Abstract
Background and Aims
Phenology is one of most sensitive traits of plants in response to regional climate warming. Better understanding of the interactive effects between warming and other environmental change factors, such as increasing atmosphere nitrogen (N) deposition, is critical for projection of future plant phenology.
Methods
A 4-year field experiment manipulating temperature and N has been conducted in a temperate steppe in northern China. Phenology, including flowering and fruiting date as well as reproductive duration, of eight plant species was monitored and calculated from 2006 to 2009.
Key Results
Across all the species and years, warming significantly advanced flowering and fruiting time by 0·64 and 0·72 d per season, respectively, which were mainly driven by the earliest species (Potentilla acaulis). Although N addition showed no impact on phenological times across the eight species, it significantly delayed flowering time of Heteropappus altaicus and fruiting time of Agropyron cristatum. The responses of flowering and fruiting times to warming or N addition are coupled, leading to no response of reproductive duration to warming or N addition for most species. Warming shortened reproductive duration of Potentilla bifurca but extended that of Allium bidentatum, whereas N addition shortened that of A. bidentatum. No interactive effect between warming and N addition was found on any phenological event. Such additive effects could be ascribed to the species-specific responses of plant phenology to warming and N addition.
Conclusions
The results suggest that the warming response of plant phenology is larger in earlier than later flowering species in temperate grassland systems. The effects of warming and N addition on plant phenology are independent of each other. These findings can help to better understand and predict the response of plant phenology to climate warming concurrent with other global change driving factors.
Keywords: Climate warming, functional group, grassland, nitrogen, reproductive phenology, temperature
INTRODUCTION
Plant phenology, the timing of plant development and growth, is one of the traits sensitive to regional climate warming (Peñuelas et al., 2002; Schwartz et al., 2006; Cleland et al., 2007). Its variation among species is important in maintaining species coexistence (Rathcke and Lacey, 1985; Cleland et al., 2007) and in determining growth dynamics of the whole plant community (Gu et al., 1998). In the past decades, a trend of earlier spring onset has been widely detected around the world (Parmesan and Yohe, 2003; Root et al., 2003; Piao et al., 2007; Pau et al., 2011). However, such an advance in plant phenology is triggered not only by temperature itself, but also by other aspects of environmental change, e.g. enhanced nitrogen (N) input via deposition. For example, a recent meta-analysis found a larger temperature sensitivity of plant phenology from long-term observations than temperature-only manipulative experiments (Wolkovich et al., 2012), suggesting that other environmental factors may enhance the temperature sensitivity of plant phenology in natural ecosystems. Thus, the interactive effects between climate warming and other factors driving global change on plant phenology are critical for improving the prediction of plant responses to future climate change.
Along with regional climate warming, the Earth's land surface has experienced approximately doubled N input as a result of the worldwide use of artificial N fertilizers (Gruber and Galloway, 2008). Given the effects of both temperature and N availability on plant activity, global warming and increasing N input have been found to interact to affect terrestrial plant growth (Majdi, 2004; Liu et al., 2011). However, it remains unclear whether and how atmospheric N deposition will alter the sensitivity of plant phenology to climate warming. To our knowledge, little effort has been made to examine the effects of an interaction between warming and N addition on plant phenology (Cleland et al., 2006; Lupi et al., 2012; Smith et al., 2012). In an annual grassland in North America, warming-advanced community greenness was dampened by N addition (Cleland et al., 2006). In an alpine tundra, N addition delayed flowering of forbs but advanced that of graminoids (Smith et al., 2012). Therefore, N addition could impact the temperature sensitivity of plant phenology differently, not only among ecosystem types but also among species within an ecosystem.
Responses of plant phenology to environmental changes have been documented to be very diverse among different species (Cleland et al., 2006; Sherry et al., 2007), exacerbating the difficulty in assessing the changes in plant phenology under concurrent climate warming and N enrichment. For example, a long-term observation of the phenology of 385 British plant species has shown that 16 % of species flowered earlier whereas 3 % of species flowered later in the 1990s compared with the previous 45 years (Fitter and Fitter, 2002). The variation in phenological trends among species has been ascribed to life form, pollination type and time of year (Fitter and Fitter, 2002). For instance, species blooming in spring are usually more sensitive to temperature increase than autumn species (Menzel, 2003; Cleland et al., 2007; Wolkovich et al., 2012). Irrespective of a large body of reports on N addition and plant growth response (Xia and Wan, 2008), only a few studies have focused on the influences of N on plant phenology. Even these studies have found that increasing N input can advance (Dewald et al., 1992; Peñuelas et al., 1995), not affect (Zhang et al., 1997) or delay phenology of individual plant species (Cleland et al., 2006). In addition, N enrichment can have various impacts on species within a plant community, e.g. it delayed flowering of grasses but slightly accelerated that in forbs in an annual grassland in North America (Cleland et al., 2006). Moreover, if species from different growing season stages and functional types have differential phenological responses, it will pose a greater challenge to assess the community-level phenological response to climate warming, N addition and their interactions.
Here, we present the results from a field study to investigate responses of plant phenology to simulated warming and N addition with four treatments, i.e. control, warming, N addition and warming plus N addition, in a semi-arid steppe in northern China since 2006. We monitored phenological times of eight species from different functional types over the entire growing seasons (May–October) from 2006 to 2009. In particular, we address the following two questions in this study. (1) How does warming in combination with N addition affect the phenology of plant species in the semi-arid steppe in northern China? (2) Are there interactive effects of warming and N addition on plant phenology in this ecosystem?
MATERIALS AND METHODS
Study site
The study was conducted in a semi-arid temperate steppe in Duolun County (42 °02′N, 116 °17′E, 1324 m a.s.l) in Inner Mongolia, China. Long-term (1953–2007) annual precipitation and temperature are 383 mm (with 90 % falling within May–October) and 2·1 °C (with monthly mean temperature ranging from –17·5 °C in January to 18·9 °C in July), respectively. The soil of the study site is classified as chestnut according to the Chinese classification or Haplic Calcisols according to the Food and Agriculture Organization (FAO) classification, with 62·75 ± 0·04 % (mean ± s.e.) sand, 20·30 ± 0·0 1 % silt and 16·9 ± 0·01 % clay. Mean bulk density is 1·31 g cm−3 and pH is 7·7. Nitrogen deposition in this area was estimated at about 20 kg ha−1 year−1 in 2005–2006 (Zhang et al., 2008).
Experimental design
The experiment used a completely randomized block design with six treatments, comprisiing control (C), daytime warming (0600–1800 h, local time), night-time warming (1800–0600 h), continuous warming (24 h; W), nitrogen addition (N) and continuous warming plus nitrogen addition (WN). The six treatments were randomly arranged into a block and replicated six times. Thus, thirty-six 3 × 4 m plots were arranged in a 6 × 6 matrix, with a 3 m distance between adjacent plots. We used C, daytime warming, night-time warming and W treatments to examine the differential impacts of day and night warming, and the treatments C, W, N and WN to test the interactive effects between warming and N addition. The C and W treatments were shared between the two sub-experiments because the warming treatment is costly. The effects of day and night warming on plant phenology were not included in this study and have been reported previously (Xia and Wan, 2012). The warmed plots have been heated continuously by using MSR-2420 infrared radiators (with a power consumption of approx. 1600 W; Kalglo Electronics Inc., Bethlehem, PA, USA) suspended 2·25 m above the ground since 23 April 2006. Across the four growing seasons from 2006 to 2009, warming treatment significantly increased the soil temperature at the depth of 10 cm by 1·8 °C [P < 0·001; two-way analysis of variance (ANOVA)]. More detailed information of the effects of warming treatments on soil microclimates can be found in Xia et al. (2010). In order to simulate the shading effects of the infrared radiator, a ‘dummy’ heater with the same shape and size was suspended 2·25 m above the ground in each control plot. N additions were spread by hand before the first rain event in the rainy season, and thr plots were treated once a year with NH4NO3 on 19 July in 2006 and 2007 and on 9 July in 2008 and 2009. Given that N effects on species composition and ecosystem production saturate at N addition rates of about 10·5 g N m−2 year−1 in this region (Bai et al., 2010), the level of N addition in this study was 10 g N m−2 year−1.
Phenological observation
Eight species, comprising five forbs (Potentilla acaulis, P. bifurca, P. tanacetifolia, Allium bidentatum, and Heteropappus altaicus), two C3 grasses (Agropyron cristatum and Stipa krylovii) and one semi-arid shrub (Artemisia frigida), were chosen for phenological observation in this study. The phenological stages of these eight species were distributed evenly throughout the entire growing season, from the earliest species (P. acaulis) in May to the latest species (A. frigida) in October. Over the four growing seasons, the eight plant species together accounted for about 78 % of the total above-ground biomass.
The changes in plant reproductive phenology with time follow a logistic growth curve (Sadras et al., 1997). As shown in Fig. 1, the reproductive phenology of both graminoid (grass) and non-graminoid (forb and semi-shrub) species can be divided into several stages (Price and Waser, 1998; Dunne et al., 2003; Sherry et al., 2007). For grasses, there were six stages: plant with flower stalks (stage 0), most culms in boot were visible (stage 0·5), presence of spikelets (stage 1), exerted anthers and styles from the spikelet florets (stage 2), dried and broken off anthers and styles (seed development; stage 3), and disarticulated seeds (stage 4). For forbs and semi-shrubs, plant phenology was divided into seven stages: plant not yet flowering (stage 0), unopened buds (stage 1), open flowers (stage 2), old flowers (post-anthesis; stage 3), initiated fruit (stage 4), expanding fruit (stage 5) and dehisced fruit (stage 6).
Fig. 1.
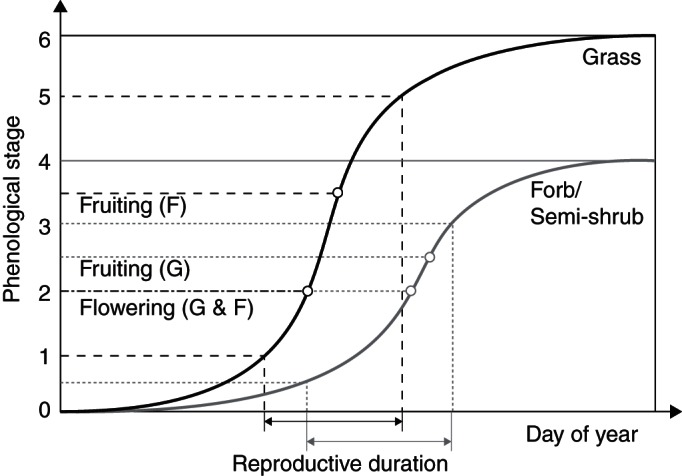
Ideal curves of phenological stage changes for graminoids (G; grey lines) and forbs and semi-shrubs (F; black lines). Parameters (K, a, b, X and m) describing the shape of the curve were obtained by fitting the observed phenological scores to eqn (1) for each species in each growing season. Details of the methods are provided in the text.
At the start of each growing season, we tagged five mature individuals for each species in each plot as soon as any of the eight species had produced obvious bud. We monitored the current stages of all individuals at weekly intervals. If a plant has more than one flower, we first recorded the current stage for each flower, and then calculated a score by averaging all flowers. By this means we obtained a single ‘phenological score’ for each individual in each plot and observation date. For example, an individual forb with one bud (stage 1), two open flowers (stage 2) and three old flowers (stage 3) received a score of 2·3. On each sampling date, the phenological scores for each species were averaged from its five individuals in each plot. We ended the observation when all individuals of a species have reached phenological stage 6 and 4 for forbs and grasses, respectively, or most of the fruits of a plant had dehisced and no more seeds dehisced in the following 2 weeks.
Phenological analysis
Since it is difficult to monitor the times of flowering and fruiting directly for all individuals, they are usually obtained by fitting some statistical models to the observed data. For example, Price and Waser (1998) fitted the observed phenological scores to a linear regression model, and obtained two parameters that describe the mean timing and duration of reproductive phenology. In this study, we fitted the observed scores to the Richards growth equation (Richards, 1959), which has been suggested to be very flexible to describe different shapes of growth data. The Richard growth equation has been successfully applied for studying plant phenology in a tallgrass prairie in North America (Sherry et al., 2007). We applied the Richards growth equation with the contraction–expansion algorithm (Gu et al., 1998) to fit phenological scores (Y) of each species against the day in Julian units (X) in each plot. The equation was described as:
| (1) |
where K is the maximum growth; a is a parameter related to the first observation date; b is the growth rate over time X in Julian days (the number of days in the Gregorian calendar year); and m is a parameter related to the curve shape. The timing of each phenological event can be calculated from Equation 1 as:
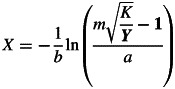 |
(2) |
We conducted the analysis to calculate the timing and duration of reproduction by the following steps. First, in each season, the sequences of calculated phenological scores from a species in each plot were fitted to Equation 1. Best parameter estimates of K, a, b, X and m were obtained for each individual from each plot in each year. Secondly, based on Equation 2, flowering time was calculated as Y = 2 for all species, and fruiting time was obtained as Y = 2·5 and Y = 3·5 for grasses and forbs/semi-shrubs, respectively (Fig. 1). Phenological duration was calculated as X between stage 0·5 (Y = 0·5) and 3 (Y = 3) for grasses, and between stage 1 (Y = 1) and 5 (Y = 5) for forbs/semi-shrubs (Fig. 1). Finally, the calculated flowering and fruiting times as well as the phenological duration were used in further statistical analyses for treatment effects. Although Equation 1 fits our data well, our method may generate some additional uncertainties in the analysed phenological times. For example, our scoring method assumes an equal increase in ‘phenological score’ between major phenological stages, e.g. from bud to opened flowers and from opened flowers to old flowers for forbs and shrubs. It would neglect the differences in temporal patterns of reproductive phenology among species. As a result, some analytical errors cannot be avoided when the analysed results are used to compare phenology among species.
Reproductive allocation
In late August of 2008, we clipped a 50 × 15 cm quadrat in each sub-plot. Above-ground biomass and fruit production of each species were measured. The dry mass of above-ground biomass and fruit production were determined by oven drying at 70 °C to constant weight. Reproductive allocation was calculated as the percentage of fruit production in above-ground biomass for each species.
Data analysis
Repeated-measures ANOVAs (RMANOVAs) were used to examine warming and N addition effects and their possible interactions on soil microclimate and plant phenology. Soil temperature and moisture in each year were first averaged to seasonal means, and then used in RMANOVA. Treatments involving warming and N addition, as well as species were treated as fixed effects, and plot was treated as a random effect. Between-group effects were evaluated as warming or N addition treatment, and within-group effects were interpreted as the effect of year. Three-way ANOVA was used to examine the effects of warming, N addition, species and their interaction on reproductive allocation in 2008. Two-way ANOVAs were used to analyse the main and interactive effects of warming and N addition on reproductive allocation for each species. The fitting of the calibrated Richards equation with the contraction–expansion algorithm (Gu et al., 1998) was carried out in Matlab (Mathworks, Natick, MA, USA) and all statistical analyses were conducted with SAS software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Climate conditions and soil microclimate
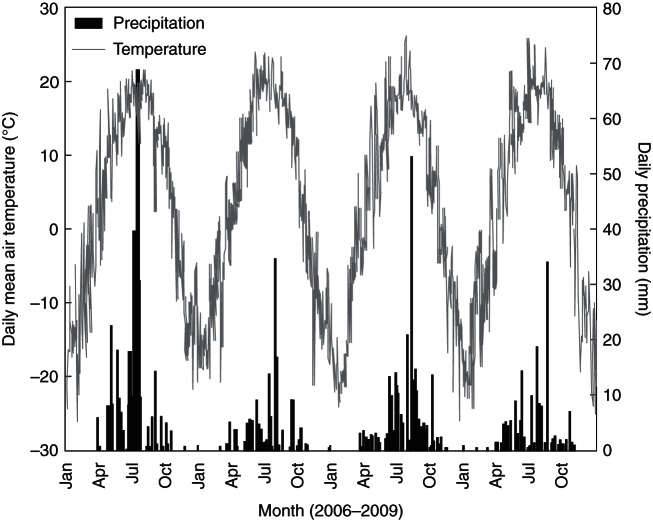
Both precipitation and air temperature fluctuated within the growing season and peaked in July and August from 2006 to 2009 (Fig. 2). The annual total precipitation from 2006 to 2009 was 423·6, 209·8, 369·8 and 196·3 mm, respectively. Most of the annual precipitation was distributed in the growing season (May–October), with 403·5, 194·0, 346·2 and 176·0 mm from 2006 to 2009. Mean annual air temperature was 3·1, 3·5, 2·7 and 3·1 °C, and the growing season mean air temperature was 13·8, 14·3, 13·3 and 14·0 °C from 2006 to 2009.
Fig. 2.
Daily precipitation and daily mean temperature from 2006 to 2009. Data are from a meteorology station close to the experimental plots (approx. 500 m).
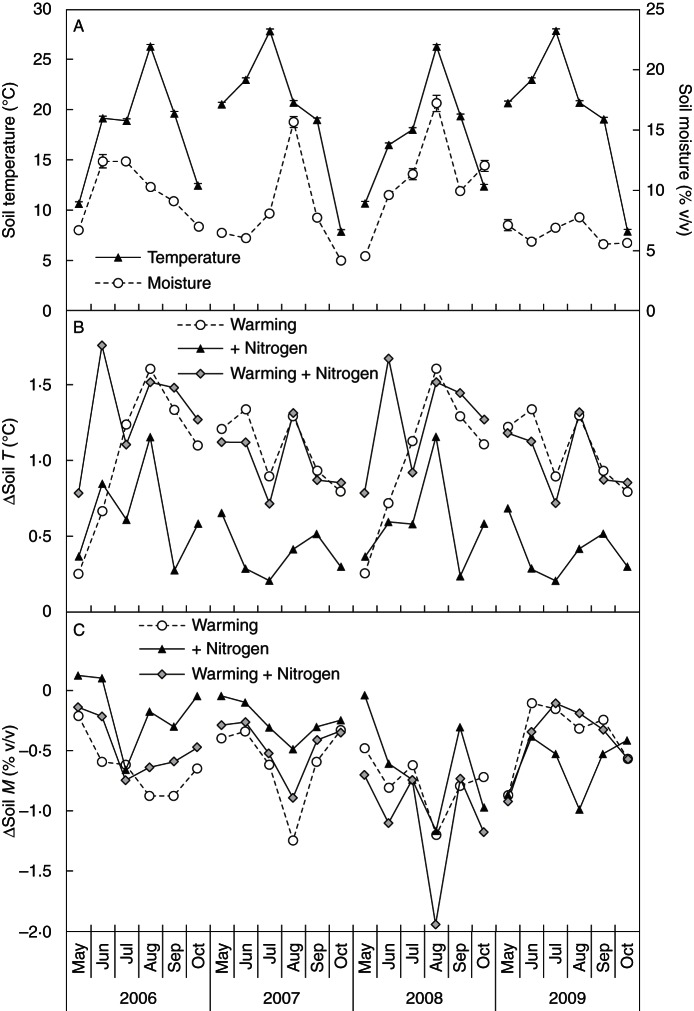
Among the four growing seasons, soil temperature at 10 cm depth in the control plots was relatively low in 2006 (17·9 ± 0·1 °C; mean ± s.e.) and 2008 (17·2 ± 0·1 °C), but high in 2007 and 2009 (both 19·9 ± 0·1 °C). In contrast, soil moisture at the depth of 0–10 cm in the control plots was relatively high in 2006 (9·64 ± 0·24 % v/v) and 2008 (10·77 ± 0·35 % v/v) but low in 2007 (8·01 ± 0·17 % v/v) and 2009 (6·44 ± 0·24 % v/v; Fig. 3A). Both warming (F = 34·00, P < 0·001; RMANOVA) and N addition (F = 4·33, P = 0·043) increased soil temperature across the four growing seasons (Fig. 3B). Warming significantly decreased soil moisture by 0·39 % v/v (absolute difference; F = 5·00, P = 0·003; RMANOVA) across the four growing seasons, while the negative effect of N addition on soil moisture (–0·21 % v/v, F = 1·44, P = 0·237) was insignificant (Fig. 3C). No interactive effect between warming and N addition on soil temperature or moisture was found.
Fig. 3.
Temporal dynamic of (A) soil temperature and moisture (0–10 cm) in the control plots, and treatment-induced changes in (B) soil temperature and (C) soil moisture in response to warming, nitrogen addition, and warming plus N addition.
Warming effects on species-level plant phenology
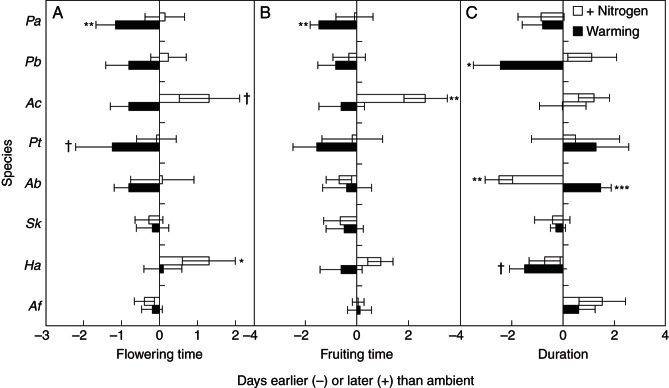
Across the eight species and four growing seasons (2006–2009), warming significantly advanced the flowering time by 0·64 d per season (Table 2), which was primarily determined by P. acaulis (1·15 d; P = 0·044) and P. tanacetifolia (1·24 d; P = 0·091; Table 3, Fig. 4A; Supplementary Data Figs S1 and S2). The fruiting time across all the species was also advanced by 0·72 d per season (Fig. 4; Table 2), which was mainly determined by P. acaulis (1·47 d; P = 0·009; Table 3, Fig. 4B; Supplementary Data Figs S1 and S2). As a result of the advance of both flowing and fruiting phenology, the reproductive duration across the eight species did not change under warming (P > 0·10; Table 1). Nevertheless, warming shortened the reproductive duration of P. bifurca (2·45 d; P = 0·023) and H. altaicus (1·50 d; P = 0·064) but extended that of A. bidentatum (1·45 d; P = 0·007; Table 3, Fig. 4C; Supplementary Data Figs S1 and S2).
Table 2.
Results (F-values) of repeated-measures ANOVA on the effects of warming (W), nitrogen addition (N), species, block, sampling year (Y), and their interactions on flowering time, fruiting time and phenological duration.
| Treatments | d.f. | Flowering time | Fruiting time | Duration |
|---|---|---|---|---|
| W | 1,160 | 9·26** | 8·95** | 0·36 |
| N | 1,160 | 1·91 | 0·77 | 0·00 |
| Species | 7,160 | 16012·9*** | 11590·6*** | 183·9*** |
| Y | 3,480 | 553·1*** | 405·0*** | 573·8*** |
| W × N | 1,160 | 1·01 | 0·22 | 0·01 |
| W × Y | 3,480 | 1·02 | 2·11 | 0·77 |
| N × Y | 3,480 | 0·40 | 0·21 | 4·95** |
| W × species | 7,160 | 0·67 | 0·65 | 1·98† |
| N × species | 7,160 | 1·26 | 2·64 | 2·03† |
| W × N × species | 7,160 | 0·60 | 0·80 | 1·40 |
| W × N × Y | 3,480 | 0·20 | 0·24 | 1·28 |
| W × species × Y | 21,480 | 0·46 | 0·98 | 1·52† |
| N × species × Y | 21,480 | 2·14** | 2·59*** | 1·36 |
| W × N × species × Y | 21,480 | 1·65* | 1·60* | 2·18** |
The degrees of freedom are shown as numerator,denominator.
†P < 0·10; *P < 0·05; **P < 0·01; ***P < 0·001.
Table 3.
Results (F-values) of repeated-measures ANOVA on the effects of warming (W), nitrogen addition (N), sampling year (Y) and their interactions on flowering time, fruiting time and reproductive duration for the eight species
| d.f. | Pa | Pb | Ac | Pt | Ab | Sk | Ha | Af | |
|---|---|---|---|---|---|---|---|---|---|
| Flowering time | |||||||||
| W | 1,20 | 4·64* | 1·51 | 1·57 | 3·16 | 2·21 | 0·20 | 0·03 | 0·09 |
| N | 1,20 | 0·08 | 0·13 | 4·24† | 0·01 | 0·02 | 0·46 | 6·08* | 0·35 |
| Y | 3,60 | 233·1*** | 386·7*** | 255·8*** | 331·6*** | 370·9*** | 2067·9*** | 33·9*** | 252·6*** |
| W × N | 1,20 | 1·25 | 0·46 | 0·69 | 0·90 | 0·65 | 0·07 | 0·96 | 0·19 |
| Y × W | 3,60 | 2·06 | 0·83 | 0·71 | 0·67 | 0·37 | 0·11 | 0·34 | 0·10 |
| Y × N | 3,60 | 7·48*** | 0·67 | 0·40 | 1·62 | 1·12 | 1·89 | 3·78* | 1·72 |
| Y × W × N | 3,60 | 1·58 | 3·44* | 0·69 | 1·15 | 2·04 | 0·07 | 2·36† | 1·12 |
| Fruiting time | |||||||||
| W | 1,20 | 8·35** | 1·01 | 0·55 | 2·83 | 0·37 | 0·55 | 1·14 | 0·05 |
| N | 1,20 | 0·04 | 0·14 | 11·06** | 0·04 | 1·25 | 1·02 | 2·53 | 0·01 |
| Y | 3,60 | 112·8*** | 112·8*** | 17·9*** | 415·8*** | 171·4*** | 1179·9*** | 15·7*** | 895·9*** |
| W × N | 1,20 | 0·38 | 0·08 | 0·54 | 0·82 | 1·80 | 0·96 | 0·96 | 0·51 |
| Y × W | 3,60 | 4·98** | 2·19† | 1·05 | 0·51 | 0·54 | 0·31 | 0·93 | 0·12 |
| Y × N | 3,60 | 4·75** | 2·57† | 2·71† | 0·93 | 2·23† | 1·32 | 2·52† | 2·31† |
| Y × W × N | 3,60 | 1·53 | 1·72 | 0·12 | 1·33 | 6·81** | 0·04 | 2·51† | 0·95 |
| Duration | |||||||||
| W | 1,20 | 0·48 | 6·08* | 0·00 | 0·97 | 9·09** | 0·17 | 3·86† | 0·29 |
| N | 1,20 | 0·56 | 1·29 | 1·53 | 0·14 | 27·12*** | 0·72 | 0·89 | 1·75 |
| Y | 3,60 | 90·2*** | 9·5*** | 562·5*** | 24·4*** | 1293·6*** | 116·6*** | 132·2*** | 604·8*** |
| W × N | 1,20 | 2·59 | 0·00 | 0·01 | 2·83 | 2·27 | 0·94 | 0·00 | 0·00 |
| Y × W | 3,60 | 8·22*** | 0·39 | 0·21 | 0·95 | 1·01 | 0·58 | 3·83* | 1·39 |
| Y × N | 3,60 | 3·39* | 5·23** | 0·90 | 0·29 | 5·17** | 0·87 | 0·88 | 0·66 |
| Y × W × N | 3,60 | 1·28 | 0·62 | 0·15 | 1·78 | 16·54*** | 0·96 | 5·73** | 0·77 |
The degrees of freedom are shown as numerator,denominator.
†P < 0·10; *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 4.
Changes in (A) flowering time, (B) fruiting time and (C) phenological duration (in days) under nitrogen addition and warming. Species are listed in order of mean time of buds first observed in control plots over the four growing seasons, beginning in April with P. acaulis and ending in October with A. frigida. Data are the mean ± s.e. for advanced (–) or delayed (+) phenology. For species' abbreviations see Table 1. †P < 0·1; *P < 0·05; **P < 0·01.
Table 1.
Information on the species examiined in this study
| Species name | Abbreviation | Life form | Pollination type | Cover (%) |
|---|---|---|---|---|
| Potentilla acaulis | Pa | Forb | Entomophilous | 3·44 |
| Potentilla bifurca | Pb | Forb | Entomophilous | 2·00 |
| Agropyron cristatum | Ac | Grass | Anemophily | 3·22 |
| Potentilla tanacetifolia | Pt | Forb | Entomophilous | 1·76 |
| Allium bidentatum | Ab | Forb | Entomophilous | 1·43 |
| Stipa krylovii | Sk | Grass | Anemophily | 4·45 |
| Heteropappus altaicus | Ha | Forb | Entomophilous | 1·43 |
| Artemisia frigida | Af | Shrub | Anemophily | 26·73 |
Cover is calculated as the average of the coverage in the control plots in late August from 2006 to 2009.
Across the eight species, warming effects on flowering time, fruiting time and reproductive duration did not vary with year (Table 2). At the species level, interactive effects between warming and year were found on the fruiting time of P. acaulis (P = 0·013) and on the reproductive durations of P. acaulis (P < 0·001) and H. altaicus (P = 0·014; Table 3). The fruiting time of P. acaulis was not affected by warming in 2006 and 2007, but advanced by 2·46 d (F = 8·45, P = 0·009) and 0·45 d (F = 3·46, P = 0·078) in 2008 and 2009, respectively (Supplementary Data Figs S1 and S2). The reproductive duration of P. acaulis was not changed by warming from 2006 to 2008, but was significantly shortened by 5·36 d in 2009 (F = 12·07, P = 0·002; Supplementary Data Figs S1 and S2). Warming showed various impacts on the reproductive duration of H. altaicus during different years, i.e. significant shortening in 2006 (–3·24 d; F = 8·92, P = 0·007) and 2007 (–3·70 d; F = 8·27, P = 0·009), extension in 2008 (+2·02 d; F = 6·60, P = 0·018) and no influence in 2009 (Supplementary Data Figs S1 and S2).
Nitrogen effects on species-level plant phenology
Although N addition showed no influence on flowering time across the eight species and the four seasons (Table 2), it significantly delayed the flowering time of H. altaicus (1·30 d; P = 0·023) and marginally postponed that of A. cristatum (1·31 d; P = 0·053; Table 3, Fig. 4A; Supplementary Data Figs S1 and S2). Similarly, N addition had no main effect on the fruiting time (Table 1) but significantly delayed that of A. cristatum (2·66 d; P = 0·003; Table 3, Fig. 4B; Supplementary Data Figs S1 and S2). For reproductive duration, no N effect was detected across all the eight species, while a significant reduction in the phenological duration of A. bidentatum (2·51 d; P < 0·001; Table 3, Fig. 4B; Supplementary Data Figs S1 and S2) was found under N treatment across the four growing seasons.
Across the eight species, the effect of N addition did not vary with year for flowering or fruiting time, but strongly depended on year for reproductive time (P = 0·002; Table 2). Across the eight species, N did not affect reproductive time in 2007 and 2009, but significantly changed reproductive time in 2006 (+1·74 d; F = 9·43, P = 0·003) and 2008 (–1·15 d; F = 4·68, P = 0·032), respectively (Supplementary Data Figs S1 and S2). At the species level, N addition and year interacted to affect the flowering time of P. acaulis (P < 0·001) and H. altaicus (P = 0·015), the fruiting times of P. acaulis (P = 0·005), and the reproductive duration of P. acaulis (P = 0·024), P. bifurca (P = 0·003) and A. bidentatum (P = 0·003) (Table 3; Supplementary Data Figs S1 and S2). The flowering time of H. altaicus was earlier in 2007 (2·15 d; F = 5·59, P = 0·028) but later in 2008 (2·15 d; F = 5·09, P = 0·035) in N plots. The flowering time of H. altaicus was marginally advanced in 2006 (1·24 d; F = 4·22, P = 0·053), not affected in 2007, but delayed in 2008 (2·39 d; F = 5·70, P = 0·027) and 2009 (2·99 d; F = 4·90, P = 0·039) by N addition (Supplementary Data Figs S1 and S2). The fruiting time of P. acaulis was significantly advanced in 2007 (2·33 d; F = 11·04, P = 0·003) but not changed in the other three years by N addition (Supplementary Data Figs S1 and S2). Nitrogen addition extended the reproductive duration of P. bifurca in 2006 (6·14 d; F = 15·83, P < 0·001) and 2007 (3·85 d; F = 3·45, P = 0·078) but not in the other three years, but shortened that of P. acaulis in 2008 (3·80 d; F = 12·67, P = 0·002) and of A. bidentatum in 2007 (3·11 d; F = 7·90, P = 0·011) and 2009 (5·29 d; F = 179·0, P < 0·001).
Interactive effects between warming and N addition on species-level phenology
Across the eight species, no interactive effect between N addition and warming was detected on flowering time, fruiting time or duration (all P > 0·10; Table 2). The additive effect between N addition and warming did not vary with year or species (all P > 0·10; Table 2). At the species level, no interactive effect between N addition and warming was detected on the flowering time, fruiting time or phenological duration of any species (all P > 0·10; Table 3). The interactive effect between N addition and warming only varied with year on the flowering time of P. bifurca (P = 0·022), the fruiting time of A. bidentatum (P < 0·001) and the reproductive durations of A. bidentatum (P < 0·001) and H. altaicus (P = 0·002; Table 3).
Variations with functional type
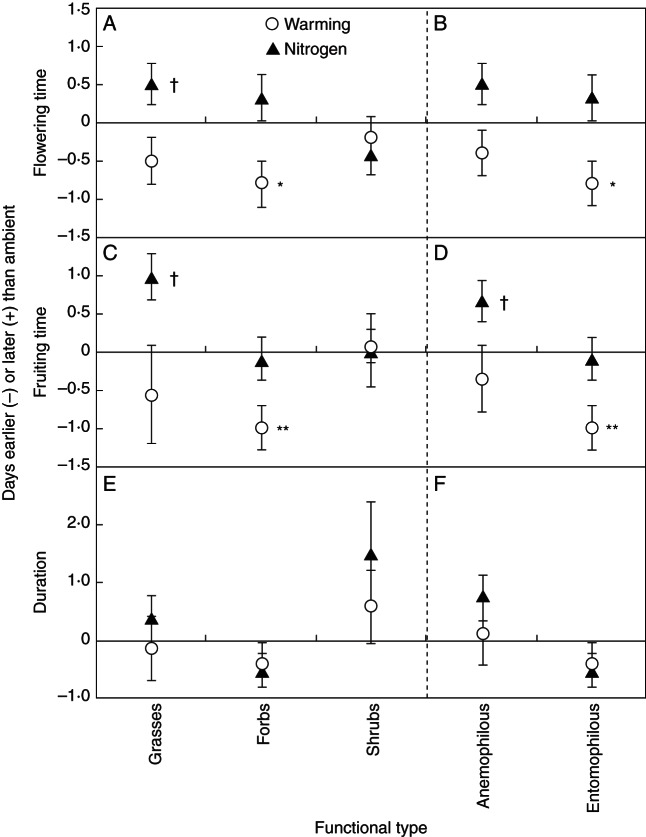
The impacts of N addition and warming varied among life forms when the eight species were divided into grasses, forbs and shrubs. N addition had no effect on any phenological event for any life form (all P > 0·10), except for a marginally significant delay effect on the flowering (F = 3·02, P = 0·098) and fruiting times (F = 3·61, P = 0·072) of grasses (Table 3, Fig. 5). Warming significantly advanced both the flowering (F = 8·03, P = 0·010) and fruiting (F = 9·51, P = 0·006) times of forbs (Fig. 5A, C) but did not affect those of grasses and shrubs (all P > 0·10; Fig. 5A, C). As a result, the phenological durations of all the three life forms were not changed by either N addition or warming (all P > 0·10; Fig. 5E). When the eight species were divided into different pollination types, none of the phenological events of anemophilous species was affected by either N addition or warming (all P > 0·10), except for a marginally significant delay effect of N addition on fruiting time (F = 3·12, P = 0·093; Fig. 5B, D). For entomophilous species, N addition did not change either the flowering or fruiting time (both P > 0·10; Fig. 5B, D), while warming advanced both the flowering (F = 8·04, P = 0·010; Fig. 5B) and fruiting (F = 9·51, P = 0·006; Fig. 5D) times, leading to no response of the phenological duration to either N addition or warming (both P > 0·1; Fig. 5F). Moreover, the responses of phenology among species also varied with the time of phenological events. Across the eight species, linear regression analyses showed that warming-induced changes in the flowering and fruiting times were positively correlated with the flowering (r2 = 0·47, P = 0·060; Fig. 6A) and fruiting (r2 = 0·49, P = 0·054; Fig. 6B) times, respectively.
Fig. 5.
Changes in (A, B) flowering time, (C, D) fruiting time and (E, F) phenological duration (in days) for different functional types under N addition and warming. †P < 0·1; *P < 0·05; **P < 0·01.
Fig. 6.
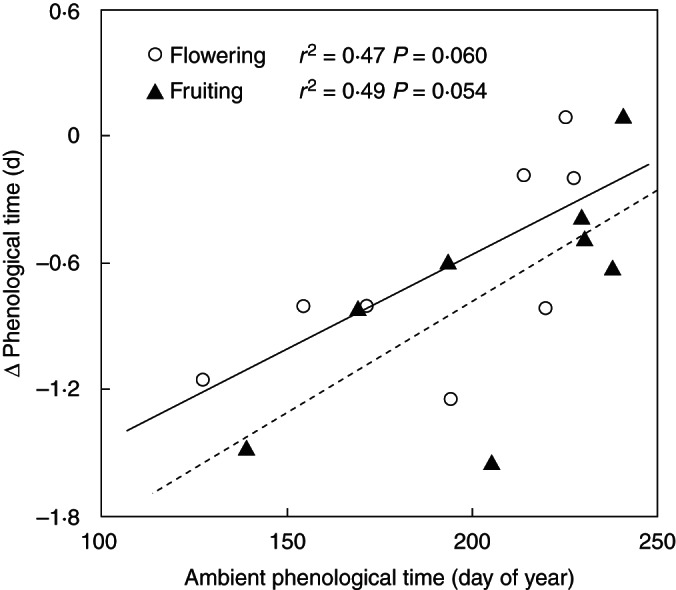
Dependence of the flowering and fruiting time responses on phenological times (day of year) under warming for all the eight species.
Reproductive allocation
Across all available species in 2008, warming had no impact on reproductive allocation, whereas N addition showed a marginally positive effect (F = 3·15, P = 0·077, three-way ANOVA; Fig. 7). At the species level, only N addition showed a marginally positive effect on the reproductive allocation of A. cristatum (F = 3·58, P = 0·065, two-way ANOVA; Fig. 7).
Fig. 7.
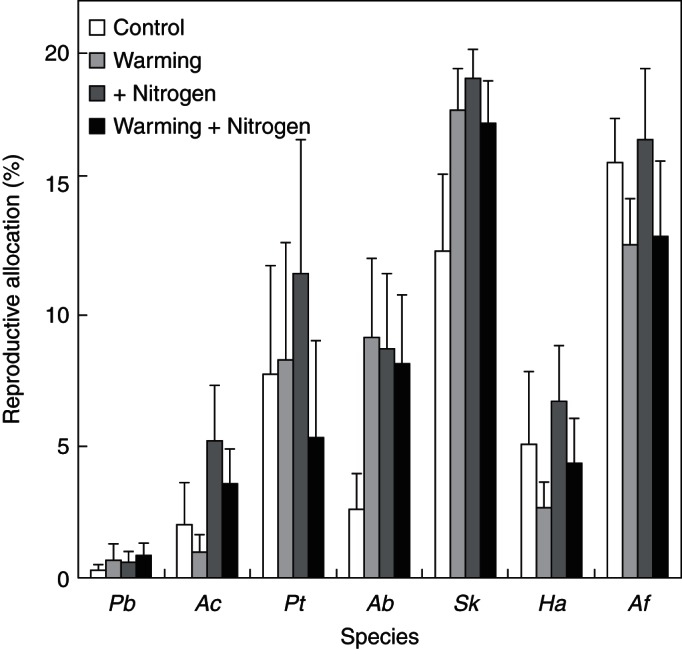
Reproductive allocation of all species under control, warming, N addition and warming plus N addition. Note that all fruits of P. acaulis had dropped before late August, so no data of reproductive allocation were obtained for P. acaulis. For species' abbreviations see Table 1.
DISCUSSION
Warming effects on plant phenology in temperate steppe in northern China
At the species level, warming advanced both the flowering and fruiting times over the four growing seasons (Fig. 4), suggesting earlier phenology of plant species under a warmer climate. These results are consistent with those of previous manipulative experiments (Price and Waser, 1998; Cleland et al., 2006; Sherry et al., 2007). Nevertheless, the 0·64 d per season advance in the flowering time in the warmed plots (increased soil temperature by 1·05 and 0·64 °C with and without N addition; Fig. 3C) is less than that reported from the grasslands in North America (Cleland et al., 2006; Sherry et al., 2007), suggesting that the effect of climate warming on plant phenology varies among ecosystems. The greater temperature sensitivity of phenology in early-blooming species, such as P. acaulis, than that in late-blooming species, such as A. frigida (Fig. 4), is in accordance with that in a sub-alpine meadow (Price and Waser, 1998). The direct evidence from manipulative experiments supports the long-term observations and remote sensing measurements which have indicated that the onset of spring had been advanced by 2–3 d, while the timing of growth senescence had been delayed by 0·3–1·6 d in the past decades (Myneni et al., 1997; Parmesan and Yohe, 2003; Root et al., 2003).
Although rising temperature led to earlier plant phenology in this ecosystem, the warming effect varied with plant functional type. In this study, warming advanced both the flowering and fruiting times of forbs but showed no impact on those of grass and shrub species (Fig. 5A, C). The observations differred from a previous study in North America which reported that both grasses and forbs flowered earlier under experimental warming (Cleland et al., 2006). In this ecosystem, forbs flowered significantly earlier than grasses and shrubs (both P < 0·001; one-way ANOVA). As an essential step in the sexual reproduction of plants, the pollination of flowers in grasslands relies on insects (entomophilous species) or wind (anemophilous species) for transfer of pollen among individual plants (Memmott et al., 2007). In this study, warming advanced both the flowering and fruiting times of entomophilous species but did not affect those of anemophilous species (Fig. 5). These results are in accordance with a previous analysis which found that insect-pollinated species were more sensitive to warming than wind-pollinated species in Britain (Fitter and Fitter, 2002). Given that the pollinators also vary seasonally, climate warming will influence the pollination success of entomophilous species and thus the seed ripening and dispersal (Fitter and Fitter, 2002), with a consequent impact on community structure and species composition in the long term.
Nitrogen effects on plant phenology in a temperate steppe in northern China
Plant species usually allocate more resources to reproduction in more N-rich soils (Tilman and Wedin, 1991). As a consequence, N addition will increase reproductive allocation and lead to a delay of fruiting time (Delph and Meagher, 1995). In this study, A. cristatum allocates more of its resources to reproduction (Fig. 7) and delays its fruiting time (Table 3; Fig. 4B) under N addition treatment. In contrast to warming, N addition tended to delay both the flowering and fruiting times of grasses slightly, but did not affect those of forbs and the shrub (Fig. 5). This suggests that when N availability increases, plant species in this N-limited ecosystem would delay grass switches in allocation from growth to reproduction (Cleland et al., 2006).
The different N responses of phenology among the life forms observed in this study are similar to the results in an annual grassland in North America (Cleland et al., 2006) and an alpine tundra (Smith et al., 2012) where N addition delayed flowering in grasses but accelerated flowering in forbs. The greater N sensitivity of flowering time in grasses than in forbs and shrubs could be ascribed to the differential N responses of growth among them. It has been widely detected that N addition benefits growth of grasses more than that of forbs and shrubs in various grassland ecosystems (Xia and Wan, 2008; Maskell et al., 2010; Stevens et al., 2010). Given that the phenology timing of grasses was later than that of forbs but earlier than that of the shrub in this ecosystem, the N-delayed phenology of grasses could benefit forbs but increase the resource competition between grasses and shrubs. In fact, in this experiment, N addition showed various impacts on different life forms, i.e. positive on grasses, neutral on forbs and negative on shrubs, across the four growing seasons (unpublished data).
Nitrogen addition showed no impact on the phenological times of entomophilous species except for a marginally significant effect on the fruiting time of anemophilous species (Fig. 5). Given that the wind pattern in this ecosystem remained relatively stable within the phenological period (June–September) for anemophilous species (Xia et al., 2010), the slight shift in the phenological times would not impact community structure and species composition under N addition in this ecosystem. Because our knowledge on N response of plant phenology is still limited, these results could help improve the understanding of phenological changes in plant community in semi-arid grassland under increasing atmospheric N deposition.
Additive effect of warming and N addition on plant phenology
Across the four growing seasons, no interactive effect between warming and N addition on any phenological event was detected for any species in this study (Tables 1 and 2), suggesting an additive effect between warming and N addition on plant phenology in this ecosystem. The absence of an interactive effect between warming and N addition in this study could be ascribed to the species-specific responses of plant phenology, i.e. the advanced effects of warming were driven by two forbs (P. acaulis and P. tanacetifolia) whereas the delayed impacts of N were not associated with a specific growth form (A. cristatum and H. altaicus; Fig. 4). At the species level, the interactive effect between warming and N addition was only detected on the fruiting time and reproductive duration of A. bidentatum in 2007 and 2009 and the reproductive durations of H. altaicus in 2008 and 2009. Our results do not confirm the findings from an experiment in North America where a negative interactive effect between warming and N addition on plant phenology was detected (Cleland et al., 2006). This suggests that temperature sensitivity of plant phenology may be differently affected by soil N availability in various regions.
Implications for ecosystem C uptake in semi-arid grassland
The greater warming responses of early-blooming species than late-blooming species observed in this study (Fig. 6A) suggest a longer growing season under climate warming at the community level. The warming-prolonged growing season at the community level in this ecosystem is consistent with those reported in many previous field observations (Menzel and Fabian, 1999; Ahas et al., 2000). The extended growing season suggests a longer period of active plant growth at the community level. It has served as one of the important mechanisms for enhancing plant growth and primary production in the northern hemisphere over the past decades (Myneni et al., 1997; Nemani et al., 2003). However, most species (five of eight) in this study did not show change in the phenological duration under climate warming (Fig. 4C), indicating that most species in this semi-arid steppe could adapt the reproductive duration by coupled shifts of starting and ending phenological times under climate warming. Our observations differ from those in northern America where experimental warming significantly extended the phenological duration of all the eight species in a sub-alpine meadow (Dunne et al., 2003) and seven of the 12 species in a tallgrass prairie (Sherry et al., 2007). Thus, it remains unclear whether climate warming can extend active growth of individual plant species in this ecosystem, which totally determines the active C uptake time at the community level.
Conclusions
Plant phenology has been shown to be highly temperature sensitive and is a biosphere indicator of climate change (Peñuelas and Filella, 2001). In this study, warming advanced both the flowering and fruiting times of plant species, leading to no impact on the reproductive duration in a temperate steppe in northern China. The phenological time of early-blooming species is advanced more than that of late-blooming species under warming, leading to a longer growing season at the community level. Although N addition affected the phenology of several species, no interactive effect between warming and N addition was found on plant reproductive phenology in this ecosystem. Our observations will facilitate long-term projections of regional plant phenology and its contribution to C cycling in the temperate steppe in northern China. This study indicates that climate warming and N addition will affect plant phenology independently of each other in the Inner Mongolian steppe, and more information about interactive effects between warming and other global change factors, e.g. CO2 elevation and precipitation changes, on plant phenology is needed.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors thank Professor Bill Shipley and two anonymous reviewers for their valuable comments on the original version of this manuscript. We also thank S. Gu and R. Sherry for their help in data analysis. and S. Niu, Z. Wang, M. Wu, Y. Li, L. Yan, Y. Zhang, W. Cheng, Z. Li, Y. Han, Z. Zhang, D. Lin and T. Li for help in field measurement. This work was financially supported by the National Natural Science Foundation of China (41030104/D0308, 30925009 and 90511006).
LITERATURE CITED
- Ahas R, Jaagus J, Aasa A. The phenological calendar of Estonia and its correlation with mean air temperature. International Journal of Biometeorology. 2000;44:159–166. doi: 10.1007/s004840000069. [DOI] [PubMed] [Google Scholar]
- Bai Y, Wu J, Clark CM, et al. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Global Change Biology. 2010;16:358–372. [Google Scholar]
- Cleland EE, Chiariello NR, Loarie SR, Mooney HA, Field CB. Diverse responses of phenology to global changes in a grassland ecosystem. Proceedings of the National Academy of Sciences, USA. 2006;103:13740–13744. doi: 10.1073/pnas.0600815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. Shifting plant phenology in response to global change. Trends in Ecology and Evolution. 2007;22:357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, et al. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- Delph LF, Meagher TR. Sexual dimorphism masks life history trade-offs in the dioecious plant Silene latifolia. Ecology. 1995;76:775–785. [Google Scholar]
- Dewald L, White TL, Duryea ML. Growth and phenology of seedlings of four contrasting slash families in ten nitrogen regimes. Tree Physiology. 1992;11:255–269. doi: 10.1093/treephys/11.3.255. [DOI] [PubMed] [Google Scholar]
- Dunne JA, Harte J, Taylor KJ. Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecological Monographs. 2003;73:69–86. [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- Gu S, Hui D, Bian A. The contraction–expansion algorithm and its use in fitting nonlinear equations. Journal of Biomathematics. 1998;13:426–434. [Google Scholar]
- Liu Q, Yin H, Chen J, Zhao C, Cheng X, Wei Y, Lin B. Belowground responses of Picea asperata seedlings to warming and nitrogen fertilization in the eastern Tibetan Plateau. Ecological Research. 2011;26:637–648. [Google Scholar]
- Lupi C, Morin H, Deslauriers A, Rossi S, Houle D. Increasing nitrogen availability and soil temperature: effects on xylem phenology and anatomy of mature black spruce. Canadian Journal of Forest Research. 2012;42:1277–1288. [Google Scholar]
- Majdi H. Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in Northern Sweden. Global Change Biology. 2004;10:182–188. [Google Scholar]
- Maskell LC, Smart SM, Bullock JM, Thompson K, Stevens CJ. Nitrogen deposition causes widespread loss of species richness in British habitats. Global Change Biology. 2010;16:671–679. [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecology Letters. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Menzel A. Phenological anomalies in Germany and their relation to air temperature and NAO. Climate Change. 2003;57:243–263. [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. [Google Scholar]
- Nemani RR, Keeling CD, Hashimoto H, et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science. 2003;300:1560. doi: 10.1126/science.1082750. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pau S, Wolkovich EM, Cook BI, et al. Predicting phenology by integrating ecology, evolution and climate science. Global Change Biology. 2011;17:3633–3643. [Google Scholar]
- Peñuelas J, Filella I. Phenology: responses to a warming world. Science. 2001;294:793. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Biel C, Estiarte M. Growth, biomass allocation, and phenology responses of pepper to elevated CO2 concentrations and different water and nitrogen supply. Photosynthetica. 1995;31:91–99. [Google Scholar]
- Peñuelas J, Filella I, Comas P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Global Change Biology. 2002;8:531–544. [Google Scholar]
- Piao S, Friedlingstein P, Ciais P, Viovy N, Demarty J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Global Biogeochemical Cycles. 2007;21 GB3018. http://dx.doi.org/10.1029/2006GB002888 . [Google Scholar]
- Price MV, Waser NM. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology. 1998;79:1261–1271. [Google Scholar]
- Rathcke B, Lacey EP. Phenological patterns of terrestrial plants. Annual Review of Ecology, Evolution, and Systematics. 1985;16:179–214. [Google Scholar]
- Richards FJ. A flexible growth function for empirical use. Journal of Experimental Botany. 1959;10:290–301. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Bange MP, Milroy SP. Reproductive allocation of cotton in response to plant and environmental factors. Annals of Botany. 1997;80:75–81. [Google Scholar]
- Schwartz MD, Ahas R, Aasa A. Onset of spring starting earlier across the Northern Hemisphere. Global Change Biology. 2006;12:343–351. [Google Scholar]
- Sherry RA, Zhou X, Gu S, et al. Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences, USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Sconiers W, Spasojevic M, Ashton I, Suding K. Phenological changes in alpine plants in response to increased snowpack, temperature, and nitrogen. Arctic, Antarctic, and Alpine Research. 2012;44:135–142. [Google Scholar]
- Stevens CJ, Duprè C, Dorland E, et al. The impact of nitrogen deposition on acid grasslands in the Atlantic region of Europe. Environmental Pollution. 2010;159:2243–50. doi: 10.1016/j.envpol.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Tilman D, Wedin D. Plant traits and resource reduction for five grasses growing on a nitrogen gradient. Ecology. 1991;72:685–700. [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, et al. Warming experiments underpredict plant phenological responses to climate change. Nature. 2012;485:494–497. doi: 10.1038/nature11014. [DOI] [PubMed] [Google Scholar]
- Xia J, Wan S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist. 2008;179:428–439. doi: 10.1111/j.1469-8137.2008.02488.x. [DOI] [PubMed] [Google Scholar]
- Xia J, Wan S. The effects of warming-shifted plant phenology on ecosystem carbon exchange are regulated by precipitation in a semi-arid grassland. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032088. e32088. http://dx.doi.org/10.1371/journal.pone.0032088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chen S, Wan S. Impacts of day versus night warming on soil microclimate: Results from a semiarid temperate steppe. Science of the Total Environment. 2010;408:2807–2816. doi: 10.1016/j.scitotenv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang S, Allen HL, Dougherty PM. Shoot and foliage growth phenology of loblolly pine trees as affected by nitrogen fertilization. Canadian Journal of Forest Research. 1997;27:1420–1426. [Google Scholar]
- Zhang Y, Zheng L, Liu X, et al. Evidence for organic N deposition and its anthropogenic sources in China. Atmospheric Environment. 2008;42:1035–1041. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.