Abstract
Background and Aims
Convergent evolution is invoked to explain similarity between unrelated organisms in similar environments, but most evaluations of convergence analyse similarity of organismal attributes rather than of the environment. This study focuses on the globular succulent plants of the Americas, the cacti, and their counterparts in Africa in the ice-plant, spurge and milkweed families. Though often held up as paragons of convergent morphological evolution, the environmental similarity of these plants has remained largely unexamined from a quantitative perspective.
Methods
Five hotspots (centres of high species diversity of globular succulents) were selected, two in Mexico and three in South Africa. Their environments were compared using niche modelling tools, randomization tests of niche similarity and multivariate analyses to test for environmental similarity.
Key Results
Although the sites selected have ‘similar’ but unrelated life forms, almost all our results highlighted more climate differences than similarities between the hotspots. Interprediction of niches within and between continents, a niche equivalence test, and MANOVA results showed significant differences. In contrast, a niche similarity test showed that the comparisons of Cuatrociénegas–Richtersveld, Huizache–Knersvlakte and Huizache–Richtersveld were similar.
Conclusions
Differences in rainfall and temperature regimes and the potential effect of edaphic factors may be involved in the differences between the hotspots. In addition, differences in structure, morphology and physiology of the globular succulents may coincide with some of the climatic dissimilarities; i.e. given convergence as the evolution of similar morphologies under similar conditions, then it may be that differing environments diagnose inconspicuous morphological differences. Moreover, although fine-scale differences between sites were found, a coarser perspective shows that these sites are clearly similar as drylands with relatively moderate drought and mild temperatures, illustrating how all studies of convergence must address the issue of how similar two entities must be before they are considered convergent.
Keywords: Adaptation, convergent evolution, environmental similarity, niche modelling, succulent plants
INTRODUCTION
Convergent evolution is a central topic in comparative biology because it is often taken as evidence of adaptation by natural selection (Harvey and Pagel, 1991; Larson and Losos, 1996). Similar attributes in unrelated groups reflect adaptative responses to similar environmental pressures, even though the initial ancestral states were different (Orians and Paine, 1983; Stearns and Hoekstra, 2000; Revell et al., 2007). Convergent evolution has been documented in many cases, including classic examples involving morphological, ecological and behavioral similarities between placental and marsupial ‘wolves’ (Werdelin, 1986; Wroe et al., 2007), the similar wing shape and size of bats and birds (Norberg, 1981), or the morphological similarity between the cacti of the Americas and spurges and milkweeds of Africa (Peet, 1978; Bennici, 2003). Because the central prediction of convergence is similar organismal structure and function in similar environmental contexts, it is crucial to evaluate the similarity of the environment just as carefully as organismal similarity.
Most convergence studies have focused on biological attributes as opposed to environmental ones, including morphology (Losos, 1992; Stayton, 2005), community structure (Parsons and Moldenke, 1975; Esler and Rundel, 1999; Melville et al., 2005), physiology (Reich et al., 1997; Meinzer, 2003) and species diversity (Schluter and Ricklefs, 1993). All these studies have provided useful information to understand convergence (Cody and Mooney, 1978; Esler and Rundel, 1999), but because they included only qualitative descriptions of the environment or coarse climatic measurements, they have left untested the crucial assumption of similar environmental pressures.
Newly available climate data allow examination of the environmental aspect of convergence predictions. Convergence in climatic conditions can be interpreted as overlap in environmental space; in contrast, divergence can be interpreted as different areas in environmental space. These spaces can be estimated from layers of climate variables, reflecting how similar the entities are in their environmental requirements. Environmental information now available (Hijmans et al., 2005) and the tools of geographic information systems (GIS) and species distribution modelling (SDM) make it possible to quantify environmental space for virtually any point on Earth, and to quantify and evaluate their similarities and differences at fine resolution in numerous variables (Kozak et al., 2008; Warren et al., 2008). These tools are able not only to compare climatic factors quantitatively, but they can also help identify environmental factors potentially driving the evolution of a given morphological trait (Kozak et al., 2008), including in a convergent fashion. By finding that dissimilar selective pressures lead to similar morphologies may help reject a hypothesis of convergence (Peet, 1978; Melville et al., 2005).
To test the hypothesis of convergence in environmental requirements quantitatively, we have selected the classic example of apparent convergent evolution between the succulent plants of the American arid regions, the cacti, and their distantly related African analogues, the milkweeds (Apocynaceae), spurges (Euphorbiaceae) and ice-plants (Aizoaceae). This case has illustrated convergent evolution in countless publications for over 100 years (a small selection of examples is presented in Table 1), but quantitative comparisons have been rare and based mainly on morphology rather than environment (Orians and Solbrig, 1977; Felger and Henrickson, 1997; Trager, 1985; Mauseth, 2004). Selection favouring low surface : volume ratios is universally invoked as a potential driving factor in the evolution of succulents. In dry and warm areas, structures with high water storage volume and low surface area across which water can be lost are thought to be favoured (Warming, 1909; Glass and Foster, 1975; Gibson and Nobel, 1986; Mauseth, 2000; Ogburn and Edwards, 2010). The apogee of this tendency is plants that approximate spheres, the shape with maximal volume per unit surface area.
Table 1.
List of selected authors citing the example of convergent evolution between American and African succulent plants
| Warming, 1909. See also Glass and Foster, 1975 | ‘The most common and extreme types are Cactaceae in America, Stapelia in South Africa, and species of Euphorbia which occur mainly in Africa. In the various genera there occur a series of shapes whose efficiency has been demonstrated …. Frequent among such shapes are those like the sphere, prism, or cylinder that combine smallness of surface with largeness of volume.’ |
| Benson, 1969, p. 3. See also Trager, 1985; Wyk and Smith, 2001. p 61 | ‘Cactus stems are succulent …. However, some plants of other families have succulent stems or leaves, and some even have the appearance of cacti. For example, the African succulent spurges (Euphorbia) seem almost identical with some cacti.’ |
| Jacobsen, 1977, p. 21 | ‘In the deserts and semideserts of the American continent, the members of the Cactaceae are the outstanding examples of adaptation to the environment. Here we find shrubby species, columnar plants and globular types. Wherever similar conditions prevail in the Old World, it will be noticed that plants from many different families have been compelled to make these modifications.’ |
| Rauh and Kendall, 1984. p 13. See also Bruyns, 2005 | ‘… the growth forms of cacti, indigenous exclusively to the New World, under similar climatic conditions are duplicated as convergents in plant groups of the Old World. This is true not only of the common columnar form but also of the considerably rarer extreme-spherical forms.’ |
| Gibson and Nobel, 1986. p. 12. See also Stearns and Hoekstra, 2000, p. 236; Futuyma, 2009, p. 134 | ‘In fact, throughout this century evolutionists have commonly cited the similarity of the stem succulent cacti of the Western Hemisphere and the succulent, cactus-like euphorbs of Africa as an example of convergent evolution.’ |
| Raven et al., 1986, p. 443 | ‘Native cacti … occur exclusively in the New World. The comparably fleshy members of the spurge and milkweed families occur mainly in desert regions in Asia and especially Africa, where they play an ecological role similar to that of the New World cacti.’ |
| Biesboer and Koukkari, 1992 | ‘Chief interest has focused on the many unusual succulent species found in the Old World. Many of these forms have converged morphologically to resemble the Cactaceae. As in the cacti, Euphorbia illustrates almost spherical forms, ridged axes, cylindrical forms, coralline forms, dwarf and arborescent forms and are often well-armed with thorns.’ |
| Ezcurra et al., 2006, p. 17 | ‘The survival strategies of desert plants present some of the most striking cases in nature of evolutionary convergence …. Such is the case of the succulent cactoid growth form, evolved in Africa from the families Euphorbiaceae, Asclepediaceae and Aizoaceae, and in the Americas from the family Cactaceae.’ |
| Mauseth, 2004. See also von Willert et al., 1992, p. 23 | ‘Succulent tissues permit certain plants to survive in arid habitats. Examples of plants with stem succulence …. occur in a number of unrelated families. Among dicots, Cactaceae and Euphorbiaceae are perhaps the most familiar examples, but stem succulence also occurs in Apocynaceae …’ |
| Eggli and Nyffeler, 2009. See also Hearn, 2009 | “The ‘cactus form’, i.e. spiny succulent photosynthetic stems such as those of New World cacti (Cactaceae) and Old World euphorbs (Euphorbiaceae), is the commonly cited textbook example for convergent adaptive evolution” |
| Ogburn and Edwards, 2009. See also Ogburn and Edwards, 2010 | ‘Characteristics such as stem based photosynthesis and a concomitant reduction of leaves, stem succulence, spines, and crassulacean acid metabolism (CAM) photosynthesis are all considered to be adaptations of cacti to water-limited environments (Gibson and Nobel, 1986), a supposition that is supported by the independent acquisition of different combinations of these traits in unrelated lineages that are also drought adapted (e.g. Euphorbiaceae, Agavaceae, Aizoaceae).’ |
| Arakaki et al., 2011 | ‘Although some 30 plant lineages have been classified as succulent, only a small subset of those are species-rich and ecologically important elements of arid and semiarid ecosystems worldwide. These lineages include the ice-plants … , the spurges … , the stapeliads … and especially the cacti.’ |
| McGhee, 2011, p. 113–114 | ‘Plants of widely differing phylogenetic lineages have repeatedly and convergently evolved succulent stems and leaves … such as the Cactaceae (cactuses), Euphorbiaceae (spurges), Aizoaceae (stone plants), and Crassulaceae (jade plants) …. The most spectacular examples of the convergent evolution of identical morphological structures to prevent dehydration are the desert adapted cactuses of the Western Hemisphere and spurges of the Eastern Hemisphere. |
Therefore, to test the hypothesis of climatic convergence, we selected the globular succulents that are found in Cactaceae, Euphorbiaceae and Apocynaceae, which are derived from stems, and those in Aizoaceae, which are derived from leaves (Fig. 1). From different ancestral states unrelated lineages have arrived at similarly globular morphologies under putatively similar selective environments. We selected succulents in general and globular ones in particular as a study system because of a remarkable unanimity in the literature regarding the selective factors behind their evolution. We are aware of no factor other than selection favouring low surface : volume ratios ever having been proposed to explain the spherical bodies of the cacti, ice-plants, spurges and milkweeds, despite their differing anatomical constructions. As a result, the cacti and Old World globular succulents represent an ideal model to test the assumption of environmental similarity implicit in the unchallenged assumption that the globular succulents represent similar responses to similar selection pressures.
Fig. 1.
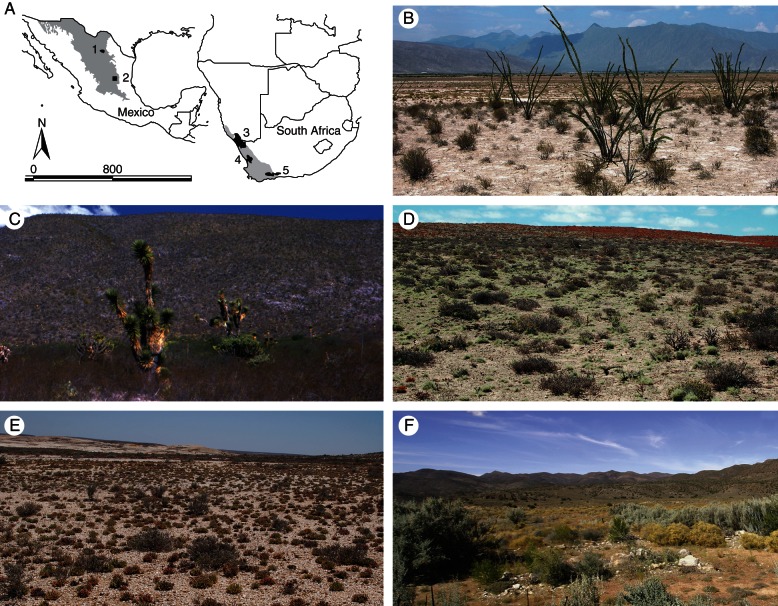
Succulent globular plants from the sites studied in Mexico and South Africa. (A–F) Mexican plants from El Huizache (A–C) and Cuatrociénegas (D–F): (A) Lophophora williamsii; (B) Epithelantha micromeris; (C) Astrophytum myriostigma; (D) Echinocereus pectinathus; (E) Mammillaria compressa; and (F) Echinocactus horizonthalonius. (G–L) South African plants from the Richtersveld (G and H), the Knersvlakte (I and J) and the Little Karoo (K and L): (G) Larryleachia cactiformis; (H) Lithops herrei; (I) Euphorbia fasciculata; (J) Conophytum calculus; (K) Euphorbia susannae; and (L) Gibbaeum heathii. Scale bars = 2 cm. Photographs: (B, F) C. Gómez, (G) J. Trager.
We present the first quantitative attempt to evaluate the pervasive assumption of convergence among globular succulent plants from a bioclimatic perspective. We selected the succulent communities of Cuatrociénegas and El Huizache in the Mexican Chihuahuan Desert, and the Knersvlakte, the Little Karoo and the Richtersveld in the Succulent Karoo, South Africa, because these areas have been singled out as the global hotspots of globular succulent plant diversity (Olson and Dinerstein, 1998; Cowling and Hilton-Taylor, 1999; Hernández et al., 2001; Wyk and Smith, 2001; see Fig. 2). In the terms of the methods used here, our prediction is that areas with high species diversity of putatively similar globular succulents should overlap in environmental space. If similar sets of environmental pressures favour similar growth forms, then the areas of highest diversity of similar globular succulents should diagnose areas of potential climatic similarity. The sites we compared in Mexico and South Africa together support the highest diversity known of globular succulents (Fig. 1). In addition to species diversity, the hotspots are also areas of some of the greatest globular succulent abundance. Although some globular taxa are soil specific (Schmiedel and Jürgens, 1999, 2004), to our knowledge, all hypotheses that have ever been presented to explain the similarity in forms between the succulents of different families on different continents involve the assumption of similar environmental conditions (Table 1; Cowling et al., 1986; Esler et al., 1999; Hernández et al., 2001; Schmiedel et al., 2010). Through our analyses we show how the use of GIS and SDM tools is ideal for detailed evaluation of environmental similarity between globular succulent hotspots in Mexico and southern Africa and discuss reasons for the overlap and lack thereof in our climate data. We conclude with points highlighted by our study that are of relevance to studies of convergence in general.
Fig. 2.
(A) Selected globular succulent hotspots in Mexico (1, 2) and southern Africa (3–5). (B) Cuatrociénegas, Mexico; (C) El Huizache, Mexico; (D) Richtersveld, southern Africa; (E) Knersvlakte, southern Africa; and (F) Little Karoo, southern Africa. Photographs: (B, C) C. Gómez.
MATERIALS AND METHODS
Study sites
To test the often-repeated assumption of environmental similarity between the sites of globular succulent occurrence (Table 1), we selected the main areas perennially singled out as the global hotspots of succulent diversity. We selected these hotspots given that globular succulent species diversity is distributed highly unevenly across the world (Hernández et al., 2001; Wyk and Smith, 2001; Hernández and Gómez-Hinostrosa, 2011), and it seems reasonable to expect that the areas of highest species diversity and abundance of globular succulents should coincide with areas of maximal environmental similarity. Two of these hotspots were in the Chihuahuan Desert, Mexico (MacMahon and Wagner, 1986; Hernández and Bárcenas, 1995; Arriaga et al., 2000; Hernández et al., 2001), and three were in the Succulent Karoo of South Africa (Olson and Dinerstein, 1998; Cowling and Hilton-Taylor, 1999; Wyk and Smith, 2001; Fig. 2; Supplementary Data 1). In Mexico, we selected the two extraordinarily cactus-rich regions known as Cuatrociénegas and El Huizache, both of which in sheer species number far exceed any other similar-sized tracts of the Americas (Desmet and Cowling, 1999; Dinerstein et al., 2000; Hernández et al., 2001). Our delimitation of these areas follows Arriaga et al. (2000) and Hernández et al. (2001). In South Africa, we selected the globular succulent hotspots known as the Richtersveld, Knersvlakte and Little Karoo (Cowling et al., 1998; Wyk and Smith, 2001; Schmiedel and Jürgens, 2004) as delimited by the Succulent Karoo Ecosystem Program (Driver et al., 2003). Although the Chihuahuan and Karoo deserts have contrasting rainfall periods, with summer versus winter rainfall, respectively (Hernández and Bárcenas, 1995; Desmet and Cowling, 1999; Wyk and Smith, 2001), their strikingly similar globular succulents (Fig. 1) and high globular species diversity has prompted frequent comparisons with American succulents and claims of convergence (Cowling, 1986; von Willert et al., 1992; Wyk and Smith, 2001).
Environmental variables, species occurrence localities, and species selection
To characterize the environmental spaces of the globular centres, we used 19 bioclimatic variables (Supplementary Data 2) from WorldClim (Hijmans et al., 2005), a global set of climate layers generated by interpolation of climate data from weather stations. The raster grids of the variables employed here had a spatial resolution of 30' (approx. 1 km2 resolution). We selected this environmental information because, in the literature, the geographic distribution, growth forms, reproduction and establishment of succulent plants are mainly attributed to climatic conditions to an overwhelming degree as compared with other factors such as soils or biological interactions (Cowling, 1986; Nobel et al., 1986; Cody, 1991; von Willert et al., 1992; Cowling and Hilton-Taylor, 1999; Hernández et al., 2001; Schmiedel et al., 2010), and because the fine resolution of these layers allowed a detailed assessment of the environmental characteristics of the globular hotspots.
To extract climatic information, we obtained occurrence localities from the BOL, HTN, MEXU and NGB herbaria (Holmgren et al., 1990) for each globular succulent species in each hotspot, and from fieldwork in the Knersvlakte and Richtersveld, South Africa, and El Huizache, Mexico. We also consulted the Pretoria Computerized Information Systems database of the South African National Biodiversity Institute and the Database of Cactus Collections from North and Central America (Hernández and Gómez-Hinostrosa, 2011). We compiled a total of 498 locality records for 89 South African species, and 821 records for 57 Mexican taxa (Supplementary Data 3). To reduce the effects of sampling bias, we removed the localities with similar co-ordinates to obtain unique records, and we ensured that the localities fell mainly in distinct grids of the bioclimatic layers.
We selected the globular succulent species based on the examples illustrated in numerous publications (Table 1). Globular succulents are found in several families, but mainly in Cactaceae, Euphorbiaceae, Apocynaceae and Aizoaceae. Regardless of the construction of the stem or leaf, selection in dry habitats favouring minimal surface area is always invoked in explaining why these species have their spherical shape (Glass and Foster, 1975; Gibson and Nobel, 1986; Mauseth, 2000; McGhee, 2011). These globular groups allowed us to test the expectation that similar globular morphologies should be found in similar selective environments. We selected the species with vegetative structures consisting of succulent organs, which are individually described as globular, semiglobular, spherical or subspherical, those with similarly wide and tall dimensions, or those wider than tall for extended periods of their life cycle. We identified species as meeting this definition based on taxonomic descriptions, specimen label information and field observations.
Modelling algorithm
A hypothesis of convergent evolution assumes that similar environmental pressures should result in similar organismal responses. We built our analyses around this assumption, which implies that the globular succulent hotspots around the world should reflect similar climate conditions. Environmental niche models are typically used to model the potential distribution of a single species, but for our question the appropriate sampling scheme was to use occurrence data for all globular species within each hotspot as the basis for our models.
We built these models using the program MaxEnt v. 3·2.1. (Phillips et al., 2006), which models based on presence records only and samples ‘pseudo-absences’ to calculate the model performance measures, making it appropriate for our study, which included only presence data. We used 70 % of records as training data and 30 % for testing the model. We constructed the models using the 19 sets of bioclimatic layers, and we also applied a principal component analysis (PCA) to the set of layers in ArcGIS 9·3 (ESRI, 2006). Although the models with the complete dataset were very similar to those generated using the PCA layers, we present results based only on the first three PCA-reduced variables (Table 2) because these reduced layers helped to compensate for possible overparameterization of models caused by multicollinearity. Moreover, we performed the statistical comparisons between sites with only the reduced dataset due to the extensive computational time required in the performance of the tests (see next section). The models generated by the complete dataset are available from the first author. We used default settings for all analyses to measure the degree to which the models differed from random using the AUC, the area under the receiver operating characteristic curve. The AUC scores are interpreted as reflecting the ability of the model to distinguish presence data from background data (Phillips et al., 2006).
Table 2.
The first three axes of the PCA of bioclimatic variables resulting from ArcGIS analysis
| PCA1: 10 = mean temperature of warmest quarter, 17 = precipitation of driest quarter |
| PCA2: 4 = temperature seasonality, 5 = max temperature of warmest month, 8 = mean temperature of wettest quarter |
| PCA3: 3 = isothermality, 9 = mean temperature of driest quarter, 13 = precipitation of wettest month, 14 = precipitation of driest month, 18 = precipitation of warmest quarter |
Tests of environmental similarity
To examine the assumption of environmental similarity between the globular hotspots of Mexico and southern Africa, we first compared the maps resulting from the MaxEnt model outputs from one globular centre against the other hotspots between continents. These comparisons gave us a starting point to evaluate the potential similarity or dissimilarity between the globular succulent centres.
Our following step was to quantify the environmental overlap between sites using the niche equivalence and similarity tests of Warren et al. (2008), expressing the results of both tests via Shoener's D score (Schoener, 1968; Warren et al., 2008), in which similarity is scaled from 0 (no overlap) to 1 (complete overlap). We used ENMTools v.1·3 (Warren et al., 2008, 2010) to implement both tests. Calculating D requires two key items of information, the modelled distribution area, i.e. preferred climate conditions of globular succulents of one area (say, Cuatrociénegas) and the model distribution of another area (e.g. the Knersvlakte). These models are expressed as probabilities, understood as the probability of finding suitable conditions for globular succulents at any given pixel within the area being examined, given how suitable or not the climate at the given pixel is. For a given site (e.g. Cuatrociénegas), we can therefore compare the probability of finding globular succulents for each pixel given the ‘native’ model based on the distribution of cacti at Cuatrociénegas px,i and compare the probability of finding globular succulents in those same pixels but using the ‘foreign’ model generated from the succulents occurring at another site, such as the Knersvlakte py,i. As implemented by ENMTools, px,i and py,i are divided by the total number of pixels so that over all px,i and py,i both sum to unity. The absolute value of the differences between px,i and py,i for a given pixel thus gives an index of how similar the climate is in that pixel with respect to globular succulents and, by summing all of them, we obtain a rough comparison of climate similarity between two sites.
For the equivalence test, we generated two distribution models based on randomizing a pooled occurrence dataset from a pair of sites. This process generates two datasets of the same size as the original ones. For each dataset, ENMTools uses MaxEnt to project the model distribution to each globular centre and, subsequently, it calculates the D statistic based on the predicted suitability scores for each pixel. We repeated this process 100 times to create a null distribution against which to compare the observed D scores. The hypothesis of equivalence was rejected when the observed values for D were significantly lower than the values expected from the critical values of the null distribution datasets (0·01) and was not when the observed values fell within those expected from the null distribution datasets. The preceding test is appealing because it is simple and similar to other comparison tests (e.g. the incongruence length difference test of molecular phylogenetics; Farris et al., 1995) and therefore provides a familiar metric by which to compare datasets. However, unless the exact points where the organisms were found in both sites are very similar climatically, the test will tend to reject the notion that the sites are identical.
The so-called similarity test is somewhat less stringent. The similarity test compares the observed values of D with those calculated from the niche model generated from the occurrence data at one hotspot with the model generated from points selected at random from within the other hotspot. In this way, we can see whether the climate of one area is statistically indistinguishable with that obtaining in the general area (as opposed to that in the exact points of occurrence) of the other hotspot. There are many possible ways of defining ‘similar’, and, like the previous one, this test offers a similarity index that is readily understood and is of familiar structure. We sampled at random from within the distribution of the ‘other’ hotspot by constructing a mask layer based on the minimum training presence value from the model predicting areas of each centre (Table 3), and we delimited the predictions using the biogeographical limits for each hotspot. We repeated this entire procedure 100 times for each site and in two directions, from site A to site B and vice versa. The expected null distribution of D scores was compared with those from the actual dataset in a two-tailed test. The hypothesis of similarity was rejected when the observed values for D were significantly lower than the critical values of the null distribution (0·01) and was not when the observed values were higher than expected from the null distribution datasets.
Table 3.
MaxEnt niche modelling results
| Location | Points | AUC test/training | Minimum training presence: logistic threshold |
|---|---|---|---|
| Knersvlakte | 126 | 0·993/0·994 | 0·101 |
| Little Karoo | 99 | 0·952/0·942 | 0·092 |
| Richtersveld | 127 | 0·972/0·970 | 0·230 |
| Cuatrociénegas | 33 | 0·994/0·994 | 0·012 |
| El Huizache | 126 | 0·987/0·976 | 0·027 |
The points column gives the number of localities used for analyses. The area under the curve (AUC) values are a measure of quality of the models. The minimum training presence shows the values used to construct the mask limits used for the similarity test.
We also tested for significant differences between the areas using a multivariate analysis of variance (MANOVA) to compare the estimates of the mean for each environmental variable in each globular centre. Instead of using information extracted from MaxEnt analyses, we extracted climate data for specimen localities directly from the Bioclim layers. We again conducted a PCA of the set of 19 climatic variables. All subsequent analyses were based on the first three PCA axes only (Table 4). Our data violated normality and homoscedasticity assumptions, so we performed a non-parametric MANOVA on the log10-transformed PCA dataset (Anderson, 2001). We performed a series of Kruskal–Wallis tests to assess which PC accounted for the overall difference detected by the non-parametric MANOVA, followed by a Behrens–Fisher post hoc test. We used the statistical software R (R Development Core Team, 2009) to perform these analyses.
Table 4.
The first three axes of the PCA from the climate data extracted for specimen localities directly from the Bioclim layers
| PCA1: 1 = annual mean temperature, 9 = mean temperature of driest quarter, 10 = mean temperature of warmest quarter, 11 = mean temperature of coldest quarter, 12 = annual precipitation, 16 = precipitation of wettest quarter, 19 = precipitation of coldest quarter |
| PCA2: 2 = mean diurnal range, 3 = isothermality, 4 = temperature seasonality, 7 = temperature annual range |
| PCA3: 15 = precipitation seasonality, 13 = precipitation of wettest month |
RESULTS
PCA MaxEnt models
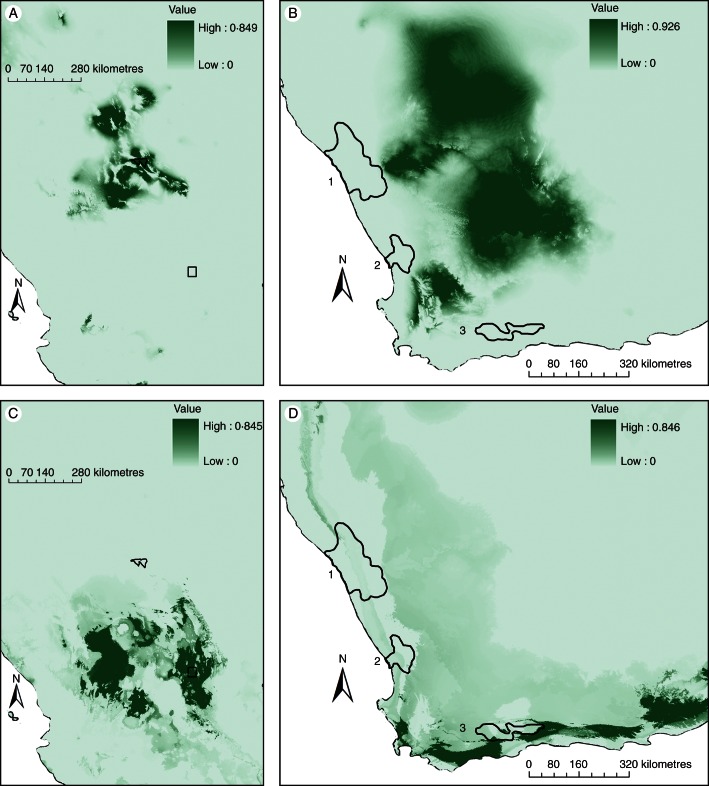
The model output for each succulent centre, based on analysis of the first three PCA axes which explained >80 % of the variance, showed AUC values indicating acceptable predictivity (Table 3). To test our hypothesis of environmental convergence between continents, we modelled the Mexican globular sites El Huizache and Cuatrociénegas and then we projected these models to the selected regions of southern Africa (Fig. 3). Our model projection from Cuatrociénegas to the African centres failed to corroborate the environmental similarity with South African hotspots (Fig. 3A, B), falling in the Western Cape region known as the Nama Karoo and extending to southern Namibia and Botswana. Our projection of the El Huizache model to South Africa predicted the southern portions of the Little Karoo and extended to the southern coast of South Africa (Fig. 3C, D), but failed to predict the two other centres.
Fig. 3.
PCA model building and projection for Cuatrociénegas and El Huizache, Mexico: (A) predictive model for the species of Cuatrociénegas; (B) model projection from Cuatrociénegas, Mexico to southern Africa; (C) predictive model for the species of El Huizache; (D) model projection from El Huizache to southern Africa. Shading indicates levels of model prediction, with scales as shown. Numbers and lines on the map of southern Africa denote (1) Richtersveld, (2) Knersvlakte and (3) Little Karoo.
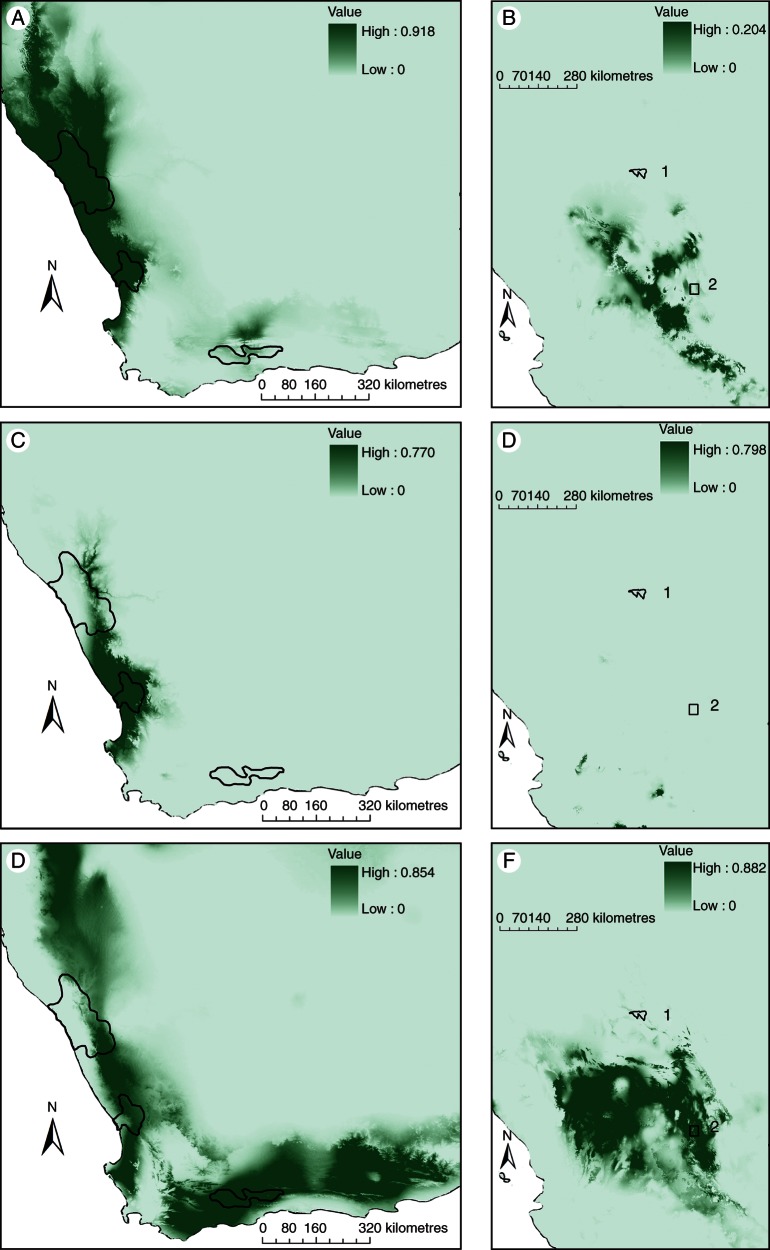
In turn, we projected the South African models to Mexico (Fig. 4). Here again, model projections from the Richtersveld and Knersvlakte failed to predict the Mexican hotspots, falling instead in small areas of the southern part of the Chihuahuan Desert and Balsas Depression respectively (Fig. 4A–D). The Little Karoo was the only comparison congruent with our main hypothesis of similarity, predicting the southern portion of the Chihuahuan and El Huizache region with striking similarity (Fig. 4E, F).
Fig. 4.
PCA model building and projection for the Richtersveld, Knersvlakte and Little Karoo, South Africa: (A) predictive model for the species of the Richtersveld; (B) model projection from the Richtersveld to Mexico; (C) predictive model for the species of the Knersvlakte; (D) model projection from the Knersvlakte to Mexico; (E) predictive model for the species of the Little Karoo; (F) model projection from the Little Karoo to Mexico. Shading indicates levels of model prediction, with scales as shown. Numbers on the map of Mexico denote (1) Cuatrociénegas (polygon) and (2) Huizache (square). Areas outlined on the map of southern Africa as is indicated in Fig. 3.
Niche equivalence test
The so-called equivalence test (Warren et al., 2008) evaluates the hypothesis that there are no significant environmental differences between the entities compared based on the climate data obtained from the exact points of occurrence of the species studied within each hotspot. The test rejected the null hypothesis of equivalence among the compared sites (Table 5).
Table 5.
Tests of niche equivalence
| Locations | na/nb | Schoener's D | P-value |
|---|---|---|---|
| Knersvlakte–Cuatrociénegas | 33/102 | 0·022 | 0·01 |
| Little Karoo–Cuatrociénegas | 33/89 | 0·028 | 0·01 |
| Richtersveld–Cuatrociénegas | 33/102 | 0·047 | 0·01 |
| Knersvlakte–El Huizache | 126/102 | 0·056 | 0·01 |
| Richtersveld–El Huizache | 126/111 | 0·072 | 0·01 |
| Little Karoo–El Huizache | 126/89 | 0·355 | 0·01 |
All pairs of comparisons between hotspots suggested that they are ecologically distinct (P ≤ 0·01). The values for D metrics are given for the PCA sets of layers. na and nb values are sample sizes for the first and second hotspot, respectively.
Niche similarity test
The similarity test evaluates whether the model from one area differs significantly from that generated based on a sampling of random points from another hotspot. The sites compared are considered similar only if the D-values are higher than the critical values of the null distributions (Warren et al., 2008, 2010). The paired comparisons that were considered similar were Cuatrociénegas–Richtersveld, Huizache–Knersvlakte and Huizache–Richtersveld (Table 6).
Table 6.
Tests of niche similarity
| Locations | na/nb | Schoener's D | Pa value | Pb value |
|---|---|---|---|---|
| Cuatrociénegas–Knersvlakte | 33/102 | 0·022 | ns | ns |
| Cuatrociénegas–Little Karoo | 33/89 | 0·028 | ≤0·01 L | ≤0·01 S |
| Cuatrociénegas–Richtersveld | 33/102 | 0·047 | ≤0·01 L | ≤0·01 L |
| El Huizache–Knersvlakte | 126/102 | 0·056 | ≤0·01 L | ≤0·01 L |
| El Huizache–Richtersveld | 126/111 | 0·072 | ≤0·01 L | ≤0·01 L |
| El Huizache–Little Karoo | 126/89 | 0·355 | ≤0·01 S | ≤0·01 L |
Results for Schoener's D metrics are given for the PCA sets of layers for all pairs of comparisons between hotspots. na and nb values are sample sizes for the first and second site. Results are given as the measured overlap between the pair followed by assessments of significance (Pa, Pb), given as Knersvlakte predicting Cuatrociénegas, and vice versa, etc. ‘S’ overlap values are smaller than the null distribution, which support niche divergence; ‘L’ overlap values are larger than the null distribution, which indicates niche similarity; ‘ns’ denotes overlap values falling within the null distribution.
MANOVA
We used a nonparametric MANOVA, Kruskal–Wallis tests and the Behrens–Fisher post hoc test as a complement to our modelling approach to evaluate the similarity between globular centres (Table 7). We applied these analyses on the first three PCA axes, which explained the 96 % of the variance (Table 4). The MANOVA results showed that at least one of the five sites differed in their mean environmental properties (F = 410·24; d.f. 4, P = 0·001), and the Behrens–Fisher post hoc tests were significant for all the comparisons (P = 0·001), thus rejecting the hypothesis of environmental similarity between hotspots.
Table 7.
Summary of multivariate analysis of variance and post hoc test
| (A) MANOVA | |||
|---|---|---|---|
| F model | d.f. | P-value | |
| 5 hotspots | 410·24 | 4 | <0·001 |
| (B) Kruskal–Wallis rank sum test | |||
|---|---|---|---|
| Kruskal–Wallis chi-squared | d.f. | P-value | |
| PC1 | 463·83 | 4 | <0·001 |
| PC2 | 330·82 | 4 | <0·001 |
| PC3 | 279·76 | 4 | <0·001 |
| (C) Behrens–Fisher | ||||||
|---|---|---|---|---|---|---|
| PC1 |
PC2 |
PC3 |
||||
| Statistic | P-value | Statistic | P-value | Statistic | P-value | |
| Knersvlakte–Huizache | –56347·13 | <0·001 | –56347·13 | <0·001 | –37·09 | <0·001 |
| Knersvlakte–Cuatrociénegas | –63835·72 | <0·001 | –3·97 | <0·001 | –11·30 | <0·001 |
| LittleKaroo–Cuatrociénegas | –415·623 | <0·001 | –74498·32 | <0·001 | 13·77 | <0·001 |
| LittleKaroo–Huizache | –80311·89 | <0·001 | –12·09 | <0·001 | 8·23 | <0·001 |
| Richtersveld–Cuatrociénegas | 158·87 | <0·001 | –73314·39 | <0·001 | –16·34 | <0·001 |
| Richtersveld–Huizache | –43·73 | <0·001 | –17·10 | <0·001 | –5·58 | <0·001 |
All pairs of comparisons between hotspots suggested that they are ecologically distinct (P ≤ 0·01).
DISCUSSION
Convergent evolution is thought to be driven by similar environmental conditions leading to similar selective pressures, for which there are similar evolutionary responses in unrelated lineages (Orians and Solbrig, 1977; Orians and Paine, 1983; Esler and Rundel, 1999; McGhee, 2011). In the case of the suggested convergence between succulent plants of the American and African drylands (Table 1), we would expect to find similar environmental conditions in the areas where globular plants are most diverse and abundant. Yet, despite their striking morphological similarity (Fig. 1) and their putatively similar environments, we found conspicuous differences in climate between the succulent hotspots of Mexico and South Africa. With some exceptions, our analyses based on distribution models (Figs 3A–D and 4A–D), niche equivalence and similarity tests (Warren et al., 2008) (Tables 5 and 6) and multivariate analyses (Table 7) suggested that globular succulent centres on different continents are in different areas of environmental space.
These differences can be illustrated by inspecting maps of model projections between continents. When we projected the climate conditions of Cuatrociénegas to southern Africa, they mainly predicted the area of the Nama Karoo, adjacent to the Succulent Karoo (Fig. 3A, B). However, the Nama Karoo is dominated by shrubs and tall stem succulents and grasses with very few globular succulents in comparison with the Succulent Karoo and Cuatrociénegas (Wyk and Smith, 2001; Hartmann, 2004, 2006). Similarly, the model projections from the South African Knersvlakte and Richtersveld did not predict the Mexican hotspots (Fig. 4A–D). Thus, despite the gross morphological similarities between Mexican and South African succulents (Fig. 1), our climate maps suggest that they occur in areas with different climates.
The statistical yardsticks we used highlighted these differences. The hotspots had generally quite different climates based on the equivalence, similarity and MANOVA tests (Tables 5–7). The niche equivalence test essentially mixes the occurrence data from two hotspots. If the two original datasets are made up of points falling in very similar climates, then the resulting projections will be similar to the originals. With this procedure, all possible pairs of intercontinental comparisons were significantly different from one another (Table 5) despite the apparent morphological similarity of their plant complements (Fig. 1). The similarity test involved comparing the observed climate of one hotspot with that found in another hotspot, as inferred from random points within it. Because it has the potential to include a greater environmental range within the other distribution, the similarity test can be considered less stringent regarding what is to be taken as similar. Accordingly, this test showed Cuatrociénegas to be similar to the Richtersveld, and El Huizache was more similar to the Richtersveld and to the Knersvlakte than we would expect given the null distribution generated (Table 6). The MANOVA results also suggested significant differences in all intercontinental comparisons (Table 7). While the succulent hotspots are no doubt similar in that they are drylands, they differ in major details of their rainfall and temperature regimes and points of similarity were fewer than the differences.
These differences among the tests inevitably raise the question of why, then, these plants look so similar, at least superficially, and why we did not find more climatic coincidence. One possibility is that the climatic variables employed do not include critical limiting factors (e.g. proximity to the ocean, water input in form of fog and dew, soil, biotic interactions, etc.) that may drive the evolution of convergent globular life forms. For example, when we projected the climate conditions of Cuatrociénegas to Africa, they fell mainly in the area of the Nama Karoo, which has tall rather than globular succulents and is adjacent to, rather than within, the globular-rich Succulent Karoo. This discrepancy in areas of prediction may be explained because both Cuatrociénegas and the Nama Karoo have summer rainfall and strong rain shadows (MacMahon and Wagner, 1986; Arriaga et al., 2000; Wyk and Smith, 2001; Fig. 3B). Another factor potentially involved in the differences between hotspots is the presence of azonal habitats such as quartz islands within the Succulent Karoo. Quartz islands are characterized by a dense layer of small quartz pebbles forming patches of variable sizes (Schmiedel and Jürgens, 1999, 2004). The environmental conditions in the quartz islands provide a selective regime abruptly distinct from the surroundings (Desmet and Cowling, 1999; Schmiedel and Jürgens, 1999). The soil affinities of cacti in Mexico are not well understood (McAuliffe, 1994; Bárcenas-Argüello et al., 2010). Some Mexican cacti are clearly soil specialists (e.g. Geohintonia and Aztekium), but for the most part the Mexican hotspots are on limestone-derived soils of wide occurrence and not edaphic islands (Hernández et al., 2001). Our results coincide with previous suggestions of differences in precipitation regime between the summer rainfall Chihuahuan Desert and the winter rainfall Succulent Karoo (Cowling et al., 1998; Desmet and Cowling, 1999; Esler and Rundel, 1999). The western coast of South Africa contrasts with the American inland deserts due to the reliability of its rainfall and its closeness to the Atlantic Ocean. This proximity mitigates the aridity of the Karoo, moderating its temperature and providing a constant supply of dew (Cowling et al., 1998; Desmet, 2007). Thus, through its largely coincident results, our study offers the first quantitative comparison between the climates of the world's main diversity centres of globular succulents and points to important differences among them.
A possible explanation for the climatic discrepancies we found is that the morphologies considered ‘similar’ in fact are differentiated either structurally or functionally in biologically important ways. While the globular groups we compared are often referred to as morphologically similar (Fig. 1 and Table 1), they are constructed in different ways (Hartmann, 2004; Mauseth, 2004). These differences could allow them to explore different sets of climatic conditions (Noble, 1982, 1989; von Willert et al., 1992). For example, the dominant group in the African hotspots is the ice-plant family (Cowling et al., 1994; Hartmann, 2004, 2006; Cowling et al., 1998), whose globular bodies are made up of leaves, whereas those of cacti are stems. Ice-plant bodies tend to be smaller than cacti, have shorter roots and short life spans, in contrast to the long-lived stems of cacti. Perhaps because of this rather frail construction, the succulent Aizoaceae have lower tolerance to drought and thermal stress than stem succulents like cacti or spurges (Noble, 1982; von Willert et al., 1992; Cowling et al., 1994; Jürgens et al., 1999). The apparently fragile construction of the globular Aizoaceae might represent an adaptation to the benign microclimate of the quartz soils (Desmet and Cowling, 1999; Schmiedel and Jürgens, 1999), to which some of them seem particularly restricted. In the case of stem succulents, their secondary growth, tough epidermal regions, deeper and woodier roots and generally larger stature may give them more marked resistance to drought and heat (Gibson and Nobel, 1986). Thus, despite their overall similarity, in that they are both globular, the morphological features of cacti probably allow them to explore a wider area of environmental space as compared with the globular Aizoaceae. For example, the mostly globular genus Mammillaria is found across a vast range from the south-western USA to northern South America, far larger than the area occupied by globular Aizoaceae. Accordingly, the model projections from cactus hotspots fell in areas drier or wetter than the Aizoaceae hotspots, such as the Nama Karoo or the southern coast of Africa, respectively. Physiologically, these groups also appear to be distinct. The Aizoaceae display significant flexibility of photosynthetic behaviour (Borland et al., 2011), while Cactaceae are obligate CAM plants. In addition, the plants classified as globular might differ when shape is studied quantitatively (Table 1 and Fig. 1). Some plants that are globular at younger developmental stages reach non-globular shapes with age, such Euphorbia obesa, perennially cited in comparisons with cacti (Glass and Foster, 1975; Trager, 1985; Gordon, 1997; McGhee, 2011). These differences in forms may translate into a lack of overlap in morphological space, implying that they may occupy different climatic conditions. Thus, the combination of structural, morphological and physiological differences could allow different groups of succulent plants to occupy distinct environments.
A final consideration, and one crucial for all studies of convergence, is the scale of analysis and what is understood by ‘similar’. Our interest was to make comparisons as fine as the available data and tools permit, and this approach pinpointed abundant differences between areas where presumably convergent life forms occur. Coarser level comparisons almost certainly would show similarities rather than differences. Taking a global perspective, the drylands of southern Africa and the Americas are certainly more similar than the prairie, Mediterranean shrublands and tropical woods that surround the succulent hotspots on both continents. Succulent globular plants can explore a wide variety of climatic conditions (Noble, 1982; Gibson and Nobel, 1986; von Willert et al., 1992), but the hotspots of globular plants seem to be drylands that have relatively predictable rainfall and moderate extremes of heat (von Willert et al., 1992; Hernández and Bárcenas, 1995; Hernández et al., 2001), as opposed to the markedly more severe Atacama, Namib or Sahara deserts. From a more global perspective, then, the globular hotspots are certainly similar in many regards.
Conclusions
Convergent evolution is the appearance of ‘similar’ morphologies under ‘similar’ selection regimes. However, there is no objective definition of ‘similar’ and any definition that we choose obeys the pragmatic considerations of a given study rather than some absolute standard of similarity. Different definitions will often yield differing results, as in the fine scale versus global comparisons discussed above. Such differences can even be found among our results. Whereas the niche equivalence test rejected their identical environment, according to the similarity test, the succulent centres of Cuatrociénegas–Richtersveld, Huizache–Richtersveld and Huizache–Knersvlakte showed similar conditions. Our pragmatic use of ‘similarity’ was to compare climate models between continents with a series of well-known metrics to discover fine-scale differences or similarities. These fine-scale climatic differences can be used to guide further morphological studies of these plants. In particular, the qualitative impression of ‘similar shape’ so frequently invoked in examples of convergence bears re-examination, e.g. via techniques such as geometric morphometrics. Such an approach would allow quantification of shape similarity and allow testing of the hypothesis that species that are closer in shape space should also be closer in niche space.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by CONACyT (grant 48322, L.O.A.C.) through a graduate scholarship, the Programa de Posgrado en Ciencias Biológicas, UNAM. For L.O.A.-C. this paper is in partial fulfillment of the requirements of the Posgrado en Ciencias Biológicas, UNAM. In addition, we are grateful for the grants from CONACyT and PAPIIT/DGAPA (nos 132404 and IN228207) and a Huntington Fellowship from The Huntington Library, Art Collections and Botanical Gardens. This work was also supported by a grant from the Research Committee of the Cactus and Succulent Society of America. We thank T. Peterson, J. Rosell and N. Martínez for helpful comments and S. Zamora and J. Rosell for statistical assistance. We thank S. Hammer for generously providing time and access to his collection; J. Trager and S. Lahmeyer at the Huntington Botanical Garden for their kindness and help in the collection of environmental and morphological data; and the staff of SANBI (South Africa) for their help and access to the PRECIS data base. We thank C. Gómez for his help with the cactus database and C. Gómez and J. Trager for photographs. We appreciate the courtesy of the curators of the herbaria consulted.
LITERATURE CITED
- Anderson M. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- Arakaki M, Christin P-A, Nyffeler R, et al. Contemporaneous and recent radiations of the world's major succulent plant lineages. Proceedings of the National Academy of Science of the USA. 2011;108:8379–8384. doi: 10.1073/pnas.1100628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga L, Espinoza JM, Aguilar C, Martínez E, Gómez L, Loa E. Regiones terrestres prioritarias de México. México: Comisión Nacional para el Conocimiento y uso de la Biodiversidad; 2000. Escala de trabajo 1:1 000 000. [Google Scholar]
- Bárcenas-Argüello ML, Gutiérrez-Castorena MC, Terrazas T, López-Mata L. Rock-soil preferences of three Cephalocereus (Cactaceae) species of tropical dry forests. Soil Science Society of America Journal. 2010;74:1374–1382. [Google Scholar]
- Bennici A. The convergent evolution in plants. Rivista Biologia. 2003;96:485–490. [PubMed] [Google Scholar]
- Benson LD. The native cacti of California. Stanford, CA: Stanford University Press; 1969. [Google Scholar]
- Biesboer DD, Koukkari WL. The taxonomy and biology of leafy spurge. Leafy Spurge Symposium and Proceedings. 1992;4:1–6. [Google Scholar]
- Borland AM, Barrera-Zambrano VA, Ceusters J, Shorrock K. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytologist. 2011;191:619–633. doi: 10.1111/j.1469-8137.2011.03781.x. [DOI] [PubMed] [Google Scholar]
- Bruyns PV. Stapeliads of southern Africa and Madagascar. Vol. 1. Hatfield, South Africa: Umdaus Press; 2005. [Google Scholar]
- Cody ML. Niche theory and plant growth form. Vegetatio. 1991;97:39–55. [Google Scholar]
- Cody ML, Mooney HA. Convergence versus nonconvergence in Mediterranean-climate ecosystems. Annual Review of Ecology and Systematics. 1978;9:265–321. [Google Scholar]
- Cowling RM. A description of the Karoo Biome Project. 1986;122:1–143. South African Scientific Programs Report No. [Google Scholar]
- Cowling RM, Hilton-Taylor C. Plant biogeography, endemism and diversity. In: Dean WRJ, Milton SJ, editors. The Karoo: ecological patterns and processes. Cambridge: Cambridge University Press; 1999. pp. 42–56. [Google Scholar]
- Cowling RM, Esler KJ, Midgley GF, Honig MA. Plant functional diversity, species diversity and climate in arid and semiarid southern Africa. Journal of Arid Environments. 1994;27:141–148. [Google Scholar]
- Cowling RM, Rundel PW, Desmet PG, Esler KJ. Extraordinary high regional-scale plant diversity in southern African arid lands: subcontinental and global comparisons. Diversity and Distribution. 1998;4:27–36. [Google Scholar]
- Desmet PG. Namaqualand: a brief overview of the physical and floristic environment. Journal of Arid Environments. 2007;70:570–587. [Google Scholar]
- Desmet PG, Cowling RM. The climate of the Karoo – a functional approach. In: Dean WRJ, Milton SJ, editors. The Karoo: ecological patterns and processes. Cambridge: Cambridge University Press; 1999. pp. 3–16. [Google Scholar]
- Dinerstein E, Olson D, Atchley J, et al. Ecoregion-based conservation in the Chihuahuan Desert: a biological assessment and biodiversity vision. 2nd edn. Washington, DC: WWF–CONABIO–The Nature Conservancy–Pronatura Noreste–ITESM; 2000. Draft report. [Google Scholar]
- Driver A, Desmet PG, Rouget M, Cowling RM, Maze KE. Succulent Karoo ecosystem plan. Biodiversity Component Technical Report. Cape Town, South Africa: Cape Conservation Unit, Botanical Society of South Africa; 2003. [Google Scholar]
- Eggli U, Nyffeler R. Living under temporarily arid conditions – succulence as an adaptive strategy. Bradleya. 2009;27:13–36. [Google Scholar]
- Esler KJ, Rundel PW. Comparative patterns of phenology and growth form diversity in two winter rainfall deserts: the Succulent Karoo and Mojave Desert ecosystems. Plant Ecology. 1999;142:97–104. [Google Scholar]
- ESRI. ArcGIS 9·3. Redlands, CA: Environmental Systems Research Institute; 2006. http://www.esri.com . [Google Scholar]
- Ezcurra E, Mellink E, Wehncke E, et al. Natural history and evolution of the world's deserts. In: Ezcurra E, editor. Global deserts outlook. Nairobi, Kenya: United Nations Environment Programme (UNEP); 2006. pp. 1–26. [Google Scholar]
- Fain MG, Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Farris JS, Kallersjo M, Kluge A, Bult C. Testing significance of incongruence. Cladistics. 1995;10:315–319. [Google Scholar]
- Felger R, Henrickson J. Convergent adaptive morphology of a Sonoran desert cactus (Peniocereus striatus) and an African spurge (Euphorbia cryptospinosa) Haseltonia. 1997;5:77–85. [Google Scholar]
- Futuyma DJ. Evolution. Sunderland, MA: Sinauer Associates; 2009. [Google Scholar]
- Gibson A, Nobel P. The cactus primer. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Glass C, Foster R. The culture of the cacti and other succulents. Cactus and Succulent Journal. 1975;48:147–154. [Google Scholar]
- Gordon DR. A history of succulent plants. Mill Valley, CA: Strawberry Press; 1997. [Google Scholar]
- Hartmann HEK. Adaptations and phytogeography in the ice-plant family (Aizoaceae): the interaction of the genetic equipment and ecological parameters. I. One leaf-pair is the plant. Bradleya. 2004;22:21–36. [Google Scholar]
- Hartmann HEK. Adaptations and phytogeography in the ice-plant family (Aizoaceae) the interaction of the genetic equipment and ecological parameters. II. Hide-and-seek: plants sunk in the ground. Bradleya. 2006;24:1–38. [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Hearn DJ. Developmental patterns in anatomy are shared among separate evolutionary origins of stem succulent and storage root-bearing growth habits in Adenia (Passifloraceae) American Journal of Botany. 2009;96:1941–1956. doi: 10.3732/ajb.0800203. [DOI] [PubMed] [Google Scholar]
- Hernández H, Bárcenas RT. Endangered cacti in the Chihuahuan Desert. I. Distribution patterns. Conservation Biology. 1995;9:1176–1188. doi: 10.1046/j.1523-1739.1995.9051169.x-i1. [DOI] [PubMed] [Google Scholar]
- Hernández HM, Gómez-Hinostrosa C. Mapping the cacti of Mexico. Succulent Plant Research. Vol. 7. Milborne Port, UK: dh Books; 2011. [Google Scholar]
- Hernández H, Gómez-Hinostrosa C, Bárcenas RT. Diversity, spatial arrangement, and endemism of Cactaceae in the Huizache area, a hot spot in the Chihuahua desert. Biodiversity and Conservation. 2001;10:1097–1112. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. Index Herbariorum. New York, NY: New York Botanical Garden; 1990. [Google Scholar]
- Jacobsen H. Lexicon of succulent plants. Poole, UK: Blandford; 1977. [Google Scholar]
- Jürgens N, Gotzmann IH, Cowling RM. Remarkable medium-term dynamics of leaf succulent Mesembryanthemaceae shrubs in the winter-rainfall desert of northwestern Namaqualand, South Africa. Plant Ecology. 1999;142:87–96. [Google Scholar]
- Kozak KH, Graham CH, Wiens JJ. Integrating GIS-based environmental data into evolutionary biology. Trends in Ecology & Evolution. 2008;23:141–148. doi: 10.1016/j.tree.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Larson A, Losos JB. Phylogenetic systematics of adaptation. In: Rose MR, Lauder GV, editors. Adaptation. San Diego, CA: Academic Press; 1996. pp. 187–220. [Google Scholar]
- Losos JB. The evolution of the convergent structure in Caribbean Anolis communities. Systematic Biology. 1992;41:403–420. [Google Scholar]
- McAuliffe JR. Landscape evolution, soil formation and ecological patterns and processes in Sonoran Desert Bajadas. Ecological Monographs. 1994;64:111–148. [Google Scholar]
- McGhee GR. Convergent evolution: limited forms most beautiful. Cambridge, MA: Massachusetts Institute of Technology Press; 2011. Vienna Series in Theoretical Biology. [Google Scholar]
- MacMahon J, Wagner FH. The Mojave, Sonoran and Chihuahuan Deserts of North America. In: Evenari M, Noy-Meier I, Goodall D, editors. Hot deserts and arid shrublands. Ecosystems of the world. Amsterdan: Elsevier; 1986. pp. 105–202. [Google Scholar]
- Mauseth JD. Theoretical aspects of surface-to-volume ratios and water-storage capacities of succulent shoots. American Journal of Botany. 2000;87:1107–1115. [PubMed] [Google Scholar]
- Mauseth JD. The structure of photosynthetic succulent stems in plants other than cacti. International Journal of Plant Science. 2004;165:1–9. [Google Scholar]
- Meinzer FC. Functional convergence in plant responses to the environment. Oecologia. 2003;134:1–11. doi: 10.1007/s00442-002-1088-0. [DOI] [PubMed] [Google Scholar]
- Melville J, Harmon LJ, Losos JB. Intercontinental community convergence of ecology and morphology in desert lizards. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2005;27:557–563. doi: 10.1098/rspb.2005.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. Low temperature tolerance and cold hardening of cacti. Ecology. 1982;63:1650–1656. [Google Scholar]
- Nobel PS. Shoot temperatures and thermal tolerance of succulent species for Haworthia and Lithops. Plant, Cell & Environment. 1989;12:643–651. [Google Scholar]
- Nobel PS, Geller GN, Kee SC, Zimmerman AD. Temperatures and thermal tolerances for cacti exposed to high temperatures near soil surface. Plant, Cell & Environment. 1986;9:279–287. [Google Scholar]
- Norberg U. Allometry of bat wings and legs and comparison with bird wings. Philosophical Transactions of the Royal Society of London Series B. 1981;292:359–398. [Google Scholar]
- Ogburn RM, Edwards EJ. Anatomical variation in Cactaceae and relatives: trait lability and evolutionary innovation. American Journal of Botany. 2009;96(391–408) doi: 10.3732/ajb.0800142. [DOI] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ. The ecological water-use strategies of succulent plants. In: Kader JC, Delseny M, editors. Advances in botanical research. Vol. 55. Burlington, MA: Academic Press; 2010. pp. 179–225. [Google Scholar]
- Olson DM, Dinerstein E. The Global 200: a representation approach to conserving the earth's most biologically valuable ecoregions. Conservation Biology. 1998;3:502–512. [Google Scholar]
- Orians GH, Paine RT. Convergent evolution at the community level. In: Futuyma DJ, Slatkin M, editors. Coevolution. Sunderland, MA: Sinauer Associates; 1983. pp. 431–458. [Google Scholar]
- Orians GH, Solbrig OT. Convergent evolution in warm desert. Stroudsburg, PA: Dowden, Hutchinson & Ross; 1977. [Google Scholar]
- Parsons DU, Moldenke AR. Convergence in vegetation structure along analogous climatic gradients in California and Chile. Ecology. 1975;56:950–957. [Google Scholar]
- Peet RK. Ecosystem convergence. American Naturalist. 1978;112:441–459. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modeling. 2006;190:231–259. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing, v.2·9.2. 2009 Vienna. http://www.R-project.org . [Google Scholar]
- Rauh W, Kendall H. The wonderful world of succulents. Washington, DC: Smithsonian Institution Press; 1984. [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE. Biology of plants. New York, NY: W.H. Freeman; 1986. [Google Scholar]
- Reich P, Walters MB, Ellsworth DS. From tropics to Tundra: global convergence in plant functioning. Proceedings of the National Academy of Science of the USA. 1997;94:13730–1374. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ, Johnson MA, Shulte JA, II, Kolbe JJ, Losos J. A phylogenetic test for adaptative convergence in rock-dwelling lizards. Evolution. 2007;61:2898–2912. doi: 10.1111/j.1558-5646.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- Schluter D, Ricklefs RE. Convergence and regional component of species diversity. In: Ricklefs RE, Schluter D, editors. Species diversity in ecological communities. Chicago, IL: University of Chicago Press; 1993. pp. 230–240. [Google Scholar]
- Schmiedel U, Jürgens N. Community structure on unusual habitat islands: quartz-fields in the Succulent Karoo, South Africa. Plant Ecology. 1999;142:57–69. [Google Scholar]
- Schmiedel U, Jürgens N. Habitat ecology of southern African quartz fields: studies on the thermal properties near the ground. Plant Ecology. 2004;170:153–166. [Google Scholar]
- Schmiedel U, Linke T, Christiaan AR, et al. Environmental and socio-economic patterns and processes in the Succulent Karoo: frame conditions for the management of this biodiversity hotspot. In: Hoffman MT, Schmiedel U, Jürgens N, editors. Biodiversity in southern Africa. Vol. 3. Göttingen: Klaus Hess Publishers; 2010. pp. 109–150. Implications for landuse and management. [Google Scholar]
- Schoener TW. The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology. 1968;49:704–726. [Google Scholar]
- Stayton T. Morphological evolution of the lizard skull: a geometric morphometrics survey. Journal of Morphology. 2005;263:47–59. doi: 10.1002/jmor.10288. [DOI] [PubMed] [Google Scholar]
- Stearns S, Hoekstra RF. Evolution: an introduction. Oxford: Oxford University Press; 2000. [Google Scholar]
- Trager J. Case of convergences. Euphorbia Journal. 1985;3:77–79. [Google Scholar]
- Warming E. Oecology of plants: an introduction to the study of plant-communities. Oxford: Clarendon Press; 1909. [Google Scholar]
- Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607–611. [Google Scholar]
- Werdelin L. Comparison of skull shape in marsupial and placental carnivores. Australian Journal of Zoology. 1986;34:109–117. [Google Scholar]
- von Willert DJ, Eller DJ, Werger BM, Brinckmann MJA, Ihlenfeldt HD. Life strategies of succulents in deserts, with special reference to the Namib Desert. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Wroe S, Clausen PD, McHenry CR, Moreno K, Cunningham E. Computer simulation of feeding behaviour in the thylacine and dingo as a novel test for convergence and niche overlap. Proceedings of the Royal Society of London Series B: Biological Sciences. 2007;274:2819–2828. doi: 10.1098/rspb.2007.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyk AE, Smith GF. Regions of floristic endemism in southern Africa: a review with emphasis on succulents. Hatfield, South Africa: Umdaus Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.