Abstract
Background and Aims
Functional groups of species interact and coevolve in space and time, forming complex networks of interacting species. A long-term study of temporal variation of an ant–plant network is presented with the aims of: (1) depicting its structural changes over a 20-year period; (2) detailing temporal variation in network topology, as revealed by nestedness and modularity analysis and other parameters (i.e. connectance, niche overlap); and (3) identifying long-term turnover in taxonomic structure (i.e. switches in ant resource use or plant visitor assemblages according to taxa).
Methods
Fieldwork was carried out at La Mancha, Mexico, and ant–plant interactions were observed between 1989 and 1991, between 1998 and 2000, and between May 2010 and 2011. Occurrences of ants on extrafloral nectaries (EFNs) were recorded. The resulting ant–plant networks were constructed from qualitative presence–absence data determined by a species–species matrix defined by the frequency of occurrence of each pairwise ant–plant interaction.
Key Results
Network variation across time was stable and a persistent nested structure may have contributed to the maintenance of resilient and species-rich communities. Modularity was lower than expected, especially in the most recent networks, indicating that the community exhibited high overlap among interacting species (e.g. few species were hubs in the more recent network, being partly responsible for the nested pattern). Structurally, the connections created among modules by super-generalists gave cohesion to subsets of species that otherwise would remain unconnected. This may have allowed an increasing cascade-effect of evolutionary events among modules. Mutualistic ant–plant interactions were structured 20 years ago mainly by the subdominant nectarivorous ant species Camponotus planatus and Crematogaster brevispinosa, which monopolized the best extrafloral nectar resources and out-competed other species with broader feeding habits. Through time, these ants, which are still present, lost their position as network hubs and diminished in their importance in structuring the network; simultaneously, plants gained in importance.
Conclusions
The long-term network analysis reveals a decrease in attended plant species richness, a notable increase in plant species participation from 1990 to 2010 (sustained by less plant taxonomic similarity in the older 1990 network), an increase in the number of ant species and a diminishing dominance of super-generalist ants. The structure of the community has remained highly nested and connected with low modularity, suggesting overall a more participative, homogeneous, cohesive interaction network. Although previous studies have suggested that interactions between ants and EFN-bearing plants are susceptible to seasonality, abiotic factors and perturbation, this cohesive structure appears to be the key for biodiversity and community maintenance.
Keywords: Ant–plant interaction interactive network, long-term variation, mutualism, nestedness, modularity, taxonomic structure
INTRODUCTION
Functional groups of species interact and coevolve in space and time, forming complex networks of interacting species (Thompson, 2005; Bascompte, 2009). As a result, entire ecological communities can be analysed as a series of complex interaction networks (Burkle and Alarcón, 2011; Aizen et al., 2012; Stouffer et al., 2012). The analysis of interaction networks, such as plant–pollinator (e.g. Bascompte et al., 2003; Bascompte, 2009; Burkle and Alarcón, 2011) and ant–plant networks (Guimarães et al., 2006, 2007; Blüthgen et al., 2007; Díaz-Castelazo et al., 2010), has revealed several patterns of interaction that are common to a wide range of free-living mutualisms. Mutualistic interactions are highly asymmetric (Vázquez and Aizen, 2004) and nested (Bascompte et al., 2003; Díaz-Castelazo et al., 2010), such that species with few partners primarily interact with hierarchical subsets of a generalized core group of partners. A few studies have explored the relationship between the architecture and functioning of ecological networks. They have found consistent relationships between the topology of the networks and their functioning, as variation in fitness attributes across populations has been correlated with higher nestedness, connectivity and clustering in mutualistic networks (but see also Rezende et al., 2007; Bastolla et al., 2009; Fontaine et al., 2011; Guimarães et al., 2011). Bascompte et al. (2006) established that the architecture of quantitative mutualistic networks, characterized by a low number of strong dependencies, high asymmetry and heterogeneity in species strength (i.e. certain species interact very frequently or with high individual participation, in contrast to other species that rarely interact), may promote community coexistence, in turn favouring the long-term persistence of species-rich assemblages, i.e. biodiversity maintenance (Bascompte and Jordano, 2007; Ings et al., 2009; Lewinsohn and Cagnolo, 2012).
Understanding the ecological and evolutionary causes and consequences of spatial and temporal variation in mutualistic interaction networks is important for addressing both basic and applied questions about community structure and function. However, the characterization of spatio-temporal variation of interactions has just begun (Olesen et al., 2008; Díaz-Castelazo et al., 2010; Burkle and Alarcón, 2011; Olesen et al., 2011; Rico-Gray et al., 2012). Ant–plant interactions are among the most temporally and spatially variable mutualistic interactions, especially regarding the species assemblages, although the outcomes of the interaction may vary as well (Rico-Gray, 1993; Bronstein, 1994; Oliveira et al., 1999; Díaz-Castelazo et al., 2004). In these interactions, plants produce food resources (e.g. extrafloral nectar) that attract and reward ants in exchange for their defence against natural enemies (Rico-Gray and Oliveira, 2007; Chamberlain and Holland, 2009). In recent years, several studies have described temporal patterns of mutualistic networks across 2–4 years of sampling (Burkle and Alarcón, 2011). To our knowledge, however, only two studies have addressed longer-term changes to network parameters in species interactions (Díaz-Castelazo et al., 2010; Olesen et al., 2011). Studies on more subtle temporal variation in mutualistic networks show a continuous switch in plant species used by animals in response to environmental disturbances and the availability of resources (Whittall and Hodges, 2007); plants can also adjust phenology and morphology to affect their visitors (Aizen and Vázquez, 2006; Kaiser-Bunbury et al., 2010). Such rewiring of interspecific interactions could not only have ecological and environmental causes (e.g. changing resource availability), but could also reflect adaptive behaviour of species for enhancing the efficiency of resource utilization (Zhang et al., 2011).
The redundant roles of species among cohesive subgroups (modules) that in turn are complementary to each other (Mello et al., 2011) is relevant to robustness (i.e. the persistence of network structure and function even though certain species are lost at the community) because of the contributions of species to nestedness. Saavedra et al. (2011) have recently found that the extinction of stronger contributors to nested topology leads to a decrease in overall network persistence. With simulations they show that the removal of a strong contributor to nestedness tends to decrease overall network persistence more than the removal of a weak contributor. In addition, strong contributors to collective persistence did not gain individual survival benefits, but were in fact the nodes most vulnerable to extinction.
Temporal stability of a Neotropical ant–plant network over a 10-year period has been previously reported for La Mancha, Veracruz, Mexico, despite changes in species composition which would potentially impact interaction structure (Díaz-Castelazo et al., 2010). Here we extended this study to: (1) examine structural changes over an additional 10-year period, thus covering the 1990–2010 interval; (2) detail temporal variation of network topology, as revealed by nestedness and modularity analysis and other parameters such as connectance and niche overlap; and (3) identify long-term turnover in taxonomic structure (genus, subfamily/family, expressed as switches in ant resource use or plant visitor assemblages according to taxa.
METHODS
Study site
Fieldwork was carried out at Centro de Investigaciones Costeras La Mancha (CICOLMA), located on the coast of the state of Veracruz, Mexico (19°36′N, 96°22′W; elevation <100 m). The climate is warm and sub-humid; a rainy season occurs between June and September, total annual precipitation is approx. 1500 mm, and mean annual temperature is 22–26 °C. The major vegetation types in the study area are tropical deciduous forest, tropical dry forest, sand dune scrub, mangrove forest, freshwater marsh and flooded deciduous forest (Moreno-Casasola, 2006).
Sampling
Ant–plant interactions were observed monthly, first between May 1989 and April 1991 (Rico-Gray, 1993), then between October 1998 and September 2000 (Díaz-Castelazo et al., 2004), and finally between May 2010 and April 2011 (hereafter 1990, 2000 and 2010, respectively) (Díaz-Castelazo et al., 2010; Sánchez-Galván et al., 2012). The study site is highly seasonal and it is important to mention that a category-3 hurricane impacted this coastal environment on September 2010, having an effect on the 2010 census (Sánchez-Galván et al., 2012).
Censuses were conducted along six trails that represent main vegetation types in the area, such as sand dune pioneers, sand dune scrub, deciduous forest, dry forest, freshwater lagoon, flooded forest and mangrove forest (Rico-Gray, 1993; Díaz-Castelazo et al., 2010). The transects varied in length, according to specific vegetation types per trial: sand dune pioneers, 638·5 m; sand dune scrub, 161·96 m; deciduous forest, 370·37 m; dry forest, 225·77 m; ecotone of freshwater marsh, deciduous forest and dune scrub, 457·66 m; mangrove forest, 171·03 m. All transects were 3 m wide. Although transect length was not equal among vegetation types, given their physiognomy (i.e. sand dune pioneer vegetation along the beach is quite sparse compared with other vegetation physiognomies such as dry forest), we performed equal numbers and seasonal repetitions of ant–plant interaction censuses within transects. We observed all plants (below 2·5 m. height, thus excluding large trees, canopy, and their associated epiphytes and vines), followed ants collecting liquids from plants, and subsequently recorded occurrences of ants on extrafloral nectaries (EFNs) strictly [based on published lists of EFN distribution within plant taxa and our knowledge of nectary morphology and position; whenever there were doubts on nectar secretion, glucose test strips (Clinistix) were used]. On each visit we noted ant species, plant species and the plant organ on which the EFNs mediating the ant–plant interaction were located. Once an individual plant was marked as visited by ants, it was subsequently re-checked throughout the study. We considered extrafloral nectar produced either on the surface of reproductive structures such as the spike, pedicel, bud, calyx or fruit, or secreted by special structures on vegetative parts, such as leaves, shoots, petioles, bracts or stems (Koptur, 1992). Ants were considered to be feeding on nectar when they were immobile, with mouthparts in contact with nectar-secreting tissues, for periods of up to several minutes. Nectar-feeding ants often showed obviously distended gasters (Rico-Gray, 1993).
Statistics and network metrics
The resulting ant–plant networks analysed here (1990, 2000 and 2010) were constructed from qualitative presence–absence data determined by a species–species matrix defined by the frequency of occurrence of each pairwise ant–plant interaction. The following analyses were performed on these three network matrices.
Network-level estimates
We estimated nestedness for each network using the metric NODF (Almeida-Neto et al., 2008). We used Aninhado 3·0 to compute NODF (Guimarães and Guimarães, 2006). The significance of NODF was estimated with a null model generating random matrices from the original matrix, null model 2 (Bascompte et al., 2003), in which the probability of interaction between any pair of species is proportional to their total number of interactions (i.e. their degree). The P-value was obtained from the proportion of random matrices that had an NODF value equal or higher than the value obtained for the real matrix. Other structural features analysed for each network included degree (number of interactions among ant and plant species) and network connectance [proportion of realized links of the total possible in each network (C= I/(P × A), where I is the total number of interactions recorded for the network, P is plant species richness and A is ant species richness]. Using the ‘Bipartite’ in software ‘R’ (Dorman et al., 2009; Dormann and Gruber, 2011), we obtained niche overlap for each trophic level using Horn's index estimated with network-level function. To provide information on the within-year variation, for the 1990 network we reported the monthly peaks of nestedness and their corresponding z-score values. The z-score values are measures of relative nestedness, the standard deviation of the 1000 replicates obtained from the null model analysis minus the nestedness value of the analysed network. We then used these values to compare within-year variation with between-year variation. Thus, we are comparing each sampling date within and between the other sampling dates.
To test whether there were ant foraging preferences which produced guilds in the community, we assessed modularity within each network (Mello et al., 2011). Modularity is a measure of how much the network is structured in cohesive subgroups of vertices (modules), in which the density of interactions is higher within than among modules. Modularity was calculated with the index M (from 0, no subgroups, to 1, totally separated subgroups) estimated with Netcarto (Guimerà et al., 2004; Guimerà and Amaral, 2005).
Temporal taxonomic turnover
We described changes in taxonomic composition of network nodes over time to depict temporal variation in ant resource use or plant visitor assemblages underlying changes in network structure. The network's temporal taxonomic turnover was described at two levels. First, we summed all interspecific interactions observed between any given pair of ant/plant genera (e.g. Camponotus/Crotalaria). The same procedure was repeated for pairs of ant/plant subfamilies and families (e.g. Formicinae/Fabaceae). Taxonomically aggregated ecological interactions were then plotted as temperature matrices describing the number of interspecific interactions observed at the two taxonomic levels mentioned above, providing a quantitative visualization for relative roles of diverse taxa in the network over time.
We described the temporal stability of taxonomic composition by comparing Jaccard's similarity index at both genus and family (for plants) or subfamily (for ants) levels between different time periods (1990, 2000 and 2010). Jaccard's similarity index between the time periods A and B was computed as follows:
| (1) |
where a is the number of genera (family or subfamily) shared between the two periods, b is the number of genera (family or subfamily) present only in the first period and c is the number of genera (family or subfamily) present only in the last period.
RESULTS
Considering all data gathered at La Mancha throughout the two decades of study (1990–2010), we recorded 54 ant species (A) in 20 genera and five subfamilies attending 76 EFN-bearing plant species (P) in 61 genera and 29 families (Supplemenatry Data Table S1), adding up to 564 distinct ant–plant associations (I) and thus leading to an overall connectance (C) of 13·7 %. Even though attended plant species richness (P) decreased from 1990 to 2010 while the number of ant species (A) increased, and turnover in species and interactions was high, connectance was relatively stable throughout the period (Fig. 1).
Fig. 1.
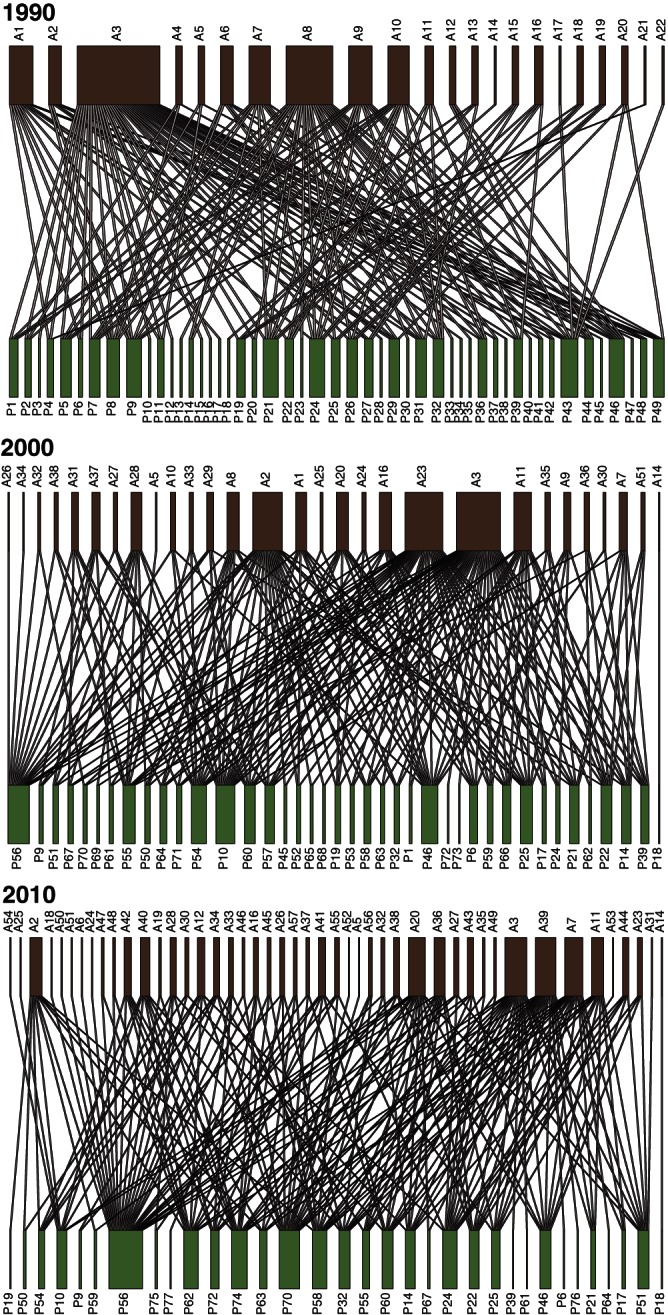
Mutualistic bipartite networks of ants and extrafloral nectary-bearing plants over a 20-years period at La Mancha, Mexico. A, ants (brown); P, plants (green). 1990: SP (plant species richness) = 49, SA (ant species richness) = 22, C (connectance) = 0·14. 2000: SP = 40, SA= 29, C = 0·175. 2010: SP = 33, SA = 46, C = 0·138. Each label is species-specific and identifies an ant (e.g. A01) or a plant (e.g. P42).
Like connectance, other network structure parameters were relatively stable across years. All ant–plant networks were significantly nested, N = 47·4 in 1990 (Nrandom= 23·02, P < 0·001), reaching a peak of N = 50·5 (Nnullmodel = 26·92, P < 0·001) in 2000, and decreasing to N = 39·4 (Nnullmodel = 21·22, P < 0·001) in 2010. The observed modularity of the 1990 network (M = 0·426) did not differ from the average modularity of random runs (Mnullmodel = 0·4, P > 0·1), while the modularity values recorded for both the 2000 (M = 0·299) and 2010 (M = 0·33) networks were significantly lower than that expected from null model simulations (Mnullmodel = 0·329, P < 0·01 for 2000 and Mnullmodel = 0·368, P < 0·05 for 2010). Thus, nestedness was higher than and modularity was often lower than or as expected by the heterogeneity of interactions across the years. Although our long-term analysis is for presence/absence data, we also provide information regarding the frequency of interactions for networks in 1990, 2000 and 2010 (Supplemenatry Data Tables S2–4). These data indicate whether an individual link in the network is supported by a single observation or by several. Overall, the higher interaction frequencies are recorded for network 2000 relative to the other years.
For the first year of our study (1990 network) all months exhibited a significantly nested network topology (P < 0·01), with NODF values ranging from 27·4 to 36·3 (P < 0·0001) from January to December, with peaks in March and June that, respectively, correspond to z-score values of 6·8 and 5·72 (Rico-Gray et al., 2012). Our whole 1990 network (year-level) exhibited a significant NODF value of 47·43 (P < 0·0001), with a z-score of 8·95; this means that even when controlling for differences in species richness, number of interactions and connectance (as z-score values are measures of relative nestedness), our whole-year nestedness is considerably higher than their monthly counterparts. We also suggest that at our study site variation in ant–plant interaction networks is lower within a year than between years (i.e. non-contiguous period of time studied).
Although network structure was relatively constant through time, the relative importance of component species varied greatly (Fig. 2). The 1990 network had a plant species niche overlap of 0·345, characterized by the prevalence of interactions between Fabaceae species, which were present in 38 % of the links. This network exhibited the lowest ant species niche overlap (0·128) with ants in all subfamilies present (the most frequent being Formicinae and Myrmicinae) (Fig. 2A). Formicine ants, mostly Camponotus spp., were the dominant group in that period, participating in 40·5 % of the links and interacting with plants across 33 genera in 19 families. Myrmicinae species, predominantly in the genera Crematogaster, Forelius and Monomorium, were responsible for 32·5 % of the links, interacting with plants in 27 genera and 13 families. Dolichoderinae species in the genera Azteca and Dorymyrmex occurred in 14 % of the interactions, being associated with 16 plant genera belonging to ten families. Pseudomyrmex species (Pseudomyrmycinae) were found in 12 % of the links, distributed in ten genera and four families (Fig. 2B). Camponotus planatus (Formicinae) and Crematogaster brevispinosa (Myrmicinae) were the species with the broadest range of interactions, acting as network hubs (Fig. 3).
Fig. 2.
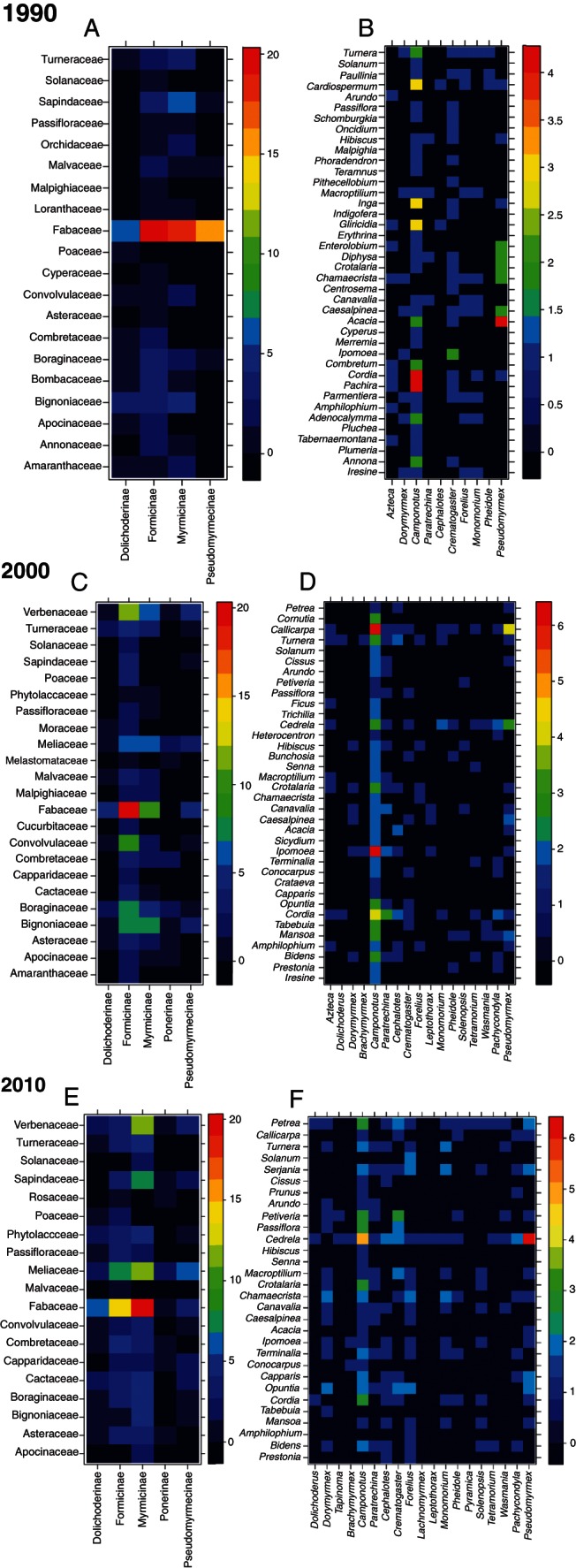
Distribution of links between ants and extrafloral nectary-bearing plants at La Mancha, Mexico. The hotter the colour, the higher is the number of species interactions between ant subfamilies and plant families (A, C, E) and between ant and plant genera (B, D, F). (A, B) 1990 network, (C, D) 2000 network, (E, F) 2010 network.
Fig. 3.
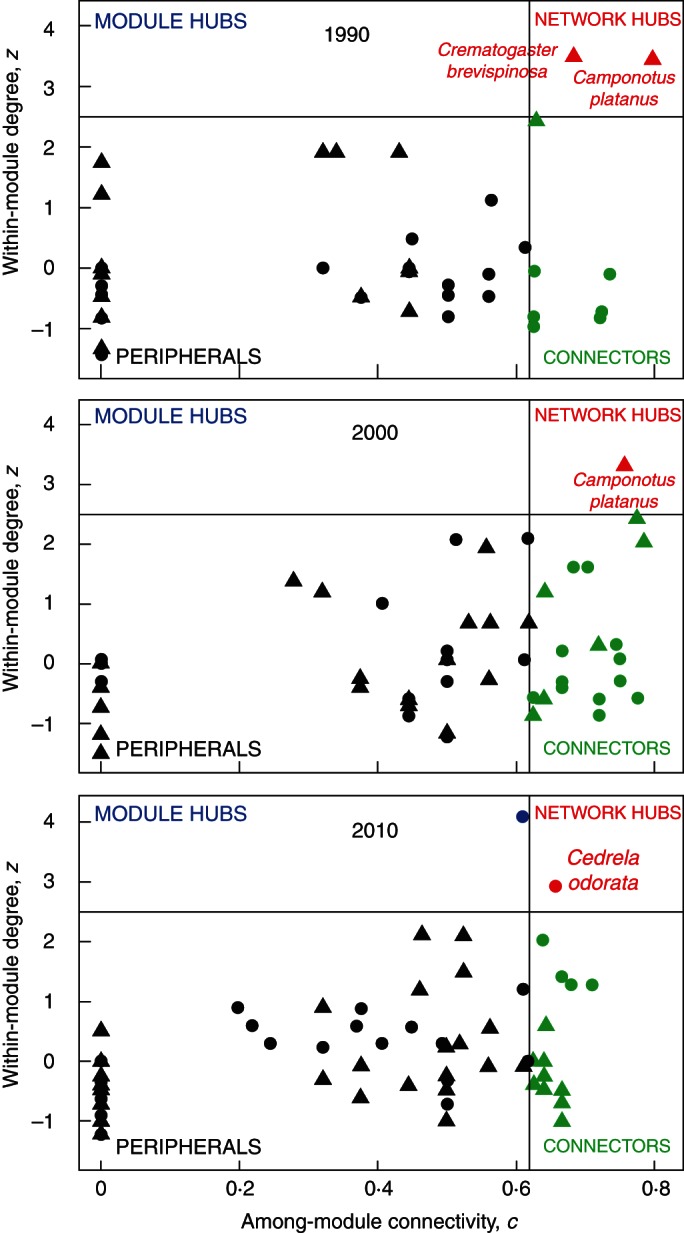
Species roles in the three networks. Among-module connectivity describes how even is the distribution of links of a given species across species in different modules. Within-module degree quantifies how large is the number of interactions of a species compared with other species in the modules.
The 2000 network exhibited a plant species niche overlap of 0·365, marked by the dominance peak of Formicine species (present in 51 % of the links), and on the plant side, a decrease in the relative participation of the Fabaceae (only 19 % of interactions). The period was also characterized by intermediate ant species niche overlap (0·195), with a high diversity of interactions with the Formicinae, including links between Camponotus, Paratrechina and Brachymyrmex and 36 plant genera in 23 families (the most frequent being Verbenaceae, Convolvulaceae, Boraginaceae and Bignoniaceae) (Fig. 2C). The only species acting as a network hub was a Formicine species (Camponotus planatus, Fig. 3). Myrmicinae were recorded in 25·6 % of the links, widespread through 24 genera in 15 plant families (the most common being Fabaceae and Bignoniaceae). Although the proportion of links involving the Dolichoderinae (8 %) and Pseudomyrmycinae (10 %) remained relatively constant, both groups expanded their range of attended plant families to 13 genera in ten families and 13 genera in eight families, respectively. Another major change in the 2000 network was the presence of the Ponerinae in 5 % of the links, comprising nine plant genera from eight plant families.
Finally, the 2010 network had the highest ant species niche overlap (0·215) and a prevalence of interactions involving Myrmicine ants (45 % of the interactions). The contributions of Forelius, Crematogaster, Cephalotes and Monomorium species were roughly equal; they interacted with 23 genera in 16 plant families, the most common being Fabaceae, Meliaceae, Sapindaceae and Verbenaceae (Fig. 2E). Although there was a decrease in the proportion of Formicinae species in these links (29 %), the subfamily remained as the one with the most taxonomically widespread interactions, embracing 26 plant genera in 17 plant families. The participation of Dolichoderinae, Pseudomyrmycinae and Ponerinae in the network links remained almost constant (approx. 10 % for the former two groups and 4·3 % for the latter), as well as their host plant ranges. The 2010 network also exhibited the lowest plant niche overlap (0·192), and plant species were more evenly visited by ants; the only species acting as a network hub was a plant (Cedrela odorata, Meliaceae; Fig. 3).
Taxonomic similarity at the genus and family level is shown in Table 1 for both plants and ants. The less similar network in terms of plant composition (at both the genus and the family level) is the 1990 network. This network is also the less similar in terms of the taxonomic composition of the ant fauna visiting EFNs.
Table 1.
Taxonomic similarity of the extrafloral nectary-bearing plants assemblage and of ant species visiting extrafloral nectaries at La Mancha, Mexico, across a 20-year period; values above the diagonals describe Jaccard's similarities at the genus level whereas values below the diagonal describe the same index at the family level
| 1990 | 2000 | 2010 | |
|---|---|---|---|
| Plants | |||
| 1990 | – | 0·24 | 0·25 |
| 2000 | 0·54 | – | 0·74 |
| 2010 | 0·5 | 0·75 | – |
| Ants | |||
| 1990 | – | 0·59 | 0·45 |
| 2000 | 0·8 | – | 0·8 |
| 2010 | 0·8 | 1 | – |
DISCUSSION
This study of ant–plant network variation across time revealed a stable connectance and a persistent nested structure. These features have been demonstrated to maintain species richness and provide community resilience to disturbance (Bascompte et al., 2006). Modularity was lower than expected, especially within the most recent networks, indicating that the networks show high overlap among interacting species. Thus, only a few species act as hubs in the network, and are partly responsible for the nested pattern (Olesen et al., 2007). Network hubs are super-generalist species which have the broadest range of interactions; they are characterized not only by their high within-module degree, but by their high among-module connectivity (Olesen et al., 2007), thus adjoining the whole community. In the 1990 network, two ant species were network hubs. In the 2000 network just one of those ant species remained as a network hub, while in the 2010 network a plant rather than ant species acted as a network hub or super-generalist. The connections that super-generalists create among modules produce cohesive subsets of species that otherwise would remain unconnected (Olesen et al., 2007; Guimarães et al., 2011). In general, this cohesive structure appears to be the key for biodiversity and community maintenance in mutualistic networks (Bascompte et al., 2003).
This study provides one of the very few cases comparing the same communities over long periods of time focusing on the dynamics of interspecific interactions. Overall, our long-term analysis has documented a decrease in the richness of EFN-bearing plant species but a significant increase in the participation of those species (more homogeneous interactions of plant species visited by ants), concomitantly with an increase in the number of ant species and a diminishing dominance of super-generalist ants through time. The structure of the community remains highly nested and connected with low modularity. This suggests that a more participative, homogeneous and cohesive interaction network has arisen. Previous studies (Díaz-Castelazo et al., 2010; Rico-Gray et al., 2012) suggest that ant–plant interactions mediated by extrafloral nectaries at the study site are susceptible to seasonality, abiotic factors and perturbation, which may explain the important change in specific links (pairwise interactions) during our three sampling periods. Complementarily, seasonality, and natural and human perturbation do not seem to affect general network structure, nestedness or connectance. These findings are in accordance with Díaz-Castelazo et al. 2010 (in the context of ant–plant interactions) and in accordance with Martínez et al. 2006 (in the context of ecosystem vulnerability), who state that relative to other sites along the Gulf of Mexico, La Mancha and the central region coasts would be considered as the least vulnerable and most resilient both to natural and to anthropogenic perturbation.
The 1990 network exhibited high numbers of interactions by the subdominant nectarivorous ant species Camponotus planatus and Crematogaster brevispinosa, which monopolized almost all the best EFN resources and which out-competed other species with broader feeding habits. Both ant species are frequent visitors to EFNs (Díaz-Castelazo et al., 2004) and are considered as solitary leaf foragers that cover foliar area at greater rates than trophobiont-tender ants, in addition to their ability to rapidly take up large loads of nectar (Davidson et al., 2004).
The presence of ruderal plants in the 2000 network, along with the increase in the richness of ant species visiting EFN-bearing plants and the arrival of some invasive ants (Díaz-Castelazo et al., 2010), established new interactions. More coexisting ant species and the presence of generalist invasive ants may help to explain the increase in ant species niche overlap (more ant species use the same plant resources). Only one of the super-generalist ant species remained as a network hub in 2000. Within the most recent network no super-generalist dominant ants were noticed to monopolize plant resources. As a result, ant species such as Cephalotes minutus (Myrmicinae) visited noticeably more plant species in 2010 than they did in 1990. This ant is normally timid, is considered a ‘generalized Myrmicinae’ in relation to resource/habitat use (Andersen, 2000) and is less dependent on nectar food (Davidson et al., 2003) than subdominant ants. These habits explain why Cephalotes minutus forages on more plants when no other ants are dominant.
Plant species niche overlap decreased noticeably as a consequence of being visited by distinct ant fauna. An extreme case is Cedrela odorata, a plant which has several features that makes it accessible to several ant species. It has numerous, small, dispersed EFNs, distributed in rachises (petioles) of compound leaves, non-lignified branches and stems and shoots. Modest amounts of nectar are secreted by these glands (N. Chavarro et al., unpubl. data). However, their quantity and distribution across the plant favours simultaneous visitation by ants, allowing them to avoid competition and perhaps facilitating their coexistence.
Overall, the 2010 network exhibited a more important contribution of plant species in structuring the mutualistic ant–plant community an increasing average degree (mean connections by node) of plant species occurring through time. This pattern may lead to an increase in convergence and complementarity for plant species as a consequence of the presence of super-generalist partners in the network (see also Guimarães et al., 2011). Our results show a large shift through time in the contribution of ant subfamilies and plant families. Regarding plants, Fabaceae, a plant family where EFNs are most abundant and diverse (McKey, 1989; Koptur, 1992; Pascal et al., 2000; Melo et al., 2010) was particularly dominant. However, it decreased in relative importance over the 20 years of the study. In the 1990 network, 19 plant genera belonged to Fabaceae, in contrast to the seven recorded in the more recent networks. Besides a reduced number of genera, a reduced abundance of plant individuals may explain the lower degree for Fabaceae: plant cover for the 2000 network was 205·6 m, versus 349·7 m in 2010 (Sánchez-Galván et al., 2012). Other plant families that interacted heavily with ants were Bignoniaceae, Verbenaceae, Convolvulaceae, Boraginaceae and Meliaceae, in increasing order of participation through time periods. These are also known as common EFN-bearing plant families that attract ant visitors (Schupp and Feener, 1991; Koptur, 1992).
With regard to ants, it is clear that the Formicinae, particularly the genus Camponotus, were associated with the majority of plant species. The extraordinary diversity of formicines foraging on plants reflects their diversification in feeding habits. Many species have proventricular adaptations that allow passive damming of sugary liquids, large crop capacities and seeping canals to nourish the midgut. These traits have turned foragers in several genera, such as Camponotus, into liquid ‘canteens’ that have allowed them to exhibit active and prolonged exploration of several environments, including open, hot habitats (Davidson et al., 2004).
Although Formicinae species are important components of the three networks, across time periods the trend was towards a decrease in their interactions with EFN-bearing plants, and an increase in the relative importance of the ant subfamily Myrmicinae. Myrmicinae (mainly the cosmopolitan genera Pheidole, Monomorium and Crematogaster) are ubiquitous and often abundant; they have generalized habits in relation to nesting and dietary requirements, and are competitive at rich food sources, including nectar (Andersen, 1995). Generalized Myrmicinae (Andersen, 2000) are considered competitive species and have a higher tolerance to a variety of stresses and disturbances; this may explain their increased importance at the more recent network, which is also the most disturbed as the presence of some invasive/tramp ant species and ruderal plants reveals, but most importantly, because important abiotic perturbation occurred during this period of time, given a high-category hurricane impacting the study site (Sánchez-Galván et al., 2012). Relative to 1990, in 2000 and 2010 an increased participation of other ant subfamilies, such as Dolichoderinae, Pseudomyrmycinae and Ponerinae, was noted. Although the proportion of links involving these three subfamilies remained constant in the most recent network, all expanded their range of attended plant taxa, perhaps because no super-generalist species dominated interactions or monopolized nectar sources. Although Pseudomyrmex was a well-represented genus in the networks, its associations tend to change from legumes in the scrub and pioneer vegetation (Fabaceae, Caesalpinoideae) to shrubs or trees in the subdeciduous forests, such as the network hub Cedrela odorata (Meliaceae).
Our study provides long-term evidence of a nested, highly cohesive structure, suggestive of a community resilient to minor disturbance, as well as to the effects of abiotic factors (i.e. hurricanes, seasonality) on specific links: plant cover loss caused by abiotic phenomena resulted in higher redundancy of ant use of EFN resources and a decrease in the number of connector species. Importantly, super-generalist species contribute to overall network cohesion (Guimarães et al., 2011) and the biological mechanisms behind this deserve future study. To understand how network structure and dynamics have implications for biodiversity and community maintenance, it is critical to continue the study of complex networks over long periods of time.
The community studied here shares structural and temporal properties with other mutualistic networks such as plant–pollinator networks which showed important daily and seasonal variation in species composition (Olesen et al., 2008), where the new species entering the network tend to interact with already well-connected species, while deviations from the trend were explained by morphological mismatching and non-overlapping phenophases. Here as well, the new species entering the community tend to interact with generalists and the taxonomic turnover is higher during the first decade, as the 1990 network exhibited the smallest taxonomic similarities especially for plant genera and families. Thus, the mechanism behind species and taxonomic shifts in ant–plant networks among decades may be vegetation changes through both human and abiotic disturbance (Díaz-Castelazo et al., 2010; Rico-Gray et al., 2012; Sánchez-Galván et al., 2012).
Furthermore, specialized mutualistic ant–plant networks, such as the symbiotic mutualisms between myrmecophilous plants and ants, are less prone to temporal variation and differ in having a modular, less generalistic structure (Guimarães et al., 2007). It is the non-generalized, mainly facultative nature of the interactions we studied that reflects the importance of seasonal variation and disturbance (convergence) more than specific adaptations or complementarity, but provides the overall resilience and richer array of community members that may maintain biodiversity.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Haydée Hernández-Yáñez and Gibrán Renoy Pérez for their help during fieldwork or in the organization of ant samples and data sheets, Flavia Marquitti for helping in modularity analysis and Roger Guimerà for providing Netcarto. This research was supported by CONACyT grants to V.R.G. and C.D.C., Universidad Veracruzana (fellowship to Haydée Hernández-Yáñez), and FAPESP (fellowship to P.R.G.J. and R.L.G.R.).
LITERATURE CITED
- Aizen MA, Vázquez DP. Flower functioning in human-altered habitats. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 159–179. [Google Scholar]
- Aizen MA, Sabatino M, Tylianakis JM. Specialization and rarity predict nonrandom loss of interactions from mutualistic networks. Science. 2012;335:1486–1489. doi: 10.1126/science.1215320. [DOI] [PubMed] [Google Scholar]
- Andersen AN. A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. Journal of Biogeography. 1995;22:15–29. [Google Scholar]
- Andersen AN. A global ecology of rainforest ants: functional groups in relation to environmental stress and disturbance. In: Agosti D, et al., editors. Ants: standard methods for measuring and monitoring biodiversity. Washington, DC: Smithsonian Institution Press; 2000. pp. 35–44. [Google Scholar]
- Almeida-Neto M, Guimarães P, Guimarães PR, Jr, Loyola RD, Ulrich W. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos. 2008;117:1227–1239. [Google Scholar]
- Bascompte J. Mutualistic networks. Frontiers in Ecology and the Environment. 2009;7:429–436. [Google Scholar]
- Bascompte J, Jordano P. Plant–animal mutualistic networks: the architecture of biodiversity. Annual Review of Ecology, Evolution and Systematics. 2007;38:567–593. [Google Scholar]
- Bascompte J, Jordano P, Melián CJ, Olesen JM. The nested assembly of plant–animal mutualistic networks. Proceedings of the National Academy of Sciences, USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Olesen JM. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. [DOI] [PubMed] [Google Scholar]
- Bastolla U, Fortuna MA, Pascual-García A, Ferrera A, Luque B, Bascompte J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature. 2009;458:1018–1021. doi: 10.1038/nature07950. [DOI] [PubMed] [Google Scholar]
- Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N. Specialization, constraints, and conflicting interests in mutualistic networks. Current Biology. 2007;17:341–346. doi: 10.1016/j.cub.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Alarcón R. The future of plant–pollinator diversity: understanding interaction networks across time, space, and global change. American Journal of Botany. 2011;98:528–538. doi: 10.3732/ajb.1000391. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. Conditional outcomes in mutualistic interactions. Trends in Ecology and Evolution. 1994;9(214–217) doi: 10.1016/0169-5347(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain SA, Holland JN. Body size predicts degree in ant–plant mutualistic networks. Functional Ecology. 2009;23:196–202. [Google Scholar]
- Davidson DW, Cook SC, Snelling RR, Chua TH. Explaining the abundance of ants in lowland tropical rainforest canopies. Science. 2003;300:969–972. doi: 10.1126/science.1082074. [DOI] [PubMed] [Google Scholar]
- Davidson DW, Cook SC, Snelling RR. Liquid-feeding performances of ants (Formicidae): ecological and evolutionary implications. Oecologia. 2004;139:255–266. doi: 10.1007/s00442-004-1508-4. [DOI] [PubMed] [Google Scholar]
- Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M. Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: richness, occurrence, seasonality and ant foraging patterns. Ecoscience. 2004;11:472–481. [Google Scholar]
- Díaz-Castelazo C, Guimarães PR, Jr, Jordano P, Thompson JN, Marquis RJ, Rico-Gray V. Changes of a mutualistic network over time: reanalysis over a 10-year period. Ecology. 2010;91:793–801. doi: 10.1890/08-1883.1. [DOI] [PubMed] [Google Scholar]
- Dormann CF, Gruber B. Package ‘bipartite’: visualising bipartite networks and calculating some ecological indices. 2011 http://cran.r-project.org/web/packages/bipartite/bipartite.pdf . [Google Scholar]
- Dormann CF, Fründ J, Blüthgen N, Gruber B. Indices, graphs and null models: analyzing bipartite ecological networks. Open Journal of Ecology. 2009;2:7–24. [Google Scholar]
- Fontaine C, Guimarães PR, Jr, Kéfi S, et al. The ecological and evolutionary implications of merging different types of networks. Ecology Letters. 2011;14:1170–1181. doi: 10.1111/j.1461-0248.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- Guimarães PR, Jr, Guimarães P. Improving the analyses of nestedness for large sets of matrices. Environmental Modelling Software. 2006;21:1512–1513. [Google Scholar]
- Guimarães PR, Jr, Rico-Gray V, Reis SF, Thompson JN. Asymmetries in specialization in ant–plant mutualistic networks. Proceedings of the Royal Society of London, Series B. 2006;273:2041–2047. doi: 10.1098/rspb.2006.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães PR, Jr, Rico-Gray V, Oliveira PS, Izzo T, Reis SF, Thompson JN. Interaction intimacy affects structure and coevolutionary dynamics in mutualistic networks. Current Biology. 2007;17:1797–1803. doi: 10.1016/j.cub.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Guimarães PR, Jr, Jordano P, Thompson JN. Evolution and coevolution in mutualistic networks. Ecology Letters. 2011;14:877–885. doi: 10.1111/j.1461-0248.2011.01649.x. [DOI] [PubMed] [Google Scholar]
- Guimerà R, Amaral LAN. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Sales-Pardo M, Amaral LAM. Modularity from fluctuations in random graphs and complex networks. Physical ReviewE70: 025101. 2004 doi: 10.1103/PhysRevE.70.025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings TC, Montoya JM, Bascompte J, et al. Ecological networks – beyond food webs. Journal of Animal Ecology. 2009;78:253–269. doi: 10.1111/j.1365-2656.2008.01460.x. [DOI] [PubMed] [Google Scholar]
- Kaiser-Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecology Letters. 2010;13:442–452. doi: 10.1111/j.1461-0248.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- Koptur S. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays EA, editor. Insect–plant interactions. Boca Raton, FL: CRC Press; 1992. Vol. IV. [Google Scholar]
- Krebs CJ. Ecological methodology. London: Addison-Wesley Longman – Educational Publishers, Inc; 1999. [Google Scholar]
- Lewinsohn TM, Cagnolo L. Keystones in a tangled bank. Science. 2012;335:1449–1451. doi: 10.1126/science.1220138. [DOI] [PubMed] [Google Scholar]
- Martínez ML, Gallego-Fernández JB, García-Franco JG, Moctezuma C, Jiménez CD. Assessment of coastal dune vulnerability to natural and anthropogenic disturbances along the Gulf of Mexico. Environmental Conservation. 2006;33:109–117. [Google Scholar]
- McKey D. Interactions between ants and leguminous plants. In: Stirton CH, Zarucchi JL, editors. Advances in legume biology. Monographs of Systematic Botany of the Missouri Botanical Garden. Vol. 29. 1989. pp. 673–718. [Google Scholar]
- Mello MAR, Marquitti FMD, Guimarães PR, Jr, ViktoriaKalko EK, Jordano P, de Aguiar MAM. The missing part of seed dispersal networks: structure and robustness of bat-fruit interactions. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0017395. e17395. http://dx.doi.org/10.1371/journal.pone.0017395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo Y, Machado SR, Alves M. Anatomy of extrafloral nectaries in Fabaceae from dry-seasonal forest in Brazil. Botanical Journal of the Linnean Society. 2010;163:87–98. [Google Scholar]
- Moreno-Casasola P. México: Instituto de Ecología, A.C. Entornos veracruzanos: la costa de La Mancha. 2006 [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. Proceedings of the National Academy of Sciences USA. 2007;104:19891–19896. doi: 10.1073/pnas.0706375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Bascompte J, Elherling H, Jordano P. Temporal dynamics of a pollination network. Ecology. 2008;89:1573–1582. doi: 10.1890/07-0451.1. [DOI] [PubMed] [Google Scholar]
- Olesen JM, Stefanescu C, Traveset A. Strong, long-term temporal dynamics of an ecological network. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0026455. e26455. http://dx.doi.org/10.1371/journal.pone.0026455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira PS, Rico-Gray V, Díaz-Castelazo C, Castillo-Guevara C. Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae) Functional Ecology. 1999;13:623–631. [Google Scholar]
- Pascal LM, Motte-Florac EF, Mckey DB. Secretory structures on the leaf rachis of Caesalpinieae and Mimosoideae (Leguminosae): implications for the evolution of nectary glands. American Journal of Botany. 2000;87:327–338. [PubMed] [Google Scholar]
- Rezende EL, Lavabre JE, Guimarães PR, Jr, Jordano P, Bascompte J. Non-random coextinctions in phylogenetically structured mutualistic networks. Nature. 2007;448:925–929. doi: 10.1038/nature05956. [DOI] [PubMed] [Google Scholar]
- Rico-Gray V. Use of plant-derived food resources by ants in the dry tropical lowlands of coastal Veracruz, Mexico. Biotropica. 1993;25:301–315. [Google Scholar]
- Rico-Gray V, Oliveira PS. The ecology and evolution of ant–plant interactions. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Rico-Gray V, Díaz-Castelazo C, Ramírez-Hernández A, Guimarães PR, Jr, Holland JN. Abiotic factors shape temporal variation in the structure of a mutualistic ant–plant network. Arthropod–Plant Interactions. 2012;6:189–295. [Google Scholar]
- Saavedra S, Stouffer DB, Uzzi B, Bascompte J. Strong contributors to network persistence are the most vulnerable to extinction. Nature. 2011;478:233–235. doi: 10.1038/nature10433. [DOI] [PubMed] [Google Scholar]
- Sánchez-Galván IR, Díaz-Castelazo C, Rico-Gray V. Effect of hurricane Karl on a plant–ant network occurring in coastal Veracruz, Mexico. Journal of Tropical Ecology. 2012;28:603–609. [Google Scholar]
- Schupp EW, Feener DH. Phylogeny, life-form, and habitat dependence of ant-defended plants in a Panamanian forest. In: Huxley CR, Cutler DF, editors. Ant–plant interactions. Oxford: Oxford University Press; 1991. pp. 175–197. [Google Scholar]
- Stouffer DB, Sales-Pardo M, Sirer MI, Bascompte J. Evolutionary conservation of species' roles in food webs. Science. 2012;335:1489–1492. doi: 10.1126/science.1216556. [DOI] [PubMed] [Google Scholar]
- Thompson JN. Coevolution: the geographic mosaic of coevolutionary arms races. Current Biology. 2005;15:R992–R994. doi: 10.1016/j.cub.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Vázquez DP, Aizen MA. Asymmetric specialization: a pervasive feature of plant-pollinator interactions. Ecology. 2004;85:1251–1257. [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hui C, Terblanche JS. An interaction switch predicts the nested architecture of mutualistic networks. Ecology Letters. 2011;14:797–803. doi: 10.1111/j.1461-0248.2011.01647.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


