Abstract
Purpose
The purpose of the current study was to explore non-linguistic learning ability in patients with aphasia, examining the impact of stimulus typicality and feedback on success with learning.
Method
Eighteen patients with aphasia and eight healthy controls participated in this study. All participants completed four computerized, non-linguistic category-learning tasks. We probed learning ability under two methods of instruction: feedback-based (FB) and paired-associate (PA). We also examined the impact of task complexity on learning ability, comparing two stimulus conditions: typical (Typ) and atypical (Atyp). Performance was compared between groups and across conditions.
Results
Results demonstrated that healthy controls were able to successfully learn categories under all conditions. For our patients with aphasia, two patterns of performance arose. One subgroup of patients was able to maintain learning across task manipulations and conditions. The other subgroup of patients demonstrated a sensitivity to task complexity, learning successfully only in the typical training conditions.
Conclusions
Results support the hypothesis that impairments of general learning are present in aphasia. Some patients demonstrated the ability to extract category information under complex training conditions, while others learned only under conditions that were simplified and emphasized salient category features. Overall, the typical training condition facilitated learning for all participants. Findings have implications for therapy, which are discussed.
Introduction
Though aphasia is a deficit primarily characterized by impairments in language, an increasing body of research has recently been dedicated to understanding the contribution of cognitive deficits of attention, concept knowledge, executive function and memory on language construction, use and rehabilitation in patients with aphasia (Erickson, Goldinger & LaPointe, 1996; Fridriksson, Nettles, Davis, Morrow, & Montgomery, 2006; Helm-Estabrooks, 2002; Hula & McNeil, 2008; Keil & Kaszniak, 2002; Lesniak, Bak, Czepiel, Seniow, & Czlonkowska, 2008; Murray, 2012; Peach, Rubin & Newhoff, 1994; Ramsberger, 2005; Zinn, Bosworth, Hoenig, & Swartwelder, 2007). Expanding upon these investigations into cognitive deficits that likely impact rehabilitation outcomes, we focus on learning ability, a skill which, to date, has received limited attention in the field of aphasia.
Researchers have identified learning ability as a central factor in rehabilitation (Ferguson, 1999; Hopper & Holland, 2005) whether improvement involves facilitating access to previously mastered information, developing compensatory strategies in light of deficits, or involves new learning (Kelly & Armstrong, 2009; Tuomiranta et al., 2011). In spite of this, only a limited number of studies have been dedicated to understanding learning in aphasia. Studies have shown that patients with aphasia are capable of demonstrating new verbal learning (Breitenstein, Kamping, Jansen, Schomacher, & Knecht, 2004; Freedman & Martin, 2001; Gupta, Martin, Abbs, Schwartz & Lipinski, 2006; Kelly & Armstrong, 2009; Marshall, Neuburger & Phillips, 1992; Tuomiranta et al. 2011) and that in addition, learning ability appears to be related to patients’ profiles of linguistic (Grossman & Carey, 1987; Gupta et al., 2006) and cognitive strengths and deficits (Freedman & Martin, 2001). Our understanding of learning in aphasia is still limited, however, particularly because all recent studies explore verbal learning. Language is the primary deficit in aphasia, so it is likely that language deficits interfere with patterns of learning when tasks are verbal or grammatically structured. We hypothesize that behavioral patterns observed during nonverbal learning tasks will shed new light on the process of learning in patients with aphasia.
As a first step towards examining this possibility, we recently explored nonlinguistic category learning in patients with aphasia and in age-matched controls (Vallila-Rohter & Kiran, 2013). Nineteen patients with aphasia and twelve healthy age-matched controls were tested as they completed non-linguistic, multi-dimensional category learning tasks. Results showed that different profiles of learning arose between healthy controls and patients with aphasia, only eleven out of nineteen patients showing learning of categories compared with across-the-board learning by control participants. Interestingly, measures of patient cognitive or linguistic abilities did not correlate with performance on learning tasks. These results highlighted that non-linguistic learning ability is affected in aphasia. The reasons for incomplete learning in the patient group, however, remain unanswered and merit further investigation.
Though little is known about nonverbal learning in aphasia, studies in other clinical populations and in healthy individuals have investigated patterns of behavior that arise during various types of nonverbal learning. Research has demonstrated that manipulations of training method, stimulus characteristics, category structure, and response selection impact learning results (Ashby, Noble, Filoteo, Waldron. & Ell, 2003; Ashby, Maddox, & Bohil, 2002; Davis, Love, & Maddox, 2009; Filoteo & Maddox, 2007; Knowlton, Squire, & Gluck, 1994; Maddox, Love, Glass, & Filoteo, 2008). Often, manipulations of task and instruction method have been found critical to promoting learning in patients with brain damage. Patients with Parkinson’s Disease (PD), for example, have shown impaired procedural-based learning, information integration and rule-based learning, particularly when stimuli pose high working memory or attention demands (Filoteo & Maddox, 2007; Filoteo, Maddox, Ing, Zizak & Song, 2005; Price, 2006). These patients show intact artificial grammar learning (Smith, Siegert, & McDowall, 2001; Reber & Squire, 1999; Witt, Nuhsman, & Deuschl, 2002) and intact information integration learning under conditions of limited complexity (Ashby et al., 2003; Filoteo, Maddox, Salmon & Song, 2005). Similarly, patients with amnesia are sensitive to instruction method, demonstrating impairments in learning that involves recall and recognition (Filoteo, Maddox, & Davis, 2001; Graf, Squire & Mandler; 1984; Knowlton, Ramus, & Squire, 1992), yet showing successful learning of probabilistic classification tasks (Knowlton et al., 1994).
The mechanism underlying the facilitation or impairment of learning for these patients is thought related to the existence of multiple memory systems that rely on different neurobiological structures and support learning in different ways. Many types of learning rely on recall of individual instances, facts or events consciously or unconsciously in order to form associations between previously unrelated stimuli. This type of learning, termed declarative or explicit learning, is thought to rely heavily on the hippocampus and medial temporal lobe structures (Seger & Miller, 2010; Squire, 1992 for review). Declarative systems are considered important for rule-learning and for paired-associate learning, in which participants store associations between cues and responses (Breitenstein et al., 2005; Squire, 1992; Warringon & Weiskrantz, 1982; Winocur & Weiskrantz, 1976). In their COmpetition between Verbal and Implicit Systems (COVIS) model, Ashby, Alfonso-Reese, Turken and Waldron, (1998) draw attention to the likely engagement of explicit processes in the early stages of many types of category learning (Ashby et al., 1998; Maddox & Ashby, 2004 for review). In these stages, learners are thought to engage logic and reasoning to form hypotheses; often verbalizeable ones. Hypotheses are then tested and results monitored, processes proposed to rely heavily on attention and working memory networks.
In contrast, unconscious systems have been thought critical for gradual learning, particularly of statistical properties, complex or abstract information, and learning via trial-by-trial feedback (Ashby et al., 1998; Keri, 2003; Knowlton, Mangels & Squire, 1996; Knowlton & Squire, 1993; Maddox & Ashby, 2004; Seger & Miller, 2010 for review). This type of learning is carried out via automatic processes that incrementally reinforce experiences (Ashby et al., 1998; Knowlton & Squire, 1993). Research suggests that unexpected rewards trigger the release of dopamine. Release of dopamine gradually strengthens the association between cues and responses (Seger and Miller, 2010; Shohamy et al., 2004; Shohamy, Myers, Kalanithi & Cluck, 2008). Feedback therefore appears to be critical to this type of learning (Ashby et al., 1998; Ashby & Crossley, 2010; Ashby & Maddox, 2011; Ashby & Valentin, 2005; Keri, 2003; Maddox & Ashby, 2004; Seger & Miller, 2010; Smith, Patalano & Jonides, 1998).
Though certain conditions are thought to emphasize the engagement of one system over another, research has suggested that these systems can interact or compete throughout learning (Ashby et al., 2008; Ashby & Crossley, 2012; Ashby & Valentin, 2005; Cincotta & Seger, 2007; Poldrack et al., 2001; Moody, Bookheimer, Vanek & Knowlton, 2004; Seger & Miller, 2010). Of particular relevance to the current study, Ashby et al., (2002) explored learning strategies employed under conditions of paired-associate (observational) and feedback training on an information integration task. Results suggested that the presence of feedback led to an effective reliance on automatic processes of information integration. In contrast, observational paradigms led to a high reliance on rule-based strategies. Researchers proposed that observation learning reduced instances of unexpected reward and therefore interfered with automatic processes of information integration. In our study, we examine the rates of successful learning when participants learn multi-dimensional categories under conditions with and without feedback.
Another factor that we explore in the current study is stimulus complexity, as complexity has been the focus of considerable research in aphasia rehabilitation. Studies in aphasia have noted generalization from complex to less complex related structures, following both syntactic (Thompson, 2001; Thomson, Ballard, & Shapiro, 1998; Thompson, Shapiro, Ballard, Jacobs, Schneider, & Tait, 1997; Thompson, Shapiro, & Roberts, 1993) and semantic therapy (Kiran, 2007; Kiran, 2008; Kiran & Thompson, 2003a, 2003b; Kiran, Sandberg & Sebastian, 2011). This observation led to the formulation of the complexity account of treatment efficacy (CATE) hypothesis (Thompson, Shapiro, Kiran & Sobecks, 2003), a hypothesis that draws attention to the potential impact of stimulus complexity on treatment outcomes and generalization patterns in aphasia.
Motivation for the development of CATE came from results obtained through aphasia treatment studies as well as from connectionist principles of generalization. In his influential paper, Plaut (1996) used connectionist modeling to explore patterns of relearning after damage. One experiment, focused on the impact of training typical or atypical words produced two major findings. First, the retraining simulation showed better learning overall of typical words than of atypical words. Second, and critical to the CATE hypothesis, training on atypical words resulted in substantial generalization to untrained typical words. Plaut posited that training of atypical exemplars highlighted feature variability within a category, simultaneously providing information about the breadth of categories and of central category tendencies. This breadth of information was lacking when models were trained only on typical words and resulted in limited generalization.
In the current study, we aim to better understand non-linguistic category learning ability in aphasia, exploring the impacts of both stimulus characteristics and instruction method on patient success with learning. We examine nonlinguistic learning ability in patients with aphasia and in healthy controls, comparing feedback-based instruction and paired associate instruction. Within these two conditions, we explore the impact of stimulus characteristics, comparing one condition in which training is designed to emphasize salient category features (typical training); and another condition in which training highlights feature variability within categories (atypical training). We will further explore whether demographic variables or standardized measures of cognitive-linguistic ability demonstrate a predictive relationship with patient scores of learning. We hypothesize that participants will learn better under feedback conditions, as research has suggested that implicit systems sensitive to feedback are better suited for complex category learning that requires information integration (Ashby et al., 2002).
We hypothesize that typical training (Typ) will result in better overall learning rates than atypical training (Atyp). Based on connectionist theories, we propose that following training in the Atyp condition, participants will show generalization of learning to typical items.
Materials and Methods
2.1 Participants
Eighteen patients (ten men) with aphasia subsequent to single left hemisphere stroke participated in this study. The mean age of participants was 61.32, SD = 12.17 (ranging from 33.7 to 77.2 years) having completed an average of 15.83 years of education, SD = 2.92 (ranging from 11 to 19 years, see Table 1). Fifteen patients were Caucasian, 2 were Black and one was of Hispanic ethnicity. Patients were tested at least six months after the onset of their stroke and had degrees of severity of aphasia that ranged from mild to severe at the time of testing, as determined by Western Aphasia Battery (WAB, Kertesz, 1982) aphasia quotients (AQ, AQs from 24.8 to 98). Our patient population represented a heterogeneous sample including patients with Conduction, Broca’s, Wernicke’s, Transcortical Motor and Anomic aphasia, classifications determined by the WAB. All patients were premorbidly right handed and were medically and neurologically stable at the time of testing. One patient participant dropped out of the study prior to completing our diagnostic test battery and therefore is missing measures of cognitive-linguistic ability and was not assigned an aphasia type.
Table 1.
Patient and control participants
| ID | Age | Gender | Ed. | MPO | Aphasia Type | Comprehension | Attn. | Mem. | Exec. | Visuospatial | BNT | AQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | 33.7 | F | 14 | 6 | Con. | 91 | WNL | Sev | Mod | WNL | 0 | 24.8 |
| Pt 2 | 49.7 | F | 18 | 24 | An. | 185 | WNL | WNL | WNL | WNL | 100 | 93.9 |
| Pt 3 | 52.7 | F | 12 | 24.8 | Wern. | 116 | WNL | Sev | Mod | WNL | 6.67 | 41.4 |
| Pt 4 | 52.7 | M | 16 | 106.8 | Con./Wern. | 142 | Mild | Sev | WNL | WNL | 6.67 | 48 |
| Pt 5 | 61 | M | 13 | 6 | An. | 192 | Mild | Mild | Sev | Mild | 80 | 91 |
| Pt 6 | 63.7 | F | 18 | 18 | An. | 143 | Mild | Sev | Sev | Mild | 13.33 | 67.7 |
| Pt 7 | 65.7 | F | 18 | 41.5 | Bro. | 120 | Mild | Sev | Sev | Mild | 0 | 28.4 |
| Pt 8 | 69.5 | M | 21 | 27.5 | Wern. | 78 | Mild | Sev | Mild | WNL | 0 | 33.8 |
| Pt 9 | 77.2 | F | 16 | 93.6 | An. | 200 | WNL | WNL | WNL | WNL | 98.3 | 98 |
| Pt 10 | 86.8 | M | 12 | 13.2 | An. | 185 | Mild | Mod | Mild | Mild | 58.33 | 88.1 |
| Pt 11 | 51.9 | M | 11 | 259.6 | An. | 175 | Mod | Sev | Mild | Mild | 31.67 | 61.3 |
| Pt 12 | 59.5 | M | 19 | 26.5 | An. | 178 | WNL | Mod | WNL | WNL | 78.33 | 82.8 |
| Pt 13 | 61 | M | 16 | 44.6 | Con. | 168 | WNL | WNL | WNL | WNL | 43.33 | 67.9 |
| Pt 14 | 63.6 | F | 16 | 64.7 | An. | 174 | WNL | Mod | Mod | WNL | 30 | 69.1 |
| Pt 15 | 67.5 | F | 12 | 28.4 | TCM | 179 | Mod | Sev | Mod | Sev | 83.3 | 82.2 |
| Pt 16 | 68 | M | 19 | 13.2 | An. | 74.3 | Mild | Mild | Mod | Mild | 30 | 74.3 |
| Pt 17 | 47.7 | M | 16 | 86 | 81.67 | |||||||
| Pt 18 | 71.9 | M | 18 | 15 | Con. | 139 | WNL | Mild | WNL | WNL | 85 | 76.7 |
|
| ||||||||||||
| Cn1 | 57.6 | F | 18 | |||||||||
| Cn2 | 57.7 | F | 18 | |||||||||
| Cn3 | 57.2 | F | 16 | |||||||||
| Cn4 | 59.7 | F | 16 | |||||||||
| Cn5 | 69.5 | M | 16 | |||||||||
| Cn6 | 70 | M | 18 | |||||||||
| Cn7 | 72.6 | F | 16 | |||||||||
| Cn8 | 58.7 | M | 16 | |||||||||
Note: Table of participants, age, gender, education (Ed.), months post onset of stroke (MPO), aphasia type, comprehension as determined by the WAB, attention (Att.), memory (Mem.), executive functions (Exec.) and visuospatial skills (Visusopatial) as determined by the cognitive linguistic quick test (CLQT). CLQT scores are within normal limits (WNL), mild, moderate (Mod) or severe (Sev). Scores are provided for the Boston Naming Test (BNT), and AQ is a patient’s aphasia quotient, an indicator of aphasia severity, higher scores representing lower degrees of impairment. Aphasia types are abbreviated as follows: Conduction (Con.), Anomic (An.), Wernicke’s (Wern.), Broca’s (Bro.) and Transcortical Motor (TCM).
Eight healthy control participants (three men) were also recruited to participate in this study. Participants had no known history of neurological disease, psychiatric disorders or developmental speech, language or learning abilities. The mean age of participants was 62.87, SD = 6.58 (ranging from 57.2 to 72.6 years) having completed an average of 16.5 years of education, SD = 1.03 (ranging from 16 to 18 years, see Table 2). One control participant, Cn 4, was left-handed. All control participants were Caucasian. We were most interested in patient patterns of learning and thus included only a small group of similarly aged healthy controls to serve as a baseline.
Table 2.
Slope scores for control and patient participants on baseline conditions, Typ and Atyp training
| Participant | FBBaseline | PABaseline | FBTyp | FBAtyp | PATyp | PAAtyp |
|---|---|---|---|---|---|---|
| Cn1 | *8.70 | *9.22 | *7.27 | *6.74 | *7.84 | −3.55 |
| Cn2 | *6.62 | *8.74 | *11.20 | *8.27 | *12.79 | *4.46 |
| Cn3 | 4.63 | *11.75 | *10.64 | *9.59 | *11.15 | *8.44 |
| Cn4 | *8.96 | *11.49 | *10.73 | −5.39 | *11.21 | 4.31 |
| Cn5 | *7.96 | *10.56 | *9.91 | *9.68 | *9.35 | *7.47 |
| Cn6 | *7.71 | *8.40 | *8.45 | *10.43 | *12.25 | *9.57 |
| Cn7 | *5.89 | *7.53 | *8.48 | *9.89 | *11.26 | *9.63 |
| Cn8 | *10.52 | *9.61 | *7.58 | *7.59 | *9.44 | *8.98 |
|
| ||||||
| Pt 1 | *10.26 | −9.07 | *10.41 | *10.84 | *10.10 | −8.29 |
| Pt 2 | *9.48 | *8.29 | *8.75 | *6.73 | *9.61 | −1.04 |
| Pt 3 | *7.66 | −9.46 | *9.89 | *10.11 | *9.719 | *9.59 |
| Pt 4 | *9.48 | *7.96 | *8.48 | −5.96 | *10.74 | −6.6 |
| Pt 5 | −1.9 | *9.57 | *9.35 | −1.5 | 0.06 | *9.35 |
| Pt 6 | −7.32 | *5.15 | 0.88 | −5.96 | *8.01 | *8.48 |
| Pt 7 | −9.74 | *9.81 | *10.00 | *8.98 | −10.11 | *9.00 |
| Pt 8 | *6.71 | *6.06 | *10.21 | 1.07 | −4.03 | 4.26 |
| Pt 9 | 1.95 | *4.87 | *10.52 | *9.09 | *11.21 | *8.01 |
| Pt 10 | 3.94 | *7.84 | *5.14 | 2.08 | *4.74 | 8.27 |
| Pt 11 | −3.33 | −2.45 | *11.39 | **6.10 | *6.88 | −4.61 |
| Pt 12 | −0.52 | 1.91 | *9.72 | 2.48 | *8.79 | −1.99 |
| Pt 13 | −0.78 | −4.37 | *10.23 | −5.76 | 3.92 | 1.69 |
| Pt 14 | 2.27 | −1.04 | −0.96 | 0.76 | *11.47 | 1.86 |
| Pt 15 | −1.17 | 3.4 | −2.36 | −4.08 | −7.42 | |
| Pt 16 | 2.55 | −2.66 | *7.58 | −2.93 | −1.77 | 2.77 |
| Pt 17 | −1.17 | 1.93 | *10.61 | −6.21 | *9.61 | −7.51 |
| Pt 18 | −0.52 | −0.76 | *10.61 | −6.212 | 3.333 | *8.29 |
Note: Slope scores indicated with an asterisk satisfied our conditions of linearity and also produced positive significant regressions with ordinal distance from prototype A. These slopes represent successful learning of categories.
2.2 Stimuli
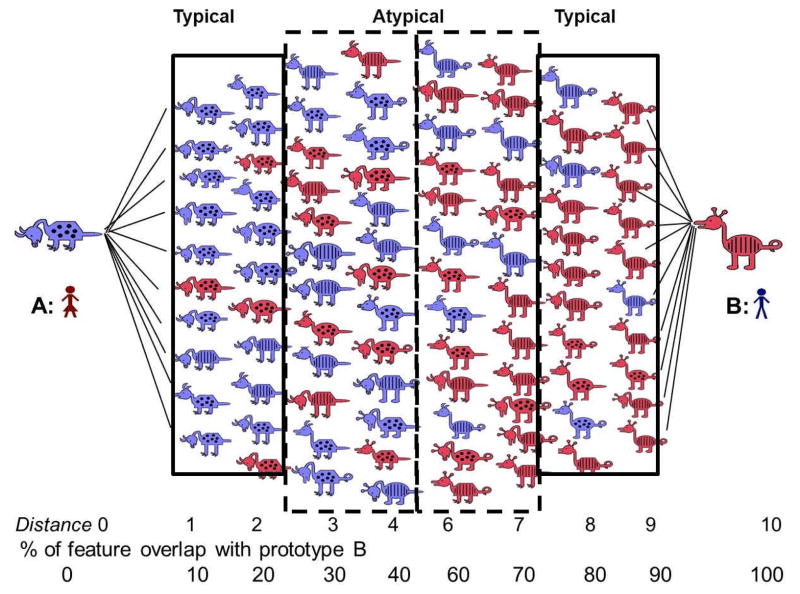
Stimuli for the current study were two sets of cartoon animals created by Zeithamova, Maddox and Schnyer (2008) and first reported in Vallila-Rohter and Kiran (2013). Each animal had one of two possible feature values for ten dimensions (i.e., neck length [long or short], tail shape [straight or curled], toes [pointed or curved], snout [round or pointed], ears [pointed or rounded], color [purple or pink], body shape [pyramidal or round], body pattern [spots or stripes], head direction [upward or downward] and leg length [long or short]). Two orthogonal categories were created per stimulus set. For each category, one animal was selected as prototype A, with the animal that differed from that prototype by all ten dimensions identified as prototype B. The remaining 1024 animals in each stimulus set were grouped by their distance from prototype A, each distance increment describing the number of features by which animals differed from prototype A. Thus, animals at distance 1 from prototype A had a nine-feature overlap with that prototype, animals at distance 2 had an eight-feature overlap with prototype A, and so forth. The binary nature of features meant that with each increasing distance increment from prototype A, animals had an increasing feature overlap with the prototypical animal of the opposite category, B (animals at distance 1 have a nine-feature overlap with prototype A and one with prototype B, animals at distance 2 have an eight-feature overlap with prototype A and two with prototype B, etc.). In this manner, two categories were established along a continuum that depended on feature overlap with each prototypical animal.
Category membership was delineated by the percentage of features shared with each of the two prototypes. All animals that shared at least six features with a prototype (60% feature overlap) were considered members of that category. Animals that were at distance 5 were not considered members of either category and were expected to be categorized with each of the two prototypes with a rate of 50%. Within a category, animals that had a high feature overlap with the prototype, meaning that they had eight to nine features in common with the prototype (80% to 90% feature overlap) were considered typical category members. Animals that matched the prototype’s features by only six to seven features (60% to 70% feature overlap) were considered atypical category members (see Figure 1).
Figure 1.
Sample animal stimuli contributed by Zeithamova et al. (2008). Animals are arranged according to the number of features with which they differ from each prototypical animal. The number of features by which an animal differs from each prototype is referred to as its distance from the prototype. Typical animals share 80% to 90% of their features with prototypes. Atypical animals share 60% to 70% of their features with prototypes.
2.3 Design and Procedures
Testing was completed in a quiet room at Boston University in the presence of a speech language pathologist over the course of up to six days (one paradigm per session for patients and up to two paradigms per session for controls). Tests were computer-based and programmed using E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA; www.pstnet.com). Patient participants completed the Western Aphasia Battery (WAB, Kertesz, 1982), the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983) and the Cognitive Linguistic Quick Test (CLQT; Helm-Estabrooks, 2001), standardized cognitive-linguistic measures. All participants completed category learning tasks for which instruction method was either feedback based (FB) or paired associate (PA) with training items which were either typical (Typ) category members or atypical (Atyp) category members. Combining these task manipulations, four conditions were established: FB Typ, FB Atyp, PA Typ, PA Atyp. Each category-learning paradigm consisted of a ten minute training phase followed by a ten minute testing phase and is described in further detail below. Of note, prior to completing these four category learning paradigms, each participant completed a baseline FB task and a baseline PA task, reported in Vallila-Rohter and Kiran (2013) and also briefly described below. Results from the current study were interpreted independently and within the context of baseline tasks. Stimulus sets and learning tasks were counterbalanced across participants, and paradigms were built such that no animal was repeatedly presented across paradigms (see Figure 2 for possible sequence of tests). At the start of testing, a speech language pathologist used illustrated pictures to explain tasks to participants. Participants were told that they would be completing multiple paradigms, each requiring them to learn to recognize animals as belonging to one of two families. They were informed that each task would have a similar overall structure, but that each was unique.
Figure 2.

Sample sequence of testing. All participants completed baseline tasks followed by the completion of four additional category-learning tasks. Task instruction, typicality, stimulus set and prototype were counterbalanced across participants.
In FB learning, animals were presented one at a time on a computer screen for 4000 msec. Participants were required to guess each animal’s affiliation, indicating their selection with a left-handed button press, “1” or “2,” corresponding to categories A and B, respectively. Participants received feedback after each trial that indicated the correct category affiliation and whether their response was correct or incorrect. If patients did not respond within the 4000 msec time-frame, a message appeared indicating that they had responded too slowly. This design encouraged gradual trial-by-trial learning through feedback.
In PA learning, animals were presented one at a time, this time with a label denoting their category affiliation. In each trial, participants were instructed to press the button that matched the indicated category as soon as the picture appeared on the screen. The overall structure and timing of the FB task was maintained in the PA condition. Animals remained on screen for 7000 msec and were followed by a 1000 msec fixation cross before advancing to the next stimulus animal. This design supports the formulation of stimulus response associations.
PA and FB instruction paradigms were similarly structured and started with a ten-minute training phase comprised of 60 categorization trials. Prototypes were never presented in training. Within these parallel task structures, we constructed two training conditions: typical (Typ) and atypical (Atyp). Recall that stimuli in each category were grouped into typical animals (animals that had an 80% to 90% feature overlap with the prototype) and atypical animals (60% to70% overlap with the prototype). Under typical training conditions, all 60 stimulus animals presented in training were typical to categories. Participants therefore saw each feature associated 24 to 30 times with one category and only 3 to 6 times with the opposite category. This condition was created in order to emphasize typical category features, increasing their salience through training. In the atypical condition, overall task structure was maintained, the only manipulation being that the 60 stimulus animals presented in training were all atypical to categories. In this condition, participants saw features associated 15 to 21 times with one category and 9 to 15 times with the opposite category. Therefore, the atypical training condition highlighted the feature variability of categories. Vallila-Rohter and Kiran (2013) FB baseline and PA baseline tasks were similarly structured to these paradigms, however in the baseline conditions the 60 stimulus items presented in training included both typical and atypical exemplars.
All training paradigms were followed by a 72-trial testing phase. Following all training conditions participants were tested on their categorization of prototypes, typical and atypical items. We were interested in examining participant abilities to learn not only animals within the training group to which they were exposed in training (typical or atypical), but whether learning generalized, such that participants showed feature matching of their responses across category items. Test items included novel animals and animals seen in training. In this phase, animals appeared one at a time on a computer screen and participants were given 4000 msec to indicate each animal’s category affiliation with a button press. No feedback was provided. Testing phases were identically structured following all conditions. Data were collected on accuracy and reaction time, though at this time only accuracy data are reported and analyzed. Accuracy rates were examined to determine whether participants learned overall category structure across tasks.
Research has shown a tendency for participant responses to probability match stimulus characteristics during probabilistic learning (Knowlton et al., 1994). For our experimental tasks therefore, successful learning is predicted to correspond to responses that match the percentage of feature overlap with prototypes (i.e., animals at distance 1 will be categorized with prototype B in 10% of trials and with prototype A in 90% of trials). This prediction is further supported by results of our previous study (Vallila-Rohter & Kiran, 2013). Percentage of B response scores (%BResp) are predicted to increase by 10% with each ordinal increase in distance from prototype A. Thus, successful learning of the category corresponds to a linearly increasing %BResp with a slope of positive ten. Chance response would produce 50% BResp across all distances from the prototype, corresponding to a slope of zero.
This model also allows us to probe the question of generalization from atypical items to typical items following training. In order to produce %BResp scores that satisfy our conditions for learning following atypical training, participants must produce categorizations with a high probability match for typical exemplars and prototypes. Therefore, successful learning following atypical training necessitates generalization from atypical exemplars to typical exemplars. Due to the nature of our task, where atypical exemplars have a 30% to 70% feature match with prototypes (close to chance response of 50%), we are unable to measure generalization from typical to atypical items.
2.4 Data Analysis
For each participant, mean accuracy scores at each distance from prototype A were first converted into a percent B response score (%BResp). This allowed us to examine responses and trends as a function of distance from prototype A. Once scores were converted to %BResp at each distance, we analyzed overall performance using a mixed model analysis of variance (ANOVA) with typicality (2 – Typ, Atyp) and instruction method (2 – FB, PA) as within-subject factors, and group (2 – controls, patients) as the between-subject factor. Main effects of group, typicality or instruction method would demonstrate that group or task manipulations impacted performance.
Next, we examined individual participant results to determine whether %BResp scores did, in fact, match the probability of feature overlap with prototype A across all distances. In order to do this, we tested scores for linearity and also examined slopes of %BResp with increasing distance. As described above, correct probability matching on our task corresponds to a linearly increasing %BResp with a slope of positive ten. Scores were tested for linearity using a method described by Cox and Wermuth (1994) and Gasdal (2012). In this method, three model regressions with different independent variables are compared. For our task, the three modeled independent variables were our distance term, the square of our distance term, and the cube of our distance term. In order to satisfy conditions of linearity, %BResp scores had to produce a significant regression between %BResp and our distance term with an alpha value <.05 that was also the greatest significance value across models. We computed a linear regression coefficient for each result. Each participant was assigned a score of learning (slope) for each training condition based on linear coefficients. Using these analyses, we were able to examine patient and control patterns of learning across conditions.
Finally, we used regression analyses to explore relationships between patient slope scores of learning, demographic information and standardized cognitive-linguistic measures. Four linear regressions were run with the independent variables: age, education, and months post onset (MPO). Each of the four linear regressions had a different dependent variable: slope score following PA Typ, PA Atyp, FB Typ and FB Atyp training. Four additional linear regressions were run, this time evaluating patient slope scores of learning and standardized measures of cognitive linguistic ability. In this regression, we explored AQ, attention, memory, executive function and visuospatial skills as determined by composite scores on the CLQT.
Results
Our 2 × 2 × 2 mixed model ANOVA yielded a significant main effect of group, F(1,23) = 14.52, p < .01, demonstrating that performance on our task differed between patients and controls. There was also a significant main effect of typicality, F(1, 23) = 11.67, p = <.01, indicating that performance varied depending on whether instruction was focused on typical or atypical exemplars. The interaction between typicality and group was non-significant, F(1, 23) = 0.46, p = .50, suggesting that stimulus typicality influenced the performance of both patients and controls. There was no significant main effect of training method, F(1,23) = 0.13, p = .72. Thus, results do not suggest an advantage of one method of instruction over another, feedback-based (FB) or paired associate (PA). Similarly, the interaction between training method and group was non-significant, F(1, 23) = 0.32, p = .57.
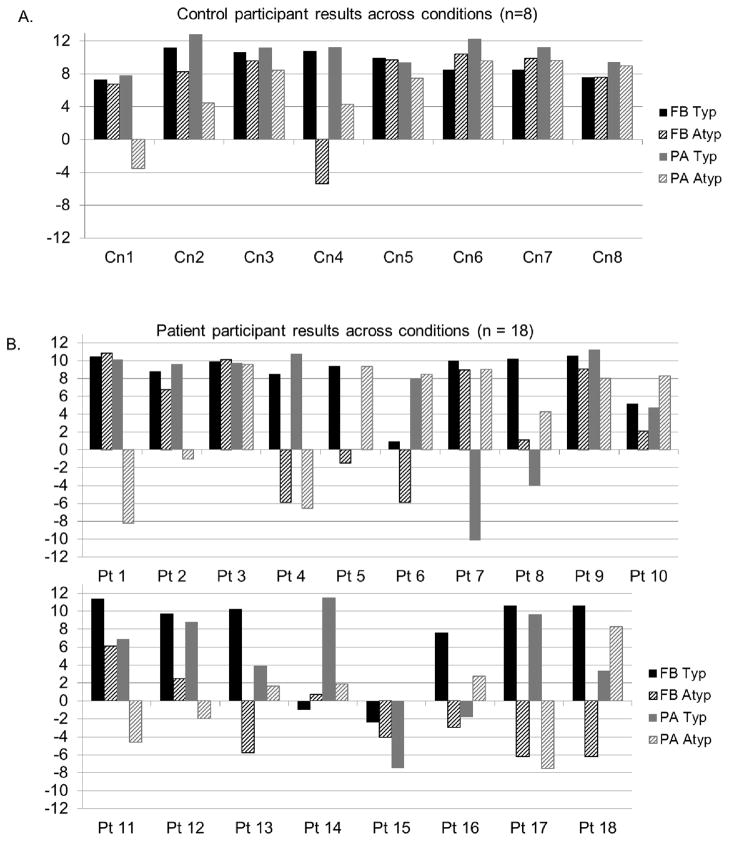
Patient and control slope scores for all four test conditions and for baseline conditions are reflected in Table 2. Recall that successful learning of categories was defined as a positive, linearly increasing %BResp with a slope approaching ten. Slope scores marked with an asterisk indicate scores that satisfied our conditions of linearity and produced significant positive regression results. Figure 3 shows sample plots of %BResp as a function of distance in which a linearly increasing %BResp with a slope approaching ten is evident.
Figure 3.
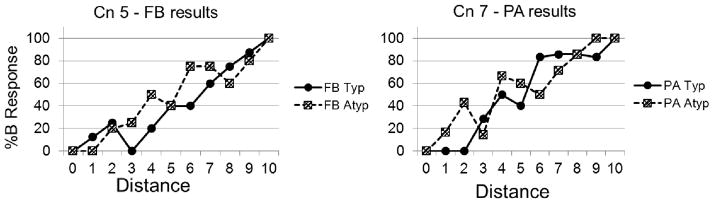
Sample plots of %Bresp as a function of distance for two control participants. Solid lines represent results for the typical training condition, while dotted lines reflect results from the atypical training condition.
An examination of individual control results revealed that six out of eight controls were able to successfully learn categories following every method of instruction; FB Typ, FB Atyp, PA Typ and PA Atyp. One control participant (Cn 1) learned under all conditions except the PA Atyp condition, and another control participant (Cn 4) learned only following typical training (see Table 2).
Upon examination of individual patient results, we found that nine out of eighteen patients with aphasia were able to learn categories under at least one atypical training condition. All nine of these patients were also able to learn categories successfully following at least one typical training condition, FB or PA. We examined the performance of these patients on our previously published baseline conditions (Vallila-Rohter & Kiran, 2013) and found that of the nine patients who learned following at least one atypical training condition, six also demonstrated successful learning of at least one baseline task.
Of the nine remaining patients who did not learn following atypical training, eight were able to learn under at least one typical training condition, FB or PA. Among these patients, only three were able to successfully learn baseline tasks from our previous study, suggesting an overall more limited ability to extract central category tendencies from training items that contain category variability. For these patients, learning occurred primarily under conditions that emphasized feature overlap between categories.
Our regression analyses exploring patient learning scores (slopes) with demographic measures produced only one significant relationship. Age was significantly related to slope scores on the PA Atyp condition (p<.01, see Table 3). Results from all other regressions of demographic measures and slope scores of learning in PA Typ, PA Atyp, FB Typ and FB Atyp conditions were non-significant. Similarly, all linear regressions between slope scores and cognitive-linguistic measures of AQ, attention, memory, executive function and visuospatial skills were non-significant (see Table 3).
Table 3.
Regression results exploring slope scores and patient demographic and linguistic variables
| Dependent Variable | Independent Variable | B | Standard Error (of B) | β | Significance |
|---|---|---|---|---|---|
| PA Typ | Age | −0.19 | 0.14 | −0.35 | 0.18 |
| Education | −0.08 | 0.59 | −0.04 | 0.89 | |
| MPO | 0.02 | 0.03 | 0.17 | 0.52 | |
| PA Typ | AQ | −0.24 | 0.18 | −0.97 | 0.21 |
| Attention | −0.02 | 0.10 | −0.16 | 0.85 | |
| Memory | 0.17 | 0.12 | 1.19 | 0.18 | |
| Executive Function | 0.18 | 0.51 | 0.19 | 0.73 | |
| Visuospatial | −0.06 | 0.26 | −0.23 | 0.82 | |
| PA Atyp | Age | 0.36 | 0.09 | 0.69 | **<0.01 |
| Education | −0.53 | 0.41 | −0.23 | 0.22 | |
| MPO | −0.04 | 0.02 | −0.38 | 0.06 | |
| PA Atyp | AQ | 0.24 | 0.15 | 1.05 | 0.14 |
| Attention | 0.12 | 0.09 | 1.05 | 0.21 | |
| Memory | −0.10 | 0.01 | −0.78 | 0.35 | |
| Executive Function | −0.32 | 0.47 | −0.35 | 0.51 | |
| Visuospatial | −0.15 | 0.25 | −0.57 | 0.56 | |
| FB Typ | Age | −0.09 | 0.09 | −0.27 | −0.30 |
| Education | 0.38 | 0.38 | 0.26 | 0.33 | |
| MPO | 0.02 | 0.02 | 0.25 | 0.36 | |
| FB Typ | AQ | −0.17 | 0.10 | −1.10 | 0.13 |
| Attention | −0.04 | 0.06 | −0.45 | 0.56 | |
| Memory | 0.08 | 0.07 | 0.87 | 0.27 | |
| Executive Function | 0.22 | 0.30 | 0.37 | 0.46 | |
| Visuospatial | 0.02 | 0.15 | 0.12 | 0.90 | |
| FB Atyp | Age | −0.09 | 0.13 | −0.18 | 0.50 |
| Education | −0.40 | 0.58 | −0.19 | 0.51 | |
| MPO | 0.00 | 0.03 | 0.01 | 0.97 | |
| FB Atyp | AQ | −0.06 | 0.17 | −0.27 | 0.72 |
| Attention | 0.06 | 0.10 | 0.55 | 0.52 | |
| Memory | −0.04 | 0.11 | −0.27 | 0.75 | |
| Executive Function | 0.56 | 0.48 | 0.63 | 0.26 | |
| Visuospatial | −0.12 | 0.25 | −0.50 | 0.63 |
p < .01
2. Discussion
In this study, we extended our previous examination of learning ability through an investigation into the impact of training method and stimulus characteristics on the non-linguistic category learning ability of patients with aphasia and a control group of healthy individuals. We compared feedback based and paired associate instruction on a multi-dimensional category learning task; conditions which researchers have posited might differentially engage learning systems through the course of learning. We posited that patients would learn better under feedback-based conditions, as researchers have found improved information integration learning under feedback conditions (Ashby et al., 2002).
For both our patients with aphasia and our healthy controls, overall learning ability was similar under paired associate and feedback-based conditions. Thus, for our task, there was no observed advantage of feedback over observational training. Our task differed from the task implemented in Ashby et al. (2002) by stimulus type and categorical rules. Training manipulations may have had a less significant impact on strategy use in our task than it did in the Ashby et al. (2002) study.
Results suggest that when patients with aphasia are able to successfully learn categories, they can do so under either paired associate or feedback conditions. These findings are in line with results from our previous study (Vallila-Rohter & Kiran, 2013). Studies conducted in other patient populations with brain damage have suggested that feedback-based and paired associate instruction significantly impact learning ability (Ashby et al., 2002, 2003; Ell, Weinstein & Ivry, 2010; Graf, Squire & Mandler, 1984; Knowlton, Ramus & Squire, 1992, Knowlton et al., 1996; Maddox, Ashby, Ing et al., 2004, Maddox et al., 2008), yet this was not the case for our patients with aphasia. In Parkinson’s disease and in amnesia, brain regions critical to feedback-based and paired associate learning, basal ganglia structures and medial temporal lobe structures respectively, are the known foci of lesions. Therefore, the observed behaviors and sensitivity to the presence or absence of feedback are supported by characteristics of the underlying neural damage. While our study does not reveal which strategies are used by patients with aphasia, results suggest that patient are able to select appropriate strategies whether instruction is paired associate or feedback-based. Diverse methods of instruction exist that have not yet been systematically explored in aphasia, and may merit further study. The diversity of lesions and profiles in aphasia may require ideal instruction methods to be identified on an individual basis.
Our second stimulus factor of interest, stimulus typicality, did impact performance on our category learning tasks. Overall, we found that the typical training condition facilitated learning for all participants. All controls learned under typical training conditions and seventeen out of eighteen patients were able to learn following typical training. These findings are supported by Plaut’s (1996) work that noted that connectionist networks relearned trained items faster when exposed to typical category exemplars than when trained on atypical category exemplars. Plaut proposed that typical training conditions highlight salient category features, limiting the complexity of training.
Regarding atypical training conditions, we first found that most control participants showed successful category learning in this condition. Successful learning following atypical training requires accurate categorization of typical items; therefore data from six control participants demonstrate support for connectionist principles that suggest that highlighting feature variability provides not only information about category breadth, but also about central category tendencies (Plaut, 1996). The majority of control participants were able to successfully extract category information in a short period of time despite high task demands. For one control (Cn4), we hypothesize that the atypical training condition was too complex for her to extract category information successfully following such a limited number of trials. For this control, learning was limited to the typical training condition in which salient category features are emphasized.
For our patient participants, only 50% were able to extract central category tendencies following training that highlighted feature variability. Examination of their results on baseline tasks showed that most of these patients also learned under baseline conditions. We propose that these patients have robust category learning mechanisms that allow them to recognize and track patterns efficiently. For the remaining patients tested, category learning was only successful under the typical training condition. These patients did not demonstrate the ability to extract central category tendencies from atypical training items, and in addition, generally did not successfully learn under baseline conditions. Thus, for seven of eighteen patients, learning was only successfully achieved when instruction highlighted feature overlap within categories. For these patients, an emphasis on central category tendencies proved critical to successful learning. We propose that for these patients, general mechanisms of learning are impaired, successful category learning occurring only under conditions which are facilitative and simplified.
In our examination of the relationship between demographic and cognitive-linguistic variables and learning scores, only age and slopes scores in the PA Atyp condition were significant. The severity of deficits, as characterized by the WAB aphasia quotient did not predict patient success with our task, suggesting that performance on our task is not directly related to severity of aphasia. We hypothesize that the aphasia-inducing strokes that each of our patients participants experienced may have differentially affected learning and language networks. Some patients may have severe language deficits within the context of a relatively persevered system for category learning while others experience mild language deficits within a more significantly impaired category learning network. One might also hypothesize that patients had different premorbid learning abilities. Though for the majority of our control group learning was consistently maintained across conditions, controls may have engaged learning strategies differently to perform the various tasks. Explicit and implicit learning systems are described to compete or interact throughout learning (Ashby & Crossley, 2012; Ashby & Valentin, 2005; Cincotta & Seger, 2007; Poldrack et a;., 2001; Moody et al., 2004; Seger & Miller, 2010). Healthy individuals may engage the learning system that is most efficient for them despite varying task demands.
Clinically, results demonstrate differential category learning abilities among patients with aphasia. Category learning depends upon the ability to detect and integrate commonalities or patterns and is considered essential towards helping us rapidly recognize and classify objects meaningfully (for review see Ashby et al., 1998; Ashby & Maddox, 2005; Keri, 2003; Seger & Miller, 2010). Current results suggest that post-stroke some patients may have difficulty engaging in such integrative processes. We do not suggest that these patients lose the ability to learn categories entirely. Our task engaged participants in very short phases of learning of complex information. It is conceivable however, that many patients with aphasia may experience difficulty in the process of integrating commonalities across stimuli.
We propose that patients who experience difficulty integrating commonalities during our task might also have difficulty integrating commonalities during therapy. Thus for these patients, therapies focused on simple targets and simple tasks that reinforce salient patterns and strategies are likely to be the most effective means of promoting improvement. Patients with general learning mechanisms that are not well-suited for extracting central category tendencies, likely do not have language learning mechanisms well-suited for extracting central category tendencies.
In contrast, we suspect that patients with a demonstrated ability to extract commonalities under conditions that highlight feature variability will translate these skills to therapy. These patients likely have general learning mechanisms suited to integrate variability and abstract patterns, mechanisms which can be recruited in therapy. We propose that these patients would be suitable candidates for therapies which include complex, variable tasks and targets.
We are limited in our predictions, as the current study involved a limited group of patients with heterogeneous profiles of aphasia. Also, we can only infer that skills demonstrated on our non-linguistic category learning task will translate to performance in actual language therapy. The next step will be to test whether predictions drawn from short, controlled non-linguistic tasks can translate to progress with therapy. In addition, there are a multitude of demands posed on patients during regular aphasia therapy than merit to be the focus of future studies.
We do propose that current results draw attention to underlying processes which have not yet been the focus of research in aphasia, yet likely contribute to outcomes with therapy. A better understanding of how these mechanisms of learning are affected in aphasia and the contribution of these processes to therapy is critical for the selection of appropriate tasks and targets for individual patients with aphasia. We suggest that only with a better understanding of the factors that contribute to successful learning in patients with aphasia, can clinicians tailor treatment to individuals, selecting targets and methods of therapy that will facilitate patient progress and improve the predictability of patient outcomes.
Figure 4.
Slope scores of learning across tasks for control participants (top panel, A) and for patient participants (lower panels, B).
References
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron E. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105(3):442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Crossley M. Interactions between declarative and procedural-learning. Neurobiology of Learning and Memory. 2010;94:1–12. doi: 10.1016/j.nlm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Maddox T. Human category learning 2.0. Annals of the New York Academy of Sciences. 2011;1224:147–161. doi: 10.1111/j.1749-6632.2010.05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT, Bohil CJ. Observational versus feedback training in rule-based and information-integration category learning. Memory and Cognition. 2002;30(5):666–677. doi: 10.3758/bf03196423. [DOI] [PubMed] [Google Scholar]
- Ashby G, Maddox T. Human category learning. Annual Review of Psychology. 2005;56:149–78. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby G, Noble S, Filoteo V, Waldron E, Ell S. Category learning deficits in Parkinson’s Disease. Neuropsychology. 2003;17(1):115–124. [PubMed] [Google Scholar]
- Ashby FG, Valentin V. Multiple systems of perceptual category learning: Theory and cognitive tests. In: Cohen H, Lefebvre C, editors. Categorization in cognitive science. New York: Elsevier; 2005. [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster A, Sommer J, Wolbers T, Knecht S. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Kamping S, Jansen A, Schomacher M, Knecht S. Word learning can be achieved without feedback: Implications to aphasia therapy. Restorative Neurology and Neuroscience. 2004;22:445–458. [PubMed] [Google Scholar]
- Cincotta CM, Seger CA. Dissociation between stratal regions while learning to categorize via observation and via feedback. Journal of Cognitive Neuroscience. 2007;118:438–442. doi: 10.1162/jocn.2007.19.2.249. [DOI] [PubMed] [Google Scholar]
- Cox DR, Wermuth N. Tests of linearity, multivariate normality and the adequacy of linear scores. Applied Statistics. 1994;43(2):347–355. [Google Scholar]
- Davis T, Love B, Maddox T. Two pathways to stimulus encoding in category learning? Memory and Cognition. 2009;37(4):394–413. doi: 10.3758/MC.37.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell S, Weinstein A, Ivry R. Rule-based categorization deficits in focal basal ganglia lesion and Parkinson’s disease patients. Neuropsychologia. 2010;48(10):2974–2986. doi: 10.1016/j.neuropsychologia.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RJ, Goldinger SD, LaPointe LL. Auditory vigilance in aphasic individuals: Detecting nonlinguistic stimuli with full or divided attention. Brain and Cognition. 1996;30:244–253. doi: 10.1006/brcg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Ferguson A. Learning in aphasia therapy: It’s not what you do but how you do it! Aphasiology. 1999;13(2):125–150. [Google Scholar]
- Filoteo V, Maddox T. Category learning in Parkinson’s disease. In: Sun MK, editor. Research Progress in Alzheimer’s Disease and Dementia. Vol. 3. New York: Nova Science Publisher; 2007. pp. 339–365. [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Zizak V, Song DD. The impact of irrelevant dimensional variation on rule-based category learning in patients with Parkinson’s disease. Journal of International Neuropsychological Society. 2005;11:503–513. doi: 10.1017/S1355617705050617. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Salmon DP, Song DD. Information-integration category learning in patients with striatal dysfunction. Neuropsychology. 2005b;19(2):212–222. doi: 10.1037/0894-4105.19.2.212. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18:193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Nettles C, Davis M, Morrow L, Montgomery A. Functional communication and executive function in aphasia. Clinical Linguistics and Phonetics. 2006;20:401–410. doi: 10.1080/02699200500075781. [DOI] [PubMed] [Google Scholar]
- Gasdal O. Analyzing cross sectional survey data using linear regression method: A ‘hands’ on introduction using ESS data. Bergen, Norway: Norwegian Social Science Data Services & European Social Survey Education Net; 2012. Retrieved from http://essedunet.nsd.uib.no/cms/topics/regression/4/3.html. [Google Scholar]
- Graf P, Squire L, Mandler G. The information that amnesic patients do not forget. Journal of Experimental Psychology: Learning, Memory and Cognition. 1984;10(1):164–178. doi: 10.1037//0278-7393.10.1.164. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carey S. Selective word-learning deficits in aphasia. Brain and Language. 1987;32:306–324. doi: 10.1016/0093-934x(87)90130-1. [DOI] [PubMed] [Google Scholar]
- Gupta P, Martin N, Abbs B, Schwartz M, Lipinski J. New word learning in aphasic patients: Dissociating phonological and semantic components. Brain and Language. 2006;99:8–9. [Google Scholar]
- Helm-Estabrooks N. Cognitive linguistic quick test. New York: The Psychological Corporation; 2001. [Google Scholar]
- Helm-Estabrooks N. Cognition and aphasia: A discussion and a study. Journal of Communication Disorders. 2002;35:171–186. doi: 10.1016/s0021-9924(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Hopper T, Holland A. Aphasia and learning in adults: Key concepts and clinical considerations. Topics in Geriatric Rehabilitation. 2005;21(4):315–322. [Google Scholar]
- Hula WD, McNeil MR. Models of attention and dual-task performance as explanatory constructs in aphasia. Seminars in Speech and Language. 2008;29(3):169–187. doi: 10.1055/s-0028-1082882. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lippincott Williams &Wilkins; 1983. [Google Scholar]
- Keil K, Kaszniak AW. Examining executive function in individuals with brain injury: A review. Aphasiology. 2002;16:305–335. [Google Scholar]
- Kelly H, Armstrong L. New word learning in people with aphasia. Aphasiology. 2009;23(12):1398–1417. [Google Scholar]
- Keri S. The cognitive neuroscience of category learning. Brain Research Reviews. 2003;43:85–109. doi: 10.1016/s0165-0173(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery. Philadelphia: Gruvne & Stratton; 1982. [Google Scholar]
- Kiran S. Semantic complexity in the treatment of naming deficits. American Journal of Speech Language Pathology. 2007 Feb;16:18–29. doi: 10.1044/1058-0360(2007/004). PMC2731154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S. Typicality of inanimate category exemplars in aphasia treatment: Further evidence for semantic complexity. Journal of Speech, Language and Hearing Research. 2008;51:1550–1568. doi: 10.1044/1092-4388(2008/07-0038). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Sandberg C, Sebastian R. Treatment of category generation and retrieval in aphasia. Journal of Speech Language and Hearing Research. 2011;54:1101–1117. doi: 10.1044/1092-4388(2010/10-0117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Thompson C. Effect of typicality on online category verification of animate category exemplars in aphasia. Brain and Language. 2003a;85:441–450. doi: 10.1016/s0093-934x(03)00064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Thompson CK. The role of syntactic complexity in treatment of sentence deficits in agrammatic aphasia: The complexity account of treatment efficacy (CATE) Journal of Speech, Language and Hearing Research. 2003b;46:591–607. doi: 10.1044/1092-4388(2003/047). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels J, Squire L. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Ramus SJ, Squire LR. Intact artificial grammar learning in amnesia: Dissociation of classification learning and explicit memory for specific instances. Psychological Science. 1992;3(3):172–179. [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: parallel brain systems for item memory and category knowledge. Science. 1993;262(5140):1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Lesniak M, Bak T, Czepiel W, Seniow J, Czlonkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dementia and Geriatric Cognitive Disorders. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- Maddox T, Asbhy G. Dissociating explicit and procedural-learning based systems of perceptual category learning. Behavioral Processes. 2004;66:309–332. doi: 10.1016/j.beproc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Maddox T, Ashby G, Ing D, Pickering A. Disrupting feedback processing interferes with rule-based but not information-integration category learning. Memory and Cognition. 2004;32(4):582–591. doi: 10.3758/bf03195849. [DOI] [PubMed] [Google Scholar]
- Maddox T, Love B, Glass B, Filoteo V. When more is less: Feedback effects in perceptual category learning. Cognition. 2008;108:578–589. doi: 10.1016/j.cognition.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Effects of facilitation and cueing on labeling of ‘novel’ stimuli by aphasic subjects. Aphasiology. 1992;6:567–583. [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton B. An implicit learning task activates medial temporal lobe in patients with Parkinson’s Disease. Behavioral Neuroscience. 2004;118(2):438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Murray L. Attention and other cognitive deficits in aphasia: Present and relation to language and communication measures. American Journal of Speech-Language Pathology. 2012;21:S51–S64. doi: 10.1044/1058-0360(2012/11-0067). [DOI] [PubMed] [Google Scholar]
- Peach R, Rubin S, Newhoff M. A topographic event-related potential analysis of the attention deficit for auditory processing in aphasia. Clinical Aphasiology. 1994;22:81–96. [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Plaut D. Relearning after damage in connectionist networks: Toward a theory of rehabilitation. Brain and Language. 1996;52:25–82. doi: 10.1006/brln.1996.0004. [DOI] [PubMed] [Google Scholar]
- Price A. Explicit category learning in Parkinson’s disease: Deficits related to impaired rule generation and selection processes. Neuropsychology. 2006;20(2):249–257. doi: 10.1037/0894-4105.20.2.249. [DOI] [PubMed] [Google Scholar]
- Ramsberger G. Achieving conversational success in aphasia by focusing on non-linguistic cognitive skills: A potentially promising new approach. Aphasiology. 2005;19(10/11):1066–1073. [Google Scholar]
- Reber PF, Squire LR. Intact learning of artificial grammars and intact category learning by patients with Parkinson’s disease. Behavioral Neuroscience. 1999;113:235–242. doi: 10.1037//0735-7044.113.2.235. [DOI] [PubMed] [Google Scholar]
- Seger C, Miller E. Category learning in the brain. Annual Review of Neuroscience. 2010;33:203–19. doi: 10.1146/annurev.neuro.051508.135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and Behavioral Reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Siegert R, McDowall J. Preserved implicit learning on both the serial reaction time task and artificial grammar in patients with Parkinson’s disease. Brain and Cognition. 2001;45:378–391. doi: 10.1006/brcg.2001.1286. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Treatment of underlying forms: A linguistic specific approach for sentence production deficits in agrammatic aphasia. In: Chapey T, editor. Language intervention strategies in adult aphasia. 4. Baltimore: Williams & Wilkins; 2001. pp. 605–628. [Google Scholar]
- Thompson CK, Ballard L, Shapiro L. Role of syntactic complexity in training wh-movements structures in agrammatic aphasia: Order for promoting generalization. Journal of the International Neuropsychological Society. 1998;4:661–674. doi: 10.1017/s1355617798466141. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Ballard KJ, Jacobs B, Schneider SL, Tait M. Training and generalized production of wh and NP movement structures in agrammatic aphasia. Journal of Speech and Hearing Research. 1997;40:228–244. doi: 10.1044/jslhr.4002.228. [DOI] [PubMed] [Google Scholar]
- Thompson C, Shapiro L, Kiran S, Sobecks J. The role of syntactic complexity in treatment of sentence deficits in agrammatic aphasia: The complexity account of treatment efficacy (CATE) Journal of Speech, Language and Hearing Research. 2003;46:591–607. doi: 10.1044/1092-4388(2003/047). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Shapiro L, Roberts M. Treatment of sentence production deficits in aphasia: A linguistic-specific approach to wh-interrogative training and generalization. Aphasiology. 1993;7:111–133. [Google Scholar]
- Tuomiranta L, Grönholm-Nyman P, Kohen F, Rautakoski P, Laine M, Martin N. Learning and maintaining new vocabulary in persons with aphasia: Two controlled case studies. Aphasiology. 2011;25(9):1030–1052. [Google Scholar]
- Vallila-Rohter S, Kiran S. Exploration into feedback and non-feedback based learning in aphasia. Neuropsychologia. 2013;51(1):79–90. doi: 10.1016/j.neuropsychologia.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warringon E, Weiskrantz L. Amnesia: A disconnection syndrome? Neuropsychologia. 1982;20(3):233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Winocur G, Weiskrantz L. An investigation into paired-associate learning in amnesic patients. Neuropsychologia. 1976;14:97–110. doi: 10.1016/0028-3932(76)90011-7. [DOI] [PubMed] [Google Scholar]
- Witt K, Nuhsman A, Deuschl G. Intact artificial grammar learning in patients with cerebellar degeneration and advanced Parkinson’s disease. Neuropsychologia. 2002;40(9):1534–1540. doi: 10.1016/s0028-3932(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Maddox WT, Schnyer DM. Dissociable prototype learning systems: evidence from brain imaging and behavior. Journal of Neuroscience. 2008;28(49):13194–201. doi: 10.1523/JNEUROSCI.2915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn S, Bosworth HB, Hoenig HM, Swartwelder HS. Executive function deficits in acute stroke. Archives of Physical Medicine and Rehabilitation. 2007;88:173–180. doi: 10.1016/j.apmr.2006.11.015. [DOI] [PubMed] [Google Scholar]