation in both groups of mice. Overall, these findings suggest that chronic elevation of anandamide levels by FAAH inhibitors is less likely to promote tolerance and dependence than chronic exposure to direct acting CB1 receptor agonists.
5.1.2. Cannabinoid withdrawal
Discontinuing THC has been reported to induce withdrawal symptoms, including drug craving, decreased appetite, sleep disturbance, anger, and aggression in humans (Haney et al., 1999a, 1999b) and wet-dog shakes, grooming, piloerection, hunched-back posture, body tremors, paw tremors, and ptosis in rats (Verberne et al., 1981; Taylor & Fennessy, 1982; Aceto et al., 1996). Although rimonabant antagonizes the acute effects of smoked marijuana in humans (Huestis et al., 2001, 2007), CB1 antagonist-precipitated withdrawal has not been demonstrated in humans, possibly because the doses tested so far have been lower than the dose required to block the acute effects of THC (Gorelick et al., 2011). CB1 antagonists generally precipitate a pronounced withdrawal syndrome in animals that have been chronically treated with cannabinoids (Aceto et al., 1995; Hutcheson et al., 1998; Costa et al., 2000). The finding that CB1 knockout mice chronically treated with THC did not exhibit precipitated withdrawal when given rimonabant (Ledent et al., 1999) indicates that somatic signs of cannabis abstinence are CB1-receptor mediated. There have been conflicting reports on the ability of rimonabant to precipitate withdrawal in rats chronically treated with anandamide (Aceto et al., 1998; Costa et al., 2000). One study of rimonabant-precipitated withdrawal in FAAH knockouts (Falenski et al., 2010) showed a markedly lower magnitude of withdrawal in mice treated subchronically with anandamide compared to mice treated with THC. Another study using FAAH knockouts (Schlosburg et al., 2009a) found that, when chronically treated with THC, these mice show rimonabant-induced withdrawal responses indistinguishable from those of wild-type mice undergoing the same treatment. However, the same study showed that FAAH inhibition by URB597 significantly attenuated rimonabant-precipitated withdrawal signs in THC-dependent wild-type mice, and that subchronic administration of URB597 did not lead to cannabinoid dependence; these properties make FAAH inhibition a promising candidate for the treatment of cannabis withdrawal.
5.1.3. Discrimination of cannabinoid effects
Discriminative-stimulus effects of CB1 agonists in animals, which are presumably analogous to the subjective effects of these drugs in humans, are pharmacologically specific. Typically, only CB1 agonists produce discriminative-stimulus effects similar to THC, and only CB1 antagonists block them (Wiley et al., 1995a, 1995b, 1995c; Jarbe et al., 2001; McMahon, 2006; Solinas et al., 2007; McMahon et al., 2008; McMahon, 2009). Investigations of the discriminative-stimulus effects of endogenous cannabinoids have produced somewhat conflicting results. In several drug-discrimination studies, anandamide did not produce THC-like effects in monkeys or rodents, or did so only at high doses that also depressed rates of responding (Burkey & Nation, 1997; Wiley et al., 1997, 1998; Jarbe et al., 2001; Wiley et al., 2004; Solinas et al., 2007; Vann et al., 2009, 2012), while other studies with rhesus monkeys have shown full THC-like effects of anandamide even at doses that did not affect rates of responding (McMahon, 2009; Stewart & McMahon, 2011). Although the reason for these discrepancies is unclear, it is likely that the weakness of anandamide’s THC-like discriminative-stimulus effects in some studies is due to anandamide’s rapid metabolic inactivation. Metabolically stable, long-lasting analogs of anandamide (i.e., methanandamide, O-1812, ACPA or AM-1346) reliably produce partial or full generalization to a THC stimulus in monkeys and rodents (Burkey & Nation, 1997; Alici & Appel, 2004; Wiley et al., 2004; Jarbe et al., 2006; McMahon, 2006; Solinas et al., 2007; McMahon et al., 2008; McMahon, 2009).
In the studies in which anandamide did not produce THC-like effects when given alone, anandamide did produce dose-related THC-like effects when its metabolic inactivation via FAAH was blocked by URB597 or the non-specific amidase inhibitor PMSF (Solinas et al., 2007; Vann et al., 2009, 2012). FAAH knockout mice can be trained to discriminate THC about as well as their wild-type littermates can (Long et al., 2009). FAAH knockouts can also be trained to discriminate anandamide from vehicle (Walentiny et al., 2011), and in these animals both THC and various doses of anandamide elicit full anandamide-like responding, which is attenuated by the CB1 antagonist/inverse agonist rimonabant. Thus, in the absence of FAAH, anandamide produces subjective effects comparable to those of THC.
In rhesus monkeys (Stewart & McMahon, 2011), URB597 did not significantly modify the discriminative stimulus effects of THC, but it potentiated the discriminative-stimulus effects of anandamide. Similarly, blockade of FAAH by the non-specific amidase inhibitor PMSF did not significantly affect THC discrimination in rats (Vann et al., 2012). Inhibition of FAAH by URB597 in the absence of exogenously administered anandamide also did not produce THC-like discriminative effects in rodents or monkeys trained to detect THC (Gobbi et al., 2005; Solinas et al., 2007; Stewart & McMahon, 2011), nor did URB597 engender CB1-like effects in a group of monkeys trained to detect the CB1 agonist AM4054 (Bergman et al., 2011). Interestingly, like both THC itself and anandamide but unlike URB597, the FAAH inhibitor AM3506 produced a THC-like discriminative stimulus when given alone (Bergman et al., 2011). Taken together, these results indicate that URB597 enhances the subjective effects of anandamide, but does not enhance or mimic the subjective effects of CB1 agonists that are not FAAH substrates. However, URB597 appears to differ from some other FAAH inhibitors, such as AM3506, that do produce subjective and behavioral effects resembling those of THC (e.g., rewarding effects in squirrel monkeys, as described below). It is unclear why these compounds, which are highly selective for FAAH over other targets (Lichtman et al., 2004; Clapper et al., 2006; Piomelli et al., 2006; Godlewski et al., 2010), would produce different discriminative effects. It is possible that these and other differences between various FAAH inhibitors are due to regional specificity or pharmacokinetic profiles; for example, AM3506 was found to inhibit FAAH in the mouse brain but not the liver (due to rapid hepatic uptake and metabolism), while URB597 was effective in both brain and liver (Godlewski et al., 2010).
5.1.4. Cannabinoid reward
For decades, attempts to obtain intravenous self-administration of THC or synthetic CB1 receptor agonists in laboratory animals were relatively unsuccessful (for review see Tanda & Goldberg, 2003; Justinova et al., 2005a). THC has still not been found to maintain persistent intravenous self-administration in mice or rats, but it has been shown to be self-administered intracranially into the cerebral ventricles (Braida et al., 2004), the VTA, and the shell of the nucleus accumbens (Zangen et al., 2006) in rats. There have also been several demonstrations of intravenous and intracerebroventricular self-administration of synthetic CB1 receptor agonists in rodents: WIN 55,212-2, CP 55,940, and HU-210 in mice (Martellotta et al., 1998; Ledent et al., 1999; Braida et al., 2001b; Navarro et al., 2001) and WIN 55,212-2 in rats (Fattore et al., 2001; Spano et al., 2004).
Robust and dose-related intravenous self-administration of THC by animals has now been established in squirrel monkeys (Tanda et al., 2000; Justinova et al., 2003, 2008b). The dose range of THC that maintained self-administration behavior in these studies (1–16 βg/kg per injection) was lower than that used in previous studies and comparable to that received from smoking a marijuana cigarette (Agurell et al., 1986; Tanda et al., 2000). Once acquired, intravenous THC self-administration responding in squirrel monkeys can be rapidly extinguished by substituting vehicle for THC or by administering the CB1 antagonist/inverse agonist, rimonabant, suggesting that the behavior is mediated by CB1 receptors (Tanda et al., 2000; Justinova et al., 2003). The opioid-receptor antagonist naltrexone can also partially reduce THC self-administration (Justinova et al., 2004), consistent with other findings of interactions between the cannabinoid and opioid systems of the brain (see Opioids section, below). Building on the procedures that were successfully used to obtain THC self-administration in squirrel monkeys, it was shown that the endocannabinoids 2-AG and anandamide, and also anandamide’s stable analog methanandamide, are also self-administered intravenously by squirrel monkeys, with or without previous experience self-administering other drugs (Justinova et al., 2005b, 2008a, 2011a, 2011b). The reinforcing effects of exogenous 2-AG, anandamide, and methanandamide in squirrel monkeys appear to be mediated by CB1 receptors, because pretreatment with rimonabant dramatically decreased self-administration of these cannabinoids.
Justinova et al. (2008a) found that in squirrel monkeys URB597 almost completely suppresses FAAH activity in brain regions involved in motivational, emotional and cognitive functions, and that this FAAH inhibition was accompanied by significant increases in anandamide levels. Enhancement of endogenous anandamide levels could conceivably produce effects similar to those of systemically administered CB1 agonists like THC. Therefore, it seems prudent that FAAH inhibitors that are being developed for therapeutic purposes should be evaluated for abuse liability. In rodents, the FAAH inhibitor URB597 shows no signs of abuse potential: it does not increase extracellular levels of dopamine in the nucleus accumbens shell (Solinas et al., 2006; Scherma et al., 2008c), does not induce conditioned place preference (Gobbi et al., 2005), and does not produce THC-like discriminative stimulus effects (Gobbi et al., 2005; Solinas et al., 2007). Most tellingly, URB597 was not self-administered over a broad range of doses in the intravenous self-administration model by squirrel monkeys that had experience self-administering either THC, anandamide, or cocaine (Justinova et al., 2008a). Moreover, unlike THC, URB597 did not reinstate extinguished drug-seeking behavior in monkeys trained with THC, anandamide, or cocaine self-administration. Finally, pretreatment with URB597 potentiated self-administration of anandamide, but did not alter the reinforcing effects of THC or cocaine. Together, these results indicate that URB597 is an effective FAAH inhibitor but does not produce reinforcing effects in animals, so it is unlikely to be abused or to provoke relapse to drug use in humans. In marked contrast with URB597, the FAAH inhibitor AM3506 does produce dose-related rewarding and discriminative-stimulus effects similar to those of THC in squirrel monkeys (Bergman et al., 2011). These findings indicate that different FAAH inhibitors can have dissimilar behavioral profiles, and that the abuse liability of other experimental FAAH inhibitors (like URB694 and PF-04457845) should be evaluated.
An alternative way to assess the rewarding effects of cannabinoids in experimental animals is with cannabinoid-induced conditioned place preference, a procedure in which rodents spend more time in a context that has been associated with administration of a cannabinoid compared to a context associated with vehicle administration. In such studies, conditioning with THC or synthetic CB1 agonists have yielded results ranging from place preference to no effect to place aversion (Lepore et al., 1995; Mallet & Beninger, 1998; Valjent & Maldonado, 2000; Braida et al., 2001a; Ghozland et al., 2002; Braida et al., 2004; Le Foll et al., 2006; Zangen et al., 2006), and the variables responsible for these divergent findings are not clear (for review see Tzschentke, 2007). Studies evaluating rewarding or aversive effects of anandamide in a place conditioning procedure showed that anandamide alone or combined with the non-specific amidase inhibitor FAAH inhibitor PMSF had no effect on place conditioning, but the combination of the FAAH inhibitor URB597 with anandamide produced significant conditioned place aversion (Mallet & Beninger, 1998; Scherma et al., 2008b). Mallet and Beninger (1998) administered anandamide intraperitoneally, which may have resulted in lower bioavailability of anandamide compared to the intravenous route used by Scherma et al. (2008b). URB597 alone does not produce conditioned place preference or aversion (Gobbi et al., 2005; Scherma et al., 2008b), which complements data from drug self-administration studies showing no motivational effects of URB597 in squirrel monkeys (Justinova et al., 2008a). Moreover, while THC can facilitate or inhibit brain stimulation reward, depending on dose (Gardner et al., 1988; Lepore et al., 1996; Vlachou et al., 2007), the synthetic CB1-agonists (WIN 55,212-2, CP 55,940, and HU-210) or FAAH inhibitors (URB597 or PMSF) mostly did not alter brain stimulation reward thresholds (Vlachou et al., 2005, 2006). The inconsistencies in place preference and brain stimulation results indicate that there is still much to be learned about the addiction-related effects of cannabinoid agonists.
5.2. Nicotine
5.2.1. Nicotine reward and relapse
The endocannabinoid system modulates the neurochemical and behavioral effects of nicotine, and cannabinoid CB1 receptors play a key role in this interaction (see reviews by Castane et al., 2005; Scherma et al., 2008a; Maldonado & Berrendero, 2010). Promising preclinical findings demonstrating CB1 modulation of nicotine’s addiction-related effects led to clinical trials with rimonabant showing that CB1 receptor antagonists/inverse agonists could be useful as therapeutic agents for smoking cessation (Fernandez & Allison, 2004). Unfortunately, the United States Food and Drug Administration could not approve rimonabant for clinical use due to increased risk of psychiatric side-effects like anxiety, depression, and suicidal ideation (Christensen et al., 2007; Rucker et al., 2007; Le Foll et al., 2009). It is not yet known whether a CB1 antagonist that lacks inverse agonist effects would be better tolerated or have efficacy for reducing smoking.
Surprisingly, given the findings that CB1 antagonism has potential as an anti-smoking strategy and that FAAH inhibition increases levels of the CB1 agonist anandamide, there is evidence that FAAH inhibition might counteract the addictive effects of nicotine. Although one study indicated that the rewarding effects of nicotine in a conditioned place preference procedure were enhanced in FAAH knock-out mice and also in wild-type mice treated with the FAAH inhibitor URB597 (Merritt et al., 2008), an extensive series of experiments in rats and monkeys has shown potentially therapeutic effects of FAAH inhibition. Scherma et al. (2008c) showed that treatment with URB597 prevented the acquisition of nicotine self-administration and the acquisition and reinstatement of nicotine-induced conditioned place preference in rats. In agreement with these behavioral findings, microdialysis and electrophysiology experiments indicate that URB597 reduces nicotine-induced dopamine elevations in the nucleus accumbens shell and blocks nicotine-induced increases in the firing rate and burst firing of VTA dopamine neurons in rats (Melis et al., 2008; Scherma et al., 2008c). While it is possible that CB1 receptors play a role in these effects of URB597, it is likely that the effects are largely due to PPAR activation, as discussed in the Peroxisome proliferator-activated receptors and addiction section, below (see also Luchicchi et al., 2010). For example, the PPAR-α antagonist MK886 prevented the effects of URB597 on nicotine-induced burst firing (although not the effects on firing rate; Melis et al., 2008), and the non-CB1, PPAR-α-activating FAAH substrates OEA and PEA (Fu et al., 2003; Lo Verme et al., 2005b) can also block addiction-related effects of nicotine on dopamine neurons (Melis et al., 2008). The finding that FAAH inhibition or deletion enhanced nicotine-induced conditioned place preference (an effect opposite to that observed in rats) might be explained by differences between rats and mice (Muldoon et al., 2012); for example, there is evidence that FAAH inhibition induces a more robust increase in endogenous PPAR ligands in rats than in mice (Fegley et al., 2005).
The effects of URB597 on the motivation to take nicotine and on the relapse-like reinstatement induced by nicotine-associated stimuli were further studied (and compared with rimonabant) by Forget et al. (2009). Rats learned to self-administer nicotine intravenously under a progressive-ratio schedule, and a break point (i.e., highest ratio completed) for obtaining nicotine was assessed as a measure of nicotine’s reinforcing efficacy. Rimonabant, but not URB597, dose-dependently and persistently reduced the break point for nicotine. This suggests that, in rats, blockade of CB1 receptors attenuates the motivation to obtain nicotine, but FAAH inhibition by URB597, which increases anandamide levels in the brain, does not. However, in this study both rimonabant and URB597 attenuated cue- and nicotine-induced reinstatement of extinguished nicotine seeking, suggesting these treatments might prevent relapse. The effect of both drugs on nicotine-induced reinstatement could be partially explained by their ability to block nicotine-induced elevations of dopamine in the nucleus accumbens shell (Cohen et al., 2002; Scherma et al., 2008c). The effects of URB597 and rimonabant on cue-induced reinstatement might be explained similarly, because nicotine-associated cues can also increase extracellular levels of dopamine in the nucleus accumbens shell (Bassareo et al., 2007). However, the effects of rimonabant and URB597 on dopamine release caused by nicotine-paired stimuli remain to be studied.
5.2.2. Nicotine withdrawal
Findings from the rodent studies described above suggest that the endocannabinoid system can modulate addiction-related effects of nicotine and that FAAH inhibitors are potentially useful agents in the treatment of tobacco dependence in humans. This possibility is further supported by a recent study showing that URB597 suppresses symptoms of nicotine withdrawal (Cippitelli et al., 2011). Rats were exposed for seven days to implanted transdermal nicotine patches to induce nicotine dependence. Removal of the patches produced a severe withdrawal syndrome with affective and somatic components. Somatic signs peaked 16 h after nicotine discontinuation (acute withdrawal), and anxiety-like symptoms peaked at 34 h. FAAH inhibition by URB597 did not modify the expression of somatic withdrawal signs, but decreased anxiety associated with protracted nicotine withdrawal (consistent with other findings that FAAH inhibition has anxiolytic effects, but not necessarily representing an interaction with nicotine-induced effects, per se). Taken together, these findings indicate that FAAH inhibitors can alleviate negative affective states associated with nicotine withdrawal and prevent nicotine- and cue-induced relapse to drug-seeking in abstinent subjects, which are major obstacles to achieving and maintaining abstinence from tobacco.
5.3. Alcohol
5.3.1. Alcohol reward
The endocannabinoid system is involved in the rewarding effects of alcohol and in relapse to alcohol abuse after a period of abstinence (for review see Rodriguez de Fonseca et al., 2005; Vengeliene et al., 2008). Rewarding effects of alcohol seem to require CB1 receptor activation, since CB1 knockout mice consumed less alcohol than wild-type mice in most studies (Crabbe et al., 2006) and did not develop a conditioned place preference for an alcohol-paired environment (Thanos et al., 2005). Generally, CB1 agonists increase (Colombo et al., 2002) and CB1 antagonist/inverse agonists decrease rodents’ oral alcohol consumption in self-administration studies (Arnone et al., 1997; Cippitelli et al., 2005). Although CB1 receptor blockade can suppress fluid and food intake (e.g., Arnone et al., 1997; McLaughlin et al., 2003), CB1 antagonist/inverse agonists were still found to decrease alcohol’s rewarding effects when this confounding factor was controlled in place-conditioning procedures by giving alcohol intraperitoneally rather than orally (Gessa et al., 2005; Lallemand & De Witte, 2006). Extracellular levels of dopamine in the nucleus accumbens shell are not increased by alcohol in CB1 knockout mice, and pharmacological blockade of CB1 receptors blocks the dopamine-releasing effects of alcohol in rats (Cohen et al., 2002; Hungund et al., 2003). These findings are consistent with results from an electrophysiology study (Perra et al., 2005) that showed that blockade of CB1 receptors by rimonabant abolishes alcohol-induced increases in the firing rate of dopamine neurons in the VTA that project to the nucleus accumbens shell.
Several studies have examined the effects of FAAH manipulations on alcohol self-administration. Genetic ablation of FAAH in mice increased alcohol preference and intake, and inhibition of FAAH by URB597 increased alcohol intake in wild-type mice (Basavarajappa et al., 2006; Blednov et al., 2007; Vinod et al., 2008). Vinod et al. (2008) found that these effects of URB597 were CB1-dependent, occurring in wild-type mice but not CB1 knockouts. Although intraperitoneal administration of URB597 did not increase alcohol consumption in Wistar or Marchigian Sardinian alcohol-preferring rats (Cippitelli et al., 2008), administration into the prefrontal cortex increased alcohol consumption in Wistar rats (Hansson et al., 2007), consistent with the hypothesis that endocannabinoid activity in this region promotes alcohol consumption (see Susceptibility to alcoholism section, below). Thus, FAAH inhibition appears to affect alcohol reward mainly through a CB1-dependent mechanism and nicotine reward mainly through a PPAR-dependent mechanism.
5.3.2. Chronic effects of alcohol
Genetic expression of the CB1 receptor was identified as being permanently affected by repeated cycles of alcohol dependence and withdrawal (Rimondini et al., 2002). Animal studies also reveal that chronic exposure to alcohol downregulates CB1 receptors (Basavarajappa et al., 1998) and that levels of CB1 receptors are decreased in drug-naive alcohol-preferring rats (Ortiz et al., 2004). Acute alcohol exposure reduces endocannabinoid levels (Ferrer et al., 2007; Rubio et al., 2007), but this is probably not a consequence of enhanced degradation by FAAH (Rubio et al., 2009). Chronic alcohol exposure is associated with increased formation of both anandamide (Basavarajappa & Hungund, 1999) and 2-AG (Basavarajappa et al., 2000). Analysis of anandamide and 2-AG levels in brains of animals treated chronically with alcohol showed increased anandamide content in the limbic forebrain and decreased content of both anandamide and 2-AG in the midbrain (Gonzalez et al., 2002, 2004b; Vinod et al., 2006). Increases in anandamide content after chronic alcohol exposure were accompanied by reduced FAAH activity in the cortex (Vinod et al., 2006). Caille et al. (2007) were the first to measure changes in endocannabinoid levels during drug self-administration in rats. They found that self-administration of alcohol increased 2-AG levels but did not alter anandamide levels in the nucleus accumbens shell; these in vivo data suggest endocannabinoid involvement in the motivational effects of ethanol.
5.3.3. Susceptibility to alcoholism
Hansson et al. (2007) found decreased expression of FAAH in the prefrontal cortex of the alcohol-preferring AA strain of rats compared to the alcohol nonpreferring ANA strain, accompanied by decreased enzyme activity and down-regulation of CB1 receptors. Rimonabant administered systemically or locally into the prefrontal cortex dose-dependently suppressed alcohol self-administration in AA rats, and local administration of URB597 into the prefrontal cortex increased alcohol self-administration in Wistar rats (Hansson et al., 2007). These data implicate FAAH in the susceptibility to high levels of voluntary alcohol intake exhibited by certain strains of rats, and they parallel findings that humans carrying a nucleotide polymorphism in the FAAH gene are more vulnerable to drug use and alcoholism (Sipe et al., 2002; see also FAAH polymorphisms and addiction section, below). Interestingly, a postmortem study demonstrated lower FAAH activity and lower levels of CB1 receptors in the ventral striatum of nonsuicidal alcohol-dependent subjects compared to nonpsychiatric controls, but alcohol-dependent subjects that committed suicide had significantly higher FAAH activity and levels of CB1 receptors in the same area compared to nonsuicidal alcohol-dependent subjects (Vinod et al., 2010). These data suggest that agents modulating endocannabinoid tone or CB1 receptor function might have therapeutic potential in the treatment of alcohol addiction and suicidal behavior.
5.3.4. Alcohol withdrawal
Cippitelli et al. (2008) found that systemic administration of URB597 had no effect on alcohol intake or relapse to alcohol seeking induced by either alcohol-associated cues, foot-shock stress or yohimbine in Wistar or Marchigian Sardinian alcohol-preferring (msP) rats. Nevertheless, URB597 completely abolished the anxiogenic response during withdrawal as measured in an elevated plus maze 12 h after acute alcohol administration. This anxiolytic effect might be beneficial in alleviating anxiety associated with alcohol withdrawal in humans.
5.4. Opioids
Numerous studies have demonstrated the existence of functional, bidirectional interactions between the endogenous cannabinoid and opioid systems. Both systems participate in the common circuits activated by addictive drugs, and both β-opioid and CB1-cannabinoid receptors are expressed in reward-related brain areas, where they share common signaling cascades (Maldonado & Rodriguez de Fonseca, 2002; Fattore et al., 2005). Research regarding the molecular mechanisms underlying cannabinoid and opioid system interactions relevant to addiction have been very fruitful, involving neurochemical approaches as well as animal models of addiction based on classic behavioral paradigms: conditioned place preference/aversion, drug self-administration, intracranial self-stimulation, withdrawal, reinstatement, tolerance and behavioral sensitization (for review see Lopez-Moreno et al., 2010). As detailed below, this research has begun to be extended to FAAH inhibition.
5.4.1. Opioid reward
Endocannabinoid involvement in the rewarding effects of opioids has been shown in several studies. For example, CB1 knockout mice have been found to develop morphine-induced place preference under certain conditions (Martin et al., 2000; Rice et al., 2002), but they have not been found to self-administer morphine (Cossu et al., 2001). The CB1 antagonist/inverse agonist rimonabant can block the development of morphine-induced conditioned place preference in rodents (Navarro et al., 2001; Singh et al., 2004). Although rimonabant does not block heroin-induced increases in extracellular dopamine levels in the nucleus accumbens shell (Tanda et al., 1997; Caillé and Parsons, 2003), it decreases heroin self-administration in rats, with more robust effects under progressive-ratio schedules than fixed-ratio 1 or fixed-ratio 5 schedules (Caillé and Parsons, 2003; De Vries et al., 2003; Solinas et al., 2003); these findings are consistent with findings that heroin self-administration is not dependent on accumbens dopamine (Shippenberg & Elmer, 1998). The reinforcing effects of heroin were enhanced by CB1 agonists (THC, WIN 55,212-2) under a progressive-ratio schedule, but THC only altered heroin self-administration under a fixed-ratio one schedule when a three-fold higher dose of THC was given, which might have produced depressant effects when combined with heroin (Solinas et al., 2005). However, in the same study, FAAH inhibition by URB597 did not alter the reinforcing efficacy of heroin under the progressive ratio schedule, and the anandamide uptake inhibitor AM404 only decreased the reinforcing efficacy of heroin; these findings led to the conclusion that modulation of the reinforcing effects of heroin produced by CB1 activation or blockade is not due to an opioid-induced release of endocannabinoids, but rather to interactions between opioid and cannabinoid receptors and their signaling pathways (Solinas et al., 2005).
5.4.2. Opioid relapse
A role of the endocannabinoid system in relapse to opioid use has also been established. Blockade of CB1 receptors can prevent heroin-induced reinstatement of heroin-seeking behavior, and CB1 agonists can reinstate heroin-seeking behavior in rats (De Vries et al., 2003; Fattore et al., 2003; Solinas et al., 2003; Spano et al., 2004; Fattore et al., 2005). Rimonabant can also block cue-induced heroin seeking in rats (De Vries et al., 2003; Alvarez-Jaimes et al., 2008). McCallum et al. (2010) studied the effects of URB597 on reinstatement of conditioned preference for a place associated with morphine, finding that this FAAH inhibitor did not alter the priming effect of re-exposure to morphine. Using a place-avoidance procedure in which a distinctive environment was associated with naloxone-precipitated opioid withdrawal, Manwell et al. (2009) found that URB597 enhanced extinction of place avoidance (consistent with other studies showing cannabinoid-induced enhancement of extinction learning; see Extinction learning section, above); but, in a similar procedure McCallum et al. (2010) found that URB597 did not alter reinstatement of place avoidance induced by re-exposure to precipitated withdrawal.
5.4.3. Opioid withdrawal
Chronic use of opiates such as morphine and heroin leads to dependence and tolerance in humans and animals. The cessation of regular morphine use or the administration of an opioid or cannabinoid CB1 receptor antagonist in morphine dependent individuals precipitates withdrawal symptoms with somatic and affective components (Navarro et al., 1998; Kosten & George, 2002). Acute administration of opioid agonists like morphine can increase anandamide levels in reward-related brain areas (Vigano et al., 2004). However, studies investigating the endocannabinoid contents of specific brain regions [including reward-related (nucleus accumbens and prefrontal cortex) and opioid withdrawal-related (locus coeruleus, periaqueductal grey and amygdala) areas] in rodents chronically exposed to morphine have not detected changes in anandamide levels (Gonzalez et al., 2003; Vigano et al., 2003; Ramesh et al., 2011). Vigano et al. (2003) did find reduced 2-AG levels in the striatum, limbic area, cortex, hippocampus and hypothalamus of morphine-treated rats and, in a later study, also in the nucleus accumbens, caudate putamen, and prefrontal cortex (Vigano et al., 2004). Ramesh et al. (2011) did not find any changes in 2-AG levels in mice treated with morphine. This suggests that changes in the endocannabinoid system might be limited to 2-AG. Nonetheless, FAAH inhibition by URB597 or PF-3845 ameliorated naloxone-precipitated morphine withdrawal symptoms in rats and mice (Ramesh et al., 2011; Shahidi & Hasanein, 2011), and FAAH knock-out mice showed an attenuated opioid withdrawal syndrome (Ramesh et al., 2011); it is not clear whether these attenuations of withdrawal effects are due to FAAH substrates being directly involved in opioid dependence or if they are to FAAH inhibition or deletion affecting other processes, such as anxiety, pain, or inflammation.
5.5. Psychostimulants
5.5.1. Cocaine
Although not without controversy, findings to date suggest that endocannabinoids acting at CB1 and possibly CB2 play a role in the direct rewarding effects of cocaine, consolidation of the cocaine addiction process, and relapse to cocaine seeking. Li et al. (2009) demonstrated that CB1 knockout mice had a significant reduction in the basal level of extracellular dopamine in the nucleus accumbens, as compared to their wild-type littermates, which displayed inhibited dopamine release after pharmacological blockade of CB1 receptors. Moreover, in this study, CB1 knockouts did not show increases in extracellular dopamine when given cocaine. Soria et al. (2005) found reduced acquisition of cocaine self-administration in CB1 knockouts. In several studies, pharmacological blockade of CB1 receptors reduced cocaine break points under a progressive-ratio schedule (Soria et al., 2005; Xi et al., 2008; Orio et al., 2009) or blocked development of cocaine-induced conditioned place preference (Chaperon et al., 1998). Recently, Xi et al. (2008, 2011) showed that blockade of CB1 receptors can inhibit cocaine-enhanced brain-stimulation reward and that activation of CB2 receptors can inhibit intravenous cocaine self-administration and cocaine-induced increases in accumbal extracellular dopamine in wild-type and CB1 knockout mice, but not in CB2 knockouts. Regarding drug- and cue-induced relapse to cocaine seeking, direct activation of CB1 receptors by CB1 agonists can induce reinstatement of cocaine seeking (De Vries et al., 2001; Spano et al., 2004; Justinova et al., 2008a), and blockade of CB1 receptors can attenuate relapse induced by re-exposure to cocaine-associated cues or cocaine itself (De Vries et al., 2001; Anggadiredja et al., 2004; Filip et al., 2006; Xi et al., 2006; Ward et al., 2009).
On the other hand, a number of studies suggest only minimal involvement of CB1 receptors in the addictive effects of cocaine. In these studies, cocaine administration induced a similar enhancement in extracellular levels of dopamine in the nucleus accumbens of both CB1 knockout and wild-type mice (Soria et al., 2005), and CB1 knockout mice were found to be capable of acquiring cocaine self-administration or developing a cocaine-induced conditioned place preference (Martin et al., 2000; Cossu et al., 2001). In rats, cocaine-induced enhancements in brain stimulation reward were unaffected by administration of a CB1 antagonist (Vlachou et al., 2003), as were cocaine-induced increases in nucleus accumbens dopamine levels (Caille & Parsons, 2006; Caille et al., 2007). Also, several other studies showed cocaine self-administration under fixed-ratio schedules was unaffected by pharmacological blockade of CB1 in mice, rats, and monkeys (Fattore et al., 1999; Tanda et al., 2000; De Vries et al., 2001; Lesscher et al., 2005; Caille & Parsons, 2006; Caille et al., 2007; Xi et al., 2008).
There are only a few studies investigating the effects of FAAH inhibition on neurochemical or behavioral effects of cocaine. Luchicchi et al. (2010) found that pretreatment with URB597 did not affect the inhibition of either firing rate or burst firing of VTA dopamine neurons induced by cocaine. In a drug-discrimination procedure (Justinova et al., 2007), FAAH inhibition had no effect on the discriminative-stimulus effects of cocaine in rats. When the effect of FAAH inhibition was studied in the intravenous self-administration model (Justinova et al., 2008a; Adamczyk et al., 2009), pretreatment with URB597 did not modify cocaine self-administration under a fixed-ratio schedule in rats or squirrel monkeys. URB597 alone did not reinstate extinguished cocaine seeking in squirrel monkeys (Justinova et al., 2008a), but dose-dependently blocked cocaine- and cue-induced reinstatement of cocaine seeking (Adamczyk et al., 2009). Thus, FAAH inhibition does not appear to alter the direct reinforcing effects of cocaine, but might provide protection against cue-induced relapse.
5.5.2. Amphetamines
Compared to cocaine, there are fewer studies investigating the role of endocannabinoids or FAAH inhibition in addiction to amphetamines [amphetamine, methamphetamine, and 3,4-methylendioxymethamphetamine (MDMA)]; but, like the cocaine studies, the findings have been inconsistent. Several studies indicate that CB1 blockade can counteract addiction-related effects of amphetamines. CB1 receptor blockade decreased methamphetamine self-administration under a fixed-ratio schedule in rats and rhesus monkeys (Vinklerova et al., 2002; Schindler et al., 2010), reduced methamphetamine self-administration into the nucleus accumbens of rats (Rodriguez et al., 2011), prevented reinstatement of extinguished methamphetamine seeking induced by re-exposure to a combination of methamphetamine and methamphetamine-associated cues in rhesus monkeys (Schindler et al., 2010), and blocked methamphetamine- and cue-induced reinstatement of methamphetamine-seeking behavior in rats (Anggadiredja et al., 2004; Hiranita et al., 2008). Consistent with these findings involving CB1 blockade, CB1 knockout mice failed to sensitize to the locomotor stimulant effects of amphetamine (Corbille et al., 2007; Thiemann et al., 2008). However, there have also been a number of studies in which CB1 receptors have been found to have little influence over the addictive effects of amphetamines. Amphetamine is self-administered in CB1 knockout mice (Cossu et al., 2001), and CB1 receptor blockade failed to attenuate methamphetamine-induced reinstatement in rats in a study by Boctor et al. (2007). In a drug-discrimination study (Barnes et al., 2006), neither activation nor blockade of CB1 receptors affected the discriminative-stimulus effects of methamphetamine in rats. Studies with MDMA showed contradictory effects as well. CB1 knockout mice failed to self-administer MDMA (Tourino et al., 2008), and blockade of CB1 receptors blocked MDMA-induced place preference (Braida et al., 2005), but CB1 blockade also increased intracerebroventricular self-administration of MDMA (Braida & Sala, 2002). In a study by Manzanedo et al. (2010), activation of CB1 receptors increased the rewarding effects of low doses of MDMA, but so did CB1 receptor blockade. In the Manzanedo et al. study, a CB1 agonist did not reinstate extinguished MDMA-induced conditioned place preference, but in a study by Daza-Losada et al. (2011), a CB1 receptor antagonist, alone or together with MDMA, did reinstate place preference.
Very few studies have examined the effects of FAAH inhibition on addiction-related effects of amphetamines. URB597 produced a CB1-mediated attenuation of behavioral sensitization to amphetamine, but failed to do so in rats that underwent olfactory bulbectomy (Eisenstein et al., 2009). This suggests that the endocannabinoid system is compromised in animals with bulbectomy-induced dopaminergic dysfunction. In a drug-discrimination procedure (Justinova et al., 2007), FAAH inhibition attenuated the discriminative-stimulus effects of methamphetamine in rats via a CB1-mediated mechanism. The effects of FAAH inhibition on rewarding effects of amphetamines have yet to be studied.
5.6. Fatty acid amide hydrolase polymorphisms and addiction
Drug addiction is a psychiatric disorder with a complex etiology that can include specific genetic variants and polymorphism in multiple genes (Nestler, 2000; Lopez-Moreno et al., 2012). Several genes linked specifically to the actions and metabolism of endogenous cannabinoids have been associated with drug addiction. One of these, CNR1, encodes for the cannabinoid CB1 receptor and likely modulates endocannabinoid and dopamine-mediated reward signaling (Filbey et al., 2010). CNR1 has been associated not only with cannabis dependence, but also with substance-use disorder phenotypes in general (e.g., Comings et al., 1997; Covault et al., 2001; Zhang et al., 2004; Ballon et al., 2006; Zuo et al., 2007; Proudnikov et al., 2010; reviews by Agrawal & Lynskey, 2009; Benyamina et al., 2011). The CNR2 gene, which encodes for the cannabinoid CB2 receptor, has received much less attention, but has been associated with alcoholism in a Japanese population (Ishiguro et al., 2007).
Another candidate that has been repeatedly associated with drug addiction is the FAAH gene, which encodes for the fatty acid amide hydrolase (for review see Lopez-Moreno et al., 2012). A missense single nucleotide polymorphism (SNP) in the FAAH DNA sequence (C385A, rs 324420) results in a FAAH variant that has normal catalytic properties but is more susceptible to proteolytic degradation (Sipe et al., 2002; Chiang et al., 2004). This can lead to reduced FAAH expression and activity in humans with an A/A genotype, leading to higher levels of anandamide and other FAAH substrates. Early reports indicated an association between the A/A genotype and problem drug use (Sipe et al., 2002; Flanagan et al., 2006), but further studies showed that those with the A/A genotype are not at greater risk for alcohol, nicotine, heroin or methamphetamine dependence (Morita et al., 2005; Iwasaki et al., 2007; Tyndale et al., 2007; Proudnikov et al., 2010). Although individuals with the A/A genotype were more likely to try cannabis, they had reduced vulnerability to the development of cannabis dependence (Tyndale et al., 2007). The A/A genotype is also associated with an elevated risk for regular use of sedatives (Tyndale et al., 2007). Individuals with one or more C alleles (C/A or C/C genotypes) reported more severe withdrawal and higher level of craving to smoke marijuana than those with the A/A genotype, and thus may be more susceptible to cannabis dependence than A allele carriers (Haughey et al., 2008; Schacht et al., 2009). Individuals with the C/C genotype also reported more intense positive effects of marijuana and more severe negative effects during withdrawal. Another study showed that individuals with the C/C genotype had greater neural response to marijuana cues in reward-related brain structures (Filbey et al., 2010), which suggest a possible dysregulation in the reward system associated with this genotype. Thus, FAAH-related genetic analysis may assist in predicting vulnerability to cannabis use disorders.
6. Peroxisome proliferator-activated receptors and addiction
Most investigations of PPAR ligands as treatments for addiction have focused on two areas: PPAR-γ activation as a potential treatment for alcoholism, and PPAR-α activation as a potential treatment for tobacco dependence. A few studies have evaluated the role of PPAR in addiction-related effects of psychostimulants (cocaine, methamphetamine, MDMA) and an opioid (morphine). Overall, the results have been promising, but this research is clearly in its earliest stages. The evidence suggests that endogenous PPAR systems modulate – and PPAR-based medications might be useful for reducing – addictive properties and other adverse effects of certain drugs.
6.1. Alcohol
Stopponi et al. (2011) studied the effects of PPAR-γ-activating thiazolidinediones (pioglitazone and rosiglitazone) in rodent models of alcoholism. They found a number of potentially therapeutic effects. During subchronic treatment, PPAR-γ activation decreased alcohol drinking in Marchigian Sardinian alcohol-preferring rats. This effect was blocked by intracerebroventricular administration of a PPAR-γ antagonist, indicating that it is mediated by PPAR-γ receptors in the brain. Furthermore, pioglitazone specifically altered alcohol’s rewarding properties, having no effect on food or saccharine self-administration, and also having no effect on alcohol or glucose metabolism.
In the same study, pioglitazone also showed promise for preventing relapse to alcohol abuse. When alcohol-abstinent rats were injected with the alpha-2 adrenoceptor antagonist yohimbine, their alcohol seeking increased; this relapse-like effect of yohimbine has been shown with a number of other drugs of abuse besides alcohol and is believed to be due to yohimbine’s stress-like effects (Shalev et al., 2010). However, when rats were treated with pioglitazone, yohimbine did not reinstate alcohol seeking. Thus, PPAR-γ activation might provide protection against stress-induced relapse, a major obstacle to abstinence. Unfortunately, pioglitazone did not protect against the relapse-inducing effects of re-exposure to an olfactory cue that had been associated with the availability of alcohol. It was not determined whether glitazone treatment would affect alcohol seeking induced by re-exposure to the pharmacological effects of alcohol (drug priming).
PPAR activation may have other beneficial effects beyond the ability to decrease alcohol consumption and relapse. Stopponi et al. (2011) found that pioglitazone reduced withdrawal symptoms in rats when subchronic intragastric alcohol administration was discontinued. PPAR-γ treatments may also provide protection against adverse effects of prenatal alcohol exposure by increasing expression and function of insulin-responsive genes, and by reducing cellular oxidative stress (de la Monte & Wands, 2010). Treatment with the PPAR-α agonist fenofibrate also had neuroprotective effects in rats that were exposed to alcohol in utero and during lactation, preventing hyperactivity and increasing attention (Marche et al., 2011). Other evidence suggests PPAR-α, δ or γ agonist treatments might reduce the severity of chronic ethanol-induced liver injury and insulin/insulin-like growth factor resistance (de la Monte et al., 2011).
6.2. Nicotine
PPAR-α ligands reduce the reinforcing effects of nicotine in a number of animal models. Initial work (Mascia et al., 2011) involved the experimental PPAR agonists WY14643 (a synthetic ligand) and methOEA (a long-lasting form of the endogenous ligand OEA). Subsequent work (Panlilio et al., 2012) has extended these findings to clofibrate, a representative of the fibrate class of medications that is widely prescribed to treat lipid disorders. All of these PPAR agonists reduce intravenous nicotine self-administration in rats and squirrel monkeys. These effects are blocked by treatment with a selective PPAR-α antagonist, and they are specific for nicotine reward, having no effect on behavior reinforced by food or cocaine. In animal models of relapse, PPAR-α agonists prevented reinstatement induced by either nicotine priming or re-exposure to nicotine-associated cues in monkeys, and it prevented reinstatement induced by a combination of nicotine priming and cues in rats. Thus, the results of these preclinical studies suggest that PPAR-α-activating treatments could be useful for reducing tobacco smoking and for maintaining abstinence by preventing relapse.
Investigations into the mechanism of these effects have taken two approaches in rats, electrophysiological (i.e., single-cell recording) and neurochemical (microdialysis and mass spectrometry). Nicotine is believed to produce rewarding effects by binding to acetylcholine receptors in the VTA, enhancing the firing of dopamine neurons that project to the nucleus accumbens shell (Mansvelder & McGehee, 2002; Ikemoto, 2007). PPAR-α agonists reduce or prevent nicotine from increasing the firing rate of dopamine neurons in the VTA (Melis et al., 2008, 2010; Panlilio et al., 2012). Consistent with this electrophysiological evidence, neurochemical evidence indicates that these treatments decrease nicotine-induced elevations of extracellular dopamine in the nucleus accumbens shell (Mascia et al., 2011; Panlilio et al., 2012). These effects are believed to be due to PPAR-α activation stimulating tyrosine kinases, which phosphorylate and negatively regulate α7 nicotinic acetylcholine receptors (Melis et al., 2010).
Demonstrations that exogenous PPAR-α agonists counteract the effects of nicotine suggest that the effects of FAAH inhibition on nicotine reward and related processes are due to FAAH inhibition enhancing levels of fatty acid amides, including OEA and PEA, that are endogenous PPAR-α agonists. However, activation of CB1 receptors, presumably by anandamide, also appears to contribute to the effects of FAAH inhibition. For example, the ability of the FAAH inhibitor URB597 to prevent nicotine’s effects on medium spiny neurons in the nucleus accumbens shell can be reduced by either the PPAR-α antagonist MK886 or by the cannabinoid CB1 antagonist/inverse agonist rimonabant (Luchicchi et al., 2010). In the VTA, URB597’s ability to reverse nicotine-induced burst firing of dopamine cells was PPAR-α-dependent, but its ability to reverse nicotine-induced increases in firing rate of these cells was CB1-dependent (Melis et al., 2010).
About 30% of smoking-attributable mortality is due to cardiovascular disease (CDC, 2008). Currently, members of one class of PPAR-α agonists, the fibrates, are prescribed to prevent cardiovascular disease associated with abnormal lipid profiles. Thus, the recent finding that clofibrate, a representative of the fibrate class, shows promise in preclinical models of nicotine dependence and relapse suggests that PPAR-α treatments might have the dual effect of reducing smoking and reducing the cardiovascular sequelae of smoking (Panlilio et al., 2012). Consistent with this possibility, fibrate treatment has been shown to attenuate nicotine-induced vascular endothelial dysfunction in rats (Chakkarwar, 2011).
6.3. Psychostimulants and opioids
To our knowledge, there has been only one study of the effects of PPAR ligands on the self-administration of a drug other than alcohol or nicotine. Mascia et al. (2011) found that – at doses that block the rewarding effects of nicotine – the PPAR-α agonist WY14643 did not alter cocaine self-administration in monkeys. They also found that WY14643 did not alter cocaine’s ability to elevate extracellular dopamine levels in the nucleus accumbens shell of rats. These findings are consistent with the finding that FAAH inhibition, which increases levels of endogenous PPAR-α agonists, did not alter self-administration of cocaine in monkeys (Justinova et al., 2008a).
Two studies have focused on the role of PPARs in sensitization to the locomotor effects of drugs of abuse (Maeda et al., 2007; Fernandez-Espejo et al., 2009), which is considered a model of neural plasticity that underlies addiction (Robinson & Berridge, 1993). Interest in the effects of PPAR ligands on locomotor sensitization arose from findings that inflammatory mediators may play a role in psychostimulant effects (Zalcman et al., 1999; Nakajima et al., 2004) and from the fact that PPAR agonists have potent anti-inflammatory properties (Varga et al., 2011). Maeda et al. (2007) found that expression (but not acquisition) of locomotor sensitization to methamphetamine in mice was prevented by treatment with the PPAR-γ agonists pioglitazone or ciglitazone and enhanced by treatment with the PPAR-γ antagonist GW9662. Fernandez-Espejo et al. (2009) also found a selective effect of a PPAR ligand on expression (but not acquisition) of locomotor sensitization, but in this case the sensitization was induced by morphine and the expression was prevented by the PPAR-α agonist WY14643. Consistent with this finding, Fernandez-Espejo et al. (2009) found that sensitization to the locomotor-activating effects of morphine was enhanced in PPAR-α-null mice compared to wild-type mice; sensitization to cocaine was not altered in PPAR-α-null mice.
Repeated methamphetamine exposure increases activity of PPAR-γ in whole brain and increases expression of PPAR-γ in nucleus accumbens (Maeda et al., 2007). In wild-type mice that received either cocaine or morphine for 1–5 days, expression of PPAR-α increased with repeated administration only in morphine-treated subjects (Fernandez-Espejo et al., 2009). These changes in PPAR are consistent with inflammation playing a modulatory role in locomotor sensitization to methamphetamine and morphine.
Other studies suggest that PPARs are involved in some of the neurotoxic effects of methamphetamine and MDMA, probably through inflammation-related mechanisms, and that pretreatment with PPARs agonists during methamphetamine exposure might protect against this toxicity. Tsuji et al. (2009) found that methamphetamine exposure drastically reduced PPAR-γ expression in mouse striatum. This and other neurotoxic effects (i.e., reductions in dopamine transporter levels and accumulation of activated microglial cells) were prevented by pretreatment with PPAR-γ ligands. Plaza-Zabala et al. (2010) found that four days of exposure to MDMA produced lasting learning and memory impairments in mice, but that pretreatment with the endogenous PPAR-α agonist OEA at a dose of 5 mg/kg/day prevented these deficits. However, a higher dose of OEA (25 mg/kg/day) increased the deficit induced by MDMA. Both doses of OEA prevented MDMA from decreasing dopamine transporter binding sites in the striatum, indicating that the cognitive deficit blocked by OEA was not mediated by striatal dopamine transporters.
7. Conclusions
FAAH and PPARs are emerging new targets for the treatment of cognitive disorders and drug addiction. FAAH inhibition might provide a means of manipulating the endogenous cannabinoid system to achieve therapeutic effects without the unwanted side effects associated with directly administering cannabinoid receptor agonists. Some of the effects of FAAH inhibition are due to its indirect effects on cannabinoid receptors, and some are due to its indirect effects on PPARs. Regarding both memory and drug reward, the possibility remains that some of the CB1 and PPAR effects of FAAH inhibition actually oppose each other. It remains to be seen whether there is an advantage to activating both systems, or whether it would be beneficial to develop compounds that will selectively enhance endogenous signaling of CB1 and PPAR independently. FAAH-based treatments have not yet been approved for human use, but preclinical studies indicate that they show promise for treating a wide range of cognitive disorders, including depression, anxiety, memory impairment, post-traumatic stress syndrome, and inflammation-related neurodegeneration. FAAH-based treatments also show promise for the treatment of addiction, particularly with cannabis and nicotine dependence, but also with a number of other drugs. Unfortunately, at least one FAAH inhibitor has been shown to be self-administered by laboratory animals, so it is clear that any potentially therapeutic drug that increases levels of the endocannabinoids anandamide or 2-AG should be evaluated for abuse potential. PPAR-based treatments are currently used to treat certain metabolic disorders (e.g., hyperlipidemia, diabetes), and these compounds or newly developed compounds might be useful for treating addiction; however, no PPAR-based treatments have yet been approved for cognitive or addiction disorders. The preliminary evidence suggests that PPAR-based treatments might be effective for treating alcohol and nicotine dependence, possibly as an aid to both the achievement of abstinence and the avoidance of relapse. However, it is clear that much remains to be done, including continued preclinical work to further understand the mechanisms underlying the effects of these treatments and refine the techniques for manipulating them, as well as clinical trials to extend the preclinical findings and bring beneficial treatments into use.
Acknowledgments
Preparation of this manuscript was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services and Department of Psychiatry, MPRC, University of Maryland School of Medicine.
Abbreviations
- AD
Alzheimer’s disease
- 2-AG
arachidonoylglycerol
- CB
cannabinoid
- COX
cyclooxygenase
- FAAH
fatty acid amide hydrolase
- FAE
fatty acid ethanolamide
- FLAT
FAAH-like anandamide transporter
- GABA
γ-aminobutyric acid
- LOX
lipoxygenase
- MAPK
mitogen-activated protein kinase
- MDMA
methylenedioxymethamphetamine
- MGL
monoacylglycerol lipase
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PPAR
peroxisome proliferator-activated receptor
- PPARs
peroxisome proliferator-activated receptors
- THC
Δ9-tetrahydrocannabinol
- TRP
transient receptor potential calcium channels
- VTA
ventral tegmental area
Footnotes
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Lowe JA, Martin BR. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol. 1995;282:R1–R2. doi: 10.1016/0014-2999(95)00447-s. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Lowe JA, Martin BR. Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther. 1996;278:1290–1295. [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Razdan RK, Martin BR. Anandamide, an endogenous cannabinoid, has a very low physical dependence potential. J Pharmacol Exp Ther. 1998;287:598–605. [PubMed] [Google Scholar]
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol. 2009;60:119–125. [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104:518–532. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol: complete substitution with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. doi: 10.1016/j.pbb.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Gomez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, et al. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol. 2008;153:216–225. doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon N, Leroy S, Roy C, Bourdel MC, Charles-Nicolas A, Krebs MO, et al. (AAT)n repeat in the cannabinoid receptor gene (CNR1): association with cocaine addiction in an African–Caribbean population. Pharmacogenomics J. 2006;6:126–130. doi: 10.1038/sj.tpj.6500352. [DOI] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C, Goldberg SR, Justinova Z. Cannabinoid CB1 receptors are not involved in the discriminative-stimulus effects of methamphetamine or cocaine in rats. Neuroscience Meeting Planner; Atlanta, GA. Society for Neuroscience; 2006. 2006 Online Program No. 689.22. [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK–N–SH cells. J Neurochem. 1999;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Benyamina A, Kebir O, Blecha L, Reynaud M, Krebs MO. CNR1 gene polymorphisms in addictive disorders: a systematic review and a meta-analysis. Addict Biol. 2011;16:1–6. doi: 10.1111/j.1369-1600.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- Bequet F, Uzabiaga F, Desbazeille M, Ludwiczak P, Maftouh M, Picard C, et al. CB1 receptor-mediated control of the release of endocannabinoids (as assessed by microdialysis coupled with LC/MS) in the rat hypothalamus. Eur J Neurosci. 2007;26:3458–3464. doi: 10.1111/j.1460-9568.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- Bergman J, Olajire S, Makriyannis A, Justinova Z, Goldberg SR. Discriminative-stimulus and reinforcing effects of FAAH inhibitors in CB-1 trained subjects. FASEB J. 2011;25:796.4. [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Boctor SY, Martinez JL, Jr., Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol. 2007;571:39–43. doi: 10.1016/j.ejphar.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosue S, Pegorini S, Sala M. 3,4 Methylenedioxymethamphetamine-induced conditioned place preference (CPP) is mediated by endocannabinoid system. Pharmacol Res. 2005;51:177–182. doi: 10.1016/j.phrs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001a;104:923–926. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol. 2001b;413:227–234. doi: 10.1016/s0014-2999(01)00766-x. [DOI] [PubMed] [Google Scholar]
- Braida D, Sala M. Role of the endocannabinoid system in MDMA intracerebral self-administration in rats. Br J Pharmacol. 2002;136:1089–1092. doi: 10.1038/sj.bjp.0704825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–3149. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, et al. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A. 2009;106:8027–8031. doi: 10.1073/pnas.0903038106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Castane A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- CDC Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Chakkarwar VA. Fenofibrate attenuates nicotine-induced vascular endothelial dysfunction in the rat. Vascul Pharmacol. 2011;55:163–168. doi: 10.1016/j.vph.2011.08.215. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, et al. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, et al. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. The fatty-acid amide hydrolase inhibitor URB597 does not affect triacylglycerol hydrolysis in rat tissues. Pharmacol Res. 2006;54:341–344. doi: 10.1016/j.phrs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, et al. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, et al. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2:161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, et al. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Colleoni M. Precipitated and spontaneous withdrawal in rats tolerant to anandamide. Psychopharmacology (Berl) 2000;149:121–128. doi: 10.1007/s002139900360. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Kranzler H. Association study of cannabinoid receptor gene (CNR1) alleles and drug dependence. Mol Psychiatry. 2001;6:501–502. doi: 10.1038/sj.mp.4000925. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17:179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- Daza-Losada M, Minarro J, Aguilar MA, Valverde O, Rodriguez-Arias M. Acute blockade of CB1 receptor leads to reinstatement of MDMA-induced conditioned place preference. Pharmacol Biochem Behav. 2011;100:33–39. doi: 10.1016/j.pbb.2011.07.011. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Pang M, Chaudhry R, Duan K, Longato L, Carter J, et al. Peroxisome proliferator-activated receptor agonist treatment of alcohol-induced hepatic insulin resistance. Hepatol Res. 2011;41:386–398. doi: 10.1111/j.1872-034X.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol. 2010;17:e390–e404. [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. Tolerance to the disruptive effects of delta(9)-THC on learning in rats. Pharmacol Biochem Behav. 2002;74:129–140. doi: 10.1016/s0091-3057(02)00966-8. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, Gonzalez S, Cavaliere P, Romero J, et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinoltolerant rats. J Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229–233. doi: 10.1016/j.tips.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Dionisi M, Alexander SP, Bennett AJ. Oleamide activates peroxisome proliferator-activated receptor gamma (PPARgamma) in vitro. Lipids Health Dis. 2012;11:51. doi: 10.1186/1476-511X-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV, Hohmann AG. Endocannabinoid modulation of amphetamine sensitization is disrupted in a rodent model of lesion-induced dopamine dysregulation. Synapse. 2009;63:941–950. doi: 10.1002/syn.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, et al. FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology. 2010;35:1775–1787. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, et al. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav. 2005;81:343–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Hashimoto A, Fecik RA, Hedrick MP, Hanus LO, Boger DL, et al. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J Pharmacol Exp Ther. 2001;299:332–342. [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fernandez JR, Allison DB. Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs. 2004;5:430–435. [PubMed] [Google Scholar]
- Fernandez-Espejo E, Ramiro-Fuentes S, Rodriguez de Fonseca F. The absence of a functional peroxisome proliferator-activated receptor-alpha gene in mice enhances motor sensitizing effects of morphine, but not cocaine. Neuroscience. 2009;164:667–675. doi: 10.1016/j.neuroscience.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, et al. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum Genet. 2006;120:581–588. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration–comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez de Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, et al. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- Gelman L, Michalik L, Desvergne B, Wahli W. Kinase signaling cascades that modulate peroxisome proliferator-activated receptors. Curr Opin Cell Biol. 2005;17:216–222. doi: 10.1016/j.ceb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK. Going nuclear in metabolic and cardiovascular disease. J Clin Invest. 2006;116:556–560. doi: 10.1172/JCI27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205. doi: 10.1177/0269881108089809. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Batkai S, Nikas SP, Cinar R, Offertaler L, et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Di Marzo V, Hernandez M, Arevalo C, Nicanor C, et al. Behavioral and molecular changes elicited by acute administration of SR141716 to delta9-tetrahydrocannabinol-tolerant rats: an experimental model of cannabinoid abstinence. Drug Alcohol Depend. 2004a;74:159–170. doi: 10.1016/j.drugalcdep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Schmid PC, Fernandez-Ruiz J, Krebsbach R, Schmid HH, Ramos JA. Region-dependent changes in endocannabinoid transmission in the brain of morphine-dependent rats. Addict Biol. 2003;8:159–166. doi: 10.1080/1355621031000117383. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Valenti M, de Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, et al. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004b;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Sesay J, Sexton CA, Riedel G, Hampson RE. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61:1016–1025. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, et al. Antagonist-elicited cannabis withdrawal in humans. J Clin Psychopharmacol. 2011;31:603–612. doi: 10.1097/JCP.0b013e31822befc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.72. http://dx.doi.org/10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, et al. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.90. http://dx.doi.org/10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Yamamoto T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology. 2008;55:1300–1306. doi: 10.1016/j.neuropharm.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, et al. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T, et al. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Ishiguro H, Higuchi S, Onaivi ES, Arinami T. Association study between alcoholism and endocannabinoid metabolic enzyme genes encoding fatty acid amide hydrolase and monoglyceride lipase in a Japanese population. Psychiatr Genet. 2007;17:215–220. doi: 10.1097/YPG.0b013e32809913d8. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. R)-methanandamide and delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with delta9-THC or (R)-methanandamide (AM-356) Psychopharmacology (Berl) 2006;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Jehl-Pietri C, Bastie C, Gillot I, Luquet S, Grimaldi PA. Peroxisomeproliferator-activated receptor delta mediates the effects of long-chain fatty acids on post-confluent cell proliferation. Biochem J. 2000;350(Pt 1):93–98. [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Barnes C, Yasar S, Vadivel SK, Makriyannis A, Piomelli D, et al. Effects of modulation of endocannabinoid levels on the discriminativestimulus effects of methamphetamine and cocaine in rats. Neuroscience Meeting Planner; San Diego, CA. Society for Neuroscience; 2007. 2007 Online Program No. 911.11. [Google Scholar]
- Justinova Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict Biol. 2011a;16:405–415. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005a;81:285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008a;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, et al. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008b;33:2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005b;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. 2011b;31:7043–7048. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992a;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992b;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13–20. doi: 10.1151/spp021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- Lallemand F, De Witte P. SR147778, a CB1 cannabinoid receptor antagonist, suppresses ethanol preference in chronically alcoholized Wistar rats. Alcohol. 2006;39:125–134. doi: 10.1016/j.alcohol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wiggins M, Goldberg SR. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of delta-9-tetrahydrocannabinol in rats. Behav Pharmacol. 2006;17:195–199. doi: 10.1097/01.fbp.0000197460.16516.81. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA, Kendall DA. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol. 2004;141:253–262. doi: 10.1038/sj.bjp.0705607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman A, Fowler CJ. Interaction of ligands for the peroxisome proliferator-activated receptor gamma with the endocannabinoid system. Br J Pharmacol. 2007;151:1343–1351. doi: 10.1038/sj.bjp.0707352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci. 1996;58:L365–L372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, Xi ZX. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology (Berl) 2009;204:1–11. doi: 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, et al. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005a;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005b;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Echeverry-Alzate V, Buhler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26:133–143. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Lopez-Jimenez A, Gorriti MA, Rodriguez de Fonseca F. Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Curr Drug Targets. 2010;11:406–428. doi: 10.2174/138945010790980312. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, et al. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15:277–288. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luconi M, Cantini G, Serio M. Peroxisome proliferator-activated receptor gamma (PPARgamma): is the genomic activity the only answer? Steroids. 2010;75:585–594. doi: 10.1016/j.steroids.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology. 2007;32:1133–1140. doi: 10.1038/sj.npp.1301213. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F. Endogenous cannabinoid and opioid systems and their role in nicotine addiction. Curr Drug Targets. 2010;11:440–449. doi: 10.2174/138945010790980358. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci. 1998;62:2431–2439. doi: 10.1016/s0024-3205(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opin Ther Targets. 2011;15:1085–1097. doi: 10.1517/14728222.2011.594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav. 2009;94:154–162. doi: 10.1016/j.pbb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Manzanedo C, Rodriguez-Arias M, Daza-Losada M, Maldonado C, Aguilar MA, Minarro J. Effect of the CB1 cannabinoid agonist WIN 55212–2 on the acquisition and reinstatement of MDMA-induced conditioned place preference in mice. Behav Brain Funct. 2010;6:19. doi: 10.1186/1744-9081-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche K, Danel T, Bordet R. Fetal alcohol-induced hyperactivity is reversed by treatment with the PPARalpha agonist fenofibrate in a rat model. Psychopharmacology (Berl) 2011;214:285–296. doi: 10.1007/s00213-010-1960-2. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Martin BR. Role of lipids and lipid signaling in the development of cannabinoid tolerance. Life Sci. 2005;77:1543–1558. doi: 10.1016/j.lfs.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Adachi H, Smart RC, Xu X, Arata J, Jetten AM. Correlation between expression of peroxisome proliferator-activated receptor beta and squamous differentiation in epidermal and tracheobronchial epithelial cells. Mol Cell Endocrinol. 1999;147:85–92. doi: 10.1016/s0303-7207(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, et al. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum AL, Limebeer CL, Parker LA. Reducing endocannabinoid metabolism with the fatty acid amide hydrolase inhibitor, URB597, fails to modify reinstatement of morphine-induced conditioned floor preference and naloxoneprecipitated morphine withdrawal-induced conditioned floor avoidance. Pharmacol Biochem Behav. 2010;96:496–500. doi: 10.1016/j.pbb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Chronic delta-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol. 2011;162:1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, et al. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68:256–264. doi: 10.1016/j.biopsych.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28:13985–13994. doi: 10.1523/JNEUROSCI.3221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Uchida N, Nomura A, Ohtani K, et al. A nonsynonymous polymorphism in the human fatty acid amide hydrolase gene did not associate with either methamphetamine dependence or schizophrenia. Neurosci Lett. 2005;376:182–187. doi: 10.1016/j.neulet.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Muldoon PP, Lichtman AH, Parsons LH, Damaj MI. The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence. Life Sci. 2012 doi: 10.1016/j.lfs.2012.05.015. http://dx.doi.org/10.1016/j.lfs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Giordano M, Cabeza R, Henriksen SJ, Mendez Diaz M, Navarro L, et al. Oleamide modulates memory in rats. Neurosci Lett. 2001;313:61–64. doi: 10.1016/s0304-3940(01)02256-x. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Chowen J, Carrera MR, del Arco I, Villanua MA, Martin Y, et al. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Genes and addiction. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gu S, Liu QR. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2012;26:92–103. doi: 10.1177/0269881111400652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Sejal P, Meozzi PA, Myers L, Tagliaferro P, et al. Methods to study the behavioral effects and expression of CB2 cannabinoid receptor and its gene transcripts in the chronic mild stress model of depression. Methods Mol Med. 2006;123:291–298. doi: 10.1385/1-59259-999-0:291. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Perez-Rial S, Palomo T, Manzanares J. Differences in basal cannabinoid CB1 receptor function in selective brain areas and vulnerability to voluntary alcohol consumption in Fawn Hooded and Wistar rats. Alcohol Alcohol. 2004;39:297–302. doi: 10.1093/alcalc/agh063. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA, Randall MD. Time-dependent vascular effects of endocannabinoids mediated by peroxisome proliferator-activated receptor gamma (PPARgamma) PPAR Res. 2009;2009:425289. doi: 10.1155/2009/425289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of delta9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun. 2005;337:824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37:1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(Suppl. 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Melis M. From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem. 2010;17:1450–1467. doi: 10.2174/092986710790980014. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Berrendero F, Suarez J, Bermudez-Silva FJ, Fernandez-Espejo E, Serrano A, et al. Effects of the endogenous PPAR-alpha agonist, oleoylethanolamide on MDMA-induced cognitive deficits in mice. Synapse. 2010;64:379–389. doi: 10.1002/syn.20733. [DOI] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, Kroslak T, Sipe JC, Randesi M, Li D, Hamon S, et al. Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: impact of long repeats of CNR1. Pharmacogenomics J. 2010;10:232–242. doi: 10.1038/tpj.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, et al. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339:173–185. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice OV, Gordon N, Gifford AN. Conditioned place preference to morphine in cannabinoid CB1 receptor knockout mice. Brain Res. 2002;945:135–138. doi: 10.1016/s0006-8993(02)02890-1. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;311:683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Boctor SY, Flores LC, Phelix CF, Martinez JL., Jr. Local pretreatment with the cannabinoid CB1 receptor antagonist AM251 attenuates methamphetamine intra-accumbens self-administration. Neurosci Lett. 2011;489:187–191. doi: 10.1016/j.neulet.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Modulation of extracellular signal-regulated kinases cascade by chronic delta 9-tetrahydrocannabinol treatment. Mol Cell Neurosci. 2004;25:355–362. doi: 10.1016/j.mcn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J Neurochem. 2005;93:984–991. doi: 10.1111/j.1471-4159.2005.03101.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J Neurochem. 2000b;75:2080–2086. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Spinello M, Zagato E, Giagnoni G, et al. Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology. 2000a;39:1331–1336. doi: 10.1016/s0028-3908(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Rubio M, de Miguel R, Fernandez-Ruiz J, Gutierrez-Lopez D, Carai MA, Ramos JA. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009;99:354–358. doi: 10.1016/j.drugalcdep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Rubio M, McHugh D, Fernandez-Ruiz J, Bradshaw H, Walker JM. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci Lett. 2007;421:270–274. doi: 10.1016/j.neulet.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- Saluja I, Granneman JG, Skoff RP. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia. 2001;33:191–204. [PubMed] [Google Scholar]
- Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: an exploratory analysis. Psychopharmacology (Berl) 2009;203:511–517. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Fadda P, Le Foll B, Forget B, Fratta W, Goldberg SR, et al. The endocannabinoid system: a new molecular target for the treatment of tobacco addiction. CNS Neurol Disord Drug Targets. 2008a;7:468–481. doi: 10.2174/187152708786927859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008b;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, et al. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008c;327:482–490. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Gilman JP, Justinova Z, Vemuri VK, Makriyannis A, et al. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633:44–49. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009a;11:342–352. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009b;11:39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132:215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S, Hasanein P. Behavioral effects of fatty acid amide hydrolase inhibition on morphine withdrawal symptoms. Brain Res Bull. 2011;86:118–122. doi: 10.1016/j.brainresbull.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl) 2011;215:665–675. doi: 10.1007/s00213-010-2162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ME, Verty AN, McGregor IS, Mallet PE. A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004;1026:244–253. doi: 10.1016/j.brainres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol. 2004;143:343–350. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol. 2011;164:655–666. doi: 10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Kendall DA, Bennett AJ. Cannabinoids and PPARalpha signalling. Biochem Soc Trans. 2006;34:1095–1097. doi: 10.1042/BST0341095. [DOI] [PubMed] [Google Scholar]
- Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;2007:23513. doi: 10.1155/2007/23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005;168:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms–a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. Time-course of the effects of chronic delta 9-tetrahydrocannabinol on behaviour, body temperature, brain amines and withdrawal-like behaviour in the rat. J Pharm Pharmacol. 1982;34:240–245. doi: 10.1111/j.2042-7158.1982.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Taylor D, Meader N, Bird V, Pilling S, Creed F, Goldberg D. Pharmacological interventions for people with depression and chronic physical health problems: systematic review and meta-analyses of safety and efficacy. Br J Psychiatry. 2011;198:179–188. doi: 10.1192/bjp.bp.110.077610. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenohrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Torres E, Gutierrez-Lopez MD, Borcel E, Peraile I, Mayado A, O’Shea E, et al. Evidence that MDMA (‘ecstasy’) increases cannabinoid CB2 receptor expression in microglial cells: role in the neuroinflammatory response in rat brain. J Neurochem. 2010;113:67–78. doi: 10.1111/j.1471-4159.2010.06578.x. [DOI] [PubMed] [Google Scholar]
- Tourino C, Ledent C, Maldonado R, Valverde O. CB1 cannabinoid receptor modulates 3,4-methylenedioxymethamphetamine acute responses and reinforcement. Biol Psychiatry. 2008;63:1030–1038. doi: 10.1016/j.biopsych.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N. Reduction of nuclear peroxisome proliferator-activated receptor gamma expression in methamphetamine-induced neurotoxicity and neuroprotective effects of ibuprofen. Neurochem Res. 2009;34:764–774. doi: 10.1007/s11064-008-9863-x. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S. Overview of the chemical families of fatty acid amide hydrolase and monoacylglycerol lipase inhibitors. Curr Top Med Chem. 2008;8:247–267. doi: 10.2174/156802608783498005. [DOI] [PubMed] [Google Scholar]
- Vann RE, Walentiny DM, Burston JJ, Tobey KM, Gamage TF, Wiley JL. Enhancement of the behavioral effects of endogenous and exogenous cannabinoid agonists by phenylmethyl sulfonyl fluoride. Neuropharmacology. 2012;62:1019–1027. doi: 10.1016/j.neuropharm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (−/−) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther. 2006;317:251–257. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJ, Taylor DA, Fennessy MR. Attenuation of delta 9-tetrahydrocannabinol-induced withdrawal-like behaviour by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 1981;73:97–98. doi: 10.1007/BF00431112. [DOI] [PubMed] [Google Scholar]
- Vigano D, Cascio MG, Rubino T, Fezza F, Vaccani A, Di Marzo V, et al. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacology. 2003;28:1160–1167. doi: 10.1038/sj.npp.1300117. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Vinklerova J, Novakova J, Sulcova A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol. 2002;16:139–143. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. WIN 55,212-2 decreases the reinforcing actions of cocaine through CB1 cannabinoid receptor stimulation. Behav Brain Res. 2003;141:215–222. doi: 10.1016/s0166-4328(02)00370-4. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology (Berl) 2005;179:498–508. doi: 10.1007/s00213-004-2050-0. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology (Berl) 2006;188:293–305. doi: 10.1007/s00213-006-0506-0. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol. 2007;18:311–319. doi: 10.1097/FBP.0b013e3282186cf2. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, et al. The endogenous cannabinoid anandamide shares discriminative stimulus effects with (9)-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–67. doi: 10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–255. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995a;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Balster RL, Martin BR. Tolerance to the discriminative stimulus effects of delta(9)-tetrahydrocannabinol. Behav Pharmacol. 1993;4:581–585. [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995b;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, et al. A comparison of the discriminative stimulus effects of delta(9)-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995c;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise LE, Harloe JP, Lichtman AH. Fatty acid amide hydrolase (FAAH) knockout mice exhibit enhanced acquisition of an aversive, but not of an appetitive, Barnes maze task. Neurobiol Learn Mem. 2009;92:597–601. doi: 10.1016/j.nlm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Savina I, Wise RA. Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res. 1999;847:276–283. doi: 10.1016/s0006-8993(99)02063-6. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Aliczki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biol Psychiatry. 2007;62:616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]