Abstract
Background and Aims
Plants endemic to areas covered by ice sheets during the last glaciation represent paradigmatic examples of rapid speciation in changing environments, yet very few systems outside the harsh arctic zone have been comprehensively investigated so far. The Galium pusillum aggregate (Rubiaceae) is a challenging species complex that exhibits a marked differentiation in boreal parts of Northern Europe. As a first step towards understanding its evolutionary history in deglaciated regions, this study assesses cytological variation and ecological preferences of the northern endemics and compares the results with corresponding data for species occurring in neighbouring unglaciated parts of Central and Western Europe.
Methods
DNA flow cytometry was used together with confirmatory chromosome counts to determine ploidy levels and relative genome sizes in 1158 individuals from 181 populations. A formalized analysis of habitat preferences was applied to explore niche differentiation among species and ploidy levels.
Key Results
The G. pusillum complex evolved at diploid and tetraploid levels in Northern Europe, in contrast to the high-polyploid evolution of most other northern endemics. A high level of eco-geographic segregation was observed between different species (particularly along gradients of soil pH and competition) which is unusual for plants in deglaciated areas and most probably contributes to maintaining species integrity. Relative monoploid DNA contents of the species from previously glaciated regions were significantly lower than those of their counterparts from mostly unglaciated Central Europe, suggesting independent evolutionary histories.
Conclusions
The aggregate of G. pusillum in Northern Europe represents an exceptional case with a geographically vicariant and ecologically distinct diploid/tetraploid species endemic to formerly glaciated areas. The high level of interspecific differentiation substantially widens our perception of the evolutionary dynamics and speciation rates in the dramatically changing environments of Northern Europe.
Keywords: Endemism, environmental change, polyploid evolution, flow cytometry, genome size, glaciation, niche differentiation, ploidy level
INTRODUCTION
The flora of Northern Europe has long been considered depauperate due to severe climatic oscillations over the Pleistocene (Comes and Kadereit, 1998; Hewitt, 2004). The extant diversity in deglaciated areas is not only reduced at the species level but the intraspecific genetic variation is also often much impoverished in comparison with that in surrounding non-glaciated areas (e.g. Schönswetter et al., 2003; Koch et al., 2006). It is assumed that only the hardiest flowering plant species may have survived in glaciated areas either on ‘islands’ protruding from the ice sheet (so-called nunataks) or on debris-covered glaciers (Fickert et al., 2007; Schneeweiss and Schönswetter, 2011; Westergaard et al., 2011; but see Parducci et al., 2012 for suggested tree survival). Otherwise, survival in peripheral ice-free refugia or post-glacial origins as a result of rapid in situ speciation have been suggested (Brochmann et al., 1996; Jakobsson et al., 2006; Skrede et al., 2006; Ståhlberg and Hedrén, 2010). Although the plant biota of Northern Europe were largely ‘wiped out’ during the last glaciation, a considerable number of endemic taxa currently restricted to deglaciated areas are now recorded, accounting for 4·5–6·3 % of the total species diversity (Brochmann et al., 2003; Jonsell and Karlsson, 2004). From the evolutionary point of view, these arctic and boreal endemics are key biodiversity components of the deglaciated landscapes and demonstrate the evolutionary potential of the northern flora. Deeper insights into the evolutionary history of these endemics are, therefore, crucial for a better understanding of patterns and processes shaping the plant diversity in changing environments.
Evolutionary pathways leading to the genesis of endemic taxa in deglaciated areas have been the subject of a long-standing debate. Whereas traditional biogeographers used the incidence of endemics in deglaciated regions as evidence for in situ nunatak survival (Löve and Löve, 1963; Dahl, 1987, 1989), more recent molecular genetic data challenged these interpretations and argued that most such species are either not sufficiently hardy for nunatak survival or are of more recent (post-glacial) origin (Brochmann et al., 2003). Alternative explanations assumed (1) survival of contemporary endemics in periglacial areas followed by their extinction in original source regions due to post-glacial environmental change, or (2) rapid in situ speciation from the new immigrants after the ice melted. The latter scenario seems to hold for several hardy ‘true arctic’ endemics that most probably originated via interspecific/interlineage hybridization and genome duplication (Brochmann et al., 2004). As a result, the majority of arctic endemics are young evolutionary products, often high allopolyploids and/or stabilized hybrids with frequent asexual reproduction, e.g. Cerastium nigrescens (Brysting and Borgen, 2000; Brysting et al., 2007), Papaver radicatum aggregate (Solstad et al., 2003), Poa jemtlandica (Brysting et al., 1997, 2000), Saxifraga opdalensis and S. svalbardensis (Steen et al., 2000). In contrast, there are no sexual outcrossing diploids among European arctic endemics (Brochmann et al., 2003).
The published evolutionary histories of endemic species from boreal and temperate parts of formerly glaciated Northern Europe are more diverse. In addition to recent allopolyploids, e.g. Arabidopsis suecica (Jakobsson et al., 2006) and Saxifraga osloensis (Brochmann et al., 1996), allopatric segregates at higher ploidy levels, e.g. Euphrasia bottnica (Jonsell, 1988), and agamospermous microspecies, e.g. Sorbus spp., Taraxacum spp. and Limonium spp. (Preston and Hill, 1997; Jonsell and Karlsson, 2004), there are also several diploid sexual endemics (Sterner, 1986; Borgen, 1987; Jonsell, 1988). In contrast to the relatively well studied northern polyploids, these diploids have been largely neglected by recent cytogeographical and/or molecular studies. Available distributional, morphological and cytological evidence indicates that the northern diploid endemics represent either (1) weakly differentiated segregates of otherwise widespread and taxonomically challenging groups (e.g. Atriplex spp., Odontites vernus complex and Euphrasia spp.: Snogerup, 1983; Elven, 1984; Borgen, 1987), or (2) relicts of a once common steppe flora with no extant geographically close relatives (e.g. some endemics of the Gotland and Öland islands; Sterner, 1986).
An outstanding case of Northern European endemics is provided by the diploid–polyploid complex of Galium pusillum (Rubiaceae). This sexual allogamous (F. Ehrendorfer, unpubl. data) aggregate encompasses approx. 27 species (the exact number depending on the particular taxonomic treatment) that are distributed from the Iberian and Balkan peninsulas to Iceland (Ehrendorfer, 1960; Ehrendorfer et al., 1976). Although the available data on the diversity of the G. pusillum aggregate in Northern Europe are still fragmentary, some unusual patterns can be discerned. First, four morphologically distinct species, at the diploid (2n = 2x = 22) and/or tetraploid (2n = 4x = 44) level, have been recognized as endemic to deglaciated parts of Northern Europe (one species is native to the arctic zone while the remainder occur in deglaciated boreal/atlantic regions; Sterner, 1944; Goodway, 1957; Ehrendorfer, 1960, 1962). Secondly, the taxa of the G. pusillum aggregate exhibit an intriguing disjunct distribution in Northern Europe, consisting of several isolated areas scattered over Denmark, southern Sweden, southern and central Norway, the UK, Ireland and Iceland. Last, but not least, the aggregate is known to have a broad ecological amplitude in this area, inhabiting a wide range of habitats such as thermophilous lime-rich vegetation, sand dunes, open forests and arctic vegetation (Sterner, 1944). These pieces of evidence indicate that the G. pusillum aggregate is a unique system, which shows a striking diversity in deglaciated parts of Northern Europe and possibly can reveal still neglected evolutionary pathways that have shaped modern plant biota.
A necessary prerequisite for a detailed molecular study of such ploidy-heterogeneous plant systems is knowledge of the geographic distribution of cytotypes. The cytogeographic data complement phylogenetic and other experimental data, and serve as a foundation for addressing questions of historical developments of modern distribution patterns, frequency of polyploid formation, ecological differentiation of cytotypes and the evolution of interploidy reproductive barriers (Suda et al., 2007). Despite some previous attempts (Sterner, 1944; Goodway, 1957; Ehrendorfer, 1960, 1962), our knowledge of the cytological and ecological diversity of the G. pusillum complex is unsatisfactory, because only small parts of the entire distribution range in Northern Europe have been researched, and cytogeographic patterns have so far been inferred from a few chromosome counts that were complemented by indirect estimates of ploidy level on the basis of pollen grain diameter.
In this study, we employed a high-throughput technique for ploidy estimation [flow cytometry (FCM)] together with a formalized analysis of ecological preferences to assess ploidy, relative genome size and niche differentiation over the entire distribution range of the G. pusillum aggregate in Northern Europe and to compare the results with a corresponding data set obtained for Central and Western European species. Specifically, we addressed the following questions. (1) What is the pattern of ploidy distribution in deglaciated areas of Northern Europe and how does it compare with ploidy variation in surrounding geographic areas (particularly in Central Europe)? (2) What is the ploidy variation at the intrapopulation level? Are there any indications of recent polyploidization events and/or interploidy gene flow? (3) What is the variation in relative DNA content at the monoploid level? Is this variation taxonomically or geographically structured? (4) How strong is the niche differentiation between different species and/or cytotypes? What are the principal ecological gradients along which different Galium species are sorted?
MATERIALS AND METHODS
Field sampling
Our sampling strategy was designed to cover the entire range of all four northern endemic species of the G. pusillum aggregate as well as of all species of the complex occurring in adjacent areas of northern Central and Western Europe (exceptions being Galium anisophyllon and G. pumilum whose ranges continue further south and were therefore covered only partially; Ehrendorfer, 1958, 1962). Plant material was collected from 2009 to 2012 in 12 countries of Central, Western and Northern Europe, i.e. Austria (17 sites), the Czech Republic (34 sites), Denmark (nine sites), France (one site), Germany (eight sites), Iceland (52 sites), Ireland (five sites), Norway (six sites), Poland (five sites), Slovakia (11 sites), Sweden (13 sites) and the UK (20 sites). In total, 181 populations of ten species from the G. pusillum aggregate were included in the study (see Supplementary Data Table S1 for locality details). Taxonomy and nomenclature follow the only complete treatment of the group in Flora Europaea (Ehrendorfer et al., 1976). Whenever possible, shoots from at least six plants per population were collected in plastic bags and stored cold until FCM analysis. To avoid collecting the same genet, the distance between the sampled individuals was at least 0·5 m. The total number of analysed Galium plants amounted to 1158. In addition, a vital Galium clump was selected at each locality and transferred to the Experimental Garden of the Institute of Botany, Academy of Sciences, Průhonice, CZ (49°59′30″N, 14°34′00″E, 320 m a.s.l.) for cultivation. Herbarium vouchers are deposited in the Herbarium of Charles University in Prague (PRC).
Environmental conditions were thoroughly characterized at 95 localities of the G. pusillum aggregate using phytosociological relevés (each covering an area of 3 × 3 m with abundant occurrence of di- or tetraploid Galium taxa; Supplementary Data Table S2). In each plot, the relative cover of all vascular plant species was characterized using a modified nine-point Braun-Blanquet scale (Braun-Blanquet, 1964) and the following environmental parameters were recorded: total vegetation cover, cover of each vegetation layer, slope inclination and orientation, proportion of bare rock and/or scree, soil depth and ‘rockiness’ (i.e. the proportion of stone particles in the soil characterized on a semi-quantitative scale). At 73 localities, mixed rhizosphere soil samples were taken from five microsites within the area of the phytosociological relevé.
Soil analysis
Soil samples were air-dried, passed through a 2 mm sieve and analysed for pH and concentrations of selected elements (C, N, K, Ca and Mg) in the Analytical Laboratory of the Institute of Botany, Průhonice, CZ. Available concentrations of Ca, Mg and K were determined with an atomic absorption spectrometer (Unicam 9200X; Unicam Ltd, Cambridge, UK) after 1 m ammonium acetate extraction (pH 7·0) for Ca and Mg, and 0·015 m ammonium fluoride extraction for K (Mehlich, 1984). Total N and C contents were determined by a combustion method (Carlo Erba NC2500 elemental analyser; CE Instruments, Milan, Italy). The results are available in Supplementary Data Table S1.
Flow cytometry
DNA ploidy levels and relative fluorescence intensities were estimated using FCM following the simplified two-step protocol (Doležel et al., 2007). Intact tissue from 1–2 young leaves from each plant was chopped together with an appropriate volume of the internal reference standard (Bellis perennis, 2C = 3·38 pg; Schönswetter et al., 2007) using a sharp razor blade in a Petri dish containing 0·5 mL of ice-cold Otto I buffer (0·1 m citric acid, 0·5 % Tween-20). The suspension was filtered through a 42 mm nylon mesh and incubated for 10 min at room temperature. Isolated nuclei were stained with 1 mL of Otto II buffer (0·4 m Na2HPO4·12H2O) supplemented with 4′,6-diamidino-2-phenylindole (DAPI) at a final concentration of 4 µg mL−1 and β-mercaptoethanol (2 µL mL−1). After a few minutes, the relative fluorescence intensity of 3000 particles was recorded using a Partec ML flow cytometer (Partec GmbH, Münster, Germany) equipped with a UV LED chip as the excitation source. Histograms were evaluated using FloMax software, ver. 2·4d (Partec). Up to five Galium plants were analysed together during the large-scale ploidy screening. Each plant was separately re-analysed whenever mixed-ploidy samples were detected, peaks were asymmetrical or the coefficient of variance (CV) of the G0/G1 Galium peak exceeded the 5 % threshold.
To assess the variation in nuclear DNA content among plants of the same ploidy level, a similar protocol was adopted but each individual was analysed separately. Despite its preferential binding to AT-rich regions of DNA, we used DAPI to fulfil this task because staining with the intercalating fluorochrome propidium iodide yielded histograms of insufficient quality (in terms of both high CVs and prominent background fluorescence). Any protocol modification, including the use of five different buffers (Loureiro et al., 2006, 2007), increasing the concentration of antioxidants, testing different plant tissues (stems, petals), incubation times and/or more filtration steps did not result in distinct improvement of propidium iodide-stained histograms. As has been shown previously (Barow and Meister, 2002), the base content varies only slightly within a family. Consequently, differences in fluorescence intensity of base-selective fluorochromes at low taxonomic levels (e.g. within a genus or a section) reflect variation in the total amount of nuclear DNA rather than variation in AT/GC base content. Nonetheless, to clarify that the amounts of nuclear DNA were estimated using a stain with base bias, we use the term ‘relative fluorescence intensity’ or ‘relative DNA content’ instead of ‘genome size’ throughout this article. More stringent criteria on the quality of the analyses were applied in this step: (1) peaks of both the sample and the internal reference standard had to be of approximately the same height; (2) a relative fluorescence intensity of 5000 particles was recorded; (3) each sample was measured at least three times on different days to minimize potential random instrumental drift; and (4) the maximum acceptable between-day variation was set at 2 %; otherwise the most remote value was discarded and the sample re-analysed. The reliability of FCM measurements (i.e. between-plant differences) was repeatedly confirmed in simultaneous runs of Galium accessions, yielding distinct fluorescence intensities.
Chromosome counts
Chromosome numbers were determined from mitotically active root tip meristems of seedlings germinated from ripe fruits collected at the original localities. Because of the small chromosome size, we used both fluorescent and light microscopy. At least five complete metaphase plates per slide were counted. Seedlings were pre-treated with a 0·002 m solution of 8-hydroxyquinoline for 2 h at room temperature and an additional 2 h at 4 °C in the darkness, fixed in ethanol:acetic acid (3:1) and stored at –20 °C until further processing. Fixed material was washed twice in citrate buffer for 10 min and enzymatically digested in a mixture of 1 % cellulase Onozuka (Serva, Heidelberg, Germany), 0·4 % pectolyase and 0·4 % cytohelicase (Sigma, Vienna, Austria) in citric buffer (Weiss-Schneeweiss et al., 2012). After incubation for 10 min at 37 °C, the samples were washed in citric buffer and stored at 4 °C for a few hours, transferred into a drop of 60 % acetic acid on a slide, and the excised meristematic tissue was gently squashed. After removing the coverslip on dry ice, preparations were air-dried and stored for 1 d at 4 °C. Staining consisted of 2 µg μL−1 DAPI dissolved in mounting antifade medium Vectashield (Vector Laboratories, Burlingame, CA, USA). After sealing with a coverslip, preparations were examined with an Axio Imager M2 (Carl Zeiss, Vienna, Austria) epifluorescent microscope.
For light microscopy, fixed material was digested in 5 m HCl for 30 min at room temperature, washed twice with tap water, and stained by Schiff's reagent in the darkness for 60 min at room temperature followed by 15 min at 4 °C. Samples were then transferred into a drop of 60 % acetic acid on a slide, and the excised meristematic tissue was gently squashed. Preparations were examined with an Axio Imager A1 light microscope (Carl Zeiss). In both protocols, images were captured with the CCD camera operated with Axiovision software (Carl Zeiss).
Data analyses
Statistical computations were performed in R, ver. 2·15·1 (http://www.r-project.org). Interspecific differences in relative fluorescence intensities were tested using one-way analysis of variance (ANOVA). A linear mixed-effect model (nlme package; Lindstrom and Bates, 1990), with species identity treated as a factor with random effect, was used to assess differences in fluorescence intensities at the monoploid level (analogous to Cx-values in analyses done with intercalating fluorochromes; Greilhuber et al., 2005) among accessions from different geographic regions.
The relevés were analysed by both unconstrained [using detrended correspondence analysis (DCA)] and constrained [using canonical correspondence analysis (CCA) with forward selection of environmental variables] ordinations in Canoco for Windows, ver. 4·5 (Lepš and Šmilauer, 2003). While the unconstrained ordination seeks the main compositional gradients in environmental data (which could be further interpreted as ecological gradients), the constrained ordination extracts the variance in species composition, which could be directly attributed to known environmental variables. Only the directly measured variables were used for calculations [i.e. Ellenberg indicator values (EIVs) were excluded because they also contain information about species composition]. Hierarchical classification of the herb layer vegetation was performed using the TWINSPAN algorithm (Hill, 1979) incorporated in JUICE 7·0 software (Tichý, 2002), by setting three pseudospecies cut-off levels (0, 5 and 25).
The EIVs (which provide estimates of environmental characteristics of the sites inferred from species composition data; Ellenberg, 1992) were calculated in JUICE 7·0 based on presence/absence data. EIVs were calculated only for relevés in which the values were available for more than one-third of herbaceous species. Differences in the values of individual environmental variables (i.e. abiotic and biotic characteristics of the relevés collected in the field, results of soil analyses and the EIVs) among localities hosting different Galium species/cytotypes were tested by simple linear models in R 2·15·1, and the Bonferroni correction for multiple tests was applied. Differences among ploidy levels, groups with different glacial history (occurring in deglaciated Nortern Europe vs. mostly unglaciated Central Europe) and their interaction were tested using linear mixed-effect models, with species identity treated as a random factor (nlme package in R).
RESULTS
Ploidy level variation and cytogeography
Four different DNA ploidy levels (2x, 4x, 6x and 8x) were detected among 1158 individuals from 181 populations of ten species belonging to the G. pusillum aggregate (Table 1). Three species were exclusively diploid (G. cracoviense, G. oelandicum and G. suecicum), two were exclusively tetraploid (G. normanii and G. sudeticum) and one was exclusively octoploid (G. pumilum). Ploidy heterogeneity was found in the remaining four species. While G. sterneri and G. valdepilosum consisted of diploid and tetraploid populations, G. fleurotii encompassed tetra- and octoploid cytotypes, and G. anisophyllon consisted of di-, tetra- and hexaploid cytotypes. Despite this cytological variation, only a single mixed-ploidy population of the same species was observed (4x + 6x G. anisophyllon, no. G179) and a sympatric growth of 4x G. valdepilosum and 8x G. pumilum was recorded at three sites (see Supplementary Data Table S1 for locality details). No odd-ploidy individuals were found. Chromosome counts confirmed the estimated ploidy levels and revealed 2n = 2x = 22 in six accessions representing G. anisophyllon, G. sterneri, G. suecicum and G. valdepilosum, 2n = 4x = 44 in four accessions representing G. sterneri and G. valdepilosum, and 2n = 8x = approx. 88 in one accession of G. pumilum (Fig. 1, Supplementary Data Table S1). Neither aneuploidy nor accessory chromosomes was observed.
Table 1.
Ploidy levels, numbers of somatic chromosomes, relative amounts of nuclear DNA and habitat preferences for the ten investigated species/15 cytotypes of the G. pusillum aggregate from Central and Northern Europe
| Species | Ploidy level | Distribution* | Habitat preferences | No. of populations/individuals | No. of chromosomes | Relative DNA content at holoploid level (mean ± s.d.)† | Relative DNA content at monoploid level (mean ± s.d.)† |
|---|---|---|---|---|---|---|---|
| Galium anisophyllon Villars | 2x | CE | Open basiphilous grasslands and basic rocks | 5/35 | 2n = 22 | 0·260 ± 0·004 | 0·130 ± 0·002 |
| 4x | CE | Basiphilous to neutral grasslands, rocks and mountain screes | 8/46 | 0·523 ± 0·010 | 0·131 ± 0·003 | ||
| 6x | CE | Basiphilous to neutral grasslands, rocks and mountain screes | 3/18 | 0·759 ± 0·007 | 0·127 ± 0·001 | ||
| Galium cracoviense Ehrendorfer | 2x | CE | Limestone rocks | 2/22 | 0·259 ± 0·002 | 0·129 ± 0·001 | |
| Galium fleurotii Jordan | 4x | BI‡ | Chalk slopes | 1/15 | 0·487 ± 0·001 | 0·122 ± 0·000 | |
| 8x | BI | Limestone rocks | 1/11 | NA | NA | ||
| Galium normanii Dahl | 4x | N | Open tundra, sub-arctic grasslands and rocks of various soil reaction | 54/166 | 0·492 ± 0·006 | 0·123 ± 0·001 | |
| Galium oelandicum (Sterner et Hylander) Ehrendorfer | 2x | N | Open vegetation in limestone flatlands (‘alvar’) | 3/22 | 0·242 ± 0·002 | 0·121 ± 0·001 | |
| Galium pumilum Murray | 8x | CE§ | Neutral to acidophilous grasslands with moderate competition | 22/119 | 2n = approx. 88 | 0·918 ± 0·004 | 0·115 ± 0·001 |
| Galium sterneri Ehrendorfer | 2x | BI | Low-competitive calciphilous grasslands and limestone rocks | 8/64 | 2n = 22 | 0·249 ± 0·005 | 0·124 ± 0·002 |
| 4x | N + BI | Low-competitive calciphilous grasslands, open tundra-like vegetation, rarely acidophilous forest margins (Norway) and dunes (Denmark) | 27/197 | 2n = 44 | 0·497 ± 0·008 | 0·124 ± 0·002 | |
| Galium sudeticum Tausch | 4x | CE¶ | Basic to neutral rocks and mountain screes | 2/35 | 0·531 ± 0·005 | 0·133 ± 0·001 | |
| Galium suecicum (Sterner) Ehrendorfer | 2x | N | Acidophilous open forests and forest margins | 10/61 | 2n = 22 | 0·240 ± 0·001 | 0·120 ± 0·001 |
| Galium valdepilosum H. Braun | 2x | CE | Open forests on various substrates (basic, acidic, serpentine) | 12/139 | 2n = 22 | 0·259 ± 0·007 | 0·130 ± 0·003 |
| 4x | CE** | Open forests on various substrates (basic, acidic, serpentine), rarely calcareous grasslands | 24/207 | 2n = 44 | 0·507 ± 0·005 | 0·127 ± 0·001 |
*N, Nordic countries (formerly glaciated); BI, the British Isles (formerly mostly glaciated); CE, Central Europe (generally remaining unglaciated although G. sudeticum and partly also G. anisophyllon occupy sites once covered by mountain glaciers).
†The fluorescence intensity of Bellis perennis (internal reference standard) was set to a unit value.
‡A single population from northern France was merged with the BI (the British Isles) group.
§Rare records from Scandinavia and the British Isles are considered adventive (Ehrendorfer, 1962).
¶Only sub-alpine populations from the Krkonoše Mountains were assigned to this species.
**Rare plants from central Denmark are referred to as an endemic subsp. slesvicense (Sterner ex Hylander) Ehrendorfer (relative DNA content of 0·511).
Fig. 1.
Mitotic chromosomes of five species from the G. pusillum aggregate stained with Schiff's reagent (A–C) and DAPI (D–F). Scale bar = 5 µm. (A) Galium valdepilosum (2n = 4x = 44, loc. G60); (B) G. anisophyllon (2n = 2x = 22, loc. G123); (C) G. valdepilosum (2n = 2x = 22, loc. G93); (D) G. suecicum (2n = 2x = 22, loc. G86); (E) G. pumilum (2n = 8x = approx. 88, loc. G106); (F) G. sterneri (2n = 4x = 44, loc. G209). See Supplementary Data Table S1 for locality details.
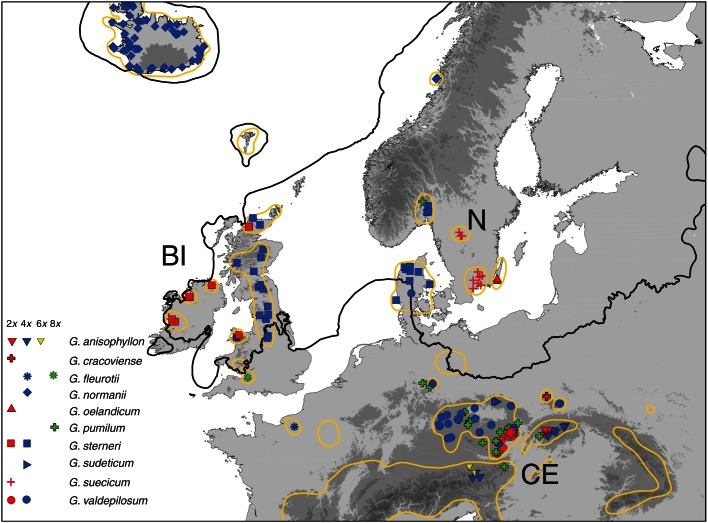
Within the studied area, di- and tetraploid taxa exhibited a rather discontinuous distribution, inhabiting only ‘islands’ of suitable habitats. Tetraploids were the most common and widespread (63 % of all populations), and occurred throughout the area under investigation (Fig. 2). In contrast, diploids were more spatially restricted and inhabited several isolated areas in Central and Northern Europe. Importantly, two exclusively diploid species (G. oelandicum and G. suecicum) and the diploid cytotype of G. sterneri were recorded only in areas that were covered by ice sheets during the last glacial maximum (LGM). Hexaploids were documented by two populations of G. anisophyllon from the Austrian Alps, while octoploids were represented by a common Central European species occasionally extending up to southern Scandinavia (G. pumilum), and by a single population of G. fleurotii from south-eastern England (Fig. 2).
Fig. 2.
Geographic location of the studied populations of the G. pusillum aggregate in Central and Northern Europe (red, diploid; blue, tetraploid; yellow, hexaploid; green, octoploid; N, Nordic countries, BI, the British Isles; CE, Central Europe). The solid black line indicates the approximate extent of the continental ice sheet during the last glacial maximum (following Ehlers et al., 2011). The distribution range of the G. pusillum complex is marked by the orange line (following Sterner, 1944; Ehrendorfer, 1958, 1962; Ehrendorfer et al., 1976; http://www.bsbi.org.uk/); the distribution of the widespread and partly adventive octoploid G. pumilum is not shown.
Homoploid differentiation in relative DNA content
In addition to ploidy variation, the accessions of the G. pusillum aggregate also exhibited a considerable variation in relative DNA content at the homoploid level. Di- and tetraploid cytotypes of different species varied 1·13- and 1·12-fold, respectively, and the interspecific differences in relative DNA content were highly significant (F5,34 = 28·3, P < 0·001 and F5,59 = 29·1, P < 0·001, respectively). The lowest relative fluorescence intensities among diploids were recorded in the Scandinavian endemics G. suecicum and G. oelandicum, whereas the highest values were possessed by the Central European G. valdepilosum and G. anisophyllon. At the tetraploid level, the French accession of G. fleurotii and the Nordic and British species G. normanii and G. sterneri were at the lower limit of the relative DNA content range, while the species of Central European mountains, G. anisophyllon and G. sudeticum, occupied the upper end (Table 1).
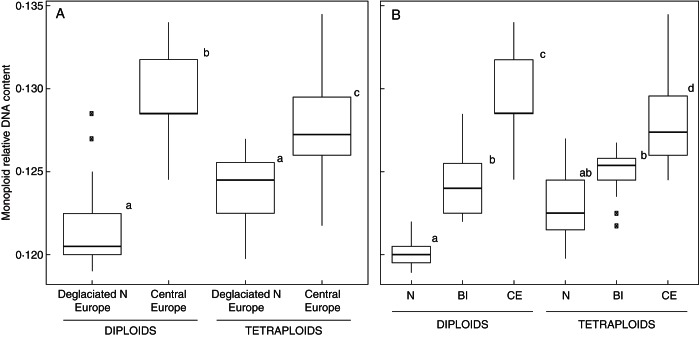
Interestingly, the pattern of relative DNA content variation in both di- and tetraploids was associated with glacial history and geography. Plants occurring in Northern Europe, covered by ice during the LGM, possessed significantly lower relative DNA contents than their counterparts from mostly unglaciated Central Europe (Table 2; Fig. 3A). When grouped according to geography, the largest differences were found between samples from Central Europe and the Nordic countries (mean relative fluorescence intensities differing by 5·7 %; Fig. 4); plants from the British Isles showed intermediate values (Fig. 3B). In contrast, the differences in relative DNA content at the monoploid level between di- and tetraploids from different regions were only marginally significant when grouped according to their glacial history and non-significant when grouped according to geographic regions (Table 2).
Table 2.
Differences in relative DNA content at the monoploid level among groups of the G. pusillum aggregate with different glacial history and geography tested using linear mixed-effect models
| Factor | d.f. | F | P |
|---|---|---|---|
| Glacial history (deglaciated N Europe/mostly unglaciated C Europe) | 1, 7 | 17·39 | 0·004 |
| Ploidy level | 1, 94 | 4·59 | 0·035 |
| Interaction glacial history × ploidy level | 1, 94 | 4·30 | 0·041 |
| Region (N/BI/CE) | 2, 91 | 34·72 | < 0·0001 |
| Ploidy level | 1, 91 | 1·98 | 0·162 |
| Interaction region × ploidy level | 2, 91 | 5·07 | 0·008 |
Species identity was treated as a factor with random effect.
Significant values (P < 0·05) are presented in bold.
Region abbreviations: N, Nordic countries; BI, the British Isles; CE, Central Europe.
Fig. 3.
Variation in relative DNA contents at the monoploid level in di- and tetraploid accessions of the G. pusillum aggregate, grouped according to their glacial history (A) and geography (B). The fluorescence intensity of Bellis perennis was set to a unit value. Different letters indicate significantly different groups at α = 0·05. Deglaciated N Europe, populations from areas covered by continental ice sheets during the last glacial maximum; C Europe, populations from generally ice-free areas of Central Europe; N, Nordic countries; BI, the British Isles; CE, Central Europe.
Fig. 4.
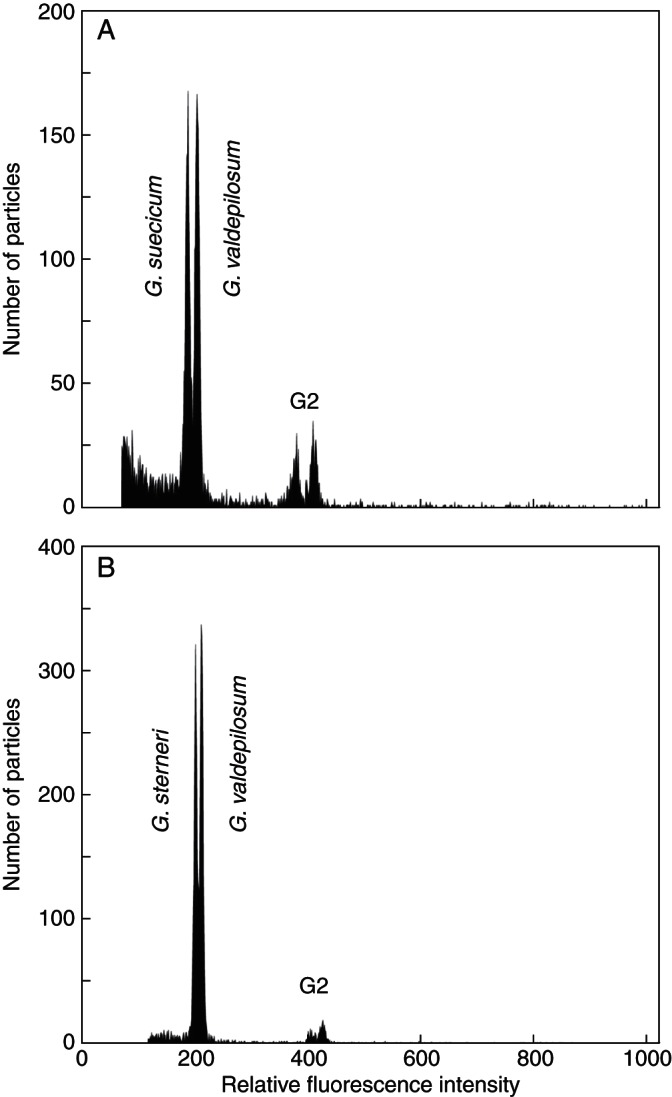
Flow cytometric histograms showing divergence in relative nuclear DNA contents between selected diploid (A) and tetraploid (B) accessions of the G. pusillum aggregate from formerly glaciated Northern Europe and unglaciated areas of Central Europe. (A) Simultaneous analysis of diploid accessions of G. suecicum (pop. G83, Sweden) and G. valdepilosum (pop. G61, the Czech Republic); difference in fluorescence intensity 8·8 %. (B) Simultaneous analysis of tetraploid accessions of G. sterneri (pop. G73, Denmark) and G. valdepilosum (pop. G36, Poland); difference in fluorescence intensity 5·2 % (see Supplementary Data Table S1 for population details). Nuclei from both samples were simultaneously isolated, stained with DAPI and analysed.
Niche differentiation
The species showed distinct habitat preferences, expressed by both biotic (i.e. floristic composition) and abiotic conditions. The DCA ordination of floristic composition identified two major groups of sites (separated along the first axis), corresponding to relevés from deglaciated Northern Europe and mostly unglaciated Central Europe (Fig. 5). The second ordination axis reflected the ecological preferences of individual species within these two major regions (e.g. basiphilous/acidophilous species, species of open forests/non-forest habitats, etc.). In ploidy-heterogeneous Galium species for which multiple relevés were available (G. sterneri and G. valdepilosum), the position of the two cytotypes overlapped but diploid cytotypes had a narrower ecological niche than their tetraploid counterparts (Fig. 5).
Fig. 5.

Habitat preferences of different species/cytotypes from the G. pusillum aggregate. The patterns in floristic composition of 95 phytosociological relevés are visualized using the detrended correspondence analysis. The first and second ordination axes explain 3·9 and 2·8 % of the total variation, respectively. (A) Relevés clustered according to the presence of different Galium species/cytotypes (open and filled symbols denote samples from formerly glaciated Northern Europe and mostly unglaciated Central Europe, respectively; red, diploids; blue, tetraploids); (B) Environmental variables influencing species composition passively projected on the sample data (centroids of samples belonging to particular species/cytotypes are highlighted). Only environmental variables chosen by the forward selection procedure and Ellenberg indicator values are projected (see the Materials and Methods for details).
The TWINSPAN hierarchical analysis largely confirmed these differences in floristic composition of the sites occupied by different Galium species (Fig. 6). Disregarding the geographically isolated Icelandic localities of G. normanii with distinct vegetation types, the major split in the data was observed between the sites of deglaciated Northern Europe vs. the generally unglaciated Central Europe. Interesting exceptions were (1) a few serpentine populations of G. valdepilosum (from open coniferous forests) and the populations of the sub-alpine G. sudeticum (both occurring in Central Europe) that formed a group close to a Scandinavian cluster of G. suecicum + G. sterneri, and (2) a single population of G. anisophyllon (G139) from a limestone sub-alpine habitat that was placed among calciphilous Northern European species. The broadest ecological range was found in G. valdepilosum, whose relevés were divided into five groups that generally reflected the gradient of basicity of the stands. As in the DCA, diploid and tetraploid accessions of the same species were intermingled and never formed separate clusters.
Fig. 6.

Hierarchical classification of vegetation data using the TWINSPAN algorithm. Population code, major geographic areas (N, Nordic countries; BI, the British Isles; CE, Central Europe), glacial history (G, formerly glaciated Northern Europe; U, mostly unglaciated Central Europe) and ploidy level (filled circle, diploid; open square, tetraploid) are displayed for individual Galium species.
Gradients of substrate basicity, light intensity and partly also nutrient levels were the major factors determining the differences in floristic composition of the Galium-occupied sites. The CCA analysis identified eight environmental parameters that significantly (F = 1·60, P = 0·001, 999 permutations) influenced species composition, namely the soil pH, concentration of Ca, inorganic N and organic C contents, cover of the tree, shrub and herb layers, and slope inclination (Fig. 7; Supplementary Data Fig. S1). In addition, significant differences were detected among species in mean EIVs for soil reaction, available nutrients, light and moisture (Table 3; Supplementary Data Fig. S1). In contrast, none of the variables tested in Table 3 differed significantly between either ploidy levels or groups of species with different glacial history (data not shown).
Fig. 7.

Box-and-whisker plots showing differences in soil pH among the sites inhabited by different 2x/4x species of the G. pusillum aggregate (diploid and tetraploid as indicated in the key). Sites of G. sterneri from different parts of its disjunct range are further divided (BI, the British Isles; Dk, Denmark; No, Norway).
Table 3.
Associations between different species from the G. pusillum aggregate and selected environmental variables tested by linear models
| Variable | d.f. | F | P |
|---|---|---|---|
| pH | 5, 67 | 4·9934 | 0·0006 |
| Ca* | 5, 64 | 5·8386 | 0·0002 |
| N* | 5, 67 | 1·8522 | 0·1146 |
| C* | 5, 67 | 1·3975 | 0·2365 |
| Cover of herb layer | 5, 81 | 5·5693 | 0·0002 |
| Slope* | 5, 81 | 10·447 | < 0·0001 |
| EIV soil reaction | 5, 79 | 6·3562 | < 0·0001 |
| EIV nutrients | 5, 81 | 4·9043 | 0·0006 |
| EIV light | 5, 81 | 11·144 | < 0·0001 |
| EIV moisture | 5, 78 | 10·429 | < 0·0001 |
P-values in bold are significant after Bonferroni correction (P < 0·05).
EIV, Ellenberg indicator value.
*Data were log-transformed before analysis.
DISCUSSION
Cytogeography of the G. pusillum complex in Northern and Central Europe
The peculiar disjunct distribution of the G. pusillum aggregate in areas covered by ice sheets during the LGM has attracted the attention of botanists for many decades. The first insights into the phenotypic and cytological diversity of the complex were gained from morphological observations (Sterner, 1944), measurements of pollen grains (Ehrendorfer, 1962) and a few chromosome counts (Goodway, 1957; Ehrendorfer, 1960). Here we have built on these pioneering studies and performed a large-scale cytotype screening (1158 individuals from 181 populations) in order to better understand the role of genome-wide processes in the evolutionary history of this group. Our FCM analyses largely confirmed earlier indications and revealed a complex pattern of di- and tetraploid species/cytotypes in both Northern and Central Europe, in addition to the occurrence of hexa- and octoploids in the latter region (Fig. 2). In previously glaciated Northern Europe, a mosaic of cytologically distinct populations was found with practically no ploidy overlap. Except for narrow zones of contact between 2x and 4x plants of G. sterneri in north Wales and north-west Scotland and the co-occurrence of 4x cytotypes of G. sterneri and G. valdepilosum subsp. slesvicense in Denmark, allopatric distribution prevails among members of the G. pusillum aggregate in Northern Europe.
The observed cytogeographic pattern is quite unusual among flowering plants and contradicts the generally acknowledged hypothesis that diploids tend to be restricted to refugia while polyploids show better abilities to re-colonize deglaciated regions (Ehrendorfer, 1980; Van Dijk and Bakx-Schotman, 1997; Parisod and Besnard, 2007). In fact, high polyploids within the G. pusillum aggregate are common in Central and Southern Europe (Fig. 2) (Ehrendorfer, 1958; Ehrendorfer et al., 1976; Krendl, 1993) but do not reach the once glaciated Northern Europe (except for occasional recent introductions of 8x G. pumilum; Ehrendorfer, 1962). There were only di- and tetraploid Galium cytotypes that spread into boreal and arctic regions after the retreat of the ice sheets. Co-occurrence of 2x and 4x cytotypes in deglaciated regions has been reported, for instance, in the Dactylorhiza maculata aggregate (Ståhlberg and Hedrén, 2010) and Parnassia palustris (Borgen and Hultgård, 2003); however, these groups do not have any higher polyploid cytotypes in more southern areas.
The G. pusillum complex also provides a remarkable system with respect to the patterns of endemism. The formerly glaciated regions of Northern Europe host several endemic taxa recognized at specific (G. normanii, G. oelandicum, G. sterneri and G. suecicum; Ehrendorfer, 1960) or sub-specific (G. valdepilosum subsp. slesvicense; Ehrendorfer, 1975) levels. This diversity is comparable with that present in the generally unglaciated regions of Central Europe, indicating little impoverishment in areas once covered by ice sheets (Fig. 2). It should be noted that the current species concept in the G. pusillum aggregate is based on classical morphology-based taxonomic studies, while the underlying pattern of genetic differentiation is still unknown. Nonetheless, on the basis of the available cytological, phenotypic and ecological evidence, we suggest that the complex in Northern Europe splits into several distinct evolutionary units (whose genetic variation is the subject of our ongoing molecular work). Therefore, and for practical reasons, we followed the only comprehensive taxonomic treatment of the group (Ehrendorfer et al., 1976) and adopted the rank of species in this study. Interestingly, two of the northern endemics are exclusively diploid (G. oelandicum and G. suecicum) and one (G. sterneri) includes both 2x and 4x cytotypes (Table 1, Fig. 2). In contrast, typical boreal-arctic endemics are (high) polyploids that have originated via recent (allo)polyploidization (e.g. Brochmann et al., 1996; Brysting and Borgen, 2000; for a review, see Brochmann et al., 2003). The only few diploid northern endemics are either weak segregates of their sister taxa occurring outside formerly glaciated regions or highly isolated relicts that lack closely related species (e.g. Elven, 1984; Sterner, 1986; for reviews, see Borgen, 1987; Jonsell and Karlsson, 2004).
Although we have not covered the entire European range of the G. pusillum aggregate, which extends to the Iberian and Balkan Peninsulas (Ehrendorfer et al., 1976), we argue that our sampling scheme is sufficient to meet the main goal, i.e. to assess cytological and ecological variation of the aggregate in its northern, formerly glaciated distribution area. This target area was here subjected to a detailed investigation, including >100 populations from all known disjunct parts of the G. pusillum aggregate range (see Fig. 2). In addition, these results were complemented by corresponding data obtained for Central European representatives of the aggregate. Our screening failed only in the case of populations from open acidophilous forests of northern Germany, probably representing diploid G. suecicum, that were documented by a few old herbarium specimens (the determination was based on fruit morphology and pollen size; Ehrendorfer, 1962). However, such plants have not been seen for approx. 70 years, and neither our own nor search attempts by local florists (M. Ristow, pers. comm.) were successful. Thus the aggregate probably has become extinct in northern Germany. In Western Europe the geographically nearest and still unsampled diploid taxon from the G. pusillum aggregate is G. timeroyi Jordan; however, it occurs as far as in the southern half of France, has a distinct morphology and prefers different (more xeric) habitats (Ehrendorfer et al., 1976).
Ecological segregation of northern endemics
The recent speciation literature emphasizes the role of ecological divergence in the development of reproductive isolation (Noor, 2003; Coyne and Orr, 2004). Clear habitat segregation was also observed among different homoploid species from the G. pusillum aggregate in both Northern and Central Europe, and can act as an efficient reproductive barrier that contributes to preserving species integrity.
Unlike the pioneering studies (Sterner, 1944; Ehrendorfer, 1962) that briefly described major vegetation units hosting different Galium species, we used a comprehensive statistical approach aimed at identifying major ecological gradients along which the populations and species/cytotypes are sorted. In particular, we found that different species from Northern Europe partitioned their niches along gradients of soil reaction (as identified by direct measurements of Ca concentration and pH, and the EIVs for soil reaction) and plant competition (as indicated by differences in tree and herb cover values as well as the EIVs for available light; Table 3, Fig. 5). Distinct ecological requirements are further manifested by allopatric distribution of investigated species that inhabit a wide scale of temperate, boreal and arctic habitats ranging from open acidophilous forests (G. suecicum) and thermophilous vegetation on limestone flatlands (so-called alvars; G. oelandicum), across various types of species-rich limestone grasslands and rocky places (G. sterneri), to bare tundra sites (G. normanii; see also Supplementary Data Fig. S2). Edaphic differentiation between closely related calciphilous and acidophilous species is a well-known evolutionary phenomenon that has triggered plant speciation in different geographic regions, including the Alps (Hungerer and Kadereit, 1998; Kadereit et al., 2011) and lowland Western Europe (Van Rossum et al., 1996, 1999). In contrast, the role of calciphilous vs. acidophilous edaphic speciation has only rarely been addressed in Northern Europe and has not been found in previous case studies (e.g. Van Rossum and Prentice, 2004).
Plant populations differing in ploidy level have often been assumed to differ also in niche requirements (Ramsey and Schemske, 1998; Soltis et al., 2010). Our results did not confirm such ploidy-specific niche differentiation within the G. pusillum aggregate (di- and tetraploid individuals of the same species occupied sites with similar environmental conditions) but suggested that genome duplication allows widening of ecological niches (as observed in G. sterneri and also in Central European populations of G. valdepilosum; Figs 5 and 7). The hypothesis of broader niches of polyploids relative to diploids has been repeatedly articulated (see Levin, 2002, and references therein) but only rarely tested explicitly (McIntyre, 2012). In contrast to most published studies that inferred niche breadth from interploidy differences in geographic distribution, we have offered quantitative evidence by a direct assessment of habitat preferences from a combination of phytosociological relevés and the quantification of selected abiotic variables.
Hypotheses on the evolutionary history of the northern endemics
Plant biota inhabiting formerly glaciated terrains have been the focus of much evolutionary research because they provide model systems for addressing questions concerning rapid speciation in changing environments. Four basic scenarios have been outlined for the origin of such boreal and arctic plants: (1) in situ survival on the ‘islands’ of suitable habitats protruding from the ice sheet (nunataks); (2) immigration from peripheral refugia and subsequent extinction in the original source areas; (3) rapid in situ post-glacial evolution via polyploidization, hybridization between independently immigrated lineages or allopatric speciation; and (iv) long-distance dispersal (Brochmann et al., 2003; Westergaard et al., 2011). Two of these have been proposed to explain the phytogeographical pattern in the Scandinavian G. pusillum aggregate. While Sterner (1944) assumed nunatak survival for at least some taxa (e.g. G. normanii and G. sterneri), Ehrendorfer (1962) hypothesized glacial survival of the ancestral lineage(s) in peripheral refugia in ice-free regions of Central Europe, followed by independent immigrations and differentiation of each of the currently endemic lineages after glacier retreat. According to this scenario, the northern ecologically distinct endemic species may still have sister taxa with rather similar habitat preferences in Central Europe.
Although detailed insights into the evolutionary history of the G. pusillum aggregate in formerly glaciated Northern Europe will require the use of molecular markers, our present data on cytotype distribution and relative genome size may rather suggest independent evolution of the Northern and Central European populations. Both di- and tetraploid taxa occurring in northern areas once covered by the ice sheet possess significantly lower relative amounts of nuclear DNA than their counterparts from Central Europe, suggesting that the Northern and Central European taxa represent two distinct lineages (Figs 1 and 3). A good example is provided by the two diploid species G. oelandicum and G. cracoviense, regarded as closely related by Ehrendorfer (1962) but differing markedly in their relative DNA content. The only exception from this pattern was an isolated tetraploid population of G. fleurotii from unglaciated northern France that possesses a relative DNA content similar to that of species from deglaciated northern areas (Table 1). In this case we can hypothesize that the Western European G. fleurotii has a common ancestor with the northern species, and possibly survived in periglacial refugia located in unglaciated parts of the British Isles, northern France and/or the exposed North Sea shelf. Importantly, our finding of divergence in relative genome size is also supported by phenotypic differences. Whereas all northern species are characterized by fruit epidermis with acute papillae, their Central European counterparts (except G. valdepilosum) have blunt-papillose fruits (Sterner, 1944; Ehrendorfer, 1962); the fruits of G. fleurotii show intermediate characters (our unpubl. obs.).
Genome size data have been repeatedly proven to be useful indicators of incipient speciation or evolutionary relatedness (e.g. Slovák et al., 2009; Dušková et al., 2010; Loureiro et al., 2010; Olšavská et al., 2012). Specifically, close relationships between genome size and genetic structure were revealed in Indian species of Curcuma (Záveská et al., 2011) or Central European taxa from the Knautia arvensis aggregate (Kolář et al., 2012). In both groups, evolutionary patterns were first hypothesized solely on the basis of genome size data (Leong-Škorničková et al., 2007, Kolář et al., 2009, respectively) and only later confirmed by molecular markers.
The lack of divergence in relative fluorescence intensities at the monoploid level between the 2x and 4x Galium cytotypes within each geographic region (Table 1) suggests in situ (auto)polyploidization from local diploids (either extant or extinct). Despite the fact that final conclusions remain open until molecular analyses have been conducted, several lines of evidence favour an autopolyploid origin over other evolutionary interpretations, such as allopolyploidy and/or independent immigrations of di- and tetraploids. For example, in the British populations of G. sterneri, the autopolyploid scenario is supported by identical relative Cx-values, as well as by great similarities in both morphology (Goodway, 1957) and ecological requirements (Fig. 5). In general, the northern tetraploids in the G. pusillum aggregate do not seem to be of very recent origin, since the 2x and 4x cytotypes are largely allopatric (Fig. 2) and no mixed-ploidy populations have been found despite extensive sampling. In addition, tetraploids occupy considerably larger geographic ranges than their diploid counterparts. In contrast, other northern polyploid endemics usually inhabit restricted areas where they often grow in sympatry with their diploid or lower polyploid progenitors, e.g. Draba cacuminum (Brochmann et al., 1992) and Saxifraga osloensis (Brochmann et al., 1996).
To sum up, our cytological and ecological data together with previous morphological studies (Sterner, 1944) revealed that the Northern European G. pusillum aggregate is a highly variable system of several geographically vicariant and ecologically distinct diploid/tetraploid species that are currently restricted to formerly glaciated areas, where they probably evolved independently from their southern counterparts. Eco-geographically differentiated diploid–polyploid plant complexes are well known from other parts of Europe (e.g. Ehrendorfer and Guo, 2006; Sonnleitner et al., 2010; Koutecký et al., 2012); however, the G. pusillum aggregate is the first example from areas formerly covered by the late Pleistocene ice sheets.
Conclusions
The present study constitutes the first step towards a better understanding of the evolutionary history of the G. pusillum aggregate in Central and Northern Europe. While the origin of the plant biota in (sub-)arctic regions has been intensively researched, much less is known about the patterns and processes that have shaped the flora of more southern deglaciated regions. Our data on ploidy and relative genome size challenge some previous evolutionary interpretations for the G. pusillum aggregate and suggest largely independent evolution of its Central vs. Northern European members. The species in both formerly glaciated and unglaciated regions show considerable eco-geographic divergence, which probably acts as a reproductive barrier and contributes to maintaining their genetic integrity. The evolution of the group in deglaciated Northern Europe has proceeded at both diploid and tetraploid levels, and has resulted in the origin of several endemic species differing in ploidy level, distribution range and habitat preferences. Thus, the complex of G. pusillum represents an exceptional case of endemism in formerly glaciated areas that substantially widens our perception of the evolutionary dynamics and speciation rates in the dramatically changing environment of Northern Europe.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are indebted to Don Cotton, Markéta Dortová, Martin Hanzl, Hana Hojerová, Adam Knotek, Eva Kolářová, Věra Koutecká, Petr Koutecký, Benjamin Ølgaard, Clemens Pachschwöll, Eliška Padyšáková, Radka Sudová, Milan Štech, Jakub Těšitel and Eliška Záveská for their help with locality information and/or sample collection. We also thank Hanna Schneeweiss and Tae-Soo Jang for their help with chromosome counts. This work was supported by the Czech Science Foundation [P506/10/0704].
LITERATURE CITED
- Barow M, Meister A. Lack of correlation between AT frequency and genome size in higher plants and the effect of nonrandomness of base sequences on dye binding. Cytometry. 2002;47:1–7. doi: 10.1002/cyto.10030. [DOI] [PubMed] [Google Scholar]
- Borgen L. Postglasial evolusjon i Nordens flora – en oppsummering. Blyttia. 1987;45:147–169. [Google Scholar]
- Borgen L, Hultgård UM. Parnassia palustris: a genetically diverse species in Scandinavia. Botanical Journal of the Linnean Society. 2003;142:347–372. [Google Scholar]
- Braun-Blanquet J. Pflanzensoziologie. Wien: Springer; 1964. [Google Scholar]
- Brochmann C, Soltis PS, Soltis DE. Multiple origins of the octoploid Scandinavian endemic Draba cacuminum: electrophoretic and morphological evidence. Nordic Journal of Botany. 1992;12:257–272. [Google Scholar]
- Brochmann C, Nilsson T, Gabrielsen TM. A classic example of postglacial allopolyploid speciation re-examined using RAPD markers and nucleotide sequences: Saxifraga osloensis (Saxifragaceae) Symbolae Botanicae Upsalienses. 1996;31:75–89. [Google Scholar]
- Brochmann C, Gabrielsen TM, Nordal I, Landvik JY, Elven R. Glacial survival or tabula rasa? The history of North Atlantic biota revisited. Taxon. 2003;52:417–450. [Google Scholar]
- Brochmann C, Brysting AK, Alsos IG, et al. Polyploidy in arctic plants. Biological Journal of the Linnean Society. 2004;82:521–536. [Google Scholar]
- Brysting AK, Borgen L. Isozyme analysis of the Cerastium alpinum–C. arcticum complex (Caryophyllaceae) supports a splitting of C. arcticum Lange. Plant Systematics and Evolution. 2000;220:199–221. [Google Scholar]
- Brysting AK, Elven R, Nordal I. The hypothesis of hybrid origin of Poa jemtlandica supported by morphometric and isoenzyme data. Nordic Journal of Botany. 1997;17:199–214. [Google Scholar]
- Brysting AK, Holst-Jensen A, Leitch I. Genomic origin and organization of the hybrid Poa jemtlandica (Poaceae) verified by genomic in situ hybridization and chloroplast DNA sequences. Annals of Botany. 2000;85:439–445. [Google Scholar]
- Brysting AK, Oxelman B, Huber KT, Moulton V, Brochmann C. Untangling complex histories of genome mergings in high polyploids. Systematic Biology. 2007;56:467–476. doi: 10.1080/10635150701424553. [DOI] [PubMed] [Google Scholar]
- Comes HP, Kadereit JW. The effect of Quaternary climatic changes on plant distribution and evolution. Trends in Plant Science. 1998;3:432–438. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Dahl E. The nunatak theory reconsidered. Ecological Bulletins. 1987;38:77–94. [Google Scholar]
- Dahl E. Nunatakk-teorien II – Endemismeproblemet. Blyttia. 1989;47:163–172. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Dušková E, Kolář F, Sklenář P, et al. Genome size correlates with growth form, habitat and phylogeny in the Andean genus Lasiocephalus (Asteraceae) Preslia. 2010;82:127–148. [Google Scholar]
- Ehlers J, Gibbard PL, Hughes PD. Quaternary glaciations – extent and chronology. A closer look. Amsterdam: Elsevier; 2011. [Google Scholar]
- Ehrendorfer F. Die geographische und ökologische Entfaltung des europäisch-alpinen Polyploidkomplexes Galium anisophyllum Vill. seit Beginn des Quartärs. Upsala Universitets Arsskrift. 1958;6:176–181. [Google Scholar]
- Ehrendorfer F. Neufassung der Sektion Lepto-Galium Lange und Beschreibung neuer Arten und Kombinationen. Sitzungsberichten der Österreichischen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Abteilung I. 1960;169:407–421. [Google Scholar]
- Ehrendorfer F. Cytotaxonomische Beiträge zur Genese der mitteleuropäischen Flora und Vegetation. Berichte der Deutschen Botanischen Gesellschaft. 1962;75:137–152. [Google Scholar]
- Ehrendorfer F. Further taxonomic notes on Rubiaceae in Europe. Plant Systematics and Evolution. 1975;124:173–178. [Google Scholar]
- Ehrendorfer F. Polyploidy and distribution. In: Lewis WH, editor. Polyploidy. Biological relevance. New York: Plenum Press; 1980. pp. 45–60. [Google Scholar]
- Ehrendorfer F, Guo YP. Multidisciplinary studies on Achillea sensu lato (Compositae-Anthemideae): new data on systematics and phylogeography. Willdenowia. 2006;36:69–87. [Google Scholar]
- Ehrendorfer F, Krendl F, Puff C. Galium L. In: Tutin TG, editor. Flora Europaea. Cambridge: Cambridge University Press; 1976. pp. 14–36. [Google Scholar]
- Ellenberg H. Zeigerwerte der Pflanzen in Mitteleuropa. 3., erweit. Aufl. Göttingen: E. Goltze. 1992 [Google Scholar]
- Elven R. Tangmelde-slekta (Atriplex L.) i Norge. Blyttia. 1984;42:15–31. [Google Scholar]
- Fickert T, Friend D, Grüninger F, Molnia B, Richter M. Did debris-covered glaciers serve as Pleistocene refugia for plants? A new hypothesis derived from observations of recent plant growth on glacier surfaces. Arctic, Antarctic, and Alpine Research. 2007;39:245–257. [Google Scholar]
- Goodway KM. The species problem in Galium pumilum. In: Lousley JE, editor. Progress in the study of the British flora. London: The Botanical Society of the British Isles; 1957. pp. 116–118. [Google Scholar]
- Greilhuber J, Doležel J, Lysák MA, Bennett MD. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359:183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MO. New York: Section of Ecology and Systematics, Cornell University. TWINSPAN – A FORTRAN program for arranging multivariate data in an ordered two-way table by classification of the individuals and attributes. 1979 [Google Scholar]
- Hungerer KB, Kadereit JW. The phylogeny and biogeography of Gentiana L. sect. Ciminalis (Adans.) Dumort.: a historical interpretation of distribution ranges in the European high mountains. Perspectives in Plant Ecology, Evolution and Systematics. 1998;1:121–135. [Google Scholar]
- Jakobsson M, Hagenblad J, Tavaré S, et al. A unique recent origin of the allotetraploid species Arabidopsis suecica: evidence from nuclear DNA markers. Molecular Biology and Evolution. 2006;23:1217–1231. doi: 10.1093/molbev/msk006. [DOI] [PubMed] [Google Scholar]
- Jonsell B. Mikroendemism i det baltiska landhöjnings-området. Blyttia. 1988;46:65–73. [Google Scholar]
- Jonsell B, Karlsson T. Endemic vascular plants in Norden. In: Jonsell B, editor. Flora Nordica – General Volume. Stockholm: Royal Swedish Academy of Sciences; 2004. pp. 139–159. [Google Scholar]
- Kadereit JW, Goldner H, Holstein N, Schorr G, Zhang L-B. The stability of Quaternary speciation: a case study in Primula sect. Auricula. Alpine Botany. 2011;121:23–35. [Google Scholar]
- Koch MA, Kiefer C, Ehrich D, Vogel J, Brochmann C, Mummenhoff K. Three times out of Asia Minor: the phylogeography of Arabis alpina L. (Brassicaceae) Molecular Ecology. 2006;15:825–839. doi: 10.1111/j.1365-294X.2005.02848.x. [DOI] [PubMed] [Google Scholar]
- Kolář F, Štech M, Trávníček P, et al. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Annals of Botany. 2009;103:963–974. doi: 10.1093/aob/mcp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolář F, Fér T, Štech M, et al. Bringing together evolution on serpentine and polyploidy: spatiotemporal history of the diploid–tetraploid complex of Knautia arvensis (Dipsacaceae) PLoS One. 2012;7:e39988. doi: 10.1371/journal.pone.0039988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutecký P, Tuleu G, Baďurová T, Košnar J, Štech M, Těšitel J. Distribution of cytotypes and seasonal variation in the Odontites vernus group in central Europe. Preslia. 2012;84:887–904. [Google Scholar]
- Krendl F. Chromosomenzahlen und geographische Verbreitung in der Gattung Galium (Sect. Leptogalium – Rubiaceae) Biosystematics and Ecology Series. 1993;4:51–112. [Google Scholar]
- Leong-Škorničková J, Šída O, Jarolímová V, et al. Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae) Annals of Botany. 2007;100:505–526. doi: 10.1093/aob/mcm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepš J, Šmilauer P. Multivariate analysis of ecological data using CANOCO. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–687. [PubMed] [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Annals of Botany. 2006;98:679–689. doi: 10.1093/aob/mcl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Annals of Botany. 2007;100:875–888. doi: 10.1093/aob/mcm152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Trávníček P, Rauchová J, et al. The use of flow cytometry in the biosystematics, ecology and population biology of homoploid plants. Preslia. 2010;82:3–21. [Google Scholar]
- Löve Á, Löve D. North Atlantic biota and their history. New York: Pergamon Press; 1963. [Google Scholar]
- McIntyre PJ. Polyploidy associated with altered and broader ecological niches in the Claytonia perfoliata (Portulacaceae) species complex. American Journal of Botany. 2012;99:655–662. doi: 10.3732/ajb.1100466. [DOI] [PubMed] [Google Scholar]
- Mehlich A. Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis. 1984;15:1409–1416. [Google Scholar]
- Noor MAF. Evolutionary biology: genes to make new species. Nature. 2003;423:699–700. doi: 10.1038/423699a. [DOI] [PubMed] [Google Scholar]
- Olšavská K, Perný M, Španiel S, Šingliarová B. Nuclear DNA content variation among perennial taxa of the genus Cyanus (Asteraceae) in Central Europe and adjacent areas. Plant Systematics and Evolution. 2012;298:1463–1482. [Google Scholar]
- Parducci L, Jørgensen T, Tollefsrud M, et al. Glacial survival of boreal trees in northern Scandinavia. Science. 2012;335:1083–1086. doi: 10.1126/science.1216043. [DOI] [PubMed] [Google Scholar]
- Parisod C, Besnard G. Glacial in situ survival in the Western Alps and polytopic autopolyploidy in Biscutella laevigata L. (Brassicaceae) Molecular Ecology. 2007;16:2755–2767. doi: 10.1111/j.1365-294X.2007.03315.x. [DOI] [PubMed] [Google Scholar]
- Preston CD, Hill MO. The geographical relationships of British and Irish vascular plants. Botanical Journal of the Linnean Society. 1997;124:1–120. [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Schneeweiss GM, Schönswetter P. A re-appraisal of nunatak survival in arctic–alpine phylogeography. Molecular Ecology. 2011;20:190–192. doi: 10.1111/j.1365-294x.2010.04927.x. [DOI] [PubMed] [Google Scholar]
- Schönswetter P, Paun O, Tribsch A, Niklfeld H. Out of the Alps: colonization of Northern Europe by East Alpine populations of the Glacier Buttercup Ranunculus glacialis L. (Ranunculaceae) Molecular Ecology. 2003;12:3373–3381. doi: 10.1046/j.1365-294x.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Schönswetter P, Suda J, Popp M, Weiss-Schneeweiss H, Brochmann C. Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Molecular Phylogenetics and Evolution. 2007;42:92–103. doi: 10.1016/j.ympev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Skrede I, Eidesen P, Portela RP, Brochmann C. Refugia, differentiation and postglacial migration in arctic–alpine Eurasia, exemplified by the mountain avens (Dryas octopetala L.) Molecular Ecology. 2006;15:1827–1840. doi: 10.1111/j.1365-294X.2006.02908.x. [DOI] [PubMed] [Google Scholar]
- Slovák M, Vít P, Urfus T, Suda J. Complex pattern of genome size variation in a polymorphic member of the Asteraceae. Journal of Biogeography. 2009;36:372–384. [Google Scholar]
- Snogerup B. Northwest European taxa of Odontites. Annales Botanici Fennici. 1983;124:1–62. [Google Scholar]
- Solstad H, Elven R, Nordal I. Isozyme variation among and within North Atlantic species of Papaver sect. Meconella (Papaveraceae) and taxonomic implications. Botanical Journal of the Linnean Society. 2003;143:255–269. [Google Scholar]
- Soltis DE, Buggs RJA, Doyle JJ, Soltis PS. What we still don't know about polyploidy. Taxon. 2010;59:1387–1403. [Google Scholar]
- Sonnleitner M, Flatscher R, García PE, et al. Distribution and habitat segregation on different spatial scales among diploid, tetraploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps. Annals of Botany. 2010;106:967–977. doi: 10.1093/aob/mcq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlberg D, Hedrén M. Evolutionary history of the Dactylorhiza maculata polyploid complex (Orchidaceae) Biological Journal of the Linnean Society. 2010;101:503–525. [Google Scholar]
- Steen SW, Gielly L, Taberlet P, Brochmann C. Same parental species, but different taxa: molecular evidence for hybrid origins of the rare endemics Saxifraga opdalensis and S. svalbardensis (Saxifragaceae) Botanical Journal of the Linnean Society. 2000;132:153–164. [Google Scholar]
- Sterner R. Galium pumilum Murr. i nordvästra Europa. Meddelanden Goteborgs Botaniska Trädgård. 1944;15:187–233. [Google Scholar]
- Sterner R. Ölands kärlväxtflora. Lund: Svensk Botanisk Tidskrift; 1986. [Google Scholar]
- Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GM, Trávníček P, Schönswetter P. Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plant Senecio carniolicus (Asteraceae) American Journal of Botany. 2007;94:1391–1401. doi: 10.3732/ajb.94.8.1391. [DOI] [PubMed] [Google Scholar]
- Tichý L. JUICE, software for vegetation classification. Journal of Vegetation Science. 2002;13:451–453. [Google Scholar]
- Van Dijk P, Bakx-Schotman T. Chloroplast DNA phylogeography and cytotype geography in autopolyploid Plantago media. Molecular Ecology. 1997;6:345–352. [Google Scholar]
- Van Rossum F, Prentice HC. Structure of allozyme variation in Nordic Silene nutans (Caryophyllaceae): population size, geographical position and immigration history. Biological Journal of the Linnean Society. 2004;81:357–371. [Google Scholar]
- Van Rossum F, De Bilde J, Lefebvre C. Barriers to hybridization in calcicolous and silicicolous populations of Silene nutans from Belgium. Belgian Journal of Botany. 1996;129:13–18. [Google Scholar]
- Van Rossum F, Meerts P, Gratia E, Tanghe M. Ecological amplitude in Silene nutans in relation to allozyme variation at the western margin of its distribution. Journal of Vegetation Science. 1999;10:253–260. [Google Scholar]
- Weiss-Schneeweiss H, Blöch C, Turner B, Villaseñor JL, Stuessy TF, Schneeweiss GM. The promiscuous and the chaste: frequent allopolyploid speciation and its genomic consequences in American daisies (Melampodium sect. Melampodium; Asteraceae) Evolution. 2012;66:211–228. doi: 10.1111/j.1558-5646.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- Westergaard KB, Alsos IG, Popp M, Engelskjøn T, Flatberg KI, Brochmann C. Glacial survival may matter after all: nunatak signatures in the rare European populations of two west-arctic species. Molecular Ecology. 2011;20:376–393. doi: 10.1111/j.1365-294X.2010.04928.x. [DOI] [PubMed] [Google Scholar]
- Záveská E, Fér T, Šída O, Leong-Škorničková J, Sabu M, Marhold K. Genetic diversity patterns in Curcuma reflect differences in genome size. Botanical Journal of the Linnean Society. 2011;165:388–401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.