Abstract
BACKGROUND
Although recent WHO guidelines recommend withdrawing stavudine (d4T) from first-line ART therapy, it remains commonly used in resource-constrained settings. In 2006, WHO recommended decreasing the dose of d4T from 40mg to 30mg to mitigate toxicities. We compared the incidence and severity of peripheral neuropathy (PN) by d4T dose in a retrospective cohort study.
METHODS
Patients’ charts from an ART-naïve population at a rural clinic in KZN, South Africa were retrospectively reviewed for signs and symptoms of incident PN and were graded for severity using the DAIDs scale. Patients enrolled prior to the WHO guideline change were enrolled if they were on d4T 40mg for at least 6 months. After the guideline change all patients were initiated on d4T 30mg.
RESULTS
A total of 475 patients were analyzed; 235 in the 40mg cohort (152.7 person-years [py]) and 240 in the 30mg cohort (244.7py). Incidence of peripheral neuropathy was 90.4/100 py (95% CI:75.9–106.8) in the 40mg cohort versus 40.5/100py (95% CI:32.9–49.3) in the 30mg group (incidence rate ratio [IRR]=0.45, p <0.0001). There was no difference in proportion of severe peripheral neuropathy cases (grade 3/4) between the cohorts; 8.3% in the 40mg group and 8.9% in the 30mg group (p=1.0). In a multivariate analysis risk of peripheral neuropathy was associated with increasing age (HR=1.65 95% CI:1.24–2.19), 40 mg dose (HR=2.1, 95% CI:1.61–2.74) and concurrent tuberculosis therapy (HR= 1.41 95% CI:1.06–1.87).
CONCLUSION
Incidence of peripheral neuropathy in the 40mg cohort was extremely high and though lower in the 30mg cohort, the rate was nonetheless unacceptably high.
INTRODUCTION
South Africa has the largest population of patients living with HIV/AIDS with approximately six million people infected accounting for 17% of the global burden of HIV infection.(1) In South Africa alone it is estimated that over one million people are on antiretroviral treatment.(2) National Antenatal HIV seroprevalence studies indicate a prevalence of 29.3% and in the province of Kwa-Zulu Natal prevalence as high as 38% (37.2 – 40.1).(3)
Stavudine (d4T) has been a critical component of combination antiretroviral therapy (cART) in resource constrained settings due to its affordability and high barrier to resistance. A survey of 23 resource limited countries found that 70% of patients on first-line cART are on a regimen containing d4T.(4) Despite its therapeutic impact, d4T has an unfavorable side-effect profile that includes peripheral neuropathy, lactic acidosis, and lipoatrophy. These clinical toxicities are linked to altered mitochondrial DNA replication. Peripheral neuropathy is a significant barrier to quality of life, and the incidence of d4T related peripheral neuropathy prior to dose reduction in patients on a regimen containing d4T is reported as high as 56%.(5)
The dose-dependent neuropathic effects of d4T were shown in a randomized trial with neuropathy observed in 6% on a dose of 0.5mg/kg/day, 15% on 1 mg/kg/day and 31% on 2 mg/kg/day.(6) Given this toxicity profile, efforts were made to assess whether a reduced dose of d4T would decrease side effects while maintaining viral efficacy. Controlled studies have shown that decreasing the dose of d4T from 40mg to 30mg twice daily leads to a significant increase in mitochondrial DNA content in peripheral blood mononuclear cells (7) and adipocytes (8) while preserving HIV virologic suppression. These studies resulted in the 2006 WHO mandate of decreasing the dose of d4T from 40mg to 30mg twice daily to mitigate toxic side effects including peripheral neuropathy. Prior to the guideline change, the WHO recommended a dose of d4T 40mg for patients with a weight greater than 60kg. Patients who weighed less than 60kg were started on d4T 30mg and if they gained weight would be dose escalated according to the guidelines.(9)
Recent WHO guidelines recommend withdrawing d4T from first-line cART therapy for medications with a more favorable side effect profile including tenofovir.(10) South African national guidelines, implemented in April 2010, also mandated a switch to alternative antiretroviral agents; however, the reality of achieving this phase out of d4T is constrained by high costs and limited supply.(11) Guidelines note that if a patient is tolerating a regimen containing d4T, they should remain on d4T. There are also many resource-limited countries where d4T remains first-line therapy.
As many people will continue on a d4T-based regimen, it is important to optimize the dose of the drug to prevent the development of peripheral neuropathy and other potential dose-dependent toxicities. The aim of this study was to determine if there has been a decrease in incidence and severity of peripheral neuropathy since the dose reduction from 40 to 30 mg and to identify factors associated with a higher risk of developing neuropathy.
METHODS
This was a retrospective cohort study of HIV–infected patients initiated on cART at Vulindlela, CAPRISA’s (Center for AIDS Programme of Research in South Africa) rural outpatient clinic in the province of Kwa-Zulu Natal. Vulindlela is a predominantly Zulu speaking community with a population of approximately 400,000 people. Clinical management of patients is based on the South African Chronic Care management and Treatment Guidelines for HIV/AIDS, which follow WHO guidelines. Charts were divided into two separate cohorts based on the timing of the government mandated dose reduction. Cohort 1 was enrolled from 2004 and treated with a cART regimen containing d4T 40mg twice daily for at least 6 months. Cohort 2 comprised patients enrolled at Vulindlela after the dose reduction strategy was introduced in November 2006 and started on a cART regimen containing d4T 30mg twice daily. Patients in Cohort 1 who had their dose of d4T reduced secondary to decrease in weight below 60kg were not reassigned to Cohort 2 and per the inclusion criteria had to be maintained on d4T 40mg for at least 6 months. Furthermore all patients in Cohort 1 were censored at the time of the guideline change in November 2006.
Based on the sample size calculation detailed below, approximately 300 patients per dosing cohort were selected from a cohort of 2,000 patients in care at Vulindlela as follows: Patients in cohort 1 were selected consecutively based on treatment initiation date from the dose reduction in April 2006 backwards to June 2004. Patients in Cohort 2 were selected consecutively from the dose reduction in November 2006 forwards to April 2009. Patients were excluded if they had a history of prior cART, history of peripheral neuropathy, less than 6 months follow up and if they had missing clinical charts. Variables collected from charts included HIV diagnosis date, antiretroviral start date, baseline CD4 cell count, baseline log viral load, development of adverse events, and antiretroviral regimen change.
The primary outcome was the development of peripheral neuropathy related to d4T. Charts were retrospectively reviewed for signs and symptoms of new onset peripheral neuropathy and were graded for severity from grades 1 to 4 using the Division of AIDS Table for Grading Adult and Pediatric Adverse Events version 1.0. Grade 1, or mild PN, was defined as minimal sensory alteration/paresthesia causing little interference with usual or functional or social activities. Grade 2, or moderate PN, was defined as sensory alteration/paresthesia causing greater than minimal interference with usual functional or social activities. Grade 3, or severe PN, was defined as sensory alteration/paresthesia causing inability to perform usual social and functional activities. Grade 4 was defined as disabling sensory alteration/paresthesia causing inability to perform basic self-care functions.(12)
Data analysis was conducted using SAS version 9.1.3. Time to peripheral neuropathy was analyzed with the use of Kaplan-Meier curves and the log-rank test. Duration in the study was calculated as the time from antiretroviral start to the first episode of peripheral neuropathy, withdrawal from study, or at 24 months of follow up, whichever occurred first. Poisson approximations were used to calculate confidence intervals for the rate of peripheral neuropathy. Proportional-hazards regression models were used to adjust for potential confounding variables in the multivariate analysis. Fisher’s exact test was used for the analysis of categorical data, and unpaired t-tests or Wilcoxon two-sample test for the analysis of continuous data. 298 patients were required in each cohort to detect a 10% difference in incidence of peripheral neuropathy between the 30mg cohort and the 40mg cohort with 80% power and an alpha of 0.5.
The study was approved by the University of KwaZulu-Natal’s Biomedical Research Ethics Committee and the Weill Cornell Medical College Institutional Review Board.
RESULTS
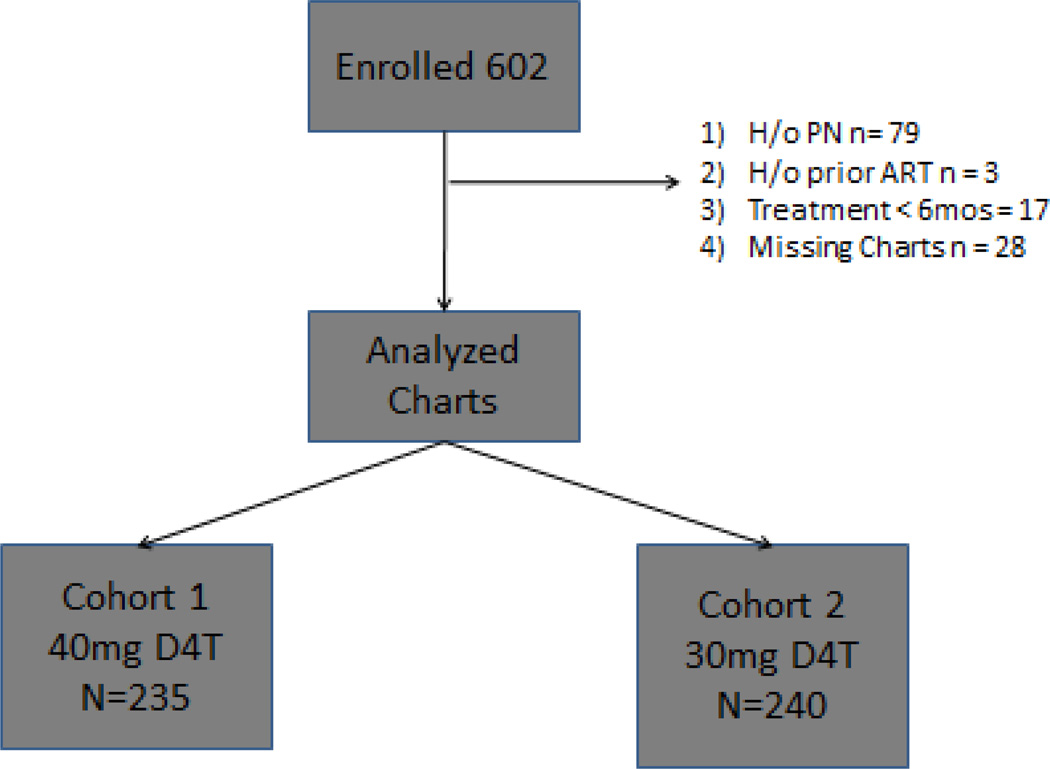
Of the 602 patients selected, 127 were excluded. Patients were excluded if they had any treatment with antiretrovirals prior to enrolment into the study (n=3). Patients were also excluded if they had a history of peripheral neuropathy (n=79), if they had less than 6 months follow up (n=17) and if they had missing clinical charts (n=28). A total of 475 patients were analyzed; 235 in the 40mg cohort (cohort 1) with 152.7 person-years (py) of follow up and 240 in the 30mg cohort (cohort 2) with 244.7 py of follow up. 48 patients in Cohort 1 were initially started on 30mg of d4T and dose escalated to 40mg when their weight met the WHO criteria for dose escalation. 18.7 of the total person years in Cohort 1 were spent on 30mg prior to the dose-escalation. The baseline characteristics of these patients are summarized in Table 1. There were no significant differences between the cohorts in age, sex, history of tuberculosis treatment, nadir CD4 cell count and log viral load at baseline. There was a significant difference between the two cohorts in patients with concurrent tuberculosis with more cases in the 30mg cohort. The mean weight of subjects was also significantly greater in the 40 mg cohort.
Table 1.
Baseline characteristics
| Variables | Cohort 1: d4T 40mg (n=235) |
Cohort 2: d4T 30mg (n=240) |
P-Value |
|---|---|---|---|
| Age (mean, SD) | 34 (8.3) | 33 (9.0) | 0.20 |
| Female Sex | 169 (71.5%) | 163 (67.2%) | 0.40 |
| Nadir CD4 count cells/uL; median (range) | 120 (2–391) | 135 (2–648) | 0.39 |
| Log HIV-1Viral Load median copies/ml (range) | 5.1 (2.6 – 6.6) | 5.1 (1.6 – 6.9) | 0.12 |
| Weight kg mean (range/SD) | 66.2 (42–111/10.5) | 56.4 (31–84/10.0) | <0.0001 |
| BMI kg/m2 (range) | 24.8 (14.5 – 49.3) | 22.8 (12.6 – 42.6) | <0.0001 |
| History of TB Treatment | 47 (20.4%) | 64 (26.6%) | 0.13 |
| Current TB Treatment | 42 (18.3%) | 95 (39.8%) | <0.0001 |
| Highest Grade of PN: | |||
| Grade1 | 60 | 20 | |
| Grade2 | 82 | 72 | |
| Grade3 | 8 | 8 | |
| Grade4 | 6 | 1 |
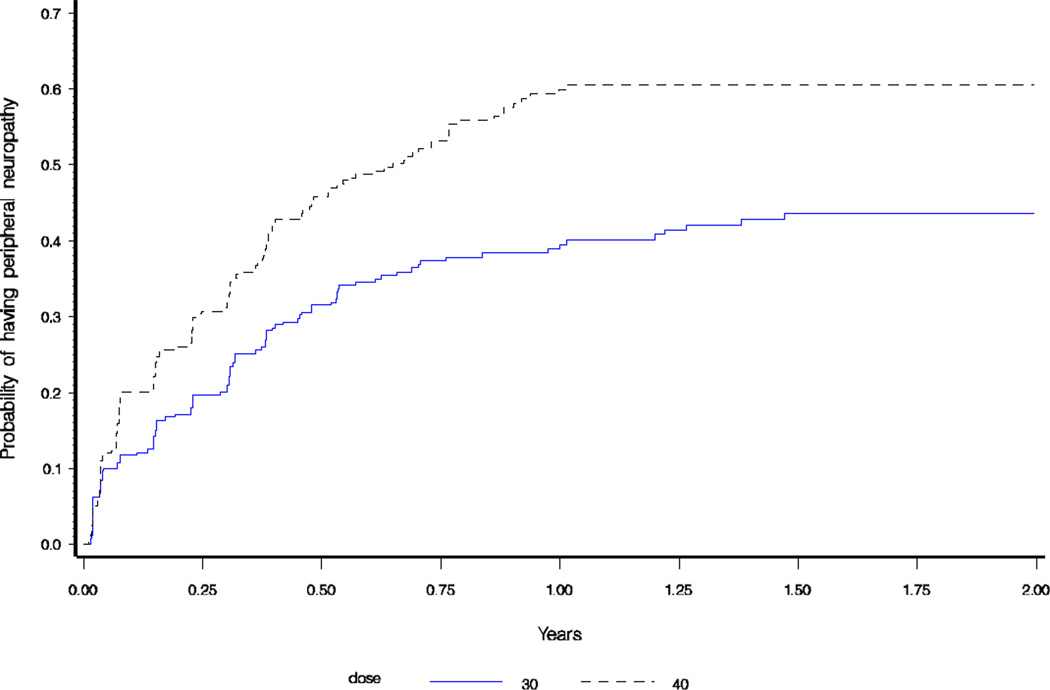
Incidence of peripheral neuropathy was 90.4/100 py (95% CI:75.9–106.8) in the 40mg cohort versus 40.5/100py (95% CI:32.9–49.3) in the 30mg group (incidence rate ratio [IRR]=0.45, p <0.0001). In patients who were started on 40mg and reduced to 30mg after the mandated dose reduction, the incidence of peripheral neuropathy was 45.2/100py (95% CI:21–54) and was not statistically different from patients initiated on 30mg alone (IRR=0.89, p=0.62). As seen in Figure 2, there was a statistically significant difference between time to developing peripheral neuropathy in the two cohorts (p <0.0001).
Figure 2.
Kaplan-Meier Failure Plot of time to peripheral neuropathy
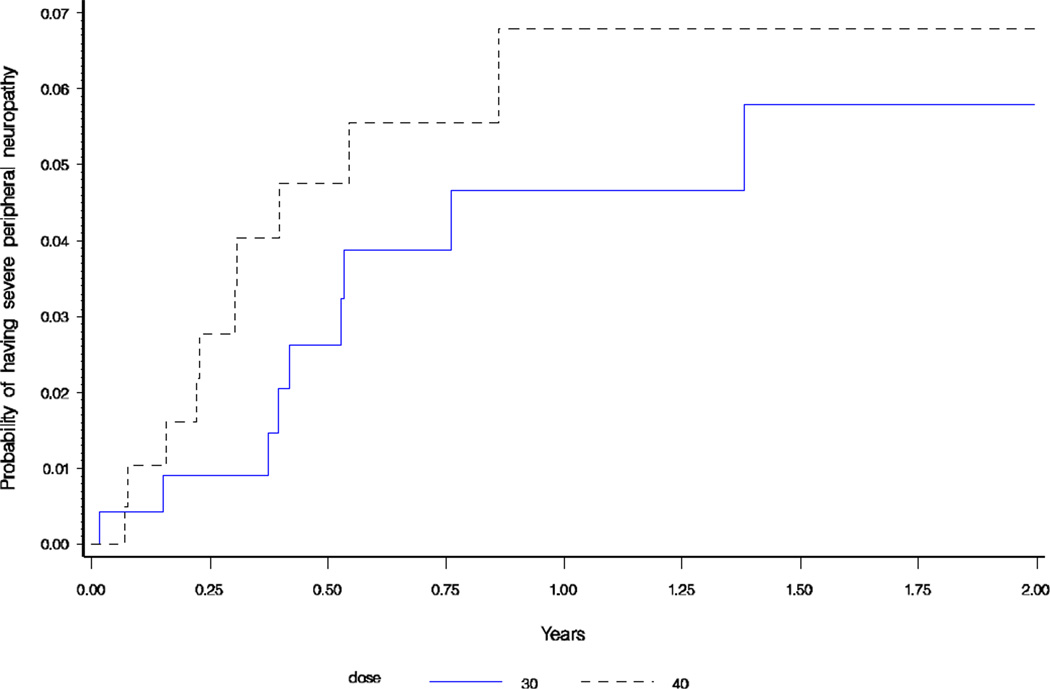
There was no difference in proportion of severe peripheral neuropathy cases (grade 3/4) between the two cohorts; 8.3% in the 40mg group and 8.9% in the 30mg group (p=1.0). As seen in Figure 3, there was no difference in time to developing severe peripheral neuropathy between the two cohorts (p=0.22).
Figure 3.
Kaplan-Meier Failure Plot of time to severe peripheral neuropathy
In univariate analysis increasing age (HR=1.78 CI:1.35–2.35) and d4T dose (HR=2.0, 95% CI: 1.58–2.61, with 30 mg dose as reference group) were the only significant predictors of the development of peripheral neuropathy. In a multivariate analysis (Table 2) an increased risk of developing peripheral neuropathy was associated with increasing age (HR=1.65 95% CI:1.24–2.19), d4T dose (HR=2.1, 95% CI:1.61–2.74) and concurrent tuberculosis therapy (HR= 1.41 95% CI:1.06–1.87); however, baseline CD4 cell count, sex, and weight were not associated with an increased risk of developing peripheral neuropathy. In univariate analysis concurrent tuberculosis was not related to an increased risk of developing peripheral neuropathy; however, in multivariate analysis concurrent tuberculosis was associated with an increased risk. This could be explained by the interaction between concurrent tuberculosis and d4T dose (p-value=0.0986). We fitted a multivariate model including the interaction term and similar results were obtained (data not shown). History of TB treatment prior to enrolment was not associated with an increased risk of developing peripheral neuropathy. During the study duration Pyridoxine was routinely given in conjunction with Isoniazid. A separate multivariate model was constructed that included log baseline viral load, which was available on 330 patients. The other covariates were not qualitatively different when the multivariate model was fitted with viral load (data not shown).
Table 2.
Multivariate analysis of risk factors for development of peripheral neuropathy
| Variable | Unadjusted Analysis |
P-Value | Adjusted Analysis | P-Value |
|---|---|---|---|---|
| Age (ref < 40) | 1.78 (1.35 to 2.35) | <0.0001 | 1.65 (1.24 to 2.19) | 0.0006 |
| Female Sex | 1.27 (0.96 to 1.67) | 0.10 | 1.23 (0.92 to 1.65) | 0.16 |
| Baseline CD4 Count cells/UL (Ref < 50) | 0.48 | 0.44 | ||
| 50–100 | 1.24 (0.85 to 1.82) | 1.22 (0.82 to 1.81) | ||
| 100–150 | 1.23 (0.86 to 1.74) | 1.27 (0.88 to 1.82) | ||
| 150–200 | 0.93 (0.63 to 1.39) | 1.02 (0.68 to 1.54) | ||
| >200 | 1.22 (0.83 to 1.80) | 1.38 (0.92 to 2.07) | ||
| Dose (ref = 30mg) | 2.03 (1.58 to 2.61) | <0.0001 | 2.10 (1.61 to 2.74) | <0.0001 |
| Past TB | 0.88 (0.65 to 1.18) | 0.39 | 0.91 (0.67 to 1.25) | 0.57 |
| Current TB Treatment | 1.12 (0.86 to 1.47) | 0.39 | 1.41 (1.06 to 1.87) | 0.02 |
| Diabetes | 1.86 (0.46 to 7.47) | 0.38 | 1.24 (0.88 to 1.82) | 0.77 |
| Weight | 1.02 (1.01 to 1.03) | 0.003 | 1.0 (0.99 to 1.02) | 0.30 |
| Viral Load copies/ml (n=330) | 1.14 (0.95 to 1.38) | 0.15 | 1.11 (0.91 to 1.34) | 0.32 |
DISCUSSION
In this retrospective cohort study in a rural clinic in South Africa, we found that use of a lower dose of d4T was associated with a lower incidence of peripheral neuropathy. Incidence of peripheral neuropathy in the 40mg cohort was extremely high and though the incidence was lower in the reduced dose cohort, the rate was nonetheless high.
There was no difference in the proportion of severe peripheral neuropathy between the two cohorts. Symptoms of peripheral neuropathy can often be permanent in patients with severe peripheral neuropathy despite discontinuation of d4T. The development of severe peripheral neuropathy despite decreased dose highlights the need for early discontinuation of d4T in patients who are maintained on d4T despite WHO recommendations.
In South Africa, a recent study looking at PN in d4T-exposed patients after the dose reduction showed similar results. Of the 395 patients enrolled with at least 6 months exposure to d4T, 57% had symptomatic PN and it was frequently associated with moderate to severe pain in the feet.(13) Like other studies, our findings show that older age is independently associated with the development of PN. A similar study in South Africa looking at PN risk factors in all patients with HIV found that 49% (n=598) of their study population was diagnosed with PN and 30% of the study population had symptomatic PN. In this study, d4T use was significantly associated with the development of PN as was older age and history of previous TB infection.(14)
It is well known that the development of peripheral neuropathy impacts quality of life and often symptom management is inadequate. Amitriptyline is often the only medication available for severe peripheral neuropathy in resource constrained settings. Despite the frequent use of amitriptyline it has not been found to be highly efficacious in clinical trials for the treatment of peripheral neuropathy.(15)
Other studies in resource constrained settings have shown that the development of peripheral neuropathy is a predictor of other toxicities including severe hyperlactatemia.(16) The SWITCH study looked at a cohort of 3,333 HIV-infected adults on cART and found the most frequent reason for switching medications was toxicity and of the individual antiretrovirals, stavudine had a significantly higher switch score than other drugs.(17) In India, a study found that 13% of 183 patients receiving a regimen containing d4T stopped therapy secondary to peripheral neuropathy.(18) Similarly a recent study in Rwanda found that of a cohort of 2,190 adults, 175 patients replaced d4T secondary to toxicity and specifically 8.0% for neuropathy.(19) Options for alternative antiretrovirals are limited in resource constrained settings and this poses a grave threat to patients who require life long treatment.
Due to the retrospective nature of this study there are several limitations that are important to note. Patients were not randomized to the two doses of d4T, and temporal trends in patient management could have affected the results. The physical exam and patient interview were not standardized between different clinicians. There was limited data available on potential confounders including history or development of diabetes mellitus, history or current alcohol abuse, and other metabolic abnormalities including vitamin B12 deficiency that can contribute to the development of peripheral neuropathy. Diabetes was only included if the diagnosis was reported by the clinician in the chart and everyone else was considered non-diabetic by default. The study is also limited because the projected sample size was not met. Due to the short duration of time (June 2004 – April 2006) that the patients were enrolled on d4T 40mg prior to the guideline change there were only a limited amount of patients (n=300) that could be enrolled in the study. After excluding patients, 235 patients in Cohort 1 and 240 patients in Cohort 2 were included in the analysis. There was a significant difference between the two cohorts in patients with concurrent tuberculosis with more cases in the 30mg cohort. It is possible that increased access to smear testing and tuberculosis treatment facilities after 2007 contributed to a case finding bias that led to this difference.
There have been few studies in resource-constrained settings looking at the incidence of d4T related peripheral neuropathy and specifically the impact of dose reduction on incidence and severity. Despite dose reduction, patients are at high risk of developing debilitating peripheral neuropathy and in many cases this neuropathy is irreversible if the offending drug is not discontinued in a timely manner. This study brings to light the importance of complying with WHO guidelines and providing acceptable alternatives to d4T despite high cost. In countries where guidelines have not changed, patients are at risk for debilitating, often irreversible side effects and efforts should be made to change country specific guidelines.
Figure 1.
Study Enrollment & Flow Diagram
Acknowledgments
Financial support: This work was supported in part by the National Institute of Allergy and Infectious Diseases (T32 AI007613 and K24 AI 78884). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health Data were presented at the XVIII International AIDS Conferenced, Vienna, Austria July 2010.
Footnotes
There are no conflicts of interest to report.
REFERENCES
- 1.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009 Sep 12;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Republic of South Africa. Pretoria: National Department of Health; 2010. Country Progress Report on the Declaration of Commitment on HIV/AIDS 2010 Report. [Google Scholar]
- 3.National Department of Health South Africa. National HIV and Syphilis Prevalence Survey South Africa 2006. Pretoria: National Department of Health; 2006. [Google Scholar]
- 4.Renaud-Thery F, Nguimfack BD, Vitoria M, Lee E, Graaff P, Samb B, Perriens J. Use of antiretroviral therapy in resource-limited countries in 2006: distribution and uptake of first- and second-line regimens. AIDS. 2007 Jul;21(Suppl 4):S89–S95. doi: 10.1097/01.aids.0000279711.54922.f0. [DOI] [PubMed] [Google Scholar]
- 5.van Oosterhout JJ, Bodasing N, Kumwenda JJ, Nyirenda C, Mallewa J, Cleary PR, de Baar MP, Schuurman R, Burger DM, Zijlstra EE. Evaluation of antiretroviral therapy results in a resource-poor setting in Blantyre, Malawi. Trop Med Int Health. 2005 May;10(5):464–470. doi: 10.1111/j.1365-3156.2005.01409.x. [DOI] [PubMed] [Google Scholar]
- 6.Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Jun 1;9(2):153–161. [PubMed] [Google Scholar]
- 7.Sanchez-Conde M, de Mendoza C, Jimenez-Nacher I, Barreiro P, Gonzalez-Lahoz J, Soriano V. Reductions in stavudine dose might ameliorate mitochondrial-associated complications without compromising antiviral activity. HIV Clin Trials. 2005 Jul-Aug;6(4):197–202. doi: 10.1310/ed57-eu48-rk6a-e5u0. [DOI] [PubMed] [Google Scholar]
- 8.McComsey GA, Lo Re V, 3rd, O'Riordan M, Walker UA, Lebrecht D, Baron E, Mounzer K, Frank I. Effect of reducing the dose of stavudine on body composition, bone density, and markers of mitochondrial toxicity in HIV-infected subjects: a randomized, controlled study. Clin Infect Dis. 2008 Apr 15;46(8):1290–1296. doi: 10.1086/529384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Geneva: World Health Organization; 2006. Addendum to 2006 WHO Guidelines on Antiretroviral Therapy for HIV Infection in Adults and Adolescents. [Google Scholar]
- 10.World Health Organization. Geneva: World Health Organization; 2009. Nov, Rapid Advice: Antiretroviral Therapy for HIV Infection in Adults and Adolescents. 2009. [Google Scholar]
- 11.National Department of Health South Africa. Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. Pretoria: National Department of Health; 2010. [Google Scholar]
- 12.Division of AIDS--University of California at Davis. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events University of California at Davis. 2004 Dec [Google Scholar]
- 13.Wadley AL, Cherry CL, Price P, Kamerman PR. HIV neuropathy risk factors and symptom characterization in stavudine-exposed South Africans. J Pain Symptom Manage. 2011 Apr;41(4):700–706. doi: 10.1016/j.jpainsymman.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Maritz J, Benatar M, Dave JA, Harrison TB, Badri M, Levitt NS, Heckmann JM. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve. 2010 May;41(5):599–606. doi: 10.1002/mus.21535. [DOI] [PubMed] [Google Scholar]
- 15.Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, Hillman S, Brizz B, Cohn DL. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA. 1998 Nov 11;280(18):1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 16.Osler M, Stead D, Rebe K, Meintjes G, Boulle A. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 2010 Feb;11(2):121–129. doi: 10.1111/j.1468-1293.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 17.Davidson I, Beardsell H, Smith B, Mandalia S, Bower M, Gazzard B, Nelson M, Stebbing J. The frequency and reasons for antiretroviral switching with specific antiretroviral associations: the SWITCH study. Antiviral Res. 2010 May;86(2):227–229. doi: 10.1016/j.antiviral.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kumarasamy N, Vallabhaneni S, Cecelia AJ, Yepthomi T, Balakrishnan P, Saghayam S, Flanigan TP, Carpenter CC, Solomon S, Mayer KH. Reasons for modification of generic highly active antiretroviral therapeutic regimens among patients in southern India. J Acquir Immune Defic Syndr. 2006 Jan 1;41(1):53–58. doi: 10.1097/01.qai.0000188123.15493.43. [DOI] [PubMed] [Google Scholar]
- 19.van Griensven J, Zachariah R, Rasschaert F, Mugabo J, Atte EF, Reid T. Stavudine- and nevirapine-related drug toxicity while on generic fixed-dose antiretroviral treatment: incidence, timing and risk factors in a three-year cohort in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010 Feb;104(2):148–153. doi: 10.1016/j.trstmh.2009.07.009. [DOI] [PubMed] [Google Scholar]