Abstract
Background and Aims
Many terrestrial orchids have an obligate requirement for mycorrhizal associations to provide nutritional support from germination to establishment. This study will investigate the ability of orchid mycorrhizal fungi (OMF) to utilize a variety of nutrient sources in the nutrient-impoverished (low organic) soils of the Southwest Australian Floristic Region (SWAFR) in order to effectively compete, survive and sustain the orchid host.
Methods
Mycorrhizal fungi representing key OMF genera were isolated from three common and widespread species: Pterostylis recurva, Caladenia flava and Diuris corymbosa, and one rare and restricted species: Drakaea elastica. The accessibility of specific nutrients was assessed by comparing growth including dry biomass of OMF in vitro on basal CN MMN liquid media.
Key Results
Each of the OMF accessed and effectively utilized a wide variety of nutrient compounds, including carbon (C) sources, inorganic and organic nitrogen (N) and inorganic and organic phosphorus (P). The nutrient compounds utilized varied between the genera of OMF, most notably sources of N.
Conclusions
These results suggest that OMF can differentiate between niches (micro-niche specialization) in a constrained, highly resource-limited environment such as the SWAFR. Phosphorus is the most limited macronutrient in SWAFR soils and the ability to access phytate by OMF indicates a characterizing functional capacity of OMF from the SWAFR. Furthermore, compared with OMF isolated from the rare D. elastica, OMF associating with the common P. recurva produced far greater biomass over a wider variety of nutritional sources. This suggests a broader tolerance for habitat variation providing more opportunities for the common orchid for recruitment and establishment at a site.
Keywords: Carbon, Ceratobasidium, nitrogen, nutrients, orchid mycorrhizal fungi, phosphorus, Sebacina, soil, terrestrial orchid, Tulasnella
INTRODUCTION
Orchids form symbiotic associations with mycorrhizal fungi that promote seed germination, plant growth and development and, in mycoheterotrophic species, a life-long nutritional dependency (Rasmussen, 2002; Smith and Read, 2008). Often lacking substantial endosperm- or seed-based nutrient reserves, orchid seeds require external nutrient inputs to germinate suppplied in nature through symbiosis with fungi (Arditti, 1967). Particularly in terrestrial species, many chlorophyllous orchids show a similar dependency on their orchid mycorrhizal fungi (OMF) partners as non-green orchids, despite their ability to assimilate carbon (C) through photosynthesis (Julou et al., 2005; Dearnaley, 2007; Tedersoo et al., 2007). The role of OMF is therefore pivotal in the nutritional support of the orchid plant, with the distribution and abundance of OMF in soil, determining niche selection by the plant and promoting, through deployment of different fungi, co-habitation of mixed orchid species (Jacquemyn et al., 2012). Thus, in developing principles for the conservation of orchids, understanding factors influencing the growth and survival of the OMF associate is essential for ensuring long-term survival and growth of the orchid (Swarts and Dixon, 2009).
OMF can live without the orchid host and grow freely as saprophytes in soil or operate as mycorrhizas on non-orchids (Julou et al., 2005). Thus, the ability of OMF to access soil nutrient sources for their growth is critical for their own survival and persistence in soil, ultimately determining the functional significance of the symbiosis, where OMF must acquire and transfer nutrients to the orchid plant. In many respects, the hyphae of OMF effectively operate as a de facto root system, ramifying mycelium through the soil exemplified by orchid species where root production is limited (Ramsay et al., 1986) or non-existent, as found in the rhizomatous heteromycotroph Rhizanthella gardnerii (Dixon et al., 1990).
The ability of OMF to utilize a range of nutrient sources and the form of those sources (mineral or organic) is poorly understood. Though various C and N sources have been investigated (Hadley, 1969, 1984; Hadley and Ong, 1978; Beyrle and Smith, 1993; Midgley et al., 2006), the data are limited on understanding the ability of OMF to utlize P, a key limiting element in terrestrial orchid hotspots such as the Mediterranean regions of southern Africa and south-west Australia (Lambers et al., 2008). Early studies demonstrated the uptake of inorganic P (Alexander et al., 1984); however, to utilize organic P requires hydrolysis of the organic compounds and release of inorganic P (Smith and Read, 2008). The information on OMF to utilize various nutrient sources is limited to early work on the fungal genera Ceratobasidium and Tulasnella (Hadley and Ong, 1978); moreover, there are no studies on the impact on fungal growth of a variety of potential sources of C, N and P for the major OMF genera (Ceratobasidium, Sebacina and Tulasnella).
The Southwest Australia Floristic region (SWAFR) is a global biodiversity hotspot (Myers et al., 2000). The region has an abundant and diverse terrestrial orchid flora with most intact bushland possessing a surprising abundance of plants from the 400 native terrestrial species, with 98 % endemic to the region (Brown et al., 2008). Orchids of the SWAFR that associate with specific mycorrhizal fungi from well-established OM genera include Ceratobasidium, Sebacina and Tulasnella (Warcup, 1971). The sources of nutrients used by OMF of orchids in the SWAFR is largely unknown, though the soils where many biodiverse communities of orchids exist are highly nutrient-impoverished, organic-deficient and leached sandy soils (podzols) (Lambers et al., 2010). In these plant communities dominated by cluster-rooting proteaceous trees and shrubs (Lambers et al., 2010), the sparse leaf litter is the major source of nutrients, including C, N and P (Kononova, 1961). For fungi in these nutrient-limited environments, the carbon sources in soil litter occur in various forms, particularly cell-wall remnants of decomposing plant litter, including cellulose, hemicellulose (xylan) and pectin. The decomposition of these polysaccharides in soil provides monosaccharides (e.g. glucose, fructose, galactose, arabinose and mannose) and disaccharides (Sparks, 1999). Nitrogen in soil litter is present in the form of inorganic N (ammonium and nitrate) and organic N (amino acids, peptides and proteins) (Chalot and Brun, 1998). Inorganic P (orthophosphate) and organic P (phosphomonoesters and phosphodiesters) in weathered parent material, plant litter or animal faeces contribute to the pool of P in soil (Beadle, 1954).
In this study, mycorrhizal fungi representing key OMF genera of the SWAFR were isolated from three common and widespread orchid species – Pterostylis recurva, Caladenia flava and Diuris corymbosa – and one rare and restricted species – Drakaea elastica. These species co-occur in the SWAFR, allowing a comparison of their ability to utilize a range of nutrients. For each OMF isolate, we compared growth on liquid media containing a variety of C, N and P sources. We assessed whether nutrient utilization by OMF varies between genera and thus explored if differences in nutrient utilization may operate to limit habitat type, distribution and abundance of the orchid partner.
MATERIALS AND METHODS
OMF were isolated from sections of the appropriately infected organs: underground stems of Drakaea elastica, Caladenia flava and Pterostylis recurva; and roots of Diuris corymbosa (Ramsay et al., 1986; Table 1). Plants were collected from bushland in the Swan Coastal Plan of SWAFR in representative habitat typical for the species (Table 1). Sections were washed under tap water and further rinsed with sterile water three times in a laminar flow cabinet. Pelotons containing mycorrhizal hyphae were teased out from the cortical cells of the sectioned tissue under a dissecting microscope and rinsed via serial dilution to sterilize. Individual pelotons were micro-pipetted onto SSE (soil solution equivalent) medium containing streptomycin (Batty et al., 2002) to inhibit bacterial growth. When fungal hyphae appeared from the pelotons, single hyphal tips were subcultured onto 6·8 g l−1 potato dextrose agar (PDA) and incubated at 21 °C.
Table 1.
Distribution, habitat description, clonal habit and mycorrhizal information for each of the taxa used in this study
| Species | Caladenia flava | Pterostylis recurva | Drakaea elastica | Diuris corymbosa |
|---|---|---|---|---|
| Conservation status | Common and widespread | Common and widespread | Rare and restricted | Common and widespread |
| Distribution |  |
 |
 |
 |
| Habitat description | Wide variety of habitats ranging from coastal woodlands, winter-wet swamps, dry eucalypt forests and granite outcrops1 | Wide variety of habitats ranging from coastal woodlands, winter-wet swamps, dry eucalypt forests and granite outcrops1 | Deep sandy soil in Banksia and Kunzea woodlands in close proximity to winter-wet swamps1 | Banksia woodlands around winter-wet swamp margins, dry eucalypt forests1 |
| Clonality | Seeder/clonal | Seeder | Seeder/clonal | Seeder/clonal |
| Infection type | Stem collar2 | Underground stem2 | Root-stem2 | Root2 |
| Mycorrhizal associate | Sebacina vermifera3,4 | Ceratobasidium sp.2,3 | Tulasnella sp.3 | Tulasnella colospora5 |
References: 1 Hoffman and Brown (1998); 2 Ramsay et al. (1986); 3 Bonnardeaux et al. (2007); 4 Swarts et al. (2010); 5 Phillips et al., (2011).
Preparation of inocula
For all treatments, two plugs of agar (inocula) were excised from the leading edge of the actively growing mycelia on PDA, using a 5-mm-diameter cork borer and plated into 30-mL tubes containing 25 mL liquid medium. For the preparation of inocula for testing C and N utilization, the isolates grown on PDA were first subcultured onto low-C (50 %) and low-N (50 %) modified Melin and Norkrans (CN MMN) agar medium to clean the cultures of nutrient carry-over and to reflect the nutrient-impoverished soils of the SWAFR (Hopper and Gioia, 2004). This medium contained (L−1): 5·0 g glucose, 0·30 g KH2PO4, 0·25 g (NH4)2HPO4, 0·14 g MgSO4.7H2O, 50 mg CaCl2, 25 mg NaCl, 3 mg ZnSO4, 12·5 mg ferric EDTA and 0·13 mg thiamine (Marx and Bryan, 1975). The pH was adjusted to 5·0–5·5 before autoclaving.
Inocula for P utilization were prepared by growing the OMF on CN MMN media agar from which P sources (NH4)2HPO4 and KH2PO4 were omitted to deplete P stored in mycelia. Double concentration of NH4NO3 (0·606 g L−1) and 164 mg L−1 KCl were added to compensate for the absence of N provided as (NH4)2HPO4 and to provide a K source for the media (Midgley et al., 2006). The composition of CN MMN media agar from which P was excluded for preparation of inocula for P treatments (L−1) was 5·0 g glucose, 0·606 g NH4NO3, 0·14 g MgSO4.7H2O, 50 mg CaCl2, 25 mg NaCl, 164 mg KCl, 3 mg ZnSO4, 12·5 mg ferric EDTA, 0·13 mg thiamine and 8 g agar. The pH was adjusted to 5·0–5·5.
Determination of exponential growth phase of the mycorrhizal fungi
The capacity of OMF to utilize C, N and P sources was assessed in the period of the exponential growth phase for each mycorrhizal fungus (Midgley et al., 2006). This was determined by growing each isolate in CN MMN liquid medium. Fungal isolates from P. recurva and C. flava were grown in straight CN MMN liquid medium, while isolates from D. elastica and D. corymbosa required a liquid medium containing a combination of vitamins, thiamine and 0·20 mg l−1 para-amino benzoic acid (PABA) (CN MMN + PABA liquid media) for growth. Fast-growing fungi from P. recurva and D. corymbosa were harvested every 2 d, while slower-growing fungi of C. flava and D. elastica were harvested every 4 d. Harvesting involved filtering liquid cultures through a 10-μm nylon mesh with the washed hyphae transferred onto pre-weighed aluminium foil, then dried for 2 h at 80 °C. The fungal growth curve was obtained by plotting biomass of the OMF against time and the exponential phase of the OMF was determined based on the growth curve.
As mycelial growth rates for the OM isolates used in this trial varied significantly, the length of incubation time was adjusted to ensure that all treatments were carried out during each isolate's exponential growth phase at the time of harvest which was standardized to approx. 5 mg dry fungal biomass. From fastest- to slowest-growing isolates, P. recurva isolates were incubated for 8 d, D. corymbosa isolates for 18 d, C. flava isolates for 20 d and D. elastica isolates for 28 d.
Carbon treatments
The ability of OMF to utilize various C sources was tested using modified CN MMN + PABA liquid media containing C sources: mannose, arabinose, rhamnose, galactose (monosaccharide), cellobiose (disaccharide), cellulose, carboxymethylcellulose (CMC), starch, pectin, xylan (polysaccharides) and tannic acid (phenolic carbon) (Sigma-Aldrich, Castle Hill, Australia). Each type of C source tested was added into liquid media from which the regular carbon source (glucose) was omitted, to ensure only one type of C source was tested at a time. A treatment with glucose as a C source was also included. For all C treatments, the final concentration of single C sources added to the liquid media was 2 g C L−1. As crystalline cellulose is insoluble, jars containing this source were agitated to ensure the cellulose remained in suspension. Growth on this substrate was determined by visual comparison to C-free controls to reduce the influence of contamination of cellulose on dry-weight measurements. For the other C treatments, the growth on the other C sources was based on the biomass production on each C substrate. There were four replicates for each C treatment. A control treatment free of C sources was included. Biomass produced on a C-free medium was subtracted from that produced on the various C compounds to obtain net growth of OMF on C sources tested.
Nitrogen treatments
The ability of OMF to utilize N sources was tested in CN MMN + PABA liquid media containing a single N source. For treatments containing one N source, individual N sources were added into basal media CN MMN + PABA liquid media from which the regular N source, ammonium (NH4)2HPO4, was omitted. For each treatment, the final N concentration was added at the same concentration of N in the CN MMN + PABA liquid media (53 mg L−1). The range of N sources tested included inorganic N (ammonium and nitrate) and organic N, including simple organic N (amino acids) and complex organic N (protein) in the form of bovine serum albumin (BSA) (Sigma-Aldrich). Amino acids used in this experiment included acidic amino acids (l-glutamic acid and l-aspartic acid), basic amino acids (l-arginine and l-histidine) and neutral amino acids (l-alanine, l-asparagine, l-glutamine, glycine and l-proline) (Sigma-Aldrich). Organic N sources were sterilized through 0·22-μm Millipore filters into autoclaved basal media as organic N is unstable to heat sterilization (autoclaving), and the pH was adjusted to 5·0. Inorganic N sources were sterilized by autoclaving. Two plugs of active fungal inocula were excised from the leading edge of the colony using a 5-mm-diameter cork borer. Inocula were added to each N treatment (liquid media containing a single type of N source in 30-mL tubes). There were four replicates for each treatment. The growth on N sources was based on the biomass production on each N substrate. As above, biomass produced on an N-free medium was subtracted from that produced on the various N compounds to obtain net biomass and growth of OMF on N sources tested.
Phosphorus treatments
The ability of OMF to utilize P sources was tested in CN MMN + PABA liquid media containing a single type of P source comprising NaH2PO4, phytic acid, and DNA supplied as sodium salt of extracted salmon testes (Sigma-Aldrich) added to basal media (CN MMN + PABA liquid media from which P sources were omitted). For P treatments, the amount of P added to basal media was at the same final total P concentration in the media (60 mg P L−1). As DNA contains C and N, the glucose content was reduced to 4·02 g L−1 and NH4NO3 was excluded from DNA treatments (based on the estimates of the C and N concentration in salmon DNA (Zubay, 1993). DNA was sterilized by immersion in 3 mL of 70 % (v/v) ethanol solution for 48 h prior to being added to autoclaved basal media. Basal media were kept at a maximum temperature of 80 °C on a heating block and stirred until DNA was completely dispersed (Leake and Miles, 1996). Phytic acid was dissolved in basal media and the pH adjusted to 5·0 before filter sterilization using a 0·22-μm Millipore membrane filter into the autoclaved basal media. A P-free treatment was included. As above, biomass produced on a P-free medium was subtracted from that produced on the various P compounds to obtain net biomass and growth of OMF on P sources tested.
Statistical analysis
All data were analysed using analysis of variance (ANOVA) with Minitab 14th Edition. Significant differences between treatments were determined by Tukey's test at 5 %.
RESULTS
Carbon treatments
The biomass of all OMF increased on all carbon sources. For each mycorrhizal type, greatest biomass was produced with xylan over any other carbon source. Other carbon sources promoting high productivity and growth included glucose, mannose, cellobiose, pectin and starch (Fig. 1). All OMF also grew on CMC to some extent. Biomass production on arabinose, rhamnose and galactose was the least for each species' isolates with the exception of P. recurva isolates which produced more than double the biomass on galactose. There was no growth observed on tannic acid (Fig. 1). Biomass production of all OMF on cellulose was greater than that on carbon-free controls.
Fig. 1.
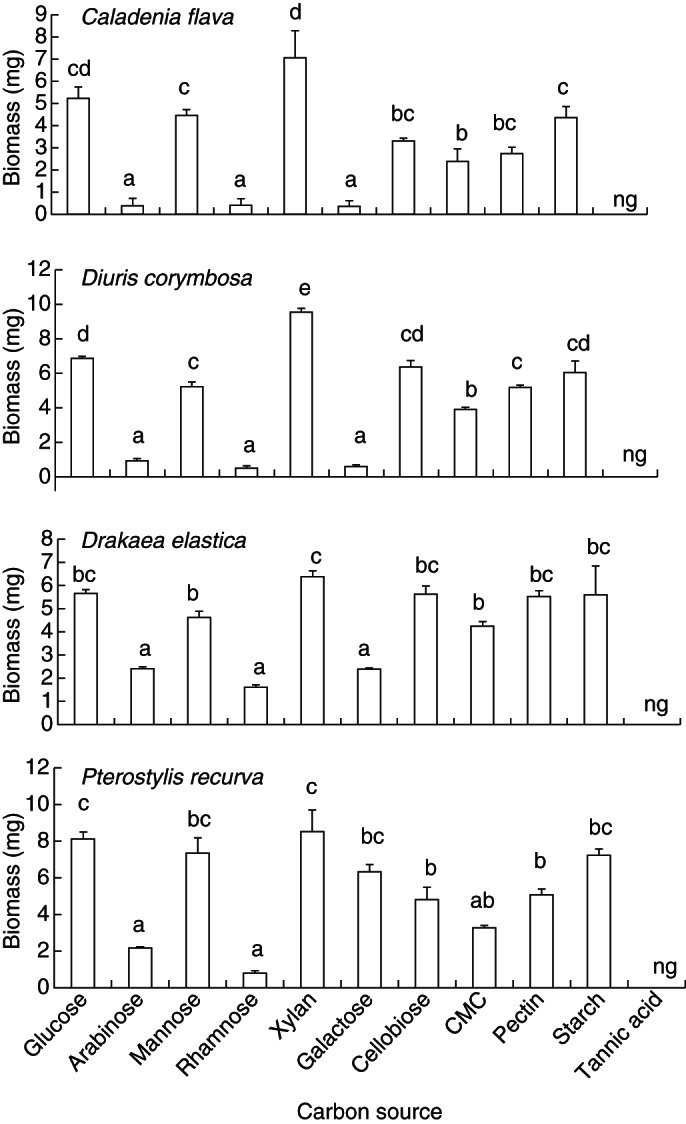
Mean biomass production (± s.e.; n = 4) of Caladenia flava isolates after 20 d, Diuris corymbosa isolates after 18 d, Drakaea elastica isolates after 28 d and Pterostylis recurva isolates after 8 d in liquid media containing 11 different C sources. Letters above columns indicate significant differences (P < 0·05); ng, no growth.
Nitrogen treatments
All OMF produced biomass on various N sources, but isolates from P. recurva produced far greater biomass than other OMF. In general, patterns of N utilization by all OMF were similar (Fig. 2), the most notable difference being the strong growth on nitrate by P. recurva isolates. Biomass production of all OMF was equally high on both acidic amino acids (aspartic acid and glutamic acid). Growth of all OMF on basic amino acid occurred for arginine, while there was no, or limited, growth on histidine. All OMF also produced biomass on neutral amino acids, alanine, asparagine, glutamine and glycine, although there was intraspecific variation in biomass production on alanine, glutamine and glycine among OMF. Pterostylis recurva isolates produced little biomass on alanine, while other OMF produced comparatively more biomass. Biomass production of C. flava on glutamine was low compared with other OMF on this amino acid. Glycine supported growth of D. corymbosa isolates, but other OMF grew poorly on glycine, or not at all (C. flava OM). Proline provided poor nutritional support for all OMF, except P. recurva isolates. All OMF produced a relatively large amount of biomass on asparagine, while they produced biomass on protein BSA in some instances.
Fig. 2.
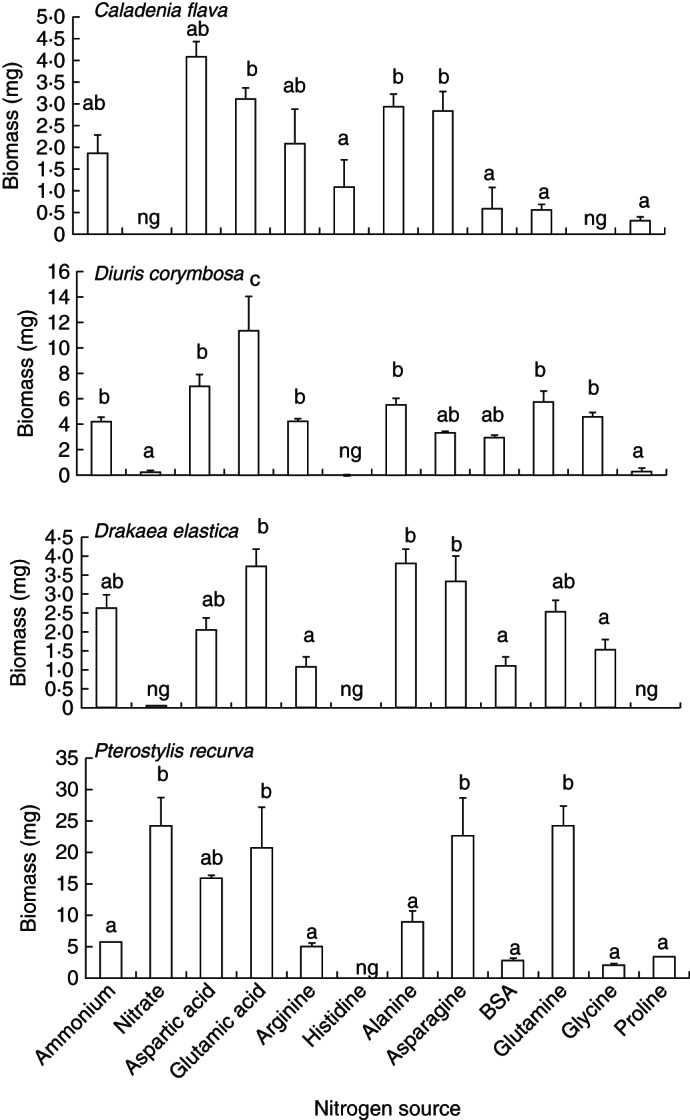
Mean biomass production (± s.e.; n = 4) of Caladenia flava isolates after 20 d, Diuris corymbosa isolates after 18 d, Drakaea elastica isolates after 28 d and Pterostylis recurva isolates after 8 d in liquid media containing 12 different N sources. Letters above columns indicate significant differences (P < 0·05); ng, no growth.
Phosphorus treatments
All OMF produced biomass on a range of P sources (Fig. 3). All OMF produced more biomass on orthophosphate and phytic acid than on DNA, with the exception of C. flava that produced little biomass on both phytic acid and DNA.
Fig. 3.
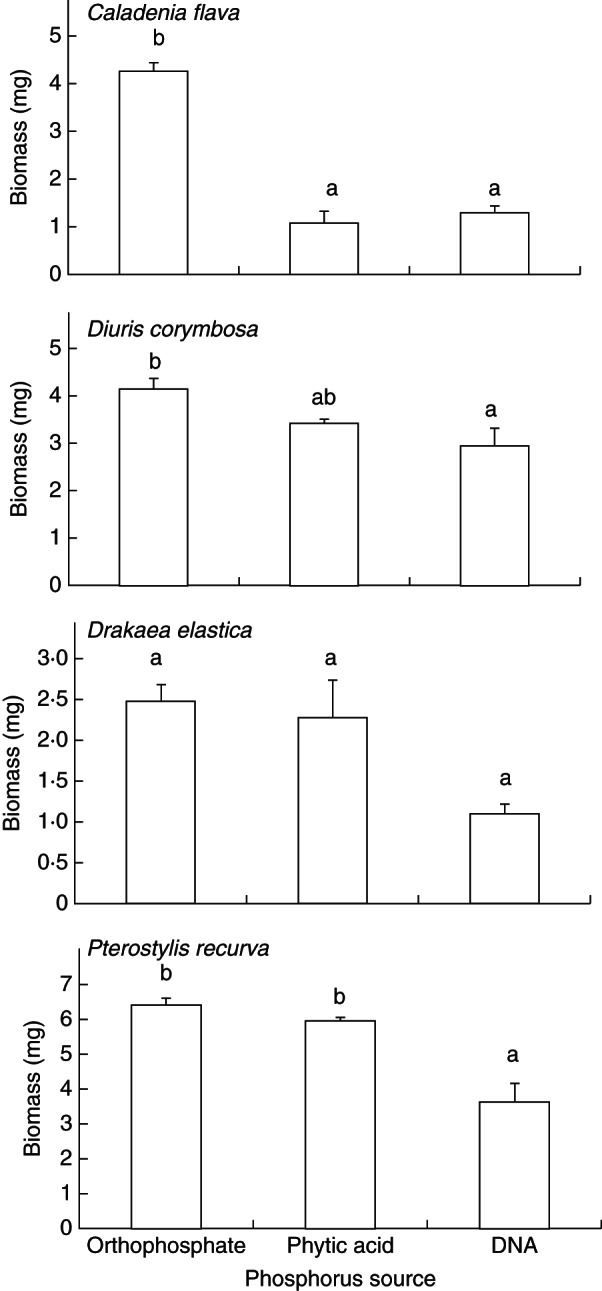
Mean biomass production (± s.e.; n = 4) of Caladenia flava isolates after 20 d, Diuris corymbosa isolates after 18 d, Drakaea elastica isolates after 28 d and Pterostylis recurva isolates after 8 d in liquid media containing three P sources. Letters above columns indicate significant differences (P < 0·05); ng, no growth.
DISCUSSION
Orchid-mycorrhizal nutrition
As free-living saprophytes in soil, OMF must effectively utilize available soil C, N and P sources for growth, survival, reproduction and persistence. Our data demonstrate the ability of OMF from different fungal genera, Ceratobasidium (from Pterostylis recurva), Sebacina (from Caladenia flava) and Tulasnella (from Diuris corymbosa and Drakaea elastica), to utilize a wide and variable menu of C, N and P sources under in vitro conditions, indicative of a capacity to access different fractions of the soil nutrient pool.
Carbon
The range of C sources utilized by OMF included glucose, mannose (monosaccharides), cellobiose (disaccharide), cellulose, CMC, xylan, pectin and starch (polysaccharides). Utilization of glucose and mannose by OMF may be related to these sugars being monosaccharides, and the relative ease whereby these simple molecules are absorbed directly through the hyphal wall and transported and metabolized for growth (Griffin, 1994; Nehls and Hampp, 2000; Guzmán et al., 2005). Cellobiose was also used as a carbon source in each isolate tested. Cellobiose can be transported directly or following cleavage into glucose molecules via an extracellular cellobiase before absorption (Griffin, 1994; Burke and Cairney, 1997).
During the establishment of the symbiotic association at seed germination, OMF must degrade the cell walls of orchid seeds which are composed of complex polysaccharides such as cellulose, hemicelluloses and pectin (Norkrans, 1963). For this to be possible, an array of extracellular enzymes (cellulase, carboxymethylcellulase, amylase, xylanase and pectinase) is required to catabolize their respective polysaccharides into simple sugars before absorption (Perotto et al., 1995; Cairney and Burke, 1996; Warren, 1996). Growth of OMF on cellulose, CMC, xylan and pectin provides evidence of the capacity of these fungi to release these extracellular enzymes and thus use these C compounds as a source of energy and carbon skeletons for seedling development.
Arabinose, rhamnose and galactose were less readily utilized by OMF as carbon sources in comparison with glucose and mannose. Although, like glucose and mannose, they are monosaccharies, the means whereby membrane transport is regulated is thought to differ, perhaps explaining this finding (Jennings, 1995). Interestingly, P. recurva isolates utilized galactose more readily than the other isolates, whilst all isolates were unable to utilize tannic acid as a carbon source, a similar result to that found in other studies (Midgley et al., 2006). Utilization of tannic acid by soil microbes, including many fungal species ( (Mingshu et al., 2006), is facilitated by tannase, which degrades tannin into glucose and gallic acid, indicating that the present OMF lack tannase even though leaf litter from families such as Mytaceae that dominant in orchid habitats in SWAFR are high in tannins.
Both the similarity and specialization of OMF for various carbon sources indicates that within the habitat where these orchid species co-occur (often within centimetres) there are multiple carbon sources where different OMF have the capacity to minimize species-to-species competition by drawing upon unique carbon constituents. Whether in nature these carbon constituents are available to the degree of providing a competitive advantage or, indeed, if this fungal growth translates to improved orchid establishment or growth remains to be investigated.
Nitrogen
In common with other OMF, the fungi in this study were able to utilize inorganic N in the form of ammonium, but not nitrate (Hadley and Ong, 1978), with the notable exception of P. recurva isolates that utilized both. Inorganic N in soil is predominantly derived from the mineralization of organic matter which releases ammonium that can subsequently be nitrified via nitrite into nitrate (Connell et al., 1995).
Various amino acids, including aspartic acid, glutamic acid, arginine, alanine and asparagine, were utilized for the growth of fungal isolates in this study. Other OMF have previously been reported to utilize these amino acids for their growth (Hadley and Ong, 1978). Fungal isolates in the present study also utilized protein (BSA) to some extent, showing that they released proteases to hydrolyse BSA into amino acids or peptides before absorption (Leake and Read, 1990). However, the degree to which free amino acids and intact protein is resident in these bushland soils is unknown, though it is likely that primary breakdown of litter by OM may release these components.
Phosphorus
Fungal isolates in the present study utilized each P source (orthophosphate, DNA and phytic acid) tested, but to varying degrees. Orthophosphate is the best P source for OMF (Colpaert et al., 1999; Ezawa et al., 2002), being the only P form that can be directly absorbed as it is exists in the soil water. Organic P must first be hydrolysed by extracellular phosphatases (phosphomonoesterases and phosphodiesterases) into orthophosphate, which can subsequently be absorbed (Bartlett and Lewis, 1973; Margesin and Schinner, 1994).
The use of organic P forms (DNA and phytic acid) by OMF in this study demonstrates their ability to produce the required enzymes for their hydrolysis prior to absorption of orthophosphate. The utilization of phytic acids indicates the release of a phytase, an extracellular enzyme, which hydrolyses phytic acid into orthophosphate. Knowledge of extracellular phytases is derived from other mycorrhizal fungi that release phytase into the medium when grown on phytic acid as the sole P source (Hilger and Krause, 1989; Antibus et al., 1992). The utilization of DNA indicates the production of extracellular phosphodiesterases to hydrolyse DNA and releases orthophosphate. Several other mycorrhizal fungi, such as ericoid mycorrhizal fungi, have previously been demonstrated to utilize DNA as a P source, through the release of phosphodiesterases (Leake and Miles, 1996; Chen et al., 1999).
Ecological implications
This study has demonstrated the variability of key OMFgenera (Ceratobasidium, Sebacina and Tulasnella), isolated from south-west Australian terrestrial orchids, to utilize a variety of C, N and P sources. Although OMF nutrient utilization data were collected under in vitro conditions, they do point to a level of ecological significance for both the mycorrhizal fungi and their orchid associates. For OMF to operate with a broad nutritional competency it must utilize a wide menu of organic and inorganic sources. This provides the fungus and, ultimately, the orchid with outstanding ability for growth and a wide range of nutritionally variable substrates. It is well established that mycorrhizal fungi have the capacity to provide, at least in the germination to early seedling establishment phases, all nutrients (carbon, nitrogen, phosphorus and other organic and inorganic supplements) and possibly growth factors, to support orchid growth. This has been established for holomycotrophic species (non-green orchids) and is demonstrated under axenic conditions (Swarts et al., 2010); however, we cannot necessarily assume complete nutrient transfer from all OMF to their mature plant hosts
The SWAFR is a subdued, stable, highly weathered, old landscape with typically nutrient-impoverished soils (Hopper and Gioia, 2004; Lambers et al., 2010). Lambers et al. (2008) discussed that plant productivity on old, weathered landscapes such as the SWAFR are more likely to be limited by P than by N, which inevitably has consequences for plant nutrient-acquisition strategies in these landscapes. Indeed, in a follow-up review, Lambers et al. (2010) found that in severely P-limited landscapes, non-mycorrhizal plants with highly specialized root structures [i.e. proteoid (cluster) roots] are more prominent (in biomass) than mycorrhizal species. The opposite is true for younger, more N-limited landscapes such as post-glacial environments (Lambers et al., 2010). Mycorrhizal species do occur in these systems, albeit not as dominant species (Lambers et al., 2010). Terrestrial orchids in the SWAFR are among these mycorrhizal species; they are small plants, many species producing a small, single leaf with a root system that is limited in development and extent or, as in the case of Drakaea, Pterostylis and Caladenia, virtually non-existent. These are, instead, strongly dependent on their mycorrhizal associates from germination to reproduction (Table. 1).
By patching into pre-existing hyphal networks, terrestrial species, particularly those taxa using a stem-based infection syndrome (Fig. 1), are equiped with a pre-made ‘fungal’ root system. The utilization of complex nutrient sources, such as polysaccharides, proteins and organic P, ensures these hyphal systems are equipped to access these nutrients, demonstrating the vital role of OMF to degrade litter and absorb released nutrients to support the growth and development of terrestrial orchids.
For mycorrhizal fungi, an ability to utilize a wide variety of nutrient sources broadens the habitat range and the ability of the orchid to grow in a wide range of soil types. This ability may allow the fungus to either increase in abundance, perhaps out-competing less efficacious fungi, or occupy different niches. Whittaker and Cairney (2001) proposed niche differentiation as a competitive strategy for Australian ectomycorrhizal fungi on the basis of differential N substrate utilization. An orchid associating with this highly competitive mycorrhizal fungus is more likely to be common and widespread as opportunities for locating an efficacious mycorrhizal fungus are substantially increased.
These are the first published data on the ability of OMF to access phytate and highlights the potential for OMF to access P in the highly P-impoverished soils of the SWAFR, enabling these orchids to grow across a broader range of environmental conditions and to co-occur with species deploying other phosphorus-acquisition strategies such as cluster roots (Lambers et al., 2010). Cluster roots are highly invasive in soils forming superficial, often impenetrable mats over many square metres under a mature proteaceous tree. Orchids of the type investigated here do grow in these mats where the orchid must compete directly for nutrients with the cluster root assemblage.
In this study, while the trends for C, N and P utilization were generally similar for each mycorrhizal type, some key differences were observed in Ceratobasidium isolated from P. recurva. First, and most notably, is the much larger biomass achieved by the isolate's mycelium over a substantially shorter time for almost every digestible nutrient source. Secondly, Ceratobasidium isolates utilized galactose and nitrate as a major nutrient source, whereas other isolates either did not or only marginally. The ability of the P. recurva mycorrhizal fungus to utilize nitrate provides this mycorrhizal fungus with a source of N that is not available to the other fungi tested in this study. The preferred habitat of P. recurva is long-term unburnt, litter-rich sites (Hoffman and Brown, 1998), where, due to the low pH, nitrate concentrations are expected to be lower than at sites with higher pH (Havill et al., 1974) (such as calcareous coastal environments) that are less common in the SWAFR (Hopper and Gioia, 2004). Though it is possible that the accessibility of either nitrate or ammonium is of no ecological advantage to P. recurva, ecological performance of the orchid may be enhanced by dual-N uptake as a result of one or both of the following. Of the four species, only P. recurva possesses a tuber buried 10–12 cm deep with a stem that supports the above-ground single shoot. All other study species are collar-infected at the soil surface (Fig. 1), or, as in Diuris, have variously infected portions of the underground root system. The underground stem of P. recurva is fully infected from just above the tuber to ground level (Fig. 1) with the mycorrhizal fungus traversing a litter-rich soil surface to mineral soil at depth. It may be that ammonium N is utilized at the surface (organic-rich layers), while deeper in the stem–soil profile, nitrate is more available (Connell et al., 1995) and the single mycorrhizal agent is capable of accessing this source of N. Such a strategy would enable P. recurva to take up nitrate N if the thick litter layers where it grows were lost, such as following fires that occur in the SWAFR.
Pterostylis recurva is the only species in the present study that is not clonal, relying on a ‘seeder’ strategy (Dixon, 1991) to recruit new plants. Utilizing a mycorrhizal fungus with wider capability for N acquisition will ensure that seed germination and seedling establishment are more probably across a broader range of nutritional niches, thereby increasing the ability of the plant to occupy a wider range of habitats.
In contrast to P. recurva, the rare species used in the present study, Drakaea elastica, as found for this genus, is confined to highly specialized micro-sites that are open, litter-poor and well-drained podzolic soils (Phillips et al., 2011). Phillips et al. (2011) observed that fungi isolated from Drakaea are among the slowest growing of all mycorrhizal associates of orchids in the SWAFR, an observation supported in this study. For Drakaea, the highly specific nature of its mycorrhizal association may reflect the relative superiority of its Tulasnella associate to compete effectively for soil nutrients/photobiotic carbon in the extremely nutrient-poor sandy soils in which it exclusively grows, whilst in high-organic-matter environments this fungus will most likely be out-competed (Phillips et al., 2011). Indeed all eight Drakaea taxa investigated associated exclusively with a narrow monophyletic clade of Tulasnella at each stage of their life cycle (Phillips et al., 2011).
Interestingly, Tulasnella isolated from Diuris corymbosa increased biomass more rapidly, yet utilized all sources of nutrients in similar proportions to Tulasnella isolated from D. elastica. Previous studies have isolated Tulasnella colospora from D. corymbosa in Western Australia (Bonnardeaux et al., 2007) and Diuris fragrantissima in Victoria, in eastern Australia (Warcup, 1973; Smith et al., 2010). It appears that Tulasnella is a common orchid mycorrhizal partner associating with taxa from other Australian genera such as Chiloglottis (Roche et al., 2010), Thelymitra and Pyrcorchis (Bonnardeaux et al., 2007) as well as northern-hemisphere genera Goodyera and Liparis (McCormick et al., 2004). Whilst the phylogenetic diversity of Tulasnella is only recently becoming understood (Roche et al., 2010; Smith et al., 2010; Phillips et al., 2011), it would seem that the genetic diversity within this mycorrhizal genus may reflect functional diversity in its habitat preference and highly compatible orchid partner associations that need to be examined further.
Mycorrhizal fungal isolated from Caladenia taxa Australia-wide almost always belong to a closely related group of lineages within the Sebacina vermifera complex (Warcup and Talbot, 1967; Bougoure et al., 2005; Bonnardeaux et al., 2007; Swarts et al., 2010; Wright et al., 2010) of Subgroup B in the order Sebacinales as recognized by Weiss et al. (2004). Within this taxonomic complex, there is significant, but as yet unresolved, genetic diversity. Two studies have demonstrated that rare Caladenia taxa associate exclusively with a phylogenetically narrow range of Sebacina isolates that are geographically restricted in distribution, whilst common Caladenia taxa associate with a broader suite of Sebacina isolates that may be less restricted in their distribution (Swarts et al., 2010; Wright et al., 2010). Inferred from this is that ecological specificity and the ability to utilize a range of nutrients may be a driver of OMF distribution, which in turn influences orchid distribution and possibly rarity if the OMF itself is rare (Swarts et al., 2010). Trends for nutrient utilization for C. flava (Sebacina) isolates were similar to those for D. corymbosa (Tulasnella). Interestingly, both taxa regularly grow in sympatry throughout their distribution ranges (Hoffman and Brown, 1998), suggesting similar nutrient requirements for phylogenetically very diverse mycorrhizal fungi. Although we only tested isolates from the common and widespread Caladenia flava, future research could compare nutrient utilization of OMF isolates from rare and geographically restricted Caladenia taxa.
Concluding remarks
This research reveals differences in the ability of three important OMF genera to access over 25 different nutrient sources. Each mycorrhizal isolate tested utilized many nutrients similarly across C, N and P sources; however, there were notable differences for some nutrients including significantly increased nitrate and galactose utilization by OMF supporting the largest growth of the study species P. recurva. In general, OMF associating with the common P. recurva produced far greater biomass over a wider variety of nutritional sources than OMF isolated from the rare D. elastica, suggesting an increased capacity by common species to differentiate between niches in a constrained, resource-limited environment such as the SWAFR. Phosphorus is the most severely limiting macronutrient in SWAFR soils and the accession of phytate by the OMF in this study indicates a unique functional capacity for OMF in this region. These results suggest a competitive advantage of nutrient acquisition via mycorrhizal fungus, enabling orchids to grow across a broader range of environmental conditions and to co-occur with species deploying other P-acquisition strategies such as cluster roots, and explaining the abundance and diversification of orchids in this biodiversity hotspot.
ACKNOWLEDGEMENTS
We thank the orchid research team at Kings Park and Botanic Garden for providing advice and technical support throughout the study, particularly Belinda Newman who provided support and guidance on the orchid conservation programme.
LITERATURE CITED
- Alexander C, Alexander IJ, Hadley G. Phosphate uptake by Goodyera repens in relation to mycorrhizal infection. New Phytologist. 1984;97:401–419. [Google Scholar]
- Antibus RK, Sinsabaugh RL, Linkins AE. Phosphatase activities and phosphorus uptake from inositol phosphate by ectomycorrhizal fungi. Canadian Journal of Botany. 1992;70:794–801. [Google Scholar]
- Arditti J. Factors affecting the germination of orchid seeds. The Botanical Review. 1967;33:1–96. [Google Scholar]
- Bartlett EM, Lewis D. Surface phosphatase activity of mycorrhizal roots of beech. Soil Biology and Biochemistry. 1973;5:249–257. [Google Scholar]
- Batty AL, Dixon KW, Brundrett MC, Sivasithamparam K. Orchid conservation and mycorrhizal associations. In: Dixon KW, Barrett RL, editors. Microorganisms in plant conservation and biodiversity. Dordrecht: Kluwer Academic Publishers; 2002. [Google Scholar]
- Beadle NCW. Soil phosphate and the delimitation of plant communities in eastern Australia. Ecology. 1954;35:370–375. [Google Scholar]
- Beyrle HF, Smith SE. The effect of carbohydrate on the development of a Cattleya hybrid in association with its mycorrhizal fungus. Mycorrhiza. 1993;3:57–62. [Google Scholar]
- Bonnardeaux Y, Brundrett M, Batty AL, Dixon KW, Koch J, Sivasithamparam K. Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research. 2007;111:51–61. doi: 10.1016/j.mycres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bougoure JJ, Bougoure DS, Cainey JWG, Dearnaley JDW. ITS–RFLP and sequence analysis of endophytes from Acianthus, Caladenia and Pterostylis (Orchidaceae) in southeastern Queensland. Mycological Research. 2005;4:452–460. doi: 10.1017/s095375620500225x. [DOI] [PubMed] [Google Scholar]
- Brown A, Dixon K, Hopper S, Dundas P. Orchids of Western Australia. University of Western Australia Press: 2008. [Google Scholar]
- Burke R, Cairney J. Purification and characterization of a β-1,4-endoxylanase from the ericoid mycorrhizal fungus Hymenoscyphus ericae. New Phytologist. 1997;135:345–352. [Google Scholar]
- Cairney JWG, Burke RM. Physiological heterogeneity within fungal mycelia: an important concept for a functional understanding of the ectomycorrhizal symbiosis. New Phytologist. 1996;134:685–695. doi: 10.1111/j.1469-8137.1996.tb04934.x. [DOI] [PubMed] [Google Scholar]
- Chalot M, Brun A. Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiology Reviews. 1998;22:21–44. doi: 10.1111/j.1574-6976.1998.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Chen A, Chambers S, Cairney J. Utilisation of organic nitrogen and phosphorus sources by mycorrhizal endophytes of Woollsia pungens (Cav.) F. Muell. (Epacridaceae) Mycorrhiza. 1999;8:181–187. [Google Scholar]
- Colpaert JV, Van Tichelen KK, Van Assche JA, Van Laere A. Short-term phosphorus uptake rates in mycorrhizal and non-mycorrhizal roots of intact Pinus sylvestris seedlings. New Phytologist. 1999;143:589–597. doi: 10.1046/j.1469-8137.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Connell M, Raison R, Khanna P. Nitrogen mineralization in relation to site history and soil properties for a range of Australian forest soils. Biology and Fertility of Soils. 1995;20:213–220. [Google Scholar]
- Dearnaley J. Further advances in orchid mycorrhizal research. Mycorrhiza. 2007;17:475–486. doi: 10.1007/s00572-007-0138-1. [DOI] [PubMed] [Google Scholar]
- Dixon K. Seeder/clonal concepts in Western Australian orchids. In: Wells TCE, Willems JH, editors. Population ecology of terrestrial orchids. The Hague: SPB Academic Publishing; 1991. [Google Scholar]
- Dixon KW, Pate JS, Kuo J. The Western Australian fully subterranean orchid Rhizanthella gardneri. In: Arditti J, editor. Orchid biology, reviews and perspectives. Portland, OR: Timber Press; 1990. Vol. V. [Google Scholar]
- Ezawa T, Smith SE, Smith FA. P metabolism and transport in AM fungi. Plant and Soil. 2002;244:221–230. [Google Scholar]
- Griffin DH. Fungal physiology. 2nd edn. New York, NY: John Wiley & Sons; 1994. [Google Scholar]
- Guzmán S, Ramos I, Moreno E, et al. Sugar uptake and sensitivity to carbon catabolite regulation in Streptomyces peucetius var. caesius. Applied Microbiology and Biotechnology. 2005;69:200–206. doi: 10.1007/s00253-005-1965-7. [DOI] [PubMed] [Google Scholar]
- Hadley G. Cellulose as a carbon source for orchid mycorrhiza. New Phytologist. 1969;68:933–939. [Google Scholar]
- Hadley G. Uptake of (14C) glucose by asymbiotic and mycorrhizal orchid protocorms. New Phytologist. 1984;96:263–273. [Google Scholar]
- Hadley G, Ong SH. Nutritional requirements of orchid endophytes. New Phytologist. 1978;81:561–569. [Google Scholar]
- Havill DC, Lee JA, Stewart GR. Nitrate utilization by species from acidic and calcareous soils. New Phytologist. 1974;73:1221–1231. [Google Scholar]
- Hilger AB, Krause HH. Growth characteristics of Laccaria laccata and Paxillus involutus in liquid culture media with inorganic and organic phosphorus sources. Canadian Journal of Botany. 1989;67:1782–1789. [Google Scholar]
- Hoffman N, Brown A. Orchids of south-west Australia. Nedlands, WA: University of Western Australia Press; 1998. revised 2nd edn with supplement. [Google Scholar]
- Hopper SD, Gioia P. The Southwest Australian Floristic Region: evolution and conservation of a global hot spot of biodiversity Annual Review of Ecology, Evolution and Systematics. 2004;35:623–650. [Google Scholar]
- Jacquemyn H, De Meester L, Jongejans E, Honnay O. Evolutionary changes in plant reproductive traits following habitat fragmentation and their consequences for population fitness. Journal of Ecology. 2012;100:76–87. [Google Scholar]
- Jennings DH. The physiology of fungal nutrition. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse M-A. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist. 2005;166:639–653. doi: 10.1111/j.1469-8137.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Kononova MM. Soil organic matter, its nature, its role in soil formation and in soil fertility, Oxford: Pergamon Press; 1961. [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE. Plant nutrient-acquisition strategies change with soil age. Trends in Ecology & Evolution. 2008;23:95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil. 2010;334:11–31. [Google Scholar]
- Leake J, Miles W. Phosphodiesterase as mycorrhizal P sources. New Phytologist. 1996;132:435–443. doi: 10.1111/j.1469-8137.1996.tb01863.x. [DOI] [PubMed] [Google Scholar]
- Leake J, Read D. Proteinase activity in mycorrhizal fungi. II. The effects of mineral and organic nitrogen sources on induction of extracellular proteinase in Hymenoscyphus ericae (Read) Korf & Kernan. New Phytologist. 1990;116:123–128. [Google Scholar]
- McCormick MK, Whigham DF, O'Neill J. Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytologist. 2004;163:425–438. doi: 10.1111/j.1469-8137.2004.01114.x. [DOI] [PubMed] [Google Scholar]
- Margesin R, Schinner F. Phosphomonoesterase, phosphodiesterase, phosphotriesterase, and inorganic pyrophosphatase activities in forest soils in an alpine area: effect of pH on enzyme activity and extractability. Biology and Fertility of Soils. 1994;18:320–326. [Google Scholar]
- Marx DH, Bryan WC. Growth and ectomycorrhizal development of loblolly pine seedlings in fumigated soil infested with the fungal symbiont Pisolithus tinctorius. Forest Science. 1975;21:245–254. [Google Scholar]
- Midgley DJ, Jordan LA, Saleeba JA, McGee PA. Utilisation of carbon substrates by orchid and ericoid mycorrhizal fungi from Australian dry sclerophyll forests. Mycorrhiza. 2006;16:175–182. doi: 10.1007/s00572-005-0029-2. [DOI] [PubMed] [Google Scholar]
- Mingshu L, Kai Y, Qiang H, Dongying J. Biodegradation of gallotannins and ellagitannins. Journal of Basic Microbiology. 2006;46:68–84. doi: 10.1002/jobm.200510600. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nehls U, Hampp R. Carbon allocation in ectomycorrhizas. Physiological and Molecular Plant Pathology. 2000;57:95–100. [Google Scholar]
- Norkrans B. Degradation of cellulose. Annual Review of Phytopathology. 1963;1:325–350. [Google Scholar]
- Perotto S, Peretto R, Faccio A, Schubert A, Varma A, Bonfante P. Ericoid mycorrhizal fungi: cellular and molecular bases of their interface with the host plant. Canadian Journal of Botany. 1995;73:S557–S568. (Suppl) [Google Scholar]
- Phillips RD, Barrett MD, Dixon KW, Hopper SD. Do mycorrhizal symbioses cause rarity in orchids? Journal of Ecology. 2011;99:858–869. [Google Scholar]
- Ramsay RR, Sivasithamparam K, Dixon KW. Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana. 1986;1:203–214. [Google Scholar]
- Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant and Soil. 2002;244:149–163. [Google Scholar]
- Roche SA, Carter RJ, Peakall R, Smith LM, Whitehead MR, Linde CC. A narrow group of monophyletic Tulasnella (Tulasnellaceae) symbiont lineages are associated with multiple species of Chiloglottis (Orchidaceae): implications for orchid diversity. American Journal of Botany. 2010;97:1313–1327. doi: 10.3732/ajb.1000049. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn. New York, NY: Academic Press; 2008. [Google Scholar]
- Smith ZF, James EA, McLean CB. Mycorrhizal specificity of Diuris fragrantissima (Orchidaceae) and persistence in a reintroduced population. Australian Journal of Botany. 2010;58:97–106. [Google Scholar]
- Sparks DL. Soil physical chemistry. 2nd edn. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- Swarts ND, Dixon KW. Terrestrial orchid conservation in the age of extinction. Annals of Botany. 2009;104:543–556. doi: 10.1093/aob/mcp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts ND, Sinclair EA, Francis A, Dixon KW. Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Molecular Ecology. 2010;19:3226–3242. doi: 10.1111/j.1365-294X.2010.04736.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Pellet P, ljalg U, Selosse M-A. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia. 2007;151:206–217. doi: 10.1007/s00442-006-0581-2. [DOI] [PubMed] [Google Scholar]
- Warcup JH. Specificity of mycorrhizal association in some Australian terrestrial orchids. New Phytologist. 1971;70:41–46. [Google Scholar]
- Warcup JH. Symbiotic germination of some Australian terrestrial orchids. New Phytologist. 1973;72:387–392. [Google Scholar]
- Warcup JH, Talbot PHB. Perfect states of Rhizoctonias associated with orchids I. New Phytologist. 1967;66:631–641. [Google Scholar]
- Warren R. Microbial hydrolysis of polysaccharides. Annual Reviews in Microbiology. 1996;50:183–212. doi: 10.1146/annurev.micro.50.1.183. [DOI] [PubMed] [Google Scholar]
- Weiss M, Selosse M-A, Rexer KH, Urban A, Oberwinkler F. Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycological Research. 2004;108:1003–1010. doi: 10.1017/s0953756204000772. [DOI] [PubMed] [Google Scholar]
- Whittaker SP, Cairney JWG. Influence of amino acids on biomass production by ericoid mycorrhizal endophytes from Woollsia pungens (Epacridaceae) Mycological Research. 2001;105:105–111. [Google Scholar]
- Wright MM, Cross R, Cousens RD, May TW, McLean CB. Taxonomic and functional characterisation of fungi from the Sebacina vermifera complex from common and rare orchids in the genus Caladenia. Mycorrhiza. 2010;20:375–390. doi: 10.1007/s00572-009-0290-x. [DOI] [PubMed] [Google Scholar]
- Zubay G. Biochemistry. Dubuque, IA: Wm. C. Brown Publishers; 1993. [Google Scholar]


