Abstract
Background and Aims
The oldest group of plants in which nectar secretions have been observed are the Polypodiopsida (ferns sensu lato). Nectaries have been reported in a dozen extant genera. The function of these nectaries has been investigated in several fern species, and in some circumstances has been demonstrated to have an antiherbivore role, attracting and maintaining biotic defence (ants and/or other predatory arthropods). This study documents foliar nectaries in Pleopeltis crassinervata, a widespread Central American epiphyte growing on a variety of trees in cloud forest areas of Veracruz, Mexico. This is a new record for this genus and species.
Methods
As previous experimental work on epiphytic species of Polypodium has demonstrated a protective role of ants for developing fronds, we conducted similar experiments (using nylon nail polish to cover nectaries rather than excluding ants with bands of sticky resin as in earlier work). The fronds of Pl. crassinervata developed over 6 weeks, at which time damage was assessed. The experiment was simultaneously conducted on a sympatric species lacking nectaries, Polypodium furfuraceum. Herbivore placement experiments were conducted with large and small caterpillars on both of these ferns.
Key Results
Fronds with nectaries covered suffered greater damage from herbivores over the course of their development, compared with fronds that had uncovered nectaries functioning normally. The parallel experiment on Po. furfuraceum showed no difference between manipulated and control fronds. Six species of ants (Brachymyrmex minutus, Crematogaster formosa, Paratrechina longicornis, Solenopsis geminata, S. picea and Wasmannia auropunctata) were observed visiting nectaries of Pl. crassinervata; most were effective in removing herbivore larvae placed on the fronds.
Conclusions
The long evolutionary history of ferns may explain why some previous studies of fern nectaries have shown little or no benefit to ferns from nectary visitors, as any coevolved herbivores are those resistant to ant defence. The results suggest that ants protect Pl. crassinervata fronds against herbivory. The presence of nectaries, and the relationship with ants, may contribute to this fern's widespread occurrence and persistence in the face of disturbance, though many other factors also play a role. Ant defence may be more likely to benefit a widespread species of disturbed habitats that encounters a wide range of non-adapted herbivores.
Keywords: Extrafloral nectar, extrasoral nectar, ferns, pteridophytes, nectaries, ant protection, Formicidae, herbivory, antiherbivore defence, Mexico, field experiment, epiphyte, cloud forest, Pleopeltis crassinervata, Polypodium furfuraceum
INTRODUCTION
No consideration of extrafloral nectaries in plants would be complete without including the non-flowering plants that also have nectaries. In this paper, we briefly review the occurrence of nectaries in ferns, and then report our experimental studies investigating the ecological role of nectaries in the biology of a species recently discovered to bear them. Fern nectaries have sometimes been called ‘extrasoral nectaries’(Wagner and Gomez, 1983; Cooper-Driver 1990), contrasting sori (clusters of sporangia that are the reproductive structures of ferns) with flowers (the reproductive structures of angiosperms). But the term ‘extrasoral’ has not caught on (Koptur et al., 1982; Koptur, 1992), perhaps because no ferns yet have been found to have nectaries in the sori on their leaves.
Ferns are the most primitive plants possessing nectaries. Modern classification of the ferns sensu lato (Pryor et al., 2001; Smith et al., 2006, 2008) recognizes three lineages leading to the Ophioglossales (grape ferns), Psilotales (whisk ferns) and Equisetales (horsetails). None of these lineages has nectaries. The remaining ferns comprise two clades, the Marattiales (the marattioid ferns) and the leptosporangiate ferns, which include all the remaining extant ferns. Nectaries are found in both of these lineages, which date to the Paleozoic (Schuettpelz and Pryer, 2008).
Among the Marattiales, nectaries are found in some tree-ferns of the genus Angiopteris (Marattiaceae) (Bonnier, 1879). Among the leptosporangiate ferns, nectaries are found in the Cyatheaceae, in the genus Cyathea [into which Cnemidaria and Hemitelia, also with nectaries (Bonnier, 1879; Arens and Smith, 1998) have been subsumed]. Within Cyathea, some species ‘primitively lack nectaries’ and the presence or absence of nectaries corresponds to recent molecular phylogenies (White and Turner, 2012). Nectaries also appear in the Dennstaedtiaceae, specifically in bracken fern (Pteridium aquilinum) (Darwin, 1877; Bonnier, 1879; Figdor, 1891; Lloyd, 1901; Lüttge, 1961; Schremmer, 1970). Nectaries have been observed in the Dryopteridaceae (Polybotrya; Koptur et al., 1982) and in many genera of Polypodiaceae: Aglaomorpha, including Photinopteris (Lüttge, 1961; Potes, 2010), Merinthosorus and Holostachyum (Zamora and Vargas, 1974), Drynaria (Zamora and Vargas, 1974; Koptur et al., 1982; Potes, 2010), Platycerium (Dümmer, 1911; Lüttge, 1961), Polypodium (Koptur et al., 1982, 1998) and Pleopeltis (the present study). Nectaries have evidently arisen independently multiple times within ferns.
Nepi et al. (2009) suggested that the nectars of ferns and gnetophytes are more similar to the extrafloral nectars of angiosperms, while pollination drops of gymnosperms are more similar in composition to floral nectars. This similarity in composition suggests that there may also be similarity in function, and new insights gained from molecular biology and proteomics of the two secretion types is reviewed by Nepi et al. (2009). Extrafloral nectar appeared many millions of years before floral nectar (Nepi et al., 2009), and apparently long before ants appeared in the fossil record (Ward, 2007); therefore, their initial function in plants did not involve ants. This gap provides some support for the ‘leaky phloem’ hypothesis of the origin of nectar (De la Barrera and Nobel, 2004), where nectaries are ‘sap valves’, and release excess sugar.
In more recent times, extrafloral nectar is involved in a variety of interactions, particularly mutualisms, between plants and beneficial insects (Koptur, 2005). Ferns do not have flowers, and so their nectaries cannot correctly be described as ‘extrafloral’; they are always on the fronds (leaves), and so are best described as ‘foliar nectaries’ or simply ‘nectaries’. The occurrence and anatomy of nectaries in ferns have been described much more often than their ecological role has been studied.
Earlier work in relatively undisturbed fragments of lower montane wet forest in Banderilla (near Xalapa, Veracruz, Mexico) was undertaken as some of the species of Polypodium known (from the literature and from cultivation) to have nectaries occurred as epiphytes there. Exclusion experiments conducted during the period of leaf expansion demonstrated that ants visiting the nectaries reduced herbivore damage to the developing fronds of Polypodium plebeium (Koptur et al., 1998), raising the possibility that other ferns with nectaries may receive similar benefits.
Nectaries in ferns are most often morphologically simple, and may be overlooked if the exudates are removed by various visitors in nature. When plants are grown in a greenhouse, or in protected areas such as gardens or nurseries where potential nectar drinkers are absent for various reasons, one may observe either droplets of nectar, or opportunistic sooty moulds (ascomycete fungi, Ophiostomales) that grow on, and consume, accumulated nectar. The presence of sooty mould may be the first indication that nectar is being produced (once honeydew from phloem-feeding insects is ruled out), and then a simple sugar detection method can reveal if nectar is the reason for the sooty mould accumulation. The observation that sooty mould occurred at the base of many fronds of Pleopeltis crassinervata, a widespread epiphyte growing on a variety of trees found in cloud forest areas of Veracruz, Mexico, prompted this study. We questioned whether the nectar from this fern functioned for ant-defence. We also studied the sympatric and closely related fern Polypodium furfuraceum, which lacks nectaries, to provide comparative data.
MATERIALS AND METHODS
Study species
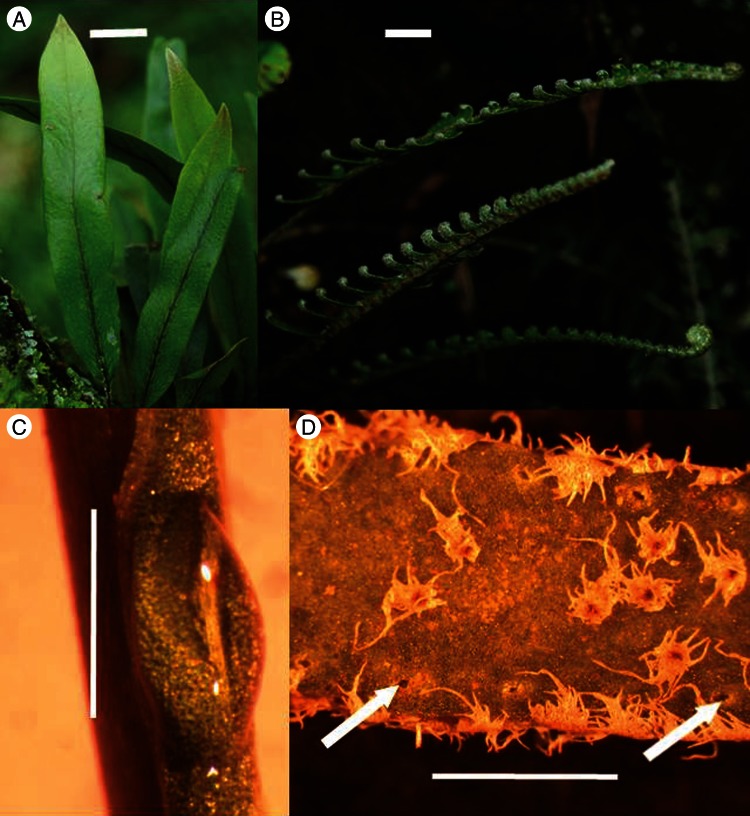
Both Pleopeltis crassinervata (Fée) Moore and Polypodium furfuraceum Schldl. & Cham. were described with material from Veracruz, and both are quite common and not endangered (Palacios-Rios, 1992). They occur together in cloud forests of Veracruz, and occur in a variety of wet to moist habitats in many states of Mexico, as well as in Guatemala. Both are epiphytes, but Pl. crassinervata fronds are simple and entire, and smaller than the fronds of Po. furfuraceum, which are pinnately lobed (Fig. 1).
Fig. 1.
(A) Epiphytic ferns growing on tree trunks: Pleopeltis crassinervata; (B) Polypodium furfuraceum; (C) nectar droplet on nectary on adaxial surface of frond lamina base (×24), Pleopeltis crassinervata; (D) abaxial surface of lamina lobe of Polypodium furfuraceum with hydathodes (×40), two of which are indicated by arrows. Scale bars: (A, B) = 1 cm; (C, D) = 1 mm.
Study site
Field work was conducted on the grounds of CECAMEV (Centro de Conservación y Educación Ambiental), known as ‘El Vivero’; this is the education centre for environmental conservation established by the General Coordination of Environment of the state of Veracruz, located across the highway from INECOL (Instituto de Ecología, A.C.) on km 2·5 of the Xalapa–Coatepec highway by Briones. The site consists of wooded remnants of lower montane wet forest (approx. 1500 m a.s.l.) interspersed with a plant nursery and the environmental education centre. Our exclusion experiments took place from early May to mid-June, when warmer temperatures lead many ferns to produce new fronds; the herbivore placement experiments in early June at the beginning of the rainy season. Both study species occurred together on trunks of trees at the forest edges and throughout the nursery.
Is it nectar?
To verify that the sooty mould we had observed was growing due to the presence of nectar and not arthropod exudates, we carefully examined many fronds, and found no larval insects whose presence might produce honeydew. On several plants with developing fronds, we wiped the leaves clean of sooty mould and waited for the accumulation of liquid in those areas. We used glucose test strips (Clinistix®; Bayer) to indicate if sugars were being secreted.
Ants associated with Pleopeltis crassinervata
On many of the host trees we observed ants on or around the ferns. We recorded observations of ants and other arthropods on the two species of ferns on a weekly basis for 1 month, and compared their occurrence on the two species. Larvae were removed from non-experimental plants and taken to the laboratory for rearing and determination. Ants and adult arthropods observed on fronds and around the plants were collected for determination, and voucher specimens were mounted.
To test the hypothesis that caterpillars will stay longer on ferns without nectaries than ferns with nectaries, we conducted herbivore placement/removal experiments. We used larvae of the common green-eyed white butterfly [Leptophobia aripa (Boisduval), Pieridae], collected in abundance from their cruciferous (Brassica oleracea cultivar) host plants in the gardens at UNCADER (Unidad de Capacitación para el Desarollo Rural) in Coatepec, Veracruz, approx. 10 km from Xalapa. Though the caterpillars of the common green-eyed white butterfly do not eat ferns, they were suitable experimental animals for the caterpillar placement experiments as they remained on the plants upon which they were placed, moving around slowly, exploring their new environment, and increasing their opportunities for encounters with ants (if present). These caterpillars were palatable to ants and wasps, and so were susceptible to attack, removal and consumption. We used two sizes of caterpillars: 2nd instar larvae (‘small’) and 5th instar larvae (‘large’). For each trial we placed a larva on a frond of the fern, and observed it for 10 min, recording its time-to-removal on the plant and its ultimate fate. We performed 14 trials on each fern species for each caterpillar size-category, for a total of 56 trials. We compared their times on the plants using univariate analysis of variance, with duration of time on the fern as the dependent variable, and caterpillar size and fern type as the independent variables.
Ant-exclusion experiments
To determine if nectaries on Pl. crassinervata might provide some antiherbivore defence, we wanted to stop ant access to nectar on some plants, and not on others, and compare the damage to the plants from herbivores. In previous studies, we used tanglefoot resin (Tanglefoot®; Contech) to prevent ant access to developing fronds (Koptur et al., 1998), but in this fern species, the small fronds are often appressed against the tree trunk allowing alternative access for ants and other insects. We chose instead to cover the nectaries with clear nylon nail polish (Sally Hansen Hard As Nails with Nylon®), a technique that has been used successfully in other systems, both pteridophytes such as bracken fern (Tempel, 1983), and angiosperms such as Chamaecrista nictitans (Ruhren and Handel, 1999) and Passiflora sp. (Apple and Feener, 2001).
We chose 20 pairs of new fronds of each fern species, one frond per plant, but paired fronds were always in close proximity to each other (chosen from plants that shared the same host tree; a total of 20 trees and 40 plants of each fern species were used). On one of each pair of Pl. crassinervata fronds, we created an experimental treatment by covering the nectaries (and the entire area at the base of the blade where the nectaries are located) with clear, nylon nail polish; on the other of the pair we painted an area halfway up the frond with the same nylon nail polish. This was done to control for any potential deterrent effect that the polish might have on arthropods, but leaving the nectaries open to secrete nectar. Other leaves on the plants were treated in the same way; the developing fronds of interest were designated by tying a short length of embroidery thread around the leaf base. Another set of experiments was performed simultaneously on Po. furfuraceum, placing polish in the same areas as the experimental and control treatments used on Pl. crassinervatum fronds, both to control for the effects of the nail polish and to provide comparative data on a fern without nectaries.
In both species, we allowed the fronds to develop until they were fully expanded and appeared mature – a period of 6 weeks. At this time we traced the fronds, tracing the amounts of damage (extrapolating from the other of the pair if large parts were missing). We measured damage in square millimeters, calculating both absolute and percentage of damage lost to herbivory. We compared damage between experimental and controls in each species using a paired t-test. Statistical analyses were performed using IBM SPSS version 19.
RESULTS
The first indication that Pleopeltis crassinervata bears nectaries was the presence of sooty mould (Ceratocystis sp.; Ophiostomales, Ascomycotina) on the adaxial surface at the base of the leaf blades. Upon closer inspection, the dark hyphae of the mould were growing in nectar secreted from glandular patch nectaries (Fig. 1B) present at the base of each simple strap-shaped frond. Leaves of this species have a variable number of nectaries – from one to four, commonly three. The nectaries appear to function on developing fronds, but the appearance of sooty mould on some mature fronds indicates that secretion may continue after the leaves are fully expanded, at least in some cases. Glucose strip-testing allowed us to verify that it is nectar secreted from the nectaries (glucose-positive indication). Polypodium furfuraceum fronds are pinnately lobed, and microscopic examination reveals many pores along the margins of the leaves (Fig. 1C). These are apparently hydathodes (for secreting water via guttation), as have been observed in many Polypodium species and other ferns. We did not find hydathodes on the fronds of Pl. crassinervata.
The most commonly encountered herbivores were sawfly larvae (Diprionidae, Hymenoptera), and we observed and collected what appeared to be two different species on leaves of both fern species; neither were successfully reared to pupation, nor were adults obtained (Fig. 2A). Geometridae larvae were observed twice, once on each fern species. We also encountered three different types of microlepidopteran larvae: two were eating leaf tissue, the third was eating sori (the clusters of sporangia on the abaxial surface of the fronds) of Pl. crassinervata. We also observed orthopteran nymphs consuming expanding fronds of Pl. crassinervata (Fig. 2B).
Fig. 2.
Insects associated with the ferns in this study: (A) sawfly larva, (B) orthopteran nymph, and (C) caterpillars (of common green-eyed white butterfly, Leptophobia aripa, Pieridae) used in placement experiments. The main image in (C) shows a large, 5th instar caterpillar, whilst the inset shows a small, 2nd instar one. Scale bars = 1 cm.
We found six species of ants on fronds of the ferns: Brachymyrmex minutus, Paratrechina longicornis, Crematogaster formosa, Solenopsis geminata, Solenopsis picea and Wasmannia auropunctata. All ant species were observed traversing the fronds of Pleopeltis, while only once did we observe Solenopsis geminata workers on Po. furfuraceum. Of these, at least three (P. longicornis, S. geminata and W. auropunctata) are exotic, tropical, cosmopolitan species – not surprising as our observations were made and experiments conducted in disturbed sites.
Both small and large caterpillars of the green-eyed white butterfly (Fig. 2C) lasted substantially less time on fronds of Pl. crassinervata than they did on fronds of Po. furfuraceum (Fig. 3); the average duration of large caterpillars on Pl. crassinervata fronds (i.e. the fern with nectaries, in the presence of ants) was 6 min, small caterpillars slightly longer (but not significantly so), versus 10 min for most of their counterparts on Po. furfuraceum fronds (i.e. without nectaries, and without ants). The only significant source of variance in the analysis of variance was fern type (F3,1 = 10·15, P<0·0001): caterpillars placed on the fern without nectaries stayed on the fronds much longer than those placed on the fern with nectaries. Neither caterpillar size nor its interaction with fern type was significant.
Fig. 3.
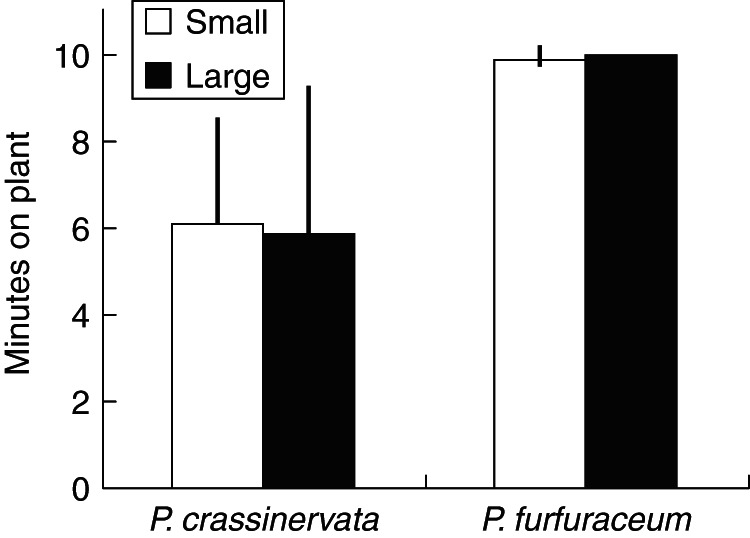
Duration of time on plants for Leptophobia aripa caterpillars placed on fronds of both fern species (mean+s.e., n = 14; 10-min maximum). Small = second instar caterpillars; large = fifth instar caterpillars. Results of univariate ANOVA: overall F3,1 = 10·15, P <.0001; fern type F1,13 = 30·4, P<0·0001; caterpillar size F1,13 = 0·002, P = 0·96; fern type × caterpillar size F1,55 = 0·062, P = 0·805.
The fates of the caterpillars in the placement experiment were drastically different between the two species of ferns: combining both sizes of caterpillars, 27 of 28 trials on Po. furfuraceum were undisturbed and the caterpillars were ‘left alone’, and only one fell prey to a wasp; while on Pl. crassinervata, seven caterpillars were killed, ten were attacked/removed, and 11 were undisturbed or ‘left alone’ (Fig. 4). All but one of the caterpillars not discovered by ants remained on the ferns on which they were placed initially; that caterpillar was nabbed by a predatory wasp. When both ant-encounter categories are combined, the requirements for expected counts greater than five are met, and the differences in caterpillar fate on ferns with versus without nectaries are highly significant (Pearson χ2 = 20·96, P<0·0001).
Fig. 4.
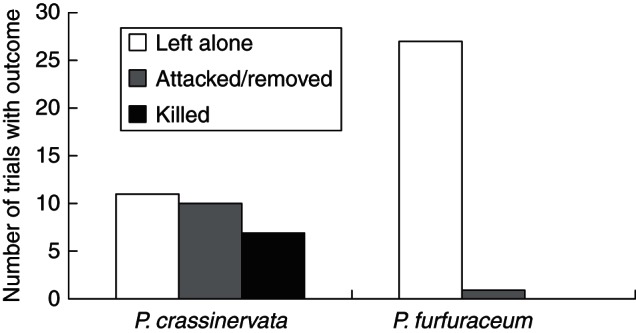
Fates of caterpillars in placement experiments on both fern species (caterpillar sizes combined). Pearson χ2 = 20·96, P<0·0001 (n = 28); both ant-encounter categories were combined to meet the requirements for expected counts for this test.
The nectary-covering ant-exclusion experiment resulted in much greater damage to fronds of Pl. crassinervata that developed with nectaries covered, compared with those that had nectaries open to secrete nectar (t = –2·72, P = 0·01; Fig. 5A). The similar experiment with Po. furfuraceum showed no significant difference between experimental and control fronds (t = 1·41, P = 0·17). Comparing percentages of leaf area damaged gave the same results (Fig. 5B).
Fig. 5.
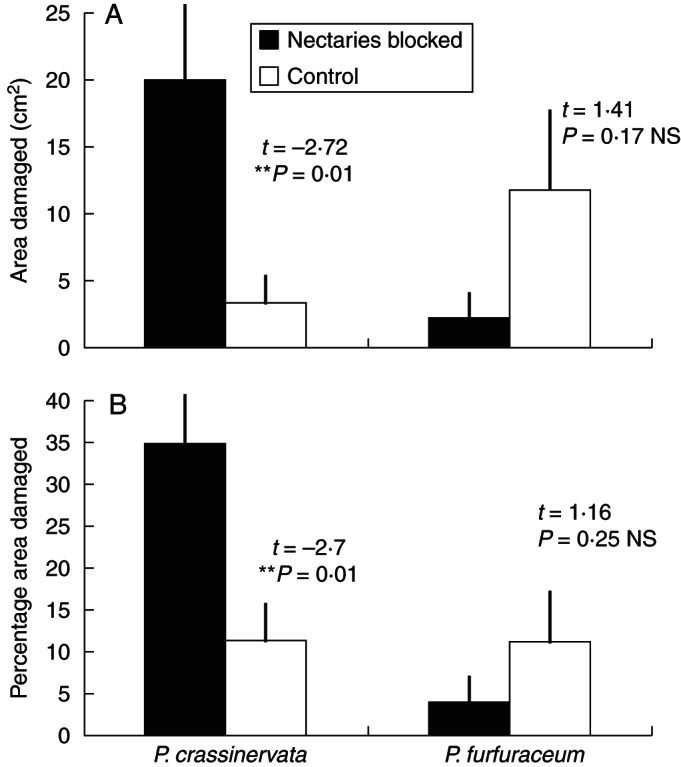
Damage to developing fronds of both fern species (means+s.e.). (A) Pleopeltis crassinervata fronds with nectaries blocked sustained much greater damage than control fronds; Polypodium furfuraceum fronds showed no significant difference between experimental and control. (B) Percentage damage, which shows the same pattern.
DISCUSSION
Our observations and experiments demonstrate that nectaries of Pleopeltis crassinervata function to promote ant visitation and thereby reduce damage to the developing fronds from herbivores. The results of parallel experiments on a sympatric species that does not bear nectaries (Polypodium furfuraceum) strengthen these conclusions, for no differences were seen between the treatments and controls on these ferns. Furthermore, caterpillars placed on fronds of this species remained indefinitely, whereas a substantial proportion of those placed on Pl. crassinervata were attacked, removed, or killed by ants associated with the plants. These two lines of evidence support the hypothesis that nectaries are of selective advantage for Pl. crassinervata ferns in the lower montane forest remnants of Veracruz.
Since Janzen's first demonstration of ant protection in Acacia cornigera (Janzen, 1966, 1967), many ant/plant interactions have been documented in Mexico (Rico-Gray, 1993; Rico-Gray et al., 1998; Cuautle and Rico-Gray, 2003; Díaz-Castelazo et al., 2004, 2010). In angiosperms, extrafloral nectaries have been shown to reduce herbivory by recruiting ants and other beneficial arthropods, providing a generalized defence that can be modified as the situation demands, increasing plant fitness in various ways, including attraction of seed dispersers and even pollinators (Rico-Gray and Oliveira, 2007).
Investigations of nectaries in ferns have been fewer, and the results of experiments more variable, leading some to conclude that fern nectaries, which appeared in the fossil record long before ants (Ward, 2007), are still functioning as merely ‘sap valves’ providing physiological release. Other predatory arthropods visit nectaries (Koptur, 2005), and they or their predecessors might have had a protective role early in fern evolution. Experiments have yet to be performed to demonstrate the benefit of eliminating excess photosynthate via ‘leaky phloem’, but perhaps by covering nectaries with an impermeable barrier to prevent leakage on experimental plants, and comparing the growth and health of control plants without their nectaries covered, we could falsify or support this hypothesis.
The widespread bracken fern (Pteridium aquilinum) has been studied in many places around the world (Cooper-Driver, 1990); the benefit from the nectaries varies with geographic location, nectary visitors and herbivores present in each location (Rashbrook et al., 1992). Heads and Lawton (1984, 1985) argued that ants on bracken may function most in repelling any new species that might colonize this vast green resource, since a number of studies have not shown any benefit to the plants from the presence of ants using ant-exclusion experiments (Tempel, 1983). It is likely that ant protectors benefit bracken fern in areas where there are aggressive ants and herbivores that are not counter-adapted, as was demonstrated with herbivore placement experiments (Heads, 1986).
Studies have suggested that other genera may benefit from ant visitors to their nectaries: the pads at the base of the pinnae of Cyathea planadae, an unusual creeping tree-fern from Colombia, are visited by ants (Arens and Smith, 1998). A recent study of the comparative anatomy of Drynaria and Aglaomorpha reviewed the distribution of nectaries in ferns and found the nectaries of Aglaomorpha to be the most specialized (Potes, 2010), perhaps in response to ant associations. These systems are promising for field and greenhouse study of the significance of nectar secretion in interactions of the ferns with other organisms. Genera previously reported to have nectaries – Holostachyum, Merinthosorus and Photinopteris – are now all included in the genus Aglaomorpha; soon the Polypodium species with nectaries will be moved to the genus Pleopeltis (A. R. Smith, pers. comm.). Even if the number of genera with nectaries is decreasing due to taxonomic revision, we hope that the number of ecological investigations into the function of nectaries in ferns will continue to increase.
We first noticed these nectaries in Pleopeltis crassinervata by observing black sooty mould accumulated in the nectary area on some specimens. The question has arisen as to the potential benefit of sooty mould to the plant. Is it wholly parasitic? Or might its presence advertise the presence of nectar from the nectaries? We have observed only that when ants are present, there is no sooty mould on the nectaries; they may clear it away or eat it in their maintenance of the nectary area, or perhaps it never accumulates. These questions provide fodder for future experiments, and more experimental work is needed in ferns with nectaries about which we have little ecological information, especially with a view to understanding their interactions with other organisms. Our work on Pl. crassinervata is one contribution to this information deficit.
Pleopeltis crassinervata is widespread in Mexico and occurs in other parts of Central America. It is, perhaps, the most resistant of all the epiphytic ferns to disturbance; in a survey of coffee plantations in Veracruz State, it was the sole species found in all forest sites and every coffee plantation surveyed (Mehltreter, 2008). It may be that nectaries provide an advantage that contributes to the tenacity of this little epiphytic fern in the face of disturbance; three of the ant species we observed on the nectaries are invasive species, and such partners may be counted upon in sites where native ants may no longer occur. However, it must be noted that Polypodium furfuraceum, without nectaries, occurred in almost as many sites (Mehltreter, 2008), and that many other factors undoubtedly play a role in the widespread distribution of both fern species studied.
ACKNOWLEDGEMENTS
We thank the Center for the International Exchange of Scholars for Fulbright-Hayes Garcia-Robles financial and logistical support, and Instituto de Ecología, A.C. for logistical support, during the sabbatical of the first author; Miguel Ángel Hernández Villanueva and John O'Neil Palenchar for field assistance; Cuauhtémoc Deloya and Jorge Valenzuela-González for access to facilities for insect work; Luis Quiroz-Robledo for initial determinations of the ants; and Beyte Barrios, Jaeson Clayborn, Ian Jones, Roxaneh Khorsand, Brigitte Marazzi, and Scott Zona for constructive comments on the manuscript. This is contribution number 247 from the Florida International University Program in Tropical Biology.
LITERATURE CITED
- Apple J, Feener DJ. Ant visitation of extrafloral nectaries of Passiflora: the effects of nectary attributes and ant behavior on patterns in facultative ant–plant mutualisms. Oecologia. 2001;127:409–416. doi: 10.1007/s004420000605. [DOI] [PubMed] [Google Scholar]
- Arens NC, Smith AR. Cyathea planadae, a remarkable new creeping tree fern from Colombia, South America. American Fern Journal. 1998;88:49–59. [Google Scholar]
- Bonnier G. Les nectaires. Étude critique, anatomique, et physiologique. Annals des Sciences Naturelles, Botanique, serie 6. 1879;8:5–212. [Google Scholar]
- Cooper-Driver GA. Defense strategies in bracken, Pteridium aquilinum L. Kuhn. Annals of the Missouri Botanical Garden. 1990;77:281–286. [Google Scholar]
- Cuautle M, Rico-Gray V. The effect of wasps and ants on the reproductive success of the extrafloral nectaried plant Turnera ulmifolia (Turneraceae) Functional Ecology. 2003;17:417–423. [Google Scholar]
- Darwin F. On the glandular bodies on Acacia sphaerocephala and Cecropis peltata serving as food for ants. With an appendix on the nectar glands of the common brake fern, Pteris aquilina. Journal of the Linnaean Society (Botany) 1877;15:398–409. [Google Scholar]
- De la Barrera E, Nobel PS. Nectar: properties, floral aspects, and speculations on origin. Trends in Plant Science. 2004;9:65–69. doi: 10.1016/j.tplants.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M. Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: richness, occurrence, seasonality and ant foraging patterns. Ecoscience. 2004;11:472–481. [Google Scholar]
- Díaz-Castelazo C, Guimarães PR, Jr, Jordano P, Thompson JN, Marquis RJ, Rico-Gray V. Changes of a mutualistic network over time: reanalysis over a 10-year period. Ecology. 2010;91:793–801. doi: 10.1890/08-1883.1. [DOI] [PubMed] [Google Scholar]
- Dümmer R. Grape sugar as an excretion in Platycerium. Annals of Botany. 1911;25:1205–1206. [Google Scholar]
- Figdor W. Ueber die extranuptialen Nectarien von Pteridium aquilinum. Oesterreichische Botanische Zeitschrift. 1891;41:293–295. [Google Scholar]
- Heads PA. Bracken, ants and extrafloral nectaries. IV. Do wood ants (Formica lugubris) protect the plant against insect herbivores? Journal of Animal Ecology. 1986;55:795–809. [Google Scholar]
- Heads PA, Lawton JH. Bracken, ants and extrafloral nectaries. II. The effect of ants on the insect herbivores of bracken. Journal of Animal Ecology. 1984;53:1015–1031. [Google Scholar]
- Heads PA, Lawton JH. Bracken, ants and extrafloral nectaries. III. How insect herbivores avoid ant predation. Ecological Entomology. 1985;10:29–42. [Google Scholar]
- Janzen DH. Coevolution between ants and acacias in Central America. Evolution. 1966;20:249–275. doi: 10.1111/j.1558-5646.1966.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Interaction of the bull's horn acacia (Acacia cornigera) with an ant inhabitant (Pseudomyrmex ferruginea) in East Mexico. Kansas University Science Bulletin. 1967;47:315–558. [Google Scholar]
- Koptur S. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays E, editor. Insect–plant interactions. Vol. 4. Boca Raton, FL: CRC Press; 1992. pp. 81–129. [Google Scholar]
- Koptur S. Nectar as fuel for plant protectors. In: Wäckers FL, van Rijn PCJ, Bruin J, editors. Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge: Cambridge University Press; 2005. pp. 75–108. [Google Scholar]
- Koptur S, Smith AR, Baker I. Nectaries in some Neotropical species of Polypodium (Polypodiaceae): preliminary observations and analyses. Biotropica. 1982;14:108–113. [Google Scholar]
- Koptur S, Rico-Gray V, Palacios-Rios M. Ant protection of the nectaried fern Polypodium plebeium in central Mexico. American Journal of Botany. 1998;85:736–739. [PubMed] [Google Scholar]
- Lloyd FE. The extra-nuptial nectaries in the common brake, Pteridium aquilinum. Science. 1901;13:885–890. doi: 10.1126/science.13.336.885. [DOI] [PubMed] [Google Scholar]
- Lüttge U. Uber die Zusammensetzung des Nektars und den Mechanismus seiner Sekretion. I. Planta. 1961;56:189–212. [Google Scholar]
- Mehltreter K. Helechos (Capitulo 6) In: Manson RH, Hernández-Ortiz V, Gallina X, Mehltreter K, editors. Agroecosistemas Cafetaleros de Veracruz: Biodiversidad, Manejo y Conservacion. Xalapa: INECOL; 2008. pp. 83–93. [Google Scholar]
- Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. Nectar and pollination drops: how different are they? Annals of Botany. 2009;104:205–219. doi: 10.1093/aob/mcp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Rios M. Tesis de Maestría en Ciencias, Universidad Nacional Autónoma de México. Las Pteridofitas del Estado de Veracruz, México. 1992 [Google Scholar]
- Potes A. Comparative anatomy of the nectaries of Aglaomorpha and Drynaria (Polypodiaceae) American Fern Journal. 2010;100:80–92. [Google Scholar]
- Pryer KM, Schneider H, Smith AR, et al. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature. 2001;409:618–622. doi: 10.1038/35054555. [DOI] [PubMed] [Google Scholar]
- Rashbrook VK, Compton SG, Lawton JH. Ant–herbivore interactions: reasons for the absence of benefits to a fern with foliar nectaries. Ecology. 1992;73:2167–2174. [Google Scholar]
- Rico-Gray V. Use of plant-derived food resources by ants in the dry tropical lowlands of coastal Veracruz, México. Biotropica. 1993;25:301–315. [Google Scholar]
- Rico-Gray V, Oliveira PS. The ecology and evolution of ant–plant interactions. Chicago, IL: University of Chicago Press; 2007. [Google Scholar]
- Rico-Gray V, García-Franco JG, Palacios-Rios M, Díaz-Castelazo C, Parra-Tabla V, Navarro JA. Geographical and seasonal variation in the richness of ant–plant interactions in México. Biotropica. 1998;30:190–200. [Google Scholar]
- Ruhren S, Handel SN. Jumping spiders (Salticidae) enhance the seed production of a plant with extrafloral nectaries. Oecologia. 1999;119:227–230. doi: 10.1007/s004420050780. [DOI] [PubMed] [Google Scholar]
- Schremmer F. Extranuptiale nektarien: Beobachtungen an Salix eleagnos und Pteridium aquilinum. Oesterreichische Botanische Zeitschrift. 1970;117:205–222. [Google Scholar]
- Schuettpelz E, Pryer KM. Fern phylogeny. In: Ranker TA, Haufler CH, editors. The biology and evolution of ferns and lycophytes. Cambridge: Cambridge University Press; 2008. pp. 395–416. [Google Scholar]
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG. A classification for extant ferns. Taxon. 2006;55:705–731. [Google Scholar]
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG. Fern classification. In: Ranker TA, Haufler CH, editors. The biology and evolution of ferns and lycophytes. Cambridge: Cambridge University Press; 2008. pp. 417–467. [Google Scholar]
- Tempel AS. Bracken fern (Pteridium aquilinum) and nectar-feeding ants: a nonmutualistic interaction. Ecology. 1983;64:1411–1422. [Google Scholar]
- Wagner WH, Gómez LD. Pteridophytes (Helechos, Ferns) In: Janzen DH, editor. Costa Rican natural history. Chicago, IL: University of Chicago Press; 1983. pp. 311–318. [Google Scholar]
- Ward PS. Phylogeny, classification, and species-level taxonomy of ants (Hymenoptera: Formicidae) Zootaxa. 2007;1668:549–563. [Google Scholar]
- White R, Turner MD. The anatomy and occurrence of foliar nectaries in Cyathea (Cyatheaceae) American Fern Journal. 2012;102:91–113. [Google Scholar]
- Zamora PM, Vargas NS. Nectary–costule association in Philippine Drynarioid ferns. Philippine Agriculture. 1974;57:72–88. [Google Scholar]