Abstract
Background and Aims
In complex communities, organisms often form mutualisms with multiple different partners simultaneously. Non-additive effects may emerge among species linked by these positive interactions. Ants commonly participate in mutualisms with both honeydew-producing insects (HPI) and their extrafloral nectary (EFN)-bearing host plants. Consequently, HPI and EFN-bearing plants may experience non-additive benefits or costs when these groups co-occur. The outcomes of these interactions are likely to be influenced by variation in preferences among ants for honeydew vs. nectar. In this study, a test was made for non-additive effects on HPI and EFN-bearing plants resulting from sharing exotic ant guards. Preferences of the dominant exotic ant species for nectar vs. honeydew resources were also examined.
Methods
Ant access, HPI and nectar availability were manipulated on the EFN-bearing shrub, Morinda citrifolia, and ant and HPI abundances, herbivory and plant growth were assessed. Ant-tending behaviours toward HPI across an experimental gradient of nectar availability were also tracked in order to investigate mechanisms underlying ant responses.
Key Results
The dominant ant species, Anoplolepis gracilipes, differed from less invasive ants in response to multiple mutualists, with reductions in plot-wide abundances when nectar was reduced, but no response to HPI reduction. Conversely, at sites where A. gracilipes was absent or rare, abundances of less invasive ants increased when nectar was reduced, but declined when HPI were reduced. Non-additive benefits were found at sites dominated by A. gracilipes, but only for M. citrifolia plants. Responses of HPI at these sites supported predictions of the non-additive cost model. Interestingly, the opposite non-additive patterns emerged at sites dominated by other ants.
Conclusions
It was demonstrated that strong non-additive benefits and costs can both occur when a plant and herbivore share mutualist partners. These findings suggest that broadening the community context of mutualism studies can reveal important non-additive effects and increase understanding of the dynamics of species interactions.
Keywords: Additive effects, Anoplolepis gracilipes, community context, extrafloral nectar, EFN, honeydew, indirect effects, invasive ants, Morinda citrifolia, mutualism, non-additive benefit, non-additive cost, simultaneous interactions
INTRODUCTION
In complex ecological communities, organisms commonly participate in mutualisms with multiple, different partners simultaneously. For example, plants can form mutualisms with pollinators, seed dispersers, mycorrhizal fungi, N-fixing bacteria, foliar endophytes and protection-conferring ants. Non-additive effects, which cannot be predicted from the outcomes of pairwise studies, may emerge among species linked by these positive interactions.
Ant protective mutualisms provide valuable models for disentangling the complex dynamics of mutualisms involving multiple groups of mutualist partners (Heil and McKey, 2003; Styrsky and Eubanks, 2007). In these mutualisms, ants protect plants from herbivores and other threats, and plants provide ants with nesting sites and/or food resources (Heil and McKey, 2003; Rico-Gray and Oliveira, 2007). Some plant species, notably those bearing extrafloral nectaries (EFNs), also host sap-sucking insects that produce honeydew, a carbohydrate-rich substance that is also highly attractive to ants. Because honeydew is processed by the herbivore's gut, it tends to contain more amino acids (Beattie, 1985) and complex sugars (Blüthgen et al., 2004) than plant nectar. Consequently, ants in some systems have shown increased preference for honeydew over nectar (Blüthgen and Fielder, 2004). Thus, EFN-bearing plants share mutualist partners (ant bodyguards) with their natural enemies [honeydew-producing insects (HPI)], setting up the possibility for complex, non-additive effects (Fig. 1).
Fig. 1.
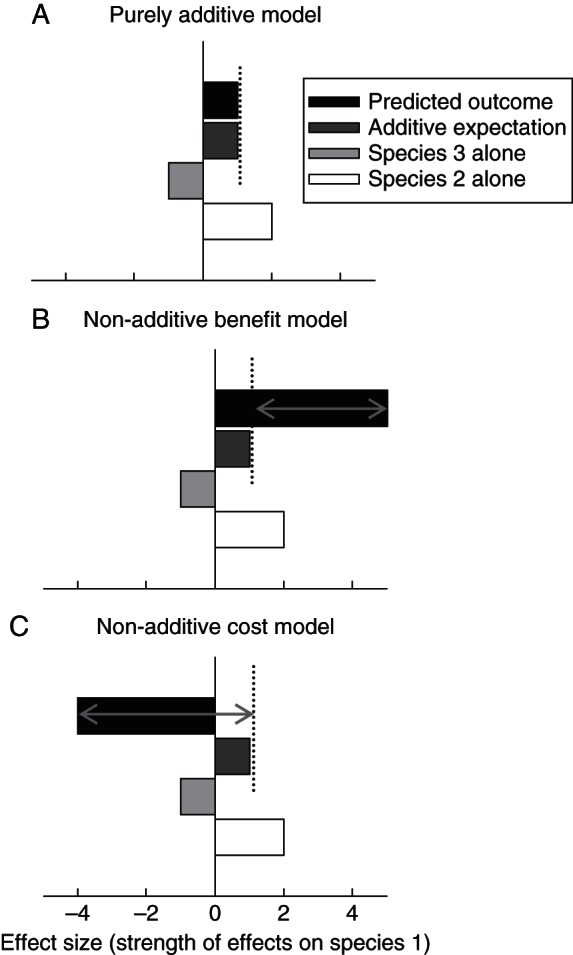
A generalized depiction of the predictions of the purely additive, non-additive benefit and non-additive cost models of outcomes of simultaneous interactions of three different species (or guilds of species). These models provided the basis for our predictions of the consequences arising from interactions among ants, extrafloral nectary (EFN)-bearing plants and honeydew-producing insects (HPI). The additive expectation (A) is the sum of the individual effects of species 2 (e.g. ants) and 3 (e.g. HPI) on species 1 (e.g. EFN-bearing plant). The black bars represent the predicted outcomes of each model. For the non-additive benefit (B) and non-additive cost models (C), the arrows depict the disparity between the predicted outcomes and the additive expectation, based upon the individual effects of species 2 and 3 on species 1.
Interactions involving ants, EFN-bearing plants and/or HPI may have important impacts on ecological communities. Extrafloral nectary-bearing plants are common, occurring in at least 3941 plant species in 745 genera and 108 families (Weber and Keeler, 2013). Ant protective mutualisms involving plants or HPI have been hypothesized to contribute to the ecological dominance of ants (Tobin, 1991; Davidson, 1997; O'Dowd et al., 2003), the success of invasive ants (Holway et al., 2002; Lach et al., 2009; Savage et al., 2009; Savage and Whitney, 2011), invasive HPI (Abbott and Green, 2007) and weedy plants (Guimaraes et al., 2006; Harvey, 2009), and the exclusion of herbivores (e.g. Goheen and Palmer, 2010) and non-myrmecophylic plants (e.g. Fredrickson et al., 2005) from some habitats.
In interactions among ants, EFN-bearing plants and HPI, multiple antagonistic and beneficial interactions simultaneously occur among all three partners, leading to potentially strong non-additive effects (Fig. 1). In addition to their individual mutualisms with ant bodyguards, EFN-bearing plants and HPI are also linked through herbivory and ant-mediated competition (or facilitation). Honeydew-producing insects also face the potential cost of predation from ant bodyguards. Thus, for EFN-bearing plants and HPI, sharing ant bodyguards could lead to either greater than additive benefits through mutualistic pathways or greater than additive costs through antagonistic pathways (Fig. 1). There are three models that predict fundamentally different outcomes for HPI and EFN-bearing plants that share the same ant bodyguards. First, non-additivity may be either absent or unimportant relative to the additive effects of these tripartite interactions (purely additive model; Fig. 1A). If, however, the effects of one partner (e.g. ants) on another (e.g. EFN-bearing plants) are either significantly more positive (non-additive benefit model; Fig. 1B) or significantly more negative (non-additive cost model; Fig. 1C) in the presence of a third partner (e.g. HPI), then more complex investigations will be required in order to understand these interactions.
The consequences of mutualisms between HPI, their EFN-bearing host plants and their shared ant bodyguards have been much less studied than is commonly assumed. In the most recent review, Styrsky and Eubanks (2007) showed that most experimental studies of interactions between ants and HPI do not manipulate both partners, but instead simply exclude ants from host plants. In fact, they identified only one study that manipulated both ants and HPI and tracked the responses of EFN-bearing plants. Buckley (1983) simultaneously manipulated the presence/absence of ants and HPI on an EFN-bearing plant and found that while ants had an individually positive influence on plant fitness, ants and HPI together reduced plant fitness. Buckley did not manipulate EFN in this study. Similarly, most experimental studies of facultative interactions between ants and EFN-bearing plants not only neglect to account for HPI (Trager et al., 2010), but also only manipulate the ant partner (e.g. Chamberlain and Holland, 2009, and references therein). Studies examining the simultaneous influences of ants and extrafloral nectar on HPI are also rare. However, Rudgers et al. (2010) recently manipulated both ants and extrafloral nectar on Gossypium thurberi plants and tracked HPI responses. Although their results varied across sites, they reported positive effects of ants on aphids that were only weakly diminished by the presence of extrafloral nectar; however, HPI were not manipulated. To our knowledge, no previous research has simultaneously manipulated all three partners and assessed the relative importance of additive and non-additive effects. This lack of study is problematic because the only way to disentangle the consequences of these interactions is to conduct simultaneous experimental manipulations of all three partners.
Furthermore, it is likely that the direction and magnitude of such effects will vary among ecological contexts, particularly with changes in the identities of the species involved. The introduction and spread of highly invasive, non-indigenous species provides a unique opportunity to investigate how shifts in species identities can alter the outcomes of complex species interactions. Across broad geographic scales and diverse habitat types, invasive ant species have been shown to respond particularly strongly to carbohydrate-rich, mutualist-derived resources (Holway et al., 2002; Ness and Bronstein, 2004; Styrsky and Eubanks, 2007). For example, we previously demonstrated that the introduction of the highly invasive ant, Anoplolepis gracilipes, to the Samoan islands was associated with EFN availability. First, the only ant species whose abundances across the Samoan Archipelago were significantly, positively correlated with the dominance of EFN-bearing plants was A. gracilipes (Savage et al., 2009). Secondly, in response to plant nectar, A. gracilipes increased aggressive behaviours towards plant-foraging insects more strongly than did less invasive ant species (Savage and Whitney, 2011). Thirdly, these shifts in ant behaviour corresponded with effects on the α- and β-diversity of local arthropod communities (Savage, 2011). Finally, across the invasive range of A. gracilipes, correlative evidence has accumulated to suggest that mutualisms with plants bearing EFNs or with HPI fuel A. gracilipes invasions and accelerate their negative, community-wide impacts (Holway et al., 2002; Hill et al., 2003; O'Dowd et al., 2003). Altogether, this evidence suggested the a priori hypothesis that A. gracilipes would have different responses to EFN-bearing plants and HPI from co-occurring, less invasive ant species (Ward et al., 2008; Savage and Whitney, 2011).
Consequently, we investigated the following questions across sites dominated by A. gracilipes and sites dominated by less invasive ant species on the island of Savai'i, Samoa. (1) What are the relative effects of plant nectar and HPI on abundances of foraging ants? Based upon our previous knowledge of A. gracilipes and other, less invasive exotic species in Samoa (Ward et al., 2008; Savage and Whitney, 2011; Savage et al., 2011), we expected that both types of carbohydrate resources would be attractive to ants. However, based upon our previous, short-term behavioural experiments, we predicted that A. gracilipes would respond more strongly to plant nectar than would other ant species. (2) Do non-additive benefits or costs emerge when HPI and EFN-bearing plants share the same ant mutualists? We expected that we would find support for the non-additive benefit model for plants, and support for the non-additive cost model for HPI. We also expected larger effects in sites dominated by A. gracilipes relative to sites with less invasive ant species, because of the ecological dominance of this invader. (3) Do modifications in ant behaviours underlie observed non-additive effects? We predicted that altered ant behaviours would be an important mechanism driving non-additive effects in this system because previous studies have shown that increasing carbohydrate resources can change the aggressive behaviours of ants (Grover et al., 2007; Ness et al., 2009; Savage and Whitney, 2011). Specifically, at sites dominated by A. gracilipes, we expected to find that EFN resources would distract ants from tending HPI, leading to declines in HPI populations in the presence of both ants and plant nectar relative to ants alone (Becerra and Venable, 1989). In contrast, we predicted that HPI would act as an ‘ant magnet’ for plants, leading to stronger positive effects of ants on plants when HPI were present (Messina, 1981). Again, we predicted that the effects at other sites would be similar in direction, but weaker in magnitude than those at sites dominated by A. gracilipes.
MATERIALS AND METHODS
Study organisms
Distributed across the tropics, Anoplolepis gracilipes has a broad breadth of diet, can form supercolonies and is considered invasive in many island groups, including Samoa (Holway et al., 2002; Lester and Tavite, 2004; Abbott, 2006; Savage et al., 2009). Its native range is currently undetermined (Wetterer, 2005). When A. gracilipes is absent from local ant assemblages in Samoa, other non-native (but less invasive) ant species are numerically dominant, in particular the species Paratrechina longicornis and Pheidole megacephala (Savage and Whitney, 2011), which we studied here. It is important to define relative invasiveness of organisms independently of any focal study. Therefore, we used the scale developed by Ward et al. (2008), based upon literature accounts of each exotic species' biological traits and ecological consequences to define the invasiveness of the dominant ants in our study (for a detailed account of this scoring approach for our system, see Savage and Whitney, 2011).
Across the Samoan Archipelago, the most common and abundant EFN-bearing plant is Morinda citrifolia (Rubiaceae; Savage, 2011). Morinda citrifolia has annular disk nectaries that produce floral, extrafloral and post-floral nectar; these are clustered together on an inflorescence (hereafter ‘nectary body’; Waki et al., 2007; Fig. 2). Morinda citrifolia plants produce nectary bodies all year round, and nectar is secreted continuously (Savage, 2011). Morinda citrifolia nectar is dominated by sucrose, which contributes an average of 72·6–88·9 % to total nectar carbohydrates (Freeman et al., 1991). We recorded an average nectar concentration of 28·06 ± 1·04 % (field refractometer), and daily nectar production per plant was 2249 ± 642 µL (Savage and Whitney, 2011). Because EFNs could not be manipulated separately from floral and post-floral nectaries (Fig. 2), the manipulations described below encompass all three types and were conducted at the scale of the nectary body.
Fig. 2.
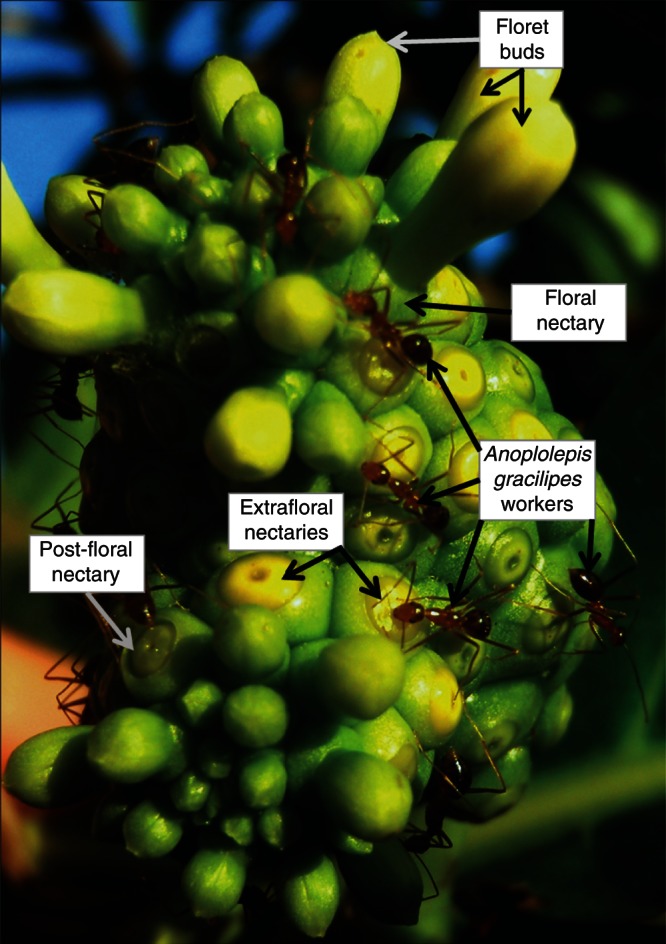
Photograph of a Morinda citrifolia nectary body with floret buds, floral nectaries, extrafloral nectaries, post-floral nectaries and Anoplolepis gracilipes workers labelled.
Honeydew-producing insects on M. citrifolia include armoured scales (Diaspididae), soft scales (Coccidae), mealybugs (Pseudococcidae) and, rarely, aphids (Aphididae). There was complete overlap of HPI genera across all sites (A. M. Savage, unpubl. res.). Furthermore, initial assessments of experimental plots prior to our manipulations (below) indicated that while plant size was significantly, negatively correlated with the abundance of HPI (P = 0·039), there was no significant difference in HPI abundance by A. gracilipes invasion status (P > 0·19).
Interactions among ants, HPI and EFN-bearing plants: plot-level mutualist exclusion experiment
Study sites
Sites were located across the island of Savaii, Samoa (see Supplementary Data Methods). Six sites had ant assemblages that were numerically dominated by A. gracilipes (>95 % of all individual ants were A. gracilipes; invasiveness score 100 %); the remaining five sites were numerically dominated by other non-native, but less invasive, ant species (<5 % of all individual ants were A. gracilipes): P. longicornis (two sites; invasiveness score 33 %) or P. megacephala (three sites; invasiveness score 83 %) (Savage and Whitney, 2011). We pooled the responses of P. longicornis and P. megacephala because previous work showed that A. gracilipes responds to nectar differently from these co-occurring, less invasive ant species in Samoa (Savage et al., 2011; Savage and Whitney, 2011) and because the responses of P. longicornis and P. megacephala were qualitatively similar.
Study sites were located on lava flows dating from 1907 to 1911, and plant communities were dominated by M. citrifolia. To increase independence, sites were separated from each other by 20 m to 73 km (mean 33·76 ± 10·12 km; median 26·25 km). To account further for spatial variation, we examined spatial autocorrelation of ant, HPI and plant responses using Mantel tests, following the protocol in Savage and Whitney (2011). There was no significant spatial autocorrelation in our results (Supplementary Data Methods).
Experimental protocol
Within each site, we established seven 4 m × 4 m plots for manipulative experiments that ran from 25 October 2007 to 27 June 2008 (including both wet and dry seasons) (Table 1). The 2 × 3 factorial design altered ant access to M. citrifolia (permitted or excluded) and the availability of carbohydrate resources (nectar reduced, HPI reduced or ambient control) (details in Table 1). In addition to the procedural controls (detailed in Table 1), we included a true control plot at each site that received no manipulations, but was sampled for arthropods prior to, and 3 and 6 months after the treatments were applied to other plots. Because procedural and true controls did not significantly differ for any of our response variables, these two control plots were pooled. Within a site, plots were located a minimum of 10 m apart (range 10 m to 67 km) to reduce the probability that ants in different plots would belong to the same nest. Plots were randomly assigned to treatments and had a minimum of three M. citrifolia plants (mean 10·1 ± 0·65 plants per plot); all M. citrifolia in plots received the treatment. Treatments were maintained every 2 weeks for 3 months (Supplementary Data Methods).
Table 1.
Experimental manipulations applied for the plot-level mutualist exclusion experiment. Treatments were applied to all M. citrifolia plants in 4 × 4 m plots. We replicated each ant treatment (reduced or control) × carbohydrate treatment (nectar reduced, HPI reduced or ambient control) combination at n = 6 sites dominated by the highly invasive ant, Anoplolepis gracilipes, and at n = 5 sites dominated by less invasive dominant exotic ants (Paratrechina longicornis and Pheidole megacephala).
| Carbohydrates |
|||
|---|---|---|---|
| Treatment | Ants | M. citrifolia nectar | Honeydew-producing insects (HPI) |
| Reduced | Paper banding material secured to the base of all M. citrifolia plants in the plot | Lightweight organza bags (1·5–2× larger than nectary bodies) secured to the base of each nectary body using a cable tie | HPI were manually removed from all stem and leaf tissues of M. ctirifolia plants using a small paintbrush every 2 weeks |
| Tree Tanglefoot pest barrier applied in a ring around the base of each M. citrifolia plant in the plot | |||
| Control | As above, except that Tree Tanglefoot was applied in a strip instead of a ring | As above, except that holes were cut in the top of each bag to allow ants access to nectaries | Stem and leaf tissue lacking HPI were brushed as above |
Response variables
Prior to treatment application, and 3 and 6 months after treatment application, we evaluated the responses of A. gracilipes, other ants, HPI and M. citrifolia plants. We visually surveyed the abundances of HPI on three M. citrifolia plants in each plot prior to sweepnet sampling. We used these data and the density of plants per plot to estimate the total abundance of HPI per plot for plots with >3 plants. We then used a canvas sweepnet to collect arthropods (Janzen and Pond, 1975; Lynch, 1981), systematically sweeping the plots from ground level to ∼2 m, using 12 sweeps per plot. We stored each sample in 70–95 % ethanol and later assessed the identity and abundances of all ants. We assessed plant size prior to experimental manipulations compared with plant size at the end of the experiment (plant growth, hereafter) by measuring the height of the main stem and the diameter at the base of the main stem (size = height × diameter). We used these data to calculate the percentage change in growth [(final size – initial size)/initial size) × 100]. Finally, during all three sampling periods, we assessed herbivory (percentage leaf surface damaged) from chewing and mining herbivores using a transparent grid (1 cm2 cells) on five haphazardly chosen leaves from each of three M. citrifolia plants per plot.
Statistical analyses: assessments of the overall responses to plot-level mutualist exclusion experiment
We conducted separate repeated measures analysis of covariance (ANCOVA) for sites dominated by A. gracilipes vs. sites dominated by other ants using SAS v. 9.1.3 (SAS Institute, 2002). Initial assessments of these data and the results from our previous studies in this system indicated that sub-ordinate ant species did not respond significantly to our treatments (see also Savage and Whitney, 2011); consequently, we focused on the response of the numerically dominant ant species at each site. The factors in the model were the ant treatment (permitted or excluded), the carbohydrate treatment (nectar reduced, HPI reduced or ambient control), time and all possible interactions. Non-additive effects were evidenced by significant interactions between the ant access and carbohydrate treatments. Initial plant size (height × diameter at base) and density of M. citrifolia plants per plot were included as covariates. Because interactions between time and the experimental factors were non-significant, we reported average abundances across both dates.
For ant data, residuals were non-normally distributed, and normality could not be obtained through transformations. Therefore, we used randomization test equivalents of ANCOVA by embedding a general linear model within an SAS randomization test macro program (Proc GLM; Cassell, 2002). Randomization tests determine P-values by comparing an observed test statistic (e.g. F-ratio) with a distribution of the test statistic that is expected under the null hypothesis (Manly, 1991). To create the expected distribution, the response variable values were pooled, permuted and randomly assigned to the treatments for 9999 iterations. For responses of plant growth, HPI abundances and herbivory, we addressed violations of model assumptions using square root (percentage plant growth and percentage herbivory) and log (n + 0·5) (HPI abundances per plot) transformations. For herbivory, we additionally included A. gracilipes invasion status in the model, along with the ant access treatment, the carbohydrate treatment and all interactions.
Statistical analyses: assessments of non-additive responses to the plot-level mutualist exclusion experiment
To characterize non-additive effects, we calculated effect sizes (ln response ratios) from least squares means following Hedges et al. (1999). For M. citrifolia plants, we assessed the independent effects of ants, HPI and their interaction on the sub-set of plants that had ambient nectar availability (i.e. we excluded from analyses the plants in which we blocked nectaries). Similarly, we assessed the relative importance of ants, nectar and their interaction for HPI abundances on the sub-set of plants with ambient (not experimentally reduced) HPI.
Effects of plant nectar on HPI-tending behaviours of ants: a plant-level, short-term assessment
Study sites
We conducted a separate, plant-level nectar gradient experiment from 16 June 2009 to 26 September 2009 (dry season) on the islands of Savaii (Independent Samoa) and Tutuila (American Samoa). These sites overlapped with the plot-level manipulative experiments (above) and our previous surveys (Savage et al., 2009). Detailed descriptions of the ant assemblages can be found in Savage and Whitney (2011). We performed spatial autocorrelation analyses using βs from within-site regressions, both across all sites and within each island (Supplementary Data Methods).
Experimental protocol
To examine the influence of plant nectar on the propensity of A. gracilipes and other dominant ants to tend HPI, we selected eight sites invaded by A. gracilipes (invasiveness score 100 %) and six sites dominated by other exotic, but less invasive ant species [P. megacephala (invasiveness score 83 %), Solenopsis geminate (invasiveness score 61 %), P. longicornis (invasiveness score 33 %), Tapinoma melanocephalum (invasiveness score 22 %) and Tetramornium bicarinatum (invasiveness score 11 %); for more information about the invasiveness scores, see Savage and Whitney (2011)]. We established 5 m × 5 m plots at each site (one plot per site), focusing on areas dominated by M. citrifolia. Within each plot, we haphazardly selected five M. citrifolia plants that were similar in size and within 1 m of each other to be used for nectar manipulations.
We manipulated nectar availability as 0, 50, 100, 150 or 200 % of ambient levels per plant. To reduce access to nectar, we used organza bags (Table 1). For plants in the 50 % treatment, we cut holes in half of the bags, and all bags had holes in the 100, 150 and 200 % above ambient nectar treatments. We used artificial nectaries (Seal-Rite microcentrifuge vials, USA Scientific, Ocala, FL, USA, filled with 500 µL of a 30 % sucrose solution) to supplement nectar levels. We inserted a 5 µL microcapillary tube into the centre of each vial to allow ants to access sucrose in the artificial nectaries. Vials were affixed to plant stems using twist ties. The 0, 50, 100, 150 and 200 % treatment levels contained 0, 0, 0, five and ten filled vials, respectively.
Response variables
We conducted ant censuses in the morning (approx. 0600–0800 h), afternoon (approx 1200–1400 h) and evening (approx. 1600–1800 h) over two consecutive days. All six censuses occurred during daylight hours due to cultural restrictions. Ant counts took approx. 5 min per plant. In addition to recording the identity and abundance of all ants (Savage and Whitney, 2011), we also tabulated the percentage of ants on the plant that were tending HPI.
Statistical analyses
We examined the relative influence of nectar availability on HPI-tending behaviours of A. gracilipes and other dominant ant species using ANCOVA (Proc GLM, SAS v. 9.1.3). The continuous factor was the nectar availability treatment (0–200 % ambient levels), site was included as a random factor nested within the fixed factor of invasion status (A. gracilipes vs. other ant species), and the response variable was the percentage of ants that were observed tending HPI. A significant interaction between invasion status and nectar availability would indicate that A. gracilipes differed in behaviour from the other ant species. Data were arcsine square-root transformed to meet model assumptions.
Results
Question 1: what are the relative effects of plant nectar and HPI on abundances of foraging ants?
Sites dominated by A. gracilipes
Plant nectar strongly and positively influenced A. gracilipes abundances, but HPI had little effect on the abundances of this species. Anoplolepis gracilipes worker abundances were 88 % lower in plots with reduced nectar availability, compared with control plots (Fig. 3A, Table 2A). However, A. gracilipes abundances were only approx. 12 % lower when HPI were reduced (non-significant), compared with the control (Fig. 3A, Table 2A).
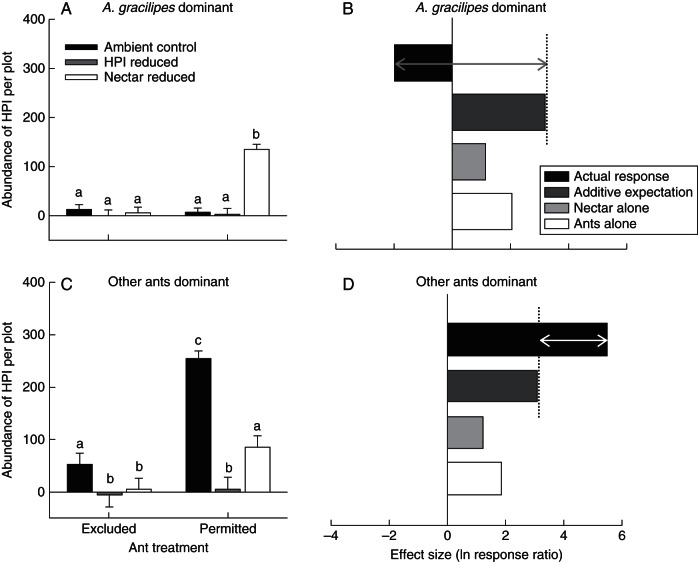
Fig. 3.
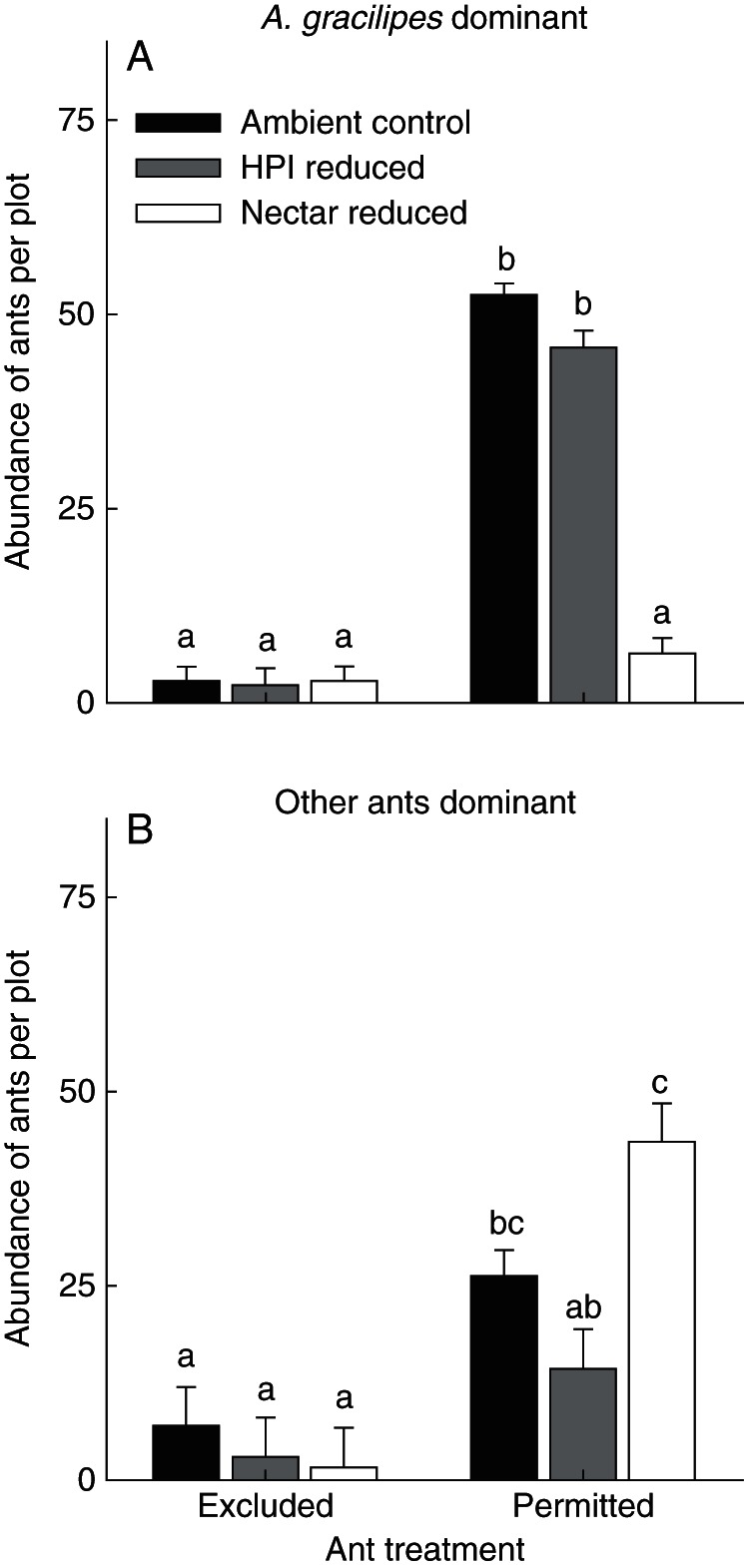
Plot (4 × 4 m)-level abundance of (A) A. gracilipes at invaded sites and (B) other dominant ants at control sites across our manipulations of access to M. citrifolia plants and availability of nectar and honeydew-producing insects (HPI). Bars represent the least square mean (LSM) + s.e. Different letters represent statistically different LSMs (P < 0·05; Tukey HSD). Sites dominated by A. gracilipes were analysed separately from sites dominated by other species.
Table 2.
Results from ANCOVA analyses for plot-level (4 × 4m) mutualist exclusion experiment at sites (A) dominated by the highly invasive species, Anoplolepis gracilipes, and (B) dominated by other exotic species that were less invasive (Paratrechina longicornis and Pheidole megacephala; see also Savage and Whitney, 2011)
| Ants per plot |
Honeydew-producing insects per plot |
% Growth of Morinda citrifolia plants |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | d.f. | F | P | d.f. | F | P | d.f. | F | P |
| (A) Dominated by Anoplolepis gracilipes | |||||||||
| Ant treatment | 1, 34 | 383·57 | < 0·0001 | 1, 24 | 24·24 | < 0·0001 | 1, 24 | 74·88 | < 0·0001 |
| Carbohydrate treatment | 2, 34 | 88·29 | < 0·0001 | 1, 24 | 14·72 | < 0·0001 | 1, 24 | 8·00 | 0·0093 |
| Ant × carbohydrate | 2, 34 | 89·51 | < 0·0001 | 1, 24 | 29·96 | < 0·0001 | 1, 24 | 8·88 | 0·0065 |
| M· citrifolia plant density | 1, 34 | 0·04 | 0·8375 | 1, 24 | 0·18 | 0·6751 | 1, 24 | 3·08 | 0·0920 |
| Initial plant size | 1, 34 | 1·11 | 0·0021 | 1, 24 | 0·09 | 0·7691 | 1, 24 | 9·40 | 0·0053 |
| (B) Dominated by other exotic species | |||||||||
| Ant treatment | 1, 27 | 37·47 | < 0·0001 | 1, 19 | 43·02 | < 0·0001 | 1, 19 | 4·14 | 0·0462 |
| Carbohydrate treatment | 2, 27 | 4·03 | 0·0295 | 1, 19 | 23·66 | 0·0001 | 1, 19 | 9·36 | 0·0065 |
| Ant × carbohydrate | 2, 27 | 5·11 | 0·0131 | 1, 19 | 6·42 | 0·0202 | 1, 19 | 11·21 | 0·0034 |
| M. citrifolia plant density | 1, 27 | 0·53 | 0·4721 | 1, 19 | 15·89 | 0·0008 | 1, 19 | 3·13 | 0·0931 |
| Initial plant size | 1, 27 | 5·16 | 0·0313 | 1, 19 | 0·02 | 0·8769 | 1, 19 | 1·72 | 0·2051 |
Significant results (P < 0·05) are highlighted in bold.
Sites dominated by other ant species
In contrast to results for A. gracilipes, plant nectar had a significant negative effect on the abundances of P. longicornis and P. megacephala, while HPI had a marginally non-significant positive effect. Specifically, abundances of these other ant species declined by approx. 45 % when HPI were reduced relative to ambient controls, but increased by approx. 66 % when plant nectar was blocked (Fig. 3B, Table 2B).
Question 2: do non-additive benefits or costs emerge when HPI and EFN-bearing plants share the same ant mutualists?
Effects of ants and plant nectar on HPI at sites dominated by A. gracilipes
The results supported the predictions of the non-additive cost model (Fig. 1C). Individually, A. gracilipes increased the abundances of HPI on M. citrifolia with ant access by >700 % compared with plants with ant exclusions (71·13 ± 5·79 vs· 8·77 ± 1·48, respectively; Table 2A; Fig. 4A, B). Similarly, the individual presence of nectar resulted in higher HPI abundances, although the magnitude of this effect was smaller than that of ants (Table 2A; Fig. 4B). In contrast to these positive individual effects, in the presence of both ants and nectar, there was an approx. 86 % reduction in the abundances of HPI (Table 2A; Fig. 4B) at sites dominated by A. gracilipes. Specifically, average abundances of HPI were only marginally greater than zero (mean 0·52 ± 10·93–11·5 ± 10·75 HPI per plot) for all treatment combinations except those with ants permitted and nectar reduced (134·84 ± 10·69; Table 2A; Fig. 4A; Tukey HSD P < 0·0001, compared with all other treatment combinations).
Fig. 4.
Influences of ants and M. citrifolia nectar on the abundance of HPI (A) at sites dominated by A. gracilipes and (C) at sites dominated by less invasive ant species. Bars represent the least square mean (LSM) + s.e. of raw data. Different letters represent statistically different log-transformed LSMs (P < 0·05; Tukey HSD). Sites dominated by A. gracilipes were analysed separately from sites dominated by other species. Graphs (B) and (D) depict the effect sizes (ln response ratios) of ants, nectar and their interaction on the abundance of HPI per plant at sites dominated by A. gracilipes and sites dominated by less invasive ant species, respectively. In these graphs, dark grey bars represent the expected additive effects of ants and nectar on HPI abundances (i.e. sum of ants alone and nectar alone) and black bars represent the realized additive + non-additive effects arising from the interaction between ants and nectar on HPI abundances per M. citrifolia plant.
Effects of ants and plant nectar on HPI at sites dominated by other ants
In support of the non-additive benefit model (Fig. 1B), we observed greater than additive effects of nectar and ants for HPI populations at non-A. gracilipes sites (Fig. 4D, Table 2B). The individual effects of ants and plant nectar were similar to those observed at sites dominated by A. gracilipes. However, the opposite pattern emerged at these sites when both ants and nectar were present, compared with sites dominated by A. gracilipes (Fig. 4C; Table 2B). Specifically, ants and nectar individually increased HPI abundances by 85 and 70 %, respectively (Fig. 4C, D). However, in the presence of both ants and plant nectar, HPI increased by >400 %, compared with plots where both ants and nectar were absent (Fig. 4C, D; ants and plant nectar ambient, 253·4 ± 16·36 HPI per plot vs. ants and nectar reduced, 0·58 ± 23·79 HPI per plot).
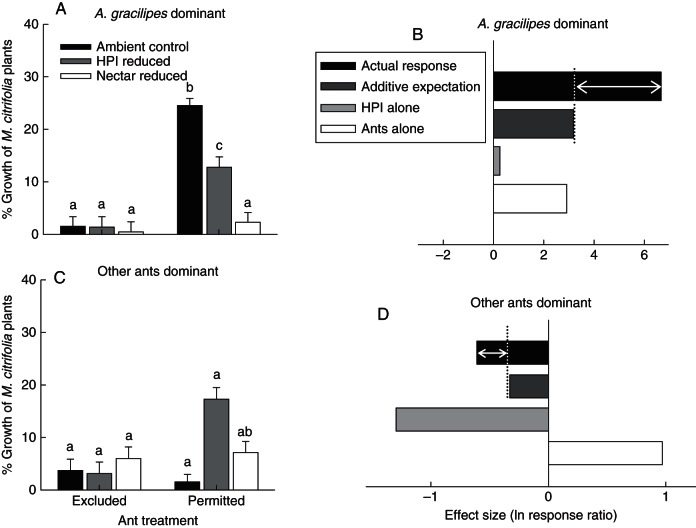
Effects of ants and HPI on M. citrifolia plants at sites dominated by A. gracilipes
At sites dominated by A. gracilipes, there were greater than additive effects of ants and HPI on the growth of M. citrifolia plants (Fig. 5B; Table 2A), thus supporting the predictions of the non-additive benefit model for plants (Fig. 1B). When ants were excluded, plants showed very little growth over 6 months. However, when ants were permitted, M. citrifolia growth was approx. 3000 % higher than in the absence of ants (Fig. 5A, B). Honeydew-producing insects had a weak, but positive effect on plant growth (Fig. 5B). These individually positive effects of ants and HPI on plant growth were amplified when both species were present. Specifically, in the absence of ants, HPI had no effect on plant growth; however, when ants were present, plants grew 92 % more in the presence of HPI than in their absence (Fig. 5A, B). Interestingly, the low growth of plants with ants permitted and reduced nectar corresponded to the highest abundances of HPI (see above) of any treatment combination at these sites. Together, these data suggest a possible cost of HPI to the plant when nectar is not available to ants.
Fig. 5.
Influences of ants and honeydew-producing insects (HPI) on the growth of M. citrifolia plants (A) at sites dominated by A. gracilipes and (C) at sites dominated by less invasive ant species (Paratrechina longicornis and Pheidole megacephala). Bars represent the least square mean (LSM) + s.e. of raw data. Different letters represent statistically different log-transformed LSMs (P < 0·05; Tukey HSD). Sites dominated by A. gracilipes were analysed separately from sites dominated by other species. Graphs (B) and (D) depict the effect sizes (ln response ratios) of ants, HPI and their interaction on the growth of M. citrifolia at sites dominated by A. gracilipes and sites dominated by less invasive ant species, respectively. In these graphs, dark grey bars represent the expected additive effects of ants and HPI on plant growth (i.e. sum of ants alone and HPI alone) and black bars represent the realized additive + non-additive effects arising from the interaction between ants and HPI on M. citrifolia growth. Arrows represent the non-additive contribution to the effects of ants and HPI on M. citrifolia growth.
Effects of ants and HPI on M. citrifolia plants at sites dominated by other ant species
Ants and HPI interactively influenced plant growth, for less than additive effects that supported the predictions of the non-additive cost model (Fig. 1C). Individually, ant presence more than doubled plant growth, and HPI significantly reduced plant growth (Fig. 5D; Table 2B). Less than additive effects emerged in the presence of both ants and HPI, with the negative influences of HPI intensifying in the presence of ants (Fig. 5D). This pattern was directly opposite to the greater than additive effects of ants and HPI on M. citrifolia plants at sites dominated by A. gracilipes.
Question 3: do modifications in ant behaviours underlie observed non-additive effects?
Effects of ant behaviours on HPI
The highly invasive ant species, A. gracilipes, showed behavioural responses to M. citrifolia nectar quite different from those of the other dominant ant species in the Samoan Archipelago (including P. longicornis and P. megacephala, in addition to other exotic ants as detailed above and in Savage and Whitney, 2011). On plants with no nectar available, on average, 40·9 ± 4·2 % of all A. gracilipes workers on plants were tending HPI; however, when nectar was increased to 200 % ambient levels, average rates of HPI tending by this species declined by approx. 96 % to 1·5 ± 4·3 % (Fig. 6). In contrast, other ant species sustained an HPI tending rate of approx. 60 % across all nectar availability levels (Fig. 6). Spatial autocorrelation analyses confirmed that ant responses to our experimental treatments were not driven by among-site spatial relationships (Supplementary Data Methods).
Fig. 6.
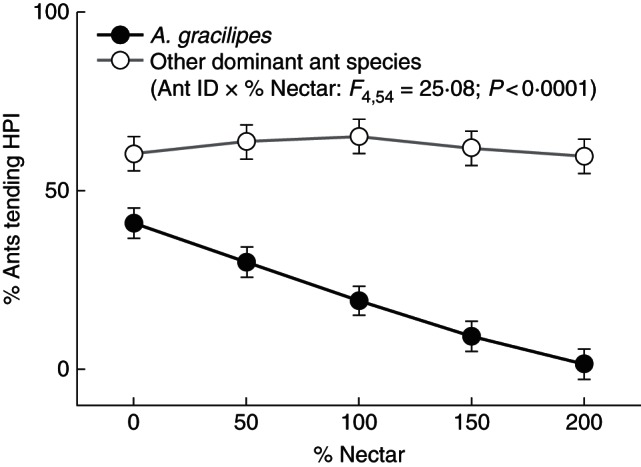
Percentage of ants tending honeydew-producing insects (HPI) across varying levels of plant nectar availability on Morinda citrifolia plants. Black circles represent ant behavioural responses of the highly invasive ant species, Anoplolepis gracilipes, and white squares represent these responses for less invasive, but dominant ant species. Error bars represent ± s.e. of the least-squares mean.
Effects of ant behaviours on M. citrifolia
Although herbivory was 40 % lower on M. citrifolia plants at sites dominated by A. gracilipes (invasion status P < 0·0001), rates of herbivory (as we measured them) did not respond to our experimental treatments (Supplementary Data Fig. S1).
DISCUSSION
Summary of results
Ants commonly participate simultaneously in mutualisms with both HPI and their EFN-bearing host plants. Consequently the three groups (ant bodyguards, HPI and EFN-bearing plants) can influence each other via direct and indirect pathways ranging from mutualistic to antagonistic. Interactions that appear beneficial in pairwise comparisons can shift towards antagonism in the presence of a third partner, and vice versa (Fig. 1). In this study, we examined these dynamics using manipulative experiments, which allowed us to evaluate empirically the potential for non-additive effects. Additionally, we examined these dynamics at sites dominated by A. gracilipes, an invasive ant with a documented preference for carbohydrate-rich foods (Holway et al., 2002; Savage et al., 2009), and sites dominated by other exotic ant species in Samoa. We found that plant nectar had a strong positive influence on plot-wide abundances of A. gracilipes, while HPI were more important for the other exotic ant species in Samoa (Fig. 3). Extrafloral nectary-bearing M. citrifolia plants benefited from the combined presence of ants and HPI, but only at sites dominated by A. gracilipes (Fig. 5). At sites dominated by other ant species, the benefits of ants to plants shifted towards antagonism in the presence of HPI (Fig. 5). In contrast, HPI benefited from the individual presence of both ants and plant nectar across sites dominated by A. gracilipes and those dominated by other ants. However, when both ants and nectar were present, abundances of HPI at A. gracilipes-dominated sites were severely depressed, whereas HPI at sites dominated by other species were greatly increased (Fig. 4). Strong reductions in the tending behaviours of A. gracilipes – but not other ant species – as nectar levels increased (Fig. 6) may underlie this among-site variability. However, we did not detect any significant differences in herbivory rates on M. citrifolia plants at any sites. Consequently, it appears that other dynamics are driving the observed patterns in plant growth responses to ants and HPI (Fig. 5; Supplementary Data Fig. S1).
The relative importance of HPI vs. plant nectar for ants
Our results suggest consistent differences among ant species in their preferences for honeydew vs. nectar resources. However, the mechanism(s) that underlie these altered preferences remain unresolved. Most studies that have compared relative feeding preferences of ants have focused on differences in preferences for protein-rich vs. carbohydrate-rich food. For example, Cook and Davidson (2006) showed that ants in the subfamilies Formicinae and Dolicoderinae have physical adaptations that allow them to store more liquid foods. However, such adaptations are unlikely to be important mechanisms underlying differences in ant choices for different types of carbohydrate-rich foods. An alternative hypothesis that ants that feed more on plant-based foods house N-fixing or recycling bacteria in their guts (e.g. Russell et al., 2009) may explain some of these variable preferences better than taxonomically based hypotheses. However, it is unclear how ants distinguish among the very similar resources provided by plants and hemipterans. Contrasting preferences of A. gracilipes and other ants for plant nectar vs. honeydew may be due to subtle differences in the composition of these foods. Although compositionally similar, nectar tends to have fewer amino acids (Beattie, 1985) and a less diverse sugar profile (Blüthgen et al., 2004) than insect honeydew. In a preference trial, Blüthgen and Fielder (2004) found that a diverse community of ants (51 species) preferred sucrose to the more complex sugars, such as melezitose and maltose, commonly found in hemipteran honeydew. They also found that many of the individual ant species preferred simple sugar baits that contained at least trace amounts of amino acids. However, amino acid preferences were much more variable among ant species, and were also conditional on a colony's nutritional status. Interestingly, Blüthgen and Fielder (2004) tested preferences of congeners of P. longicornis and P. megacephala; both displayed a significant preference for a sugar–amino acid mixture that mimicked the composition of honeydew over sucrose (they did not test Anoplolepis). These patterns are consistent with our finding that these species preferred honeydew to M. citrifolia nectar, which is largely composed of sucrose (approx. 73–89 % sucrose; Freeman et al., 1991). In contrast, Engel et al. (2001) examined the effects of HPI on Lasius niger ants that tended extrafloral nectar. They first reared ants on a diet of protein (from crickets) and honeydew (from two aphid species that were not part of the experiment). Ants were then allowed access to EFN-bearing Vicia faba plants in the presence and absence of honeydew-producing Aphis fabae colonies. They demonstrated that ants tended nectaries regardless of the presence of HPI. This species showed a preference for nectar over honeydew; analyses of relative composition revealed that the plant nectar had more sugars and fewer amino acids than honeydew. Finally, Gibb and Cunningham (2009) surveyed canopies of Australian Acacia and Eucalyptus trees, which included both nectar and honeydew resources. They found that the behaviourally dominant ants in a diverse assemblage tended HPI rather than nectaries. This result may suggest a consistent preference for honeydew in ants that are strongly competing for resources, although it does not explain the competitive dominance of nectar-preferring A. gracilipes at sites where they dominate. Experimental manipulations at broader spatial scales will be required in order to determine the strength of these correlative patterns. Altogether, current data suggest strong differences among ant species in their responses to alternative sources of carbohydrates. Due to the varied roles of ants in their ecosystems, these differences could cascade to influence entire communities.
The strong response of A. gracilipes to nectar availability, at both the plant and plot level, provides additional support for the hypothesis that this species in particular, and highly invasive ants in general, prefer carbohydrate-rich foods (Holway et al., 1999). Anoplolepis gracilipes has a pantropical distribution (Wetterer 2005). When it displays invasive characteristics, A. gracilipes commonly associates with species that produce carbohydrate-rich food, although the majority of past studies have focused on HPI rather than nectar as sources of carbohydrates. For example, when A. gracilipes were introduced to Christmas Island, they remained at low population densities during a lag phase that lasted >70 years (O'Dowd et al., 2003). In the 1990s, this species began displaying invasive characteristics. Within about 10 years, A. gracilipes had killed up to one-third of the island's endemic red land crabs (Gecarcoidea natalis). Furthermore, A. gracilipes actively tended scale insects, and correlative evidence suggests that the combination of increased carbohydrate resources for the ants and population expansion of scale insects led to the death of native canopy trees (O'Dowd et al., 2003). Similar interactions among HPI and A. gracilipes invasions have been recorded in South Africa (Sivakumar et al., 2013), Seychelles (Hill et al., 2003), Tokelau (Lester and Tavite, 2004) and Japan (Tanaka et al., 2011). In contrast to results at these other invaded locations, our findings suggest that M. citrifolia nectar is a more important resource to A. gracilipes than is honeydew. In these studies of other invaded habitats, EFNs were either not present on host plants or not considered in the study, which may explain the disparity. Perhaps A. gracilipes protect HPI intensively only in the absence of plant-derived carbohydrates, and, in the presence of EFN-bearing plants, this species may shift to collecting extrafloral nectar and defending nectaries rather than HPI. Conversely, variation in abundances of HPI or protein requirements of A. gracilipes colonies could lead to these types of responses. Whatever the mechanism, our results suggest that the community-level consequences of A. gracilipes invasion will depend on whether sites have resident, EFN-bearing plants. This hypothesis is supported by our own data showing that A. gracilipes have positive effects on HPI abundances only when plant nectar is reduced (Savage et al., 2011) and that the abundance of A. gracilipes per site is strongly and positively associated with the dominance of EFN-bearing plants across the Samoan Archipelago (Savage et al., 2009).
One counterintuitive result from our study was that the plot-wide abundances of the less invasive ant species actually increased when plant nectar was reduced, despite reductions of HPI populations on plants with nectar reduced. Although unlikely, it is possible that there were cryptic HPI on these plants (such as camouflaged scale insects), that were not detected in our surveys. We consider this unlikely, because we intensively surveyed plant surfaces for the presence of these honeydew producers. However, we only surveyed three plants per plot during each sampling period, so we cannot discount the possibility that other plants in the plot hosted higher abundances of HPI. Alternatively, it is possible that the small populations of HPI on the plants became a more valuable resource to ants when other sources of carbohydrate-rich food became scarce, leading to a greater investment in workers to defend small HPI populations on plants rather than engaging in other behaviours. Increasing sucrose-rich nectar resources may also have led local ant populations to become protein starved (Ness et al., 2009), causing colonies to invest more workers in foraging for insect prey. Our study was longer in duration (6 months) than many studies of ant protective mutualisms, so we may have been observing dynamics that are not apparent at the scale of days or weeks.
Support for non-additive benefit vs. non-additive cost models depended upon ant identity and focal species (HPI vs. M. citrifolia plants)
Plant growth responses at sites dominated by A. gracilipes supported the predictions of the non-additive benefit model, while HPI responses at these sites supported the predictions of the non-additive cost model (Fig. 1). Anoplolepis gracilipes responded more strongly to plant nectar than to the presence of HPI (Fig. 3); furthermore, increasing plant nectar availability reduced ants' propensity to tend HPI (Fig. 6). Increasing plant nectar not only distracted ants from tending HPI (see Introduction) but also reduced the abundance of HPI on plants, via an antagonistic, non-additive effect of ants plus nectar (Fig. 4). Together, these dynamics led to a strong, greater than additive effect of ants and HPI on M. citrifolia plant growth (Fig. 5). These dynamics suggest that A. gracilipes workers may be attracted to plants with HPI and then distracted from HPI as nectar levels increase, and that EFN-bearing plants can benefit from these shifts in ant behaviours (Becerra and Venable, 1989). In a similar study, Itzhak Martinez et al. (2011) assessed the effects of plant nectar on Tapinoma erraticum tending behaviours towards HPI (aphids) for two different EFN-bearing plants. In a short-term experiment (8 d), they detected similar nectar distraction of T. erraticum from HPI on one of the host plants, although the consequences for plant growth and HPI abundances were not assessed.
In direct contrast to our findings at sites dominated by A. gracilipes, responses of M. citrifolia plants at sites dominated by less invasive ant species supported the predictions of the non-additive cost model; meanwhile, the responses of HPI confirmed the predictions of the non-additive benefit model (Fig. 1). Although ant presence increased plant growth when HPI were experimentally reduced, HPI had a negative, independent influence on plant growth, and the combined effects of ants and HPI were more strongly negative than the additive expectation (Fig. 5). Thus, plants with ants grew the least under ambient nectar and ambient HPI, which corresponded to the highest abundances of HPI (Fig. 4). Although conducted on a plant that did not bear EFNs, Grinath et al. (2012) also simultaneously manipulated both ants and HPI and tracked plant responses. They found that in the absence of ants, HPI had weak negative effects on plant fitness; however, when ants were allowed on plants, the negative effects of HPI were amplified, for a net negative effect of ants on plants despite their role in reducing the abundances of chewing herbivores. In contrast to the response of A. gracilipes, other dominant, non-native ants in Samoa actually declined in the presence of nectar, but increased in the presence of HPI (Fig. 3). Also, in contrast to A. gracilipes, the two less invasive ant species examined here did not alter tending behaviours as nectar levels increased, supporting the hypothesis that HPI could distract ants from tending EFNs (Becerra and Venable, 1989; Fig. 6). Support for the HPI distraction hypothesis was also provided by previous research in Brazil. Del Claro and Oliveira (1993) manipulated plant nectar levels and found that Camponotus spp. consistently tended HPI across all nectar levels.
In support of the non-additive benefit model (for HPI) at sites dominated by less invasive ant species, both ants and nectar had a positive effect on HPI abundances when they were present alone. When both resources were present together, HPI abundances were larger than the additive expectation (Fig. 4). Although more study is needed, these results suggest that variation in ant identity is a key factor in determining the likelihood that these multispecies interactions will benefit HPI vs. their EFN-bearing host plants. In a recent study, Katayama and Suzuki (2010) found that plants with EFNs attracted more ants than those without EFNs, regardless of the presence of small aphid aggregations. Although aphid tending rates were lower than EFN tending rates on these plants, aphids ultimately benefited from the presence of EFN-tending ants because coccinelid adults spent less time attacking aphid colonies on plants in which ants tended EFNs. This study underscores the importance of considering multiple community members in studies of ant protective mutualisms. Future work investigating dynamics among M. citrifolia plants and exotic ants should also assess the identity and abundance of other predacious insects. Nevertheless, these findings, combined with our results, suggest that longer term studies (on the scale of weeks, months or seasons, rather than days) are needed to assess the non-additive influences of ants and plant nectar on HPI populations.
Conclusions
Partner sharing is a relatively common phenomenon in mutualistic associations, but has received little attention in the ecological literature. Recent review and network approaches have highlighted the diversity of partners of a similar type (e.g. multiple protective ant species or multiple plant–pollinator interactions) (Bascompte et al., 2006; Melián et al., 2009; Sazima et al., 2010; Jones et al., 2012), but many organisms that share partners represent quite different ecological functional groups or trophic positions (such as plants and HPI); these interactions are not well represented by current approaches, such as bipartite network models or models of cheaters and intraguild mutualisms (Crowley and Cox, 2011; Jones et al., 2012). In this study, we examined dynamics among HPI, their EFN-bearing host plants and their shared ant bodyguards in the context of purely additive, non-additive benefit and non-additive cost models. We found no support for the purely additive model. However, there was strong support for both non-additive models. The direction and magnitude of these effects varied with the identity of the ant partner as well as the identity of the guild of carbohydrate-producing mutualist partners (HPI vs. EFN-bearing plants). Anoplolepis gracilipes displayed very different responses to carbohydrate-rich, mutualist-derived resources than co-occurring less invasive, but exotic ant species in Samoa. More studies that investigate the responses of ants with varying levels of invasiveness (from native species to highly invasive) will allow us to determine if these findings are generalizable to successful invaders.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Ken Whitney for substantial advice during the project development and data analysis phases of these experiments. Thanks also to Jeremy Caves, Kevin Dougherty, Rebecca Sandidge and Mark Schmaedick for field assistance, and to Rosalie Berg and Ryan Gravolet for their laboratory assistance. Samoan land owners graciously allowed us access to their private property to conduct these experiments and were very hospitable. We also thank Judith Bronstein, Brigitte Marazzi, Suzanne Koptur, Jeffery Karron and the anonymous reviewers for their thoughtful comments on previous drafts of this manuscript. This work was supported by the National Geographic Society grant 8237-07, by the Godwin Assistant Professorship to J.A.R. and by a Rice University Wray-Todd Fellowship to A.M.S.
LITERATURE CITED
- Abbott KL. Spatial dynamics of supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes on Christmas Island, Indian Ocean. Diversity and Distributions. 2006;12:101–110. [Google Scholar]
- Abbott KL, Green PT. Collapse of an ant-scale mutualism in a rainforest on Christmas Island. Oikos. 2007;116:1238–1246. [Google Scholar]
- Bascompte J, Jordano P, Olesen JM. Assymetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. [DOI] [PubMed] [Google Scholar]
- Beattie AJ. The evolutionary ecology of ant–plant mutualisms. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Becerra JXI, Venable DL. Extrafloral nectaries: a defense against ant–homoptera mutualisms? Oikos. 1989;55:276–280. [Google Scholar]
- Blüthgen N, Fielder K. Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. Journal of Animal Ecology. 2004;73:155–166. [Google Scholar]
- Blüthgen N, Gottsberger G, Fielder K. Sugar and amino acid composition of ant-tended nectar and honeydew sources from an Australian rainforest. Austral Ecology. 2004;29:418–429. [Google Scholar]
- Buckley RC. Interaction between ants and membracid bugs decreases growth and seed set of host plant bearing extrafloral nectaries. Oecologia. 1983;58:132–136. doi: 10.1007/BF00384553. [DOI] [PubMed] [Google Scholar]
- Cassell DL. Proceedings of the 27th Annual SAS User's Group International Conference. Cary, NC: SAS Institute; 2002. A randomization-test wrapper for SAS PROCS. [Google Scholar]
- Chamberlain SA, Holland JN. Quantitative synthesis of context-dependency in ant–plant protection mutualisms. Ecology. 2009;90:2384–2392. doi: 10.1890/08-1490.1. [DOI] [PubMed] [Google Scholar]
- Cook SC, Davidson DW. Nutritional and functional biology of exudates-feeding ants. Entomologia Experimentalis et Applicata. 2006;118:1–10. [Google Scholar]
- Crowley PH, Cox JJ. Intraguild mutualism. Trends in Ecology and Evolution. 2011;26:627–633. doi: 10.1016/j.tree.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Davidson DW. The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biological Journal of the Linnaean Society. 1997;61:153–181. [Google Scholar]
- Del Claro K, Oliveira PS. Ant–homoptera interaction: do alternative sugar sources distract tending ants? Oikos. 1993;68:202–206. [Google Scholar]
- Engel V, Fischer MK, Wackers FL, Volkl W. Interactions between extrafloral nectaries, aphids and ants: are there competition effects between plant and Homopteran sugar sources? Oecologia. 2011;129:577–584. doi: 10.1007/s004420100765. [DOI] [PubMed] [Google Scholar]
- Frederickson ME, Greene MJ, Gordon DM. Ecology: ‘Devil's gardens’ bedevilled by ants. Nature. 2005;437:495–496. doi: 10.1038/437495a. [DOI] [PubMed] [Google Scholar]
- Freeman CE, Worthington RD, Jackson MS. Floral nectar sugar compositions of some south and southeast Asian species. Biotropica. 1991;23:568–574. [Google Scholar]
- Goheen JR, Palmer TM. Defensive plant-ants stabilize megaherbivore-driven landscape change in an African savanna. Current Biology. 2010;20:1–5. doi: 10.1016/j.cub.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Gibb H, Cunningham SA. Does the availability of arboreal honeydew determine the prevalence of ecologically dominant ants in restored habitats? Insectes Sociaux. 2009;56:405–412. [Google Scholar]
- Grinath JB, Inouye BD, Underwood N, Billick I. The indirect consequences of a mutualism: comparing positive and negative components of the net interaction between honeydew-tending ants and host plants. Journal of Animal Ecology. 2012;81:494–502. doi: 10.1111/j.1365-2656.2011.01929.x. [DOI] [PubMed] [Google Scholar]
- Grover CD, Kay AD, Monson JA, Marsh TC, Holway DA. Linking nutrition and behavioural dominance: carbohydrate scarcity limits aggression and activity in Argentine ants. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2951–2957. doi: 10.1098/rspb.2007.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes PR, Jr, Raimundo RLG, Bottcher C, Silva RR, Trigo JR. Extrafloral nectaries as a deterrent mechanism against seed predators in the chemically protected weed Crotalaria pallida (Leguminosae) Austral Ecology. 2006;31:776–782. [Google Scholar]
- Harvey AW. Extrafloral nectaries in Kudzu, Pueraria montana (Lour.) Merr., and Groundnut, Apios americana Medicus (Fabaceae) Castanea. 2009;74:360–371. [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Heil M, McKey D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology, Evolution, and Systematics. 2003;34:425–453. [Google Scholar]
- Hill M, Holm K, Vel T, Shah NJ, Matyok P. Impact of the introduced yellow crazy ant, Anoplolepis gracilipes, on Bird Island, Seychelles. Biodiversity and Conservation. 2003;12:1969–1984. [Google Scholar]
- Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. Causes and consequences of ant invasions. Annual Review of Ecology and Systematics. 2002;33:181–203. [Google Scholar]
- Itzhak Martinez JJ, Cohen M, Mgocheki N. The response of an aphid tending ant to artificial extrafloral nectaries on different host plants. Arthropod Plant Interactions. 2011;5:185–192. [Google Scholar]
- Janzen DH, Pond CM. A comparison, by sweep sampling, of the arthropod fauna of secondary vegetation in Michigan, England and Costa Rica. Transactions of the Royal Society of London. 1975;127:33–50. [Google Scholar]
- Jones EI, Bronstein JL, Ferrière R. The fundamental role of competition in the ecology and evolution of mutualisms. Annals of the New York Academy of Sciences. 2012;1256:66–88. doi: 10.1111/j.1749-6632.2012.06552.x. [DOI] [PubMed] [Google Scholar]
- Katayama N, Suzuki N. Extrafloral nectaries indirectly protect small aphid colonies via ant-mediated interactions. Applied Entomology and Zoolology. 2010;45:505–511. [Google Scholar]
- Lach L, Hobbs RJ, Majer JD. Herbivory-induced extrafloral nectar increases native and invasive ant survival. Population Ecology. 2009;51:237–243. [Google Scholar]
- Lester PJ, Tavite A. Long-legged ants, Anoplolepis gracilipes (Hymenoptera: Formicidae), have invaded Tokelau, changing composition and dynamics of ant and invertebrate communities. Pacific Science. 2004;58:391–401. [Google Scholar]
- Lynch JF. Seasonal, successional, and vertical segregation in a Maryland ant community. Oikos. 1981;37:183–198. [Google Scholar]
- Manly BFJ. Randomization and Monte Carlo methods in biology. London: Chapman and Hall; 1991. [Google Scholar]
- Melián CJ, Bascompte J, Jordano P, Krivan V. Diversity in a complex ecological network with two interaction types. Oikos. 2009;118:122–130. [Google Scholar]
- Messina FJ. Plant protection as a consequence of an ant–membracid mutualism: interactions on goldenrod (Solidago Sp.) Ecology. 1981;62:1433–1440. [Google Scholar]
- Ness JH, Bronstein JL. The effects of invasive ants on prospective ant mutualists. Biological Invasions. 2004;6:445–461. [Google Scholar]
- Ness JH, Morris WF, Bronstein JL. For ant-protected plants, the best defense is a hungry offense. Ecology. 2009;90:2823–2831. doi: 10.1890/08-1580.1. [DOI] [PubMed] [Google Scholar]
- O'Dowd DJ, Green PT, Lake PS. Invasional ‘meltdown’ on an oceanic island. Ecology Letters. 2003;6:812–817. [Google Scholar]
- Rico-Gray V, Oliveira PS. The ecology and evolution of ant–plant mutualisms. Chicago, IL: University of Chicago Press; 2007. [Google Scholar]
- Rudgers JA, Savage AM, Rua MA. Geographic variation in a facultative mutualism: consequences for local arthropod composition and diversity. Oecologia. 2010;163:985–996. doi: 10.1007/s00442-010-1584-6. [DOI] [PubMed] [Google Scholar]
- Russell JA, Moreau CS, Goldman-Huertas B, Fujiwara M, Lohman DJ, Pierce NE. Bacterial gut symbionts tightly linked with the evolution of herbivory in ants. Proceedings of the National Academy of Sciences, USA. 2009;106:21236–21241. doi: 10.1073/pnas.0907926106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS version 9.1.3. Cary, NC: SAS Institute; 2003. [Google Scholar]
- Savage AM. Rice University. Department of Ecology and Evolutionary Biology; 2011. Anoplolepis gracilipes invasion of the Samoan Archipelago: can mutualisms with native species amplify ecological consequences? PhD Dissertation. [Google Scholar]
- Savage AM, Whitney KD. Mutualistic, trait-mediated indirect interactions in invasions: a highly invasive ant has unique behavioural responses to plant nectar. Ecosphere. 2011;2:106. http://dx.doi.org/10.1890/ES11-00145.1 . [Google Scholar]
- Savage AM, Rudgers JA, Whitney KD. Elevated dominance of extrafloral nectary-bearing plants is associated with increased abundances of an invasive ant and reduced native ant richness. Diversity and Distributions. 2009;15:751–761. [Google Scholar]
- Savage AM, Johnson SD, Whitney KD, Rudgers JA. Do invasive ants respond more strongly to carbohydrate availability than co-occurring non-invasive ants? A test along an active Anoplolepis gracilipes invasion front. Austral Ecology. 2011;36:310–319. [Google Scholar]
- Sazima C, Guimarães PR, Dos Reis SF, Sazima I. What makes a species central in a cleaning mutualism network? Oikos. 2010;119:1319–1325. [Google Scholar]
- Sivakumar T, Chandrika M, Babu M. Incidence and management of the leaf hopper, Busoniomimus manjunathi, on Malabar Tamarind, Garcinia cambogia. African Journal of Agricultural Research. 2013;8:145–147. [Google Scholar]
- Styrsky JD, Eubanks MD. Ecological consequences of interactions between ants and honeydew-producing insects. Proceedings of the Royal Society B: Biological Sciences. 2007;274:151–164. doi: 10.1098/rspb.2006.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Ohnishi H, Tatsuta H, Tsuji K. An analysis of mutualistic interactions between exotic ants and honeydew producers in the Yanbaru district of Okinawa Island, Japan. Ecological Research. 2011;26:931–941. [Google Scholar]
- Tobin JE. A neotropical rainforest canopy ant community: some ecological considerations. In: Huxley CR, Cutlter DF, editors. Ant–plant interactions. Oxford: Oxford University Press; 1991. pp. 236–238. [Google Scholar]
- Trager MD, Bhotika S, Hostetler JA, Andrade GV, Rodriguez-Cabal MA, McKeon CS, Osenberg CW, Bolker BMM. Benefits for plants in ant-plant protective mutualisms: a meta-analysis. PLoS ONE. 2010;5:e14308. doi: 10.1371/journal.pone.0014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki J, Olkpul T, Komolong MK. A descriptor list for morphological characterization of Noni (Morinda citrifolia L.) South Pacific Journal of Natural Science. 2007;10:61–66. [Google Scholar]
- Ward DF, Stanley MC, Toft RJ, Forgie AS, Harris RJ. Assessing the risk of invasive ants: a simple and flexible scorecard approach. Insectes Sociaux. 2008;55:360–363. [Google Scholar]
- Weber MG, Keeler KH. The phylogenetic distribution of extrafloral nectaries in plants. Annals of Botany. 2013;111:1251–1261. doi: 10.1093/aob/mcs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterer JK. Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae) Sociobiology. 2005;45:77–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.