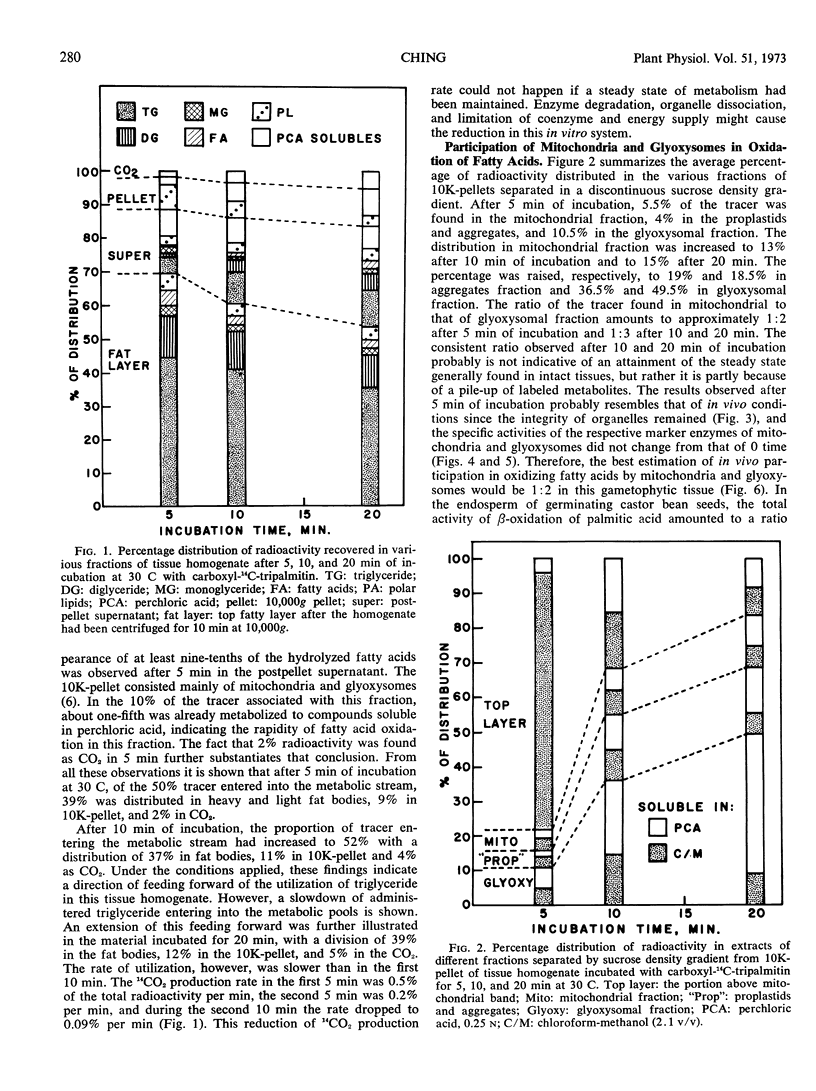
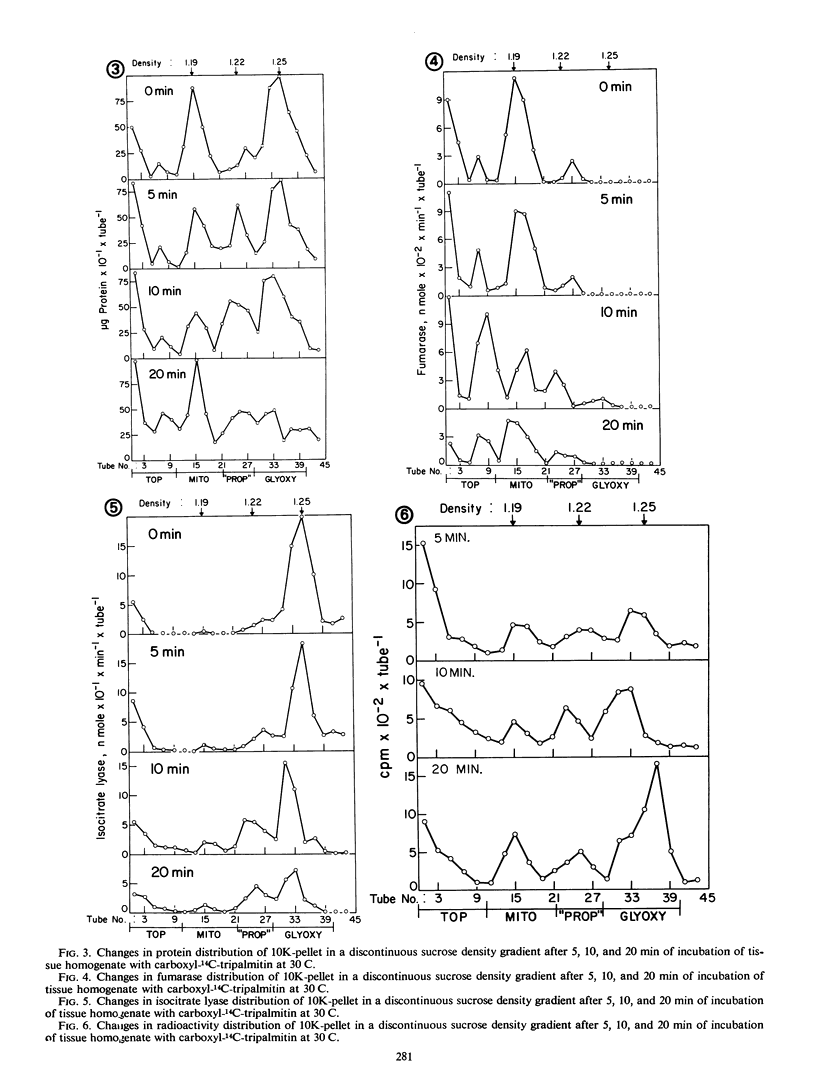
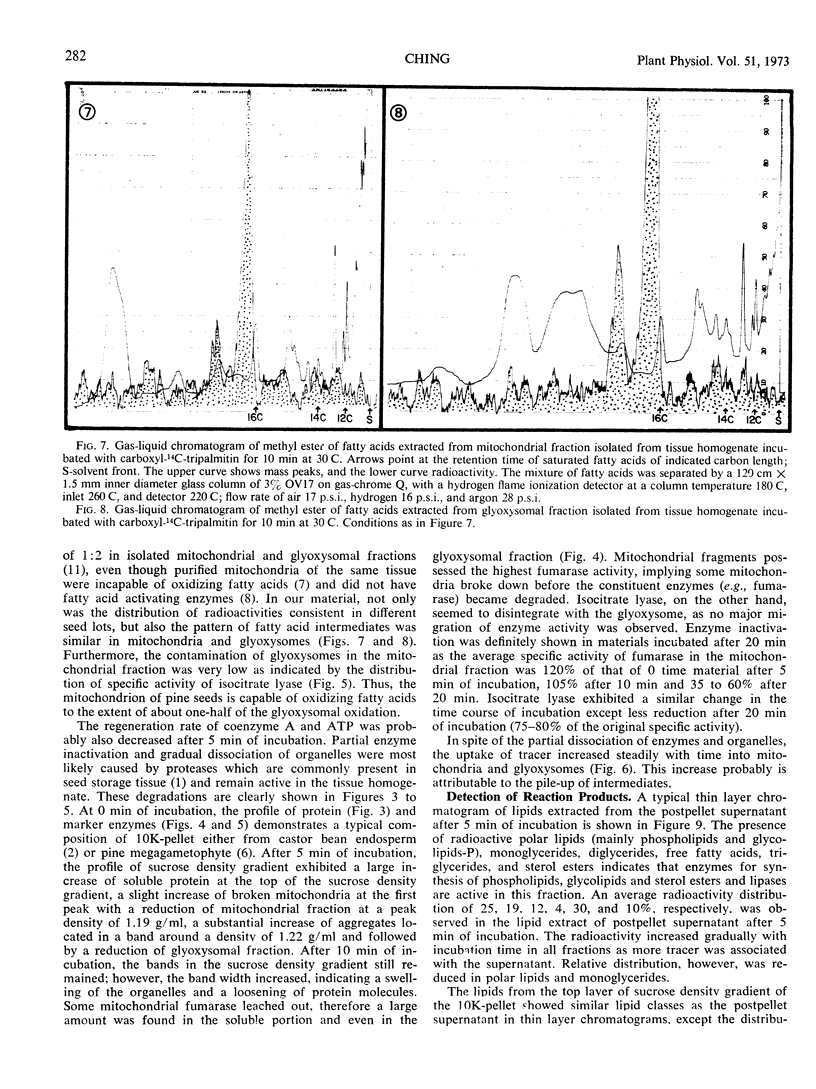
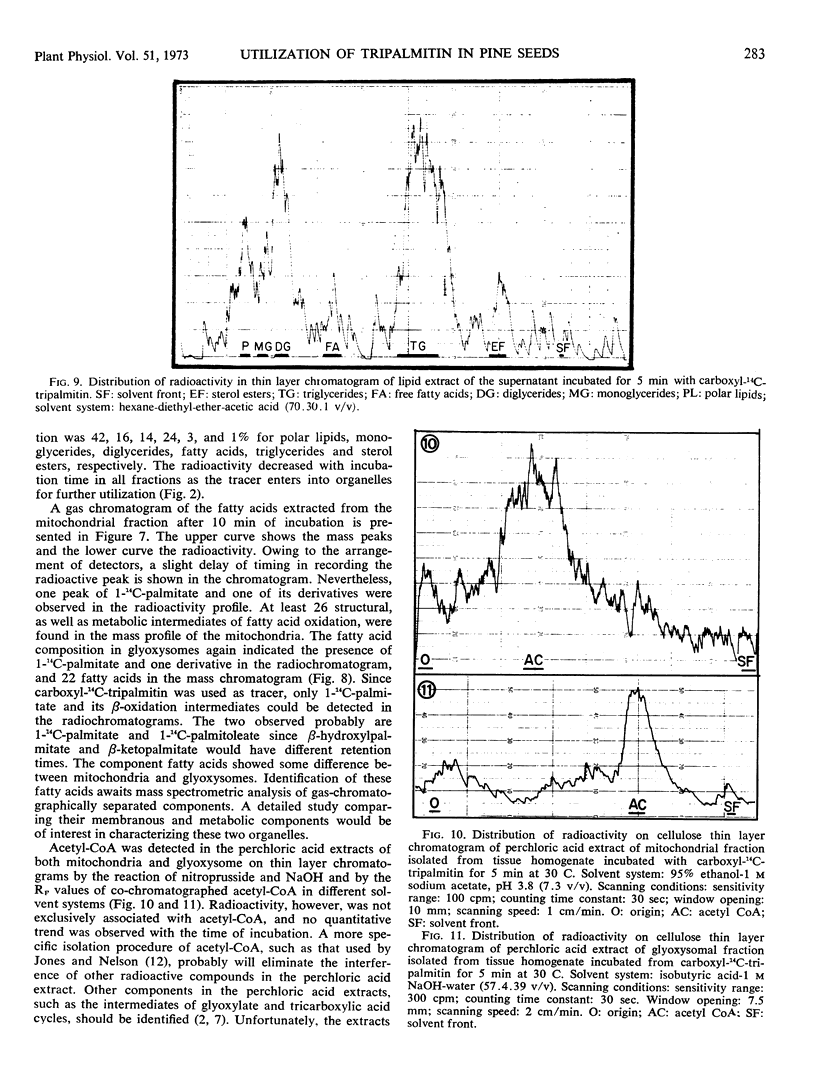
Abstract
A tissue homogenate of megagemetophyte of germinating seeds of Jeffrey pine (Pinus Jefferii Grev. and Balf.) was incubated with sonication-dispersed and albumin-carried 14C-tripalmitin in order to elucidate the sequential and quantitative role of cellular organelles in utilizing lipid reserve in seeds. After 5 minutes at 30 C, 25% of the tracer was localized in the fat body fraction, 9% in the pellet containing mitochondria and glyoxysomes, 14% in the supernatant, and 2% was found as CO2. Radioactivity increased with time of incubation in the latter three fractions indicating the forward direction of utilization. Fat bodies contained mainly lipases and hydrolyzed the tracer to palmitate with diglyceride and monoglyceride as intermediates. About two-thirds of the palmitate had left the fat bodies in 5 minutes and entered the pellet fraction within which the tracer was distributed 1:2 in mitochondria and glyoxysomes, respectively. Longer incubation reduced the ratio to 1:3 while both organelles acquired more radioactive intermediates. Labeled acetyl-CoA and intermediate of β-oxidation were found in both organelle-containing fractions. The supernatant fraction contained radioactive diglycerides, monoglycerides, palmitate, sterol esters, and phospholipids, indicating lipase activity and direct utilization of fatty acid for the synthesis of sterol esters and polar lipids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Fang S. C. Differential incorporation of acetate and glucose in maturing Douglas fir seeds. Lipids. 1969 Nov;4(6):522–525. doi: 10.1007/BF02531035. [DOI] [PubMed] [Google Scholar]
- Ching T. M. Fat Utilization in Germinating Douglas Fir Seed. Plant Physiol. 1963 Nov;38(6):722–728. doi: 10.1104/pp.38.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Intracellular distribution of lipolytic activity in the female gametophyte of germinating Douglas fir seeds. Lipids. 1968 Nov;3(6):482–488. doi: 10.1007/BF02530890. [DOI] [PubMed] [Google Scholar]
- Cooper T. G. Activation of fatty acids in castor bean endosperm. J Biol Chem. 1971 Jun 10;246(11):3451–3455. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Farstad M., Bremer J., Norum K. R. Long-chain acyl-CoA synthetase in rat liver. A new assay procedure for the enzyme, and studies on its intracellular localization. Biochim Biophys Acta. 1967 Mar 15;132(2):492–502. doi: 10.1016/0005-2744(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn V., Howell R. W., Hanson J. B. Fat Metabolism in Germinating Soybeans. I. Physiology of Native Fat. Plant Physiol. 1960 Nov;35(6):854–860. doi: 10.1104/pp.35.6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg O., Prusiner S. B., Cannon B., Ching T. M., Eisenhardt R. H. Metabolic control in isolated brown fat cells. Lipids. 1970 Feb;5(2):204–209. doi: 10.1007/BF02532470. [DOI] [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Passeron S., Savageau M. A., Harary I. Optimal conditions for palmitate oxidation by rat heart homogenates. Arch Biochem Biophys. 1968 Oct;128(1):124–128. doi: 10.1016/0003-9861(68)90014-3. [DOI] [PubMed] [Google Scholar]
- Stanley R. G., Conn E. E. Enzyme Activity of Mitochondria from Germinating Seedlings of Sugar Pine (Pinus Lambertiana Dougl.). Plant Physiol. 1957 Sep;32(5):412–418. doi: 10.1104/pp.32.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]


