Abstract
Background and Aims
‘Human-red’ flowers are traditionally considered to be rather unpopular with bees, yet some allogamous species in the section Oncocyclus (genus Iris, Iridaceae) have evolved specialized interactions with their pollinators, a narrow taxonomic range of male solitary bees. The dark-red, tubular flowers of these irises are nectarless but provide protective shelters (i.e. a non-nutritive form of reward) primarily to male solitary bees (Apidae, Eucerini) that pollinate the flowers while looking for a shelter. An earlier study on orchids suggested that species pollinated predominantly by male solitary bees produce significantly larger amounts and larger numbers of different n-alkenes (unsaturated cuticular hydrocarbons). Whether or not this also applies to the Oncocyclus irises and whether pollinators are attracted by specific colours or scents of these flowers is unknown.
Methods
Using Iris atropurpurea, recording of pollinator preferences for shelters with different spatial parameters was combined with analyses of floral colours (by spectrophotometry) and scents (by gas chromatography–mass spectrometry) to test the hypotheses that (a) pollinators significantly prefer floral tunnels facing the rising sun (floral heat-reward hypothesis), and that (b) flowers pollinated predominantly by male solitary bees produce significantly larger amounts and larger numbers of unsaturated cuticular hydrocarbons (n-alkenes) in their floral scent (preadaptation to sexual-deception hypothesis).
Key Results
Male bees do not significantly prefer shelters facing the rising sun or with the presence of high absolute/relative amounts and numbers of n-alkenes in the floral scent.
Conclusions
The results suggest that the flowers of I. atropurpurea probably evolved by pollinator-mediated selection acting primarily on floral colours to mimic large achromatic (‘bee-black’) protective shelters used preferentially by male solitary bees, and that pollinator visits are presumably not the result of an odour-based sexual stimulation or motivated by an increased morning floral heat reward in tunnels facing the rising sun.
Keywords: Shelter mimicry, floral evolution, pollinator preferences, floral scents, floral colours, Iris atropurpurea, Oncocyclus
INTRODUCTION
Flowering plants around the world deploy a spectacular diversity of floral forms, colours and scents, all interacting in varying degrees, to entice foraging insects that inadvertently move pollen between or within flowers and bring about pollination in the process (Proctor et al., 1996). Bees in particular, with approx. 20 000 species described worldwide (Michener, 2007) and their tight interdependence with flowering plants, are key to the sexual reproduction of both wild plants and cultivated crops (Free, 1993; Proctor et al., 1996; Biesmeijer et al., 2006; Klein et al., 2007; Potts et al., 2010). Bees use combinations of visual, olfactory and tactile floral traits to locate floral resources (von Frisch, 1919; Lacher, 1964; Vareschi, 1971; Barth, 1985; Kevan and Lane, 1985; Menzel, 1985; Dobson, 1994; Giurfa et al., 1996; Whitney et al., 2009), and although we still know very little about their innate preferences for colours and scents (see, for example, Dötterl and Vereecken, 2010), a series of experimental studies has reported that bees show distinct sensory biases for violet- and blue-coloured flowers (Lunau et al., 1996; Keasar et al., 1997; Raine and Chittka, 2007). Although these innate preferences might become less prevalent as the foraging bees build up individual experience with other flowers (Gumbert, 2000; Chittka et al., 2001), certain combinations of hue, saturation and contrast of floral colours that we perceive as yellow, blue, violet and white are traditionally associated with the attraction of bees (Lunau and Maier, 1995; Proctor et al., 1996).
By contrast, red flowers as we perceive them (‘human-red’) have classically been associated with the pollination by hummingbirds (trochilophily) or by butterflies (psychophily) (Faegri and Van der Pijl, 1979; Proctor et al., 1996; Fenster et al., 2004, and references therein) and have been suggested repeatedly to go unnoticed by bees (but see Lack and Kevan, 1987; Irwin and Brody, 1999; Briscoe and Chittka, 2001). Experimental evidence from recent studies indicates that changes in a few genes with large phenotypic effect (e.g. the change from red to another colour), when combined with variation in external ecological factors (e.g. pollinator efficiency or visitation rates), have the potential to drive shifts in pollination ‘syndromes’ from melittophily to trochilophily (Schemske and Bradshaw, 1999; Bradshaw and Schemske, 2003; Wilson et al., 2006; Thomson and Wilson, 2008, and references therein). These results suggest that red, as a colour, has a filtering effect on pollinators, discouraging bees (see also Raven, 1972; Castellanos et al., 2004; Lunau et al., 2011) whose visual receptors are more sensitive to other wavelengths, namely to the UV-blue-green range of the spectrum (see also Spaethe et al., 2001; Wilson et al., 2006). Bees have long been considered to be red-blind because pure red colours usually absorb light across the 30- to 630-nm range and reflect light in wavelengths where the bees' discrimination capabilities (and the relative excitation of their green visual receptor) are comparatively very low (e.g. Chittka et al., 1992). However, the analysis of floral colours from a human perspective has limitations, and several studies have challenged this theory of the bees' red-blindness. For example, it has been reported that a significant proportion of the ‘human-red’ floral colours might actually reflect sufficient UV or blue light to be perceived as coloured by bees (Menzel and Shmida, 1993; Chittka et al., 1994; Chittka and Waser, 1997). Furthermore, several studies have provided evidence that pure red flowers (i.e. those with a reflectance restricted to longer wavelengths) seen against a green foliage background might provide sufficient achromatic contrast to be detected by the bees' green receptor (Chittka and Waser, 1997; Kevan et al., 2001; Reisenman and Giurfa, 2008; Martinez-Harms et al., 2010).
Among the few ‘human-red’ flowers that have evolved specialized interactions with bees are species belonging to the section Oncocyclus of the genus Iris (Iridaceae). These plants are endemic to dry, Mediterranean-type climates, particularly to the semi-desert areas of the Middle-East, Turkey and the Caucasus (Mathew, 1989). The tunnel-like flowers of the Oncocyclus irises are large and some species display dark-red petals with a characteristic black disc marking the entrance of the floral tunnels (Fig. 1). The flowers do not produce nectar but provide protective shelters (i.e. a non-nutritive form of reward) primarily to male solitary bees, particularly by eucerine bees (Apidae, Eucerini), that pollinate the flowers during periods of overcast weather or late in the afternoon while looking for an overnight shelter (Sapir et al., 2005; Monty et al., 2006). The same bees that pollinate the Oncocyclus irises can also be found in ‘natural’ shelters, i.e. in rock crevices, under flat stones or in hollow wood stems, at exactly the same time of day and/or under the same climatic conditions (N. J. Vereecken, pers. obs.). These male eucerine bees generally ignore the flowers altogether during daytime when they visit flowers of other plants for the collection of pollen and nectar. These circumstances led to the hypothesis that the Oncocyclus irises mimic protective shelters used by male solitary bees (Sapir et al., 2005), a phenomenon also observed in the Mediterranean orchid genus Serapias (Vöth, 1980; Dafni et al., 1981; Vereecken et al., 2012).
Fig. 1.
Shelter mimicry in Iris atropurpurea (section Oncocyclus, genus Iris): (A) detail of a flower of I. atropurpurea at the study site of Netanya; (B, C) males of Synhalonia spectabilis (Hymenoptera, Apidae) inside (B) and at the entrance (C) of the floral tube of I. atropurpurea; (D) male of Megachile (Chalicodoma) sicula (Hymoptera, Megachilidae) leaving a floral tube of I. atropurpurea. The white pollen grains are visible on the body of the male bees in (B) and (D). All photographs by N. J. Vereecken.
Both Dafni et al. (1981) and Sapir et al. (2006) observed that the temperature in the floral tunnels of shelter-mimicking species reached a maximum of 2·5 °C above that of the ambient air in the morning, and Sapir et al. (2006) therefore suggested that the dark flowers of the Oncocyclus irises had evolved to gather heat when exposed to solar radiation early in the morning. Sapir et al. (2006) postulated that this increased morning floral heat was used as a reward by the pollinators, which significantly preferred east-facing floral tunnels (i.e. facing the rising sun) as shelters. Although the floral heat-reward hypothesis in Oncocyclus irises is based on little experimental evidence (see below), it has become popular in the literature and in modern textbooks on pollination biology (see, for example, Willmer, 2011). In a recent study on the floral scents of various species of Mediterranean orchids, including representatives of the orchid genus Serapias, Schiestl and Cozzolino (2008) suggested that species pollinated predominantly by male solitary bees produce significantly larger amounts and higher numbers of different n-alkenes (unsaturated cuticular hydrocarbons) than the species pollinated either primarily by female bees or other insects. These compounds are virtually absent in floral scents of plants pollinated by female bees and other pollinators, but they are found in significant amounts (and high numbers of different compounds) in the floral scents of a wide range of Ophrys orchids where they are key pollinator (male bees) attractants (Schiestl and Cozzolino, 2008; Vereecken and McNeil, 2010). Schiestl and Cozzolino (2008) therefore suggested that the specific attraction of male bees could be driven by the increased absolute amounts and numbers of these compounds in the floral scents, and that the presence of these compounds in floral scents may be a possible pre-adaptation to the evolution of pollination sexual deception (sexual mimicry). Whether or not this phenomenon also applies to the Oncocyclus irises pollinated primarily by male eucerine bees has not been investigated so far.
We investigated the adaptive significance of floral traits in Iris atropurpurea, a member of the section Oncocyclus. We combined field surveys of pollinators with investigations of floral colours (by spectrophotometry), and scents [by gas chromatography–mass spectrometry (GC-MS)] to assess the adaptive significance of phenotypic characteristics as to the following. (a) How do pollinators perceive the floral colours of I. atropurpurea? (b) Do pollinators preferentially visit floral tunnels facing the rising sun? (c) Do pollinators exhibit preferences for floral tunnels emitting specific blends of compounds in their floral scent? In light of our results, we provide an alternative explanation for the evolution of dark-red colours in Oncocyclus irises, and we re-examined the role of floral scents in these highly specialized interactions with male solitary bees as pollinators.
MATERIALS AND METHODS
Study species and sites
Iris atropurpurea Baker belongs to the section Oncocyclus and is endemic to the coastal plains of Israel and usually blooms in early spring, from late January to early April. Individual plants reach up to 40 cm in height and bear three large, dark-red flowers which are 7–9 cm in diameter (Lynch, 1904; Mathew, 1989). Observations were conducted in populations of Iris atropurpurea within the central coastal plains of Israel (32·25 °N, 34·85 °E) between February and March 2011. All the experiments were carried out in March 2011.
Surveys of solitary bees sheltering in flowers
To investigate if night-sheltering male bees preferentially visited east-facing floral tunnels, we monitored flowers throughout March at Yaqum and Netanya in 2011. We focused on night-sheltering visits because prior observations showed that bees rarely sheltered in flowers during the daytime (Sapir et al., 2005), unless during periods of overcast weather. The tunnels of each flower were sampled for night-sheltering male bees either 1 h before sunset or shortly after sunrise. Each observer examined all open flowers in a designated area of the population and over 30 and 60 min. When bees were found in the floral tubes, those flowers were tagged and the following information was recorded: the number of individuals per species of bee per tunnel, the orientation of the occupied tunnels (eight compass bearings), and the number of flowers surveyed. A sample of bees was collected from flowers and deposited individually into labelled vials for later identification.
In a second experiment, three separate patches of I. atropurpurea at Yaqum were selected in the centre of the population to determine if the same individuals returned to the same flowers on consecutive nights. Seven flowers in each patch were tagged and colour coded pink, green and orange, and then sampled for bees. The experimental patches were approx. 50 m2. Each evening over a 5-d period, those bees found in flowers were marked on the abdomen with fluorescent markers (pens), corresponding to the colour of the tagged flowers in each designated experiential patch. Each patch was sampled for marked bees using ultraviolet light during consecutive nights.
The voucher specimens of bees collected during this study are deposited in the collection of the Laboratory of Pollination Ecology, Institute of Evolution, University of Haifa, Israel.
Chemical analyses
In both the Yaqum and Netanya populations, flowers hosting male bees in the same tunnels over consecutive nights were tagged and the falls (lower petals) were collected for floral scent analysis. We extracted the odour compounds from the falls of control unvisited (n = 26) and visited (n = 23) tunnels in 2 mL of n-hexane (HPLC grade) for 2 min to characterize the patterns of cuticular hydrocarbons and their derivatives (for details on the method, see Mant et al., 2005). All samples were then stored at –20 °C.
Prior to the analyses, 50 ng of n-octadecane (n-C18) were added as an internal standard and the extracts were analysed by GC-MS on a Finnigan Trace Ultra GC coupled to a Finnigan POLARIS Q ion trap mass. We used an Agilent DB-5 MS column (30 m length × 0·25 mm diameter × 25 µm film thickness). Aliquots (1 µL) of the extracts were injected in splitless mode at 50 °C (1 min, followed by a programmed increase of oven temperature to 300 °C at a rate of 10 °C min−1; helium was used as carrier gas.
We identified the compounds by comparisons of the mass spectrum and retention time of known reference substances (purchased from Dr Ehrenstorfer GmbH, Germany), and the double-bond positions in mono-unsaturated compounds were assigned by DMDS derivatization according to Buser et al. (1983) and Dunkelblum et al. (1985).
All compounds found in the floral odour were used for the statistical analyses. Their absolute amounts were calculated by dividing the peak area of each compound by the peak area of the internal standard and multiplied with the internal standard amount. Relative proportions (%) were calculated by summing the absolute amounts of all compounds; absolute amounts of individual compounds were then divided by the sum and multiplied by 100.
Spectral measurements of flowers
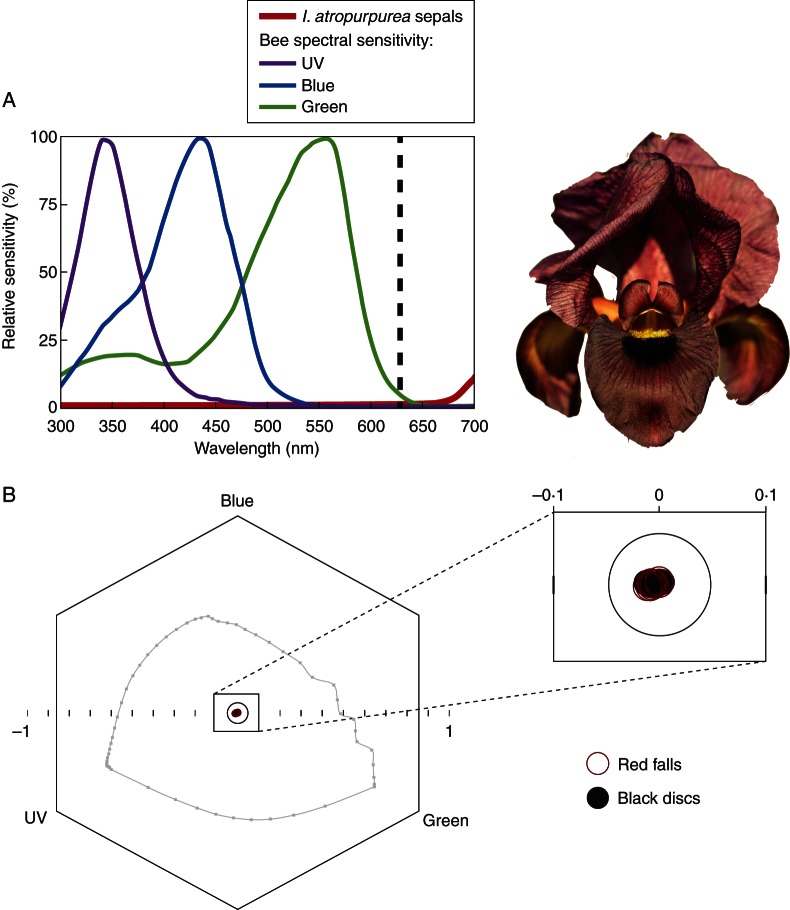
We used a portable spectrophotometer (AVASPEC-2048-USB2-UA; Avantes, Eerbeek, The Netherlands) equipped with a Xenon light source (AVALIGHT-XE; Avantes) to measure the relative reflectance (in %, 300–700 nm in 5-nm steps) of ten flowers of Iris atropurpurea picked randomly at the study site of Yaqum. The spectrophotometer was calibrated with a white standard (WS-2; Avantes). We measured the reflectance of the black disc that delimits the entrance of the floral tube as well as that of the dark-red falls (see Figs 1A and 2A). We used the spectral sensitivity functions of the honeybee (Apis mellifera) because they are representative of a wide taxonomic spectrum of higher Hymenoptera, and they are largely consistent within the Apoidea (bees sensu lato) (Peitsch et al., 1992; Chittka and Kevan, 2005). We plotted the mean relative reflectance (in %) of the red falls together with the relative spectral sensitivity curves (in %) across the 300–700 nm range (see Fig. 5A). We then converted the raw data (relative reflectance measurements of red sepals and black discs) into individual loci in the bee colour hexagon (Chittka, 1992) by using the honeybee receptor-sensitivity curves and a mid-grey adaptation background of 30 % reflectance across all wavelengths (300–700 nm). We assessed and quantified the colour and achromatic contrasts between the dark-red sepals and the black discs by calculating (a) pairwise Euclidean distances between loci and (b) the mean Euclidean distance between the species centroids in the bee colour hexagon. The Euclidean distance between any two loci indicates the perceived colour difference or contrast between the stimuli, and threshold values of hexagon units for colour discrimination usually range between 0·062 (Dyer and Chittka, 2004a, b; Dyer et al., 2008) and 0·100 (Chittka et al., 1997) for bees.
Fig. 5.
Spectral analysis of Iris atropurpurea flowers. (A) Mean relative reflectance (in %) as a function of wavelength for sepals of I. atropurpurea (red curve, n = 20) against the spectral sensitivity functions of the UV, blue and green receptors of bees drawn from Chittka and Kevan (2005). The vertical dashed line indicates wavelengths above which bees have virtually no discrimination capability (no green receptor excitation). (B) Loci of the black discs and the dark-red colours of falls (lower sepals) of I. atropurpurea in the bee colour hexagon. All loci included in the 0·1 hexagon unit centre are considered achromatic from the bees' perspective, and the thin grey line illustrates loci of pure monochromatic lights at the background intensity.
Statistical analyses
Because the variances in our data were not homogeneous, we used a Mann–Whitney U-test with a Bonferroni correction (α = 0·05 divided by the number of comparisons) to compare both the absolute (in ng) and the relative amounts (in % of the total blend) of n-alkanes vs. n-alkenes vs. the compounds belonging to other categories in the floral scent extracts. We then compared the mean absolute amounts of odour compounds emitted by visited vs. non-visited flowers of I. atropurpurea by using Student's t-test. These tests were made using the SPSS 17·0 statistical software.
Because the multivariate normality of variances of our ‘individuals × compounds’ matrix was not met [Shapiro–Wilk normality test, W = 0·1273, P-value = 1·654 × 10−15, test in the mvnormtest package (Jarek, 2012) in R (R Core Team, 2012)], we made two multivariate non-parametric analyses based on Multiple Response Permutation Procedures (MRPP) with the average Bray–Curtis distances weighted to group size and 999 permutations (Mielke and Berry, 2001; McCune and Grace, 2002) to test H0 of no difference in floral scent (relative amounts, in %) according (a) to the orientation of the floral tubes (eight compass bearings) and (b) to the flower visitation patterns observed under natural conditions (visited vs. non-visited flowers). The MRPP tests were performed with the vegan package (version 2·0-5) (Oksanen et al., 2012) in R (R Core Team, 2012).
To detect floral scent compounds with presences statistically associated with specific orientations of the floral tubes or with visitation patterns observed under natural conditions (visited vs. non-visited flowers), we used an Indicator Species Analysis with 999 random permutations. The computed Indicator Value of each compound reflects both its relative abundance (specificity) and its relative frequency (fidelity), and the associated P-values indicated if specific compounds are significant indicators of certain groups (here, the orientation of the floral tubes or with visitation patterns observed). The Indicator Species Analysis was carried out with the indicspecies package (De Caceres and Legendre, 2009) in R (R Core Team, 2012).
To visualize the floral scent dissimilarities among samples associated with different groups (the orientation of the floral tubes visits vs. visitation patterns observed), we used a non-metric multi-dimensional scaling (nMDS) ordination based on a matrix of Bray–Curtis dissimilarities calculated on the relative proportions of odour compounds (in % of the total blend). The appropriateness of the nMDS results was assessed by comparing, in a Shepard diagram, the distances among samples in the ordination plot with the original distances, and the stress value generated by the nMDS analysis reflects how well the ordination summarizes the observed distances among the samples. The nMDS analysis was performed with the vegan package (version 2·0-5; Oksanen et al., 2012) in R (R Core Team, 2012).
We used Rayleigh's Z-test of uniformity to assess the significance of the mean resultant length which determines whether the distribution of sheltering male bees in floral tunnels with different orientation is random or non-random (i.e. are the azimuths of the distribution clumped in a particular direction?). We also used the same approach to test whether the occupancy of floral shelters by male solitary bees follow a unimodal distribution with a specified mean direction (here, the floral tunnels pointing towards the east) and unknown mean resultant length. All directional statistical analyses were performed with the circular package (version 0·4-3; Agostinelli and Lund, 2011) in R (R Core Team, 2012). We also tested the null hypothesis of a random distribution of male bees in floral tunnels with a G-test for goodness-of-fit (likelihood ratio or log-likelihood test). The randomly expected numbers were calculated using the theoretical expectation that male bees have an equal likelihood of visiting floral tunnels of each orientation (McDonald, 2009). We used the ratio between the total number of male bees observed and the number of orientations of the floral tunnels (690/8 = 86·25) as an expected value, and the significance level was set to α = 0·05. We tested the 28 possible comparisons by performing χ2 post-hoc tests, and to avoid Type I errors, we used a Bonferroni correction by dividing α = 0·05 by the number of comparisons (28), yielding a critical value of 0·00178 against which all P-values were tested.
RESULTS
Pollinators of Iris atropurpurea
The tunnels of 5934 individual flowers of I. atropurpurea were surveyed for sheltering male bees, and we observed aggregates of up to 12 sheltering male bees in a single flower (mean of all the flowers checked over the entire season = 2·03 ± 1·85 s.d. and 1·53 ± 1·50 s.d. in Yaqum and Netanya, respectively). Of those flowers sampled for bee preferences in relation to orientation of tunnels, 553 individuals were recorded. From those flowers sampled over 33 mornings/evenings, 5·7 % contained bees. Between 16 % and 19 % of flowers sampled hosted male bees in the same tunnels over 2–4 consecutive nights in the Netanya and Yaqum populations. During the peak of aggregation, between 23 and 28 March 2011 (i.e. towards the end of the flowering season), 59 % of those bees marked returned to the same flowers in experimental patches (marked bees; n = 17; colour-coded flowers; n = 21), i.e. bees marked pink returned to pink patches and bees marked orange returned to orange-tagged flowers. In two cases, two bees marked orange returned to the same tagged flowers in the pink patch and one individual marked pink was found in a flower in the green patch. The remainder of flowers sampled in the three patches had either bees with no marks or no bees in tunnels. Despite the small sample size, this result suggests that the same individual bees returned to the same flowers over consecutive nights, but this behaviour was only observed towards the end of the flowering season.
Among the male solitary bees collected at our study sites, we recorded ten species belonging to the Eucerini tribe, including three previously undescribed species (see also Watts et al., 2013). Males of Synhalonia spectabilis (Hymenoptera, Apidae, Eucerini; Fig. 1) were by far the most frequent sheltering individuals of all bee species collected across sites and seasons, and the most frequent species found to form sheltering aggregations in the floral tubes. We occasionally found other Hymenoptera in the flowers, including males of the mason bee Megachile (Chalicodoma) sicula (Hymenoptera, Megachilidae, Megachilini) (Fig. 1), and, more rarely, workers of the social wasp Vespa germanica (Hymenoptera, Vespidae) and of the bumblebee Bombus terrestris (Hymenoptera, Apidae), a non-native species introduced in the study region for the pollination of agricultural crops (see Dafni and Shmida, 1996).
Floral scent differentiation, shelter orientation and pollinator preferences
The odour extracts of fresh individual falls (lower sepals) of I. atropurpurea consisted primarily of mixtures of long, straight-chained n-alkanes ranging from 19 to 33 carbon atoms and, to a lesser extent, of their associated n-alkenes. Collectively, they represent on average 90·03 % (± 4·87 %) of all compounds identified in the floral scent extracts. We found that the mean (± s.e.) total absolute amounts (in ng) and relative amounts (in % of the total blend) of n-alkenes were significantly lower than those of both their corresponding n-alkanes and of two unknown compounds (marked as ‘other’) (Mann–Whitney U-test with Bonferroni correction, two-tailed P < 0·0001; Fig. 2).
Fig. 2.
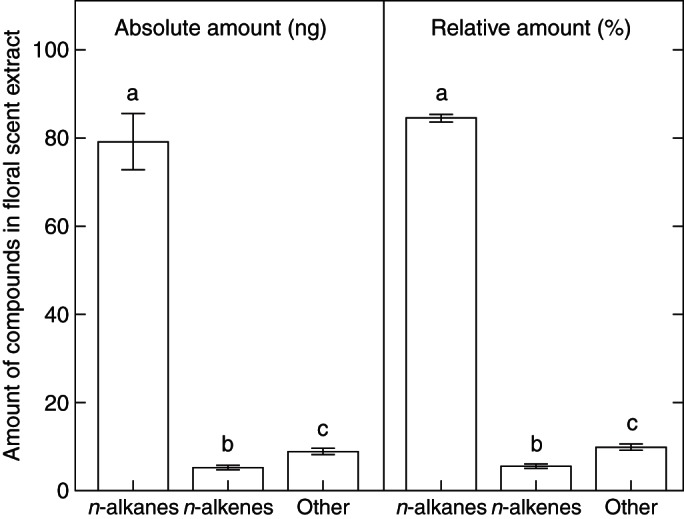
Mean (± s.e.) absolute (left) and relative (right) amounts of n-alkanes (saturated hydrocarbons), n-alkenes (unsaturated hydrocarbons) and other classes of compounds in the floral scent extracts of Iris atropurpurea. Different letters indicate significant differences (Mann–Whitney U-tests with Bonferroni correction, α = 0·0167).
We found no significant difference in the total absolute amounts of odour compounds emitted by individual falls that were visited vs. those that were not visited (Student's t-test, P = 0·427). Our MRPP analysis indicates that there are no differences in floral scent (relative amounts, in %) according (a) to the orientation of the floral tubes (eight compass bearings) (MRPP, A = 0·0038, δobs = 0·2434, δexp = 0·2443, P = 0·414) and (b) to the flower visitation patterns observed under natural conditions (visited vs. non-visited flowers) (MRPP, A = –0·0025, δobs = 0·2449, δexp = 0·2443, P = 0·527). The Indicator Species Analysis failed to detect any significant association between any compound within the blends of floral scents and the orientation of the floral tubes or the visitation patterns observed. Likewise, the nMDS ordination did not produce discrete clusters of samples according to any category. The nMDS biplots produced were characterized by high linear and non-metric fits (R2 = 0·948 and 0·986, respectively) and a low stress value (0·12), indicating that the ordination summarizes well the observed chemical distances among samples (Fig. 3).
Fig. 3.
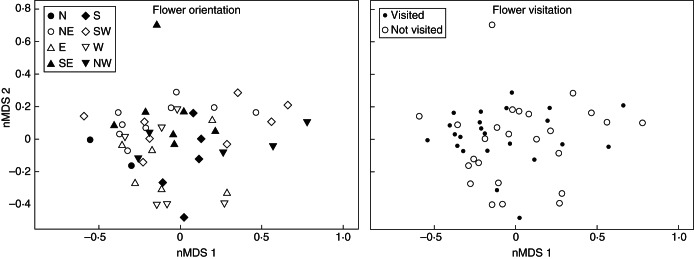
Non-metric multi-dimensional scaling (nMDS) ordination biplots based on a matrix of Bray–Curtis dissimilarities calculated on the relative proportions of odour compounds (in % of the total blend). The samples are plotted according to the orientation of the floral tubes (left, eight compass bearings) and to their visitation patterns (right, visited versus not visited). The results show that there is no difference in floral scent (relative amounts, in %) according to the orientation of the floral tubes or to the flower visitation patterns observed under natural conditions (visited versus non-visited flowers); see Results section for details. Stress value = 0·12.
We recorded a total of 690 male solitary bees at Yaqum and at Netanya in 2011 to test the biological null hypothesis that male bees visit the floral tunnels randomly, without regard to their orientation. Our results indicate that this hypothesis is rejected (Rayleigh's Z = 0·1979, P < 0·0001, angular deviation = 1·323; angular variance = 1·751, small mean resultant length = 0·1246), and that east-facing tunnels do not host significantly more sheltering male bees (Rayleigh's test of an unimodal probability distribution with a specified mean direction to the east and unknown mean resultant length, Z = –0·1228, P > 0·05; Fig. 4). These results are also supported by the G- test for goodness-of-fit (likelihood ratio or log-likelihood test; Supplementary Data Fig. S1).
Fig. 4.
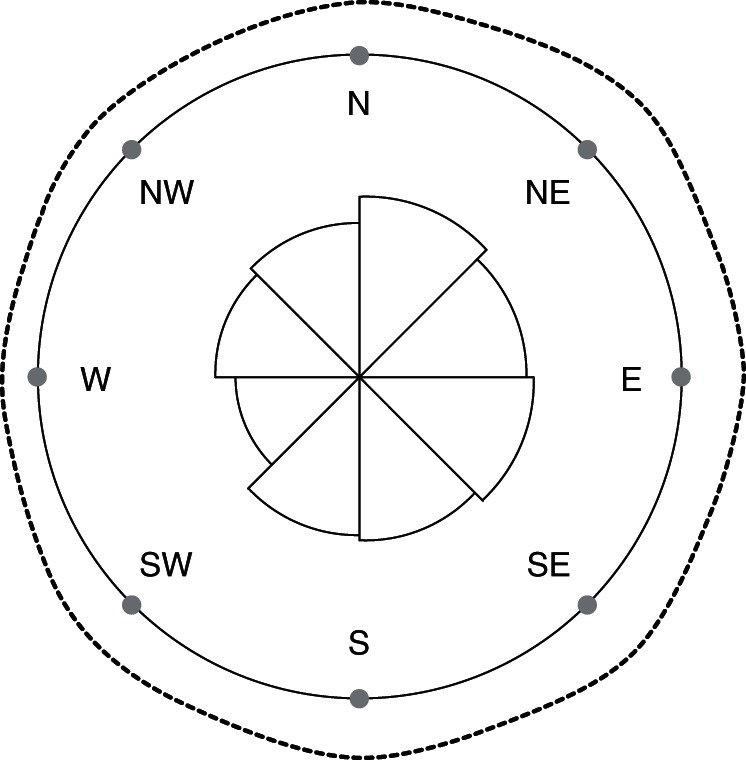
Rose diagram illustrating the orientation preferences of sheltering male bees, illustrating total number of male bees found in floral tunnels of Iris atropurpurea flowers facing eight different compass bearings. Data were pooled across the Yaqum and Netanya populations for 2011, representing a total of 690 male bee specimens. The dotted circle represents the kernel density estimate of the observed counts of male bees in floral tunnels of each orientation. Our results reject the null hypothesis of random visitation of floral tunnels by sheltering male bees (Rayleigh's Z-test of uniformity: Z = 0·19797, P < 0·0001, angular deviation = 1·323; angular variance = 1·751, small mean resultant length = 0·1246) but also rejects the hypothesis that east-facing tunnels host significantly more sheltering male bees (Z = –0·1228, P > 0·05). For additional analyses, see Supplementary Data Fig. S1.
Spectral measurements of flowers
Our analysis of the spectral reflectance of I. atropurpurea shows that the red flowers absorb light strongly between 300 and 640 nm and have a slightly increasing reflectance between 640 and 700 nm where the values reach just above 10 % (mean 10·63 %; Fig. 5A). The colour loci of the dark-red falls (lower petals) and the black discs were found to overlap greatly near the 0·1 hexagon units centre of the hexagonal bee colour space (Fig. 5B), indicating that they appear similar and almost achromatic (‘bee black’) from the bees' perspective. Indeed, the mean colour contrast measured between the spectral reflectance of the dark-red falls and the black discs of I. atropurpurea was found to amount to 0·045 hexagon units, i.e. below the lower threshold value of hexagon units for colour discrimination (Dyer and Chittka, 2004a, b; Dyer et al., 2008), suggesting that the male bees are unlikely to be able to discriminate between these two visual stimuli based on colour. All achromatic contrasts calculated with different background types were also equivalent (Table 1), suggesting that pollinators are unlikely to use achromatic contrasts to discriminate between the dark-red falls and the black discs of the flowers.
Table 1.
Mean (± s.d.) achromatic contrasts (L-receptor contrasts) of the red falls (n = 20) and the black discs (n = 20) of Iris atropurpurea at the study site of Yaqum
| Background type | Achromatic contrasts of red falls | Achromatic contrasts of black discs |
|---|---|---|
| Green foliage measured in situ | 0·48 (± 0·01) | 0·45 (± 0·01) |
| Mid-grey (uniformly 30 %) | 0·48 (± 0·01) | 0·47 (± 0·02) |
| Black discs | 0·48 (± 0·005) | - |
DISCUSSION
Male eucerine bees and shelter mimicry
To date, pollination by shelter mimicry is known only from the Euro-Mediterranean region and its neighbouring areas where it has evolved independently in at least three groups of flowering plants. Besides the 10+ representative species in the section Oncocyclus of the genus Iris (Iridaceae) (Sapir and Shmida, 2002; Sapir et al., 2005), this mimicry system is also the main pollination strategy adopted by 20+ species in the orchid genus Serapias (most outcrossing species) (Bellusci et al., 2008), as well as in the orchid genus Ophrys where O. helenae, a narrow endemic species from northern Greece and Albania, is the only species in the genus pollinated by shelter mimicry rather than by sexual deception (Paulus and Gack, 1993; Vereecken et al., 2012). A common characteristic of all these shelter-mimicking species is that they all attract primarily male eucerine bees as pollinators, and results from other studies indicate that some shelter mimics occasionally exploit male solitary bees belonging to the same species. For example, males of Synhalonia rufa (= Tetralonia berlandi) have been reported to pollinate both I. atropurpurea (Fig. 1; Sapir and Shmida, 2002; Sapir et al., 2005; Watts et al., 2013) and O. helenae (Paulus and Gack, 1993; Vereecken et al., 2012). Likewise, and males of Megachile (Chalicodoma) sicula reported to occasionally shelter in flowers of I. atropurpurea (Fig. 1; Watts et al., 2013) also pollinate the shelter flowers of Serapias levantina (alongside other male eucerine bees such as Eucera decipiens) in the coastal plains of Israel, in natural populations located just a few hundreds of metres away from our study sites of I. atropurpurea (N. J. Vereecken, pers. obs.). Our own investigations on the behavioural ecology of solitary bees in the Mediterranean basin during the past decade suggest that there is no evidence that the sleeping behaviour of eucerine bees differs from that of other wild bees, and that male eucerine bees are therefore not more likely than any other group of bees to exploit flowers as protective shelters.
We predict that more in-depth pollinator surveys will reveal extensive pollinator sharing among shelter-mimicking species, and we suggest that the floral convergence in form and function among shelter-mimicking orchids and irises represents a striking and new pollination syndrome. Male solitary bees are sometimes found sheltering overnight in aggregations or as singletons in tubular or bowl-shaped flowers of other plant species, particularly during their short reproductive period and in those plants that are visited by females of oligolectic species (i.e. pollen specialists) and used as ‘rendez-vous’ sites for mating (Westrich, 1989; Müller et al., 1997). Although male bees might also contribute to the plants' reproductive success in those cases (see, for example, Cane et al., 2011), these plants are generally also pollinated during the day by a wide taxonomic range of foraging insects. By contrast, male solitary bees, particularly eucerine bees, are the key pollinators of shelter-mimicking species, and diurnal visits by foraging pollinators are rare in most cases (Monty et al., 2006; but see Watts et al., 2013).
Pollinator preferences and floral evolution
Our investigations into the pollinators' preferences indicate that east-facing floral tunnels are not the most attractive to sheltering male bees (Fig. 4), and that the visitation patterns of male bees as specialized pollinators does not require the production of particular blends of chemical compounds (n-alkenes or fatty acids) in this Iris species. Indeed, we found no significant difference in floral scent chemistry (absolute and relative amounts of compounds in the total blends) between visited and non-visited flowers, or among flowers with different orientation (Fig. 3). The analysis of the floral colours from the pollinators' perspective also reveals that the dark-red flowers absorb light all across the UV range of the spectrum to 630nm, and that they are therefore presumably perceived as uniformly black (achromatic) by the male solitary bees (Fig. 5).
The chemistry of the female sex pheromones of eucerine bees, including S. spectabilis, the most frequent pollinator of I. atropurpurea, is still virtually unexplored. Nevertheless, our results suggest that unlike the hypothesis presented by Schiestl and Cozzolino (2008) for the evolution of pollination by male solitary bees in orchids, specialized attraction of male eucerine bees in shelter-mimicking irises is presumably not the result or by-product of an odour-based sexual stimulation. Indeed, the floral scent of I. atropurpurea lacks the high amounts of polar compounds emitted by O. tenthredinifera, and n-alkenes, a major class of sexual attractants for male solitary bees (Ayasse et al., 2001; Vereecken and Schiestl, 2008; Vereecken and McNeil, 2010), and also key pollinator attractants in the floral scent of most Ophrys species investigated so far (reviewed by Vereecken and McNeil, 2010; Vereecken et al., 2010; Ayasse et al., 2011), are only produced in trace amounts in I. atropurpurea. Furthermore, our floral-colour analyses, the similar and unique suites of floral traits shared by I. atropurpurea and other shelter-mimicking species with closely related pollinator species support the hypothesis that male solitary bees do not approach these flowers during patrolling flights in search of females, but instead visit and pollinate them when looking for a suitable dark shelter or a cavity that would protect them overnight or during overcast conditions.
Consequently, we challenge the view that the specialized attraction of male bees requires the presence of high absolute/relative amounts and numbers of n-alkenes in the floral scent, and hypothesize that the dark-red, ‘bee-black’ flowers in I. atropurpurea and many other Oncocyclus irises have presumably been shaped by selection for dark shelters mediated by shelter-seeking male solitary bees. We speculate that the unusually large size of individual flowers might have evolved to facilitate their detection by pollinators via chromatic or achromatic contrasts with the vegetation background (see, for example, Chittka et al., 1994; Kevan et al., 2001; Martínez-Harms et al., 2010; Streinzer et al., 2009; Spaethe et al., 2010), and we hypothesize that other parameters than the floral tunnel orientation, such as flower density, flower size and/or the structure and proximity of the surrounding vegetation might influence their overall patterns of flower visitation within populations (S. Watts, unpubl. res.).
Morning floral heat as pollinator reward?
The earlier observations by Dafni et al. (1981) that the temperature of shelter-mimicking flowers increases by to a maximum of 2·5 °C in the morning has led Sapir et al. (2006) to suggest that the dark flowers of the Oncocyclus irises had evolved to gather heat, and that pollinators might use this floral heat as a ‘reward’ to increase their body temperature and thereby leave the flowers earlier in the morning. The results presented by Sapir et al. (2006) are based on a survey of individual plants for the presence of sheltering male solitary bees over three nights, at a single site, and using four compass bearings. Based on very small numbers of male bee specimens observed, Sapir et al. (2006) concluded that pollinators sheltered ‘more frequently’ (no statistics presented) in east-facing floral tunnels. Similar results were presented by Monty et al. (2006) in a study on I. cedretii, another representative of the section Oncocyclus, but their interpretation was not backed up by statistical tests either.
In an attempt to demonstrate that sheltering male bees might indeed benefit from the increased floral temperature and accelerated emergence time of male bees earlier in the morning, Sapir et al. (2006) reported that male bees forced to shelter overnight in artificial white cones became active and flew away significantly later than the ones that were forced to shelter in the Iris flowers (Sapir et al., 2006). However, these authors never accounted for the use of white cones as models and how they translate into real-world components of the bees' habitat, and, most importantly, they found no significant difference in the early morning emergence time of male eucerine bees between those sheltering overnight in the floral tunnels and those spending the night on the ground (Sapir et al., 2006). The lack of evidence for an early emergence of male bees sheltering in the Iris flowers was also reported by Monty et al. (2006), who confirmed statistically that male eucerine bees that shelter in the Iris flowers became active significantly later and at significantly higher ambient air temperatures than the ones that had spent the night exposed on the ground or hidden in the vegetation.
Our data, based on surveys of 690 specimens recorded across the whole 2011 season from different populations demonstrate that male bees are not randomly distributed among floral tunnels of I. atropurpurea with different orientations (Fig. 4), and that male bees do not significantly prefer to shelter in tunnels facing the rising sun (Fig. 4 and Supplementary Data Fig. S1). In fact, our results show that the number of bees observed in east-facing tunnels is not significantly different from those found in N–NE–SE–S–SW-facing ones, and that only tunnels facing the west are significantly less visited than the east-facing ones (Fig. 4 and Supplementary Data Fig. S1). These results therefore contradict the results presented by Sapir et al. (2006). We think that our approach involving surveys of several thousands of plants across the whole flowering season, in two populations, with several hundreds of male eucerine bee specimens observed and more compass bearings (eight compared with four in Sapir et al., 2006) is statistically and ecologically robust and very valuable as a test of the ‘morning-floral-heat-reward’ hypothesis. In light of our results and the conflicting ideas described above, we therefore recommend that the ‘morning-floral-heat-reward’ hypothesis is laid to rest and the pollination biology of I. atropurpurea and the Oncocyclus irises is looked at from a more integrative and experimentally robust view angle.
Although we acknowledge that, under certain circumstances and in a foraging context, a 2·5 °C difference between the ambient air and the flowers might be of biological significance (see, for example, Dyer et al., 2006; Rands and Whitney, 2008; both in a foraging context), we think that the available data on the behavioural ecology of the nectarless Oncocyclus irises and their pollinators support the alternative scenario that floral heat represents a weak, ephemeral and therefore negligible ‘reward’ from the pollinators' perspective. In the shelter mimicry context, male solitary bees generally visit the Oncocyclus flowers late in the afternoon or early in the evening when they settle down for the night: at this time of the day, differences in temperatures between the ambient air and the floral tunnels are weak (<1 °C on average, see Sapir et al., 2006). There is therefore a disconnect between the time when the flowers are visited and the time when Sapir et al. (2006) suggested the bees would get a heat reward from the flowers (i.e. in the morning). Thus, it seems obvious to us that male solitary bees do not just choose warmer flowers but favour flowers whose traits (colour, shape, size, etc.) are perceived by the bees as if they could provide a protective shelter.
Solitary bees that are active in early spring usually have a medium to large body size, and dark-bodied and densely haired species (hairiness provides insulation) like S. spectabilis, the most frequent pollinator of I. atropurpurea generally become active earlier at low ambient air temperatures (e.g., in early spring) because they have faster warm-up rates (as high as 10–15 °C min−1 according to Stone and Willmer, 1989), and retain higher levels of thoracic heat at low temperatures since flight muscles generate more power per unit mass (reviewed by Willmer and Stone, 2004). This general principle stems from both experimental studies and observations that the wild bee fauna of the northern hemisphere is dominated by large-bodied, darker and hairier species in spring, while smaller-bodied, lighter-coloured and hairless species are comparatively more abundant in summer (Westrich, 1989; Stone and Willmer, 1989; Müller et al., 1997; Willmer and Stone, 2004). Finally, we think it is worth mentioning that the sandy-gravel coastal plains of Israel represent a very thermophilous region of the Mediterranean where the average daily temperature of the ambient air can be higher than 20 °C in February–March, with temperatures above 10 °C during the night at this time of the year and quickly raising after sunrise, allowing the insects to become active soon after the first rays of sunlight reach the surrounding vegetation. Increased floral heat might therefore be of behavioural significance under colder climates, but we think it is unlikely in the ecological and geographical context presented here.
Concluding remarks
Our study provides the first functional analysis of floral traits associated with a shelter–mimicry system in the Oncocyclus irises. Although it is often thought that achromatic flowers might be avoided by bees and be preferred by other types of pollinators (see Lunau et al., 2011, and references therein), we suggest that the large, tunnel-like, dark-red flowers of I. atropurpurea (all perceived as ‘bee-black’; Fig. 1) is likely to have evolved by pollinator-mediated selection primarily to mimic hollow (dark) protective shelters used preferentially by male solitary bees. The direct outcome (or by-product) of this phenomenon is that the dark flowers gather heat when exposed to solar radiation early in the morning, but the slight increase of temperature in the floral tubes compared with that of the ambient air seems irrelevant to the male eucerine bees. The recent discovery of a sexually deceptive species in the section Oncocyclus of the genus Iris (Vereecken et al., 2012) raises important questions on the relative role of floral colours and scents in the evolution of pollinator specialization, from the still relatively generalized shelter–mimicry strategy to the most extreme case of sexual deception.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was financially supported by the Belgian FRS-FNRS (Fonds National pour la Recherche Scientifique) through a post-doctoral grant (‘Chargé de Recherches’) and several travel grants (2008–2012) to N. J. Vereecken. We are grateful to J. Ollerton, P. G. Kevan, S. P. M. Roberts, M. Streinzer, J. Spaethe, Y. Sapir and S. Risch for fruitful discussions during the preparation of this study, to C. Agostinelli, J. H. McDonald and G. Elvers for statistical advice, and to three anonymous referees for their helpful comments on an earlier version of the manuscript. We thank B. Segal and N. Bensoussan for field assistance. The experiments comply with the current laws of the country in which the experiments were performed.
LITERATURE CITED
- Agostinelli C, Lund U. R package ‘circular’: circular statistics (version 0·4-3) 2011 https://r-forge.r-project.org/projects/circular/ [Google Scholar]
- Ayasse M, Paxton RJ, Tengö J. Mating behavior and chemical communication in the order Hymenoptera. Annual Review of Entomology. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- Ayasse M, Stökl J, Francke W. Chemical ecology and speciation in sexually deceptive orchids. Phytochemistry. 2011;72:1667–1677. doi: 10.1016/j.phytochem.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Barth FG. Insects and flowers: the biology of a partnership. Princeton, NJ: Princeton University Press; 1985. [Google Scholar]
- Bellusci F, Pellegrino G, Palermo AM, Musacchio A. Phylogenetic relationships in the orchid genus Serapias L. based on noncoding regions of the chloroplast genome. Molecular Phylogenetics and Evolution. 2008;47:986–991. doi: 10.1016/j.ympev.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Briscoe A, Chittka L. The evolution of color vision in insects. Annual Review of Entomology. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Buser H-R, Arn H, Guerin P, Rauscher S. Determination of double bond position in monounsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Analytical Chemistry. 1983;55:818–822. [Google Scholar]
- Cane JH, Sampson BJ, Miller SA. Pollination value of male bees: the specialist bee Peponapis pruinosa (Apidae) at Summer Squash (Cucurbita pepo) Environmental Entomology. 2011;40:614–620. doi: 10.1603/EN10084. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. “Anti-bee” and “pro-bird” changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology. 2004;17:876–885. doi: 10.1111/j.1420-9101.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- Chittka L. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A. 1992;170:533–543. [Google Scholar]
- Chittka L, Kevan PG. Flower colour as advertisement. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge, Canada: Enviroquest; 2005. pp. 157–196. [Google Scholar]
- Chittka L, Waser NM. Why red flowers are not invisible to bees. Israel Journal of Plant Sciences. 1997;45:169–183. [Google Scholar]
- Chittka L, Beier W, Hertel H, Steinmann E, Menzel R. Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in hymenoptera. Journal of Comparative Physiology A. 1992;170:545–563. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. Ultraviolet as a component of flower reflections, and the colour perception of the hymenoptera. Vision Research. 1994;34:1489–1508. doi: 10.1016/0042-6989(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Chittka L, Gumbert A, Kunze J. Foraging dynamics of bumble bees: correlates of movements within and between plant species. Behavioural Ecology. 1997;8:239–249. [Google Scholar]
- Chittka L, Spaethe J, Schmidt A, Hickelsberger A. Adaptation, constraint, and chance in the evolution of flower color and pollination color vision. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge: Cambridge University Press; 2001. pp. 106–126. [Google Scholar]
- Dafni A, Shmida A. The possible ecological implications of the invasion of Bombus terrestris (L.) (Apidae) at Mt Carmel, Israel. In: Matheson A, Buchman SL, O'Toole C, Westrich P, Williams IH, editors. The conservation of bees. London: Academic Press; 1996. pp. 183–200. [Google Scholar]
- Dafni A, Ivri Y, Brantjes NBM. Pollination of Serapias vomeracea Briq. (Orchidaceae) by imitation of holes for sleeping solitary male bees (Hymenoptera) Acta Botanica Neerlandica. 1981;30:69–73. [Google Scholar]
- De Caceres M, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- Dobson HEM. Floral volatiles in insect biology. In: Bernays EA, editor. Insect–plant interactions. London: CRC Press; 1994. pp. 47–81. [Google Scholar]
- Dötterl S, Vereecken NJ. The chemical ecology and evolution of bee–flower interactions: a review and perspectives. Canadian Journal of Zoology. 2010;88:668–697. [Google Scholar]
- Dunkelblum E, Tan SH, Silk PJ. Double bond location in monounsaturated fatty acids by dimethyl disulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of 4 Lepidoptera. Journal of Chemical Ecology. 1985;11:265–277. doi: 10.1007/BF01411414. [DOI] [PubMed] [Google Scholar]
- Dyer A, Chittka L. Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a study case. Journal of Comparative Physiology A. 2004a;190:105–114. doi: 10.1007/s00359-003-0475-2. [DOI] [PubMed] [Google Scholar]
- Dyer A, Chittka L. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften. 2004b;91:224–227. doi: 10.1007/s00114-004-0508-x. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Bees associate warmth with floral colour. Nature. 2006;442:525. doi: 10.1038/442525a. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Spaethe J, Prack S. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. Journal of Comparative Physiology A. 2008;194:617–627. doi: 10.1007/s00359-008-0335-1. [DOI] [PubMed] [Google Scholar]
- Faegri K, Van der Pijl L. The principles of pollination biology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Free JB. Insect pollination of crops. London: Academic Press; 1993. [Google Scholar]
- von Frisch K. Über den Geruchssinn der Bienen und seine blütenbiologische Bedeutung. Zoologische Jahrbuecher (Physiol.) 1919;37:1–238. [Google Scholar]
- Giurfa M, Vorobyev M, Kevan P, Menzel R. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. Journal of Comparative Physiology A. 1996;178:699–709. [Google Scholar]
- Gumbert A. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology. 2000;48:36–43. [Google Scholar]
- Irwin RE, Brody AK. Nectar-robbing bumble bees reduce the fitness of Ipomopsis aggregata (Polemoniaceae) Ecology. 1999;80:1703–1712. [Google Scholar]
- Jarek S. mvnormtest: normality test for multivariate variables. 2012 R package version 0·1–9. http://cran.r-project.org/web/packages/mvnormtest/ . [Google Scholar]
- Keasar T, Bilu Y, Motro U, Shmida A. Foraging choices of bumblebees on equally rewarding artificial flowers of different colors. Israel Journal of Plant Sciences. 1997;45:223–233. [Google Scholar]
- Kevan PG, Lane MA. Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Sciences of the USA. 1985;82:4750–4752. doi: 10.1073/pnas.82.14.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevan PG, Chittka L, Dyer AG. Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. Journal of Experimental Biology. 2001;204:2571–2580. doi: 10.1242/jeb.204.14.2571. [DOI] [PubMed] [Google Scholar]
- Klein A-M, Vaissière BE, Cane JH, et al. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society of London B. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacher V. Elektrophysiologische Untersuchungen an einzelnen Rezeptoren für Geruch, Kohlendioxyd, Luftfeuchtigkeit und Temperatur auf den Antennen der Arbeitsbiene und der Drohne (Apis mellifica L.) Zeitschrift für vergleichende Physiologie. 1964;48:587–623. [Google Scholar]
- Lack AJ, Kevan PG. The reproductive biology of a distylous tree, Sarcotheca celebica (Oxalidaceae) in Sulawesi, Indonesia. Botanical Journal of the Linnean Society. 1987;95:1–8. [Google Scholar]
- Lunau K, Maier EJ. Innate colour preferences of flower visitors. Journal of Comparative Physiology A. 1995;177:1–19. [Google Scholar]
- Lunau K, Wacht S, Chittka L. Colour choices of naive bumble bees and their implications for colour perception. Journal of Comparative Physiology A. 1996;178:477–489. [Google Scholar]
- Lunau K, Papiorek S, Eltz T, Sazima M. Avoidance of achromatic colours by bees provides a private niche for hummingbirds. Journal of Experimental Biology. 2011;214:1607–1612. doi: 10.1242/jeb.052688. [DOI] [PubMed] [Google Scholar]
- Lynch RI. The book of the iris. London: J. Lane; 1904. [Google Scholar]
- McCune B, Grace JB. Analysis of ecological communities. Gleneden Beach, OR: MjM Software Design; 2002. [Google Scholar]
- McDonald JH. Handbook of biological statistics. 2nd edn. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- Mant JG, Brändli C, Vereecken NJ, Schulz C, Francke W, Schiestl FP. Cuticular hydrocarbons as source of the sex pheromone in Colletes cunicularius (Hymenoptera: Colletidae) and the key to its mimicry by the sexually deceptive orchid Ophrys exaltata (Orchidaceae) Journal of Chemical Ecology. 2005;31:1765–1787. doi: 10.1007/s10886-005-5926-5. [DOI] [PubMed] [Google Scholar]
- Martínez-Harms J, Palacios AG, Márquez N, Estay P, Arroyo MTK, Mpodozis J. Can red flowers be conspicuous to bees Bombus dahlbomii and South American temperate forest flowers as a case in point. Journal of Experimental Biology. 2010;213:564–571. doi: 10.1242/jeb.037622. [DOI] [PubMed] [Google Scholar]
- Mathew B. The iris. London: BT Batsford; 1989. [Google Scholar]
- Menzel R. Learning in honey bees in an ecological and behavioral context. In: Hölldobler B, Lindauer M, editors. Experimental behavioral ecology and sociobiology. Stuttgart, Germany: Gustav Fischer Verlag; 1985. pp. 55–74. [Google Scholar]
- Menzel R, Shmida A. The ecology of flower colors and the natural color vision of insect pollinators: the Israeli flora as a study case. Biological Reviews. 1993;68:81–120. [Google Scholar]
- Michener CD. The bees of the world. Baltimore, MD: Johns Hopkins University Press; 2007. [Google Scholar]
- Mielke PW, Berry KJ. Permutation methods: a distance function approach. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- Monty A, Saad L, Mahy G. Bimodal pollination system in rare endemic Oncocyclus irises (Iridaceae) in Lebanon. Canadian Journal of Botany. 2006;84:1327–1338. [Google Scholar]
- Müller A, Krebs A, Amiet F. Bienen, Mitteleuropäische Gattungen, Lebensweise, Beobachtung. Augsburg, Switzerland: Natur Buch Verlag; 1997. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, et al. vegan: Community Ecology Package. 2012 R package version 2·0–5. http://cran.r-project.org/web/packages/vegan/index.html . [Google Scholar]
- Paulus HF, Gack C. Schlafplatzmimikry bei der mediterranen Orchidee Ophrys helenae. Verhandlungen der Deutsche Zoologisches Gesellschaft Salzburg. 1993;86:267. [Google Scholar]
- Peitsch D, Fietz A, Hertel H, de Souza J, Fix Ventura D, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. Portland, OR: Timber Press; 1996. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. 2012 R Foundation for Statistical Computing, Vienna: http://www.r-project.org/ [Google Scholar]
- Raine NE, Chittka L. The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. PLoS ONE. 2007;2:e556. doi: 10.1371/journal.pone.0000556. http://dx.doi.org/10.1371/journal.pone.0000556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands SA, Whitney HM. Floral temperature and optimal foraging: is heat a feasible floral reward for pollinators? PLoS ONE. 2008;3:e2007. doi: 10.1371/journal.pone.0002007. http://dx.doi.org/10.1371/journal.pone.0002007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven PH. Why are bird-visited flowers predominantly red? Evolution. 1972;26:674. doi: 10.1111/j.1558-5646.1972.tb01975.x. [DOI] [PubMed] [Google Scholar]
- Reisenman CE, Giurfa M. Chromatic and achromatic stimulus discrimination of long wavelength (red) visual stimuli by the honeybee Apis mellifera. APIS. 2008;2:137–146. [Google Scholar]
- Sapir Y, Shmida A. Species concepts and ecogeographical divergence of Oncocyclus irises. Israel Journal of Plant Sciences. 2002;50:119–127. [Google Scholar]
- Sapir Y, Shmida A, Ne'eman G. Pollination of the Oncocyclus irises (Iris: Iridaceae) by night-sheltering male bees. Plant Biology. 2005;7:417–424. doi: 10.1055/s-2005-837709. [DOI] [PubMed] [Google Scholar]
- Sapir Y, Shmida A, Ne'eman G. Morning floral heat as a reward to the pollinators of the Oncocyclus irises. Oecologia. 2006;147:53–59. doi: 10.1007/s00442-005-0246-6. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD., Jr Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences of the USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Cozzolino S. Evolution of sexual mimicry in the orchid subtribe orchidinae: the role of preadaptations in the attraction of male bees as pollinators. BMC Evolutionary Biology. 2008;8:27. doi: 10.1186/1471-2148-8-27. http://dx.doi.org/10.1186/1471-2148-8-27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebees: fFlower size and color affect search time and flight behavior Proceedings of the National Academy of Sciences of the USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Streinzer M, Paulus HF. Why sexually deceptive orchids have colored flowers. Communative and Integrative Biology. 2010;3:139–141. doi: 10.4161/cib.3.2.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone GN, Willmer PG. Warm-up rates and body temperatures in bees: the importance of body size, thermal regime and phylogeny. Journal of Experimental Biology. 1989;147:303–328. [Google Scholar]
- Streinzer M, Paulus HF, Spaethe J. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. Journal of Experimental Biology. 2009;212:1365–1370. doi: 10.1242/jeb.027482. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Wilson P. Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. International Journal of Plant Sciences. 2008;169:23–38. [Google Scholar]
- Vareschi E. Duftunterschiede bei der Honigbiene – Einzelzell-Ableitungen und Verhaltensreaktionen. Zeitschrift für vergleichende Physiologie. 1971;75:143–173. [Google Scholar]
- Vereecken NJ, McNeil JN. Cheaters and liars: chemical mimicry at its finest. Canadian Journal of Zoology. 2010;88:725–752. [Google Scholar]
- Vereecken NJ, Schiestl FP. The evolution of imperfect floral mimicry. Proceedings of the National Academy of Sciences of the USA. 2008;105:7484–7488. doi: 10.1073/pnas.0800194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken NJ, Cozzolino S, Schiestl FP. Hybrid floral scent novelty drives pollinator shift in sexually deceptive orchids. BMC Evolutionary Biology. 2010;10:103. doi: 10.1186/1471-2148-10-103. http://dx.doi.org/10.1186/1471-2148-10-103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken NJ, Wilson CA, Hötling S, Schulz S, Banketov SA, Mardulyn P. Pre-adaptations and the evolution of pollination by sexual deception: Cope's rule of specialisation revisited. Proceedings of the Royal Society of London B. 2012;279:4786–4794. doi: 10.1098/rspb.2012.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöth W. Können Serapias blüten Nesttäuschblumen sein? Die Orchidee. 1980;31:159–162. [Google Scholar]
- Watts S, Sapir Y, Segal B, Dafni A. The endangered Iris atropurpurea (Iridaceae) in Israel: honey bees, night-sheltering male bees and female solitary bees as pollinators. Annals of Botany. 2013;111:395–407. doi: 10.1093/aob/mcs292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich P. Die Wildbienen Baden-Württembergs. Spezieller Teil: Die Gattungen und Arten. Stuttgart, Germany: Eugen Ulmer GmbH & Co; 1989. [Google Scholar]
- Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science. 2009;323:130–133. doi: 10.1126/science.1166256. [DOI] [PubMed] [Google Scholar]
- Willmer PG. Pollination and floral ecology. Princeton, USA: Princeton University Press; 2011. [Google Scholar]
- Willmer PG, Stone GN. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Advances in the Study of Behavior. 2004;34:347–466. [Google Scholar]
- Wilson P, Castellanos MC, Wolfe AD, Thomson JD. Shifts between bee- and bird-pollination among penstemons. In: Waser NM, Ollerton J, editors. Specialization and generalization in pollination systems. Chicago, IL: University of Chicago Press; 2006. pp. 47–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.