Abstract
Major depression represents one of the most disabling illnesses and current treatments are only partially effective. All antidepressant agents modulate the monoamine system, which likely accounts for the similar efficacy profile of available treatments. Herein we will summarize the current state of depression therapeutics and assess the antidepressant development pipeline. Antidepressant response rates in controlled trials are estimated at ~54% and real-world effectiveness data suggests a somewhat lower rate. Response rates are lower still in patients who have not responded to previous treatment attempts and meaningful advancements will likely come only from identification of mechanistically novel agents. Monoaminergic agents largely dominate the antidepressant development pipeline, however the glutamate neurotransmitter system represents a bright spot on the antidepressant horizon. We review in detail findings regarding the antidepressant effects of the glutamate N-methyl-d-aspartate receptor antagonist ketamine in order to highlight the promise of novel agents as future treatments for major depression.
Keywords: Major depressive disorder, MDD, Treatment-resistant depression, TRD, Antidepressant, Drug discovery, Psychopharmacology, Glutamate, Ketamine, N-methyl-d-aspartate receptor, NMDAR, Mood disorders, Psychiatry
Introduction
Major depressive disorder (MDD) represents one of the most disabling medical illnesses worldwide. According to the World Health Organization (WHO), unipolar depressive disorders account for 65.5 million disability-adjusted life years (DALYs) and rank third among leading causes of global disease burden (1). Among all mental, neurological and substance-use disorders, unipolar depressive disorders rank first, followed by alcohol-use disorders (23.7 DALYs) and schizophrenia (16.8 DALYs) (2). Identifying more effective treatments for MDD represents a critical component required to meet this large public health challenge; increasing access to care and identifying risk and protective factors for depressive disorders represent additional essential components.
Current antidepressant therapeutics target aspects of the monoamine neurotransmitter systems in the brain and are often only partially effective for MDD. A major goal of current neuropharmacology research is to identify safe and more effective treatments for depression by targeting neural systems and chemical messengers outside of the monoamine system (3–6). In the current review we will analyze the current state of depression therapeutics and summarize the efficacy and effectiveness of available antidepressants. We will then examine the pharmaceutical antidepressant development pipeline and ask the question, “Is there anything really novel on the antidepressant horizon?”
We suggest that the glutamate system represents a promising avenue for antidepressant drug development and highlight this bright spot on the antidepressant horizon. We will review in detail current evidence for the antidepressant action of the glutamate N-methyl-d-aspartate (NMDA) receptor (NMDAR) antagonist ketamine as a touchstone for glutamatergic antidepressant agents. We will briefly review molecular mechanisms hypothesized to underlie ketamine’s antidepressant effect and conclude with a discussion of future research directions.
Depression Therapeutics: Current State of the Art
The discovery of drugs in the 1950’s that had a selective mitigating effect on core symptoms of mood disorders represents a landmark event in modern psychiatry and the advent of psychopharmacology for major depression has led to symptom relief for untold numbers of patients. Despite more than 60 years of clinical and basic science research related to depression and antidepressant agents, however, there are critical limitations among current treatment options. All antidepressant agents currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of MDD primarily act to modulate components of the monoamine neurotransmitter system in the brain and generally increase the intra-synaptic availability of serotonin, norepinephrine or dopamine. Below we review the efficacy and effectiveness data for current antidepressants.
In recent years there has been considerable debate regarding the efficacy and effectiveness of antidepressant medications (7–9). A recent meta-analysis including more than one hundred randomized, placebo-controlled trials (RCTs) of antidepressant agents and 27,127 patients with MDD yielded an average response rate of 54% for FDA-approved antidepressants, compared to 37% for placebo (9). The pooled drug-placebo relative response rate was 1.42 (1.38–1.48) with a number needed to treat (NNT) of 8. These results are similar to a previously published meta-analysis of antidepressant efficacy trials including 44,240 patients with MDD, which yielded average response rates of 54.3% and 37.9% for active drug and placebo, respectively (10). These and other study studies have generally not found meaningful differences in efficacy between different classes of antidepressants or between individual agents (11). The meta-analysis by Undurraga did find a small but statistically significant advantage of older agents – include the tricyclic antidepressants (TCAs) – over newer agents (9). The recently published psychopharmacology update on the treatment of depressive disorders by the Canadian Network for Mood and Anxiety Treatments (CANMAT) found that all second-generation antidepressants have Level 1 evidence to support efficacy and tolerability and found a lack of meaningful difference between treatment options (12). The findings of these large meta-analyses support the conclusion that currently antidepressants are significantly but only modestly efficacious in the treatment of MDD.
Important factors to consider when interpreting outcomes from antidepressant RCTs include the potential role of publication biases (7) and the influence of baseline depression severity on observed efficacy (8). Turner et al found a mean weighted effect size for antidepressants of 0.41 (Hedges’ g) based on published RCTs compared to a mean effect size of 0.31 when unpublished data from the FDA was included, representing a 32% difference (7). Regarding the influence of baseline depression severity on apparent efficacy, Fournier at al found a mean effect size of 0.47 (Cohen’s d) – corresponding to a NNT of 4 – among patients with depression severity that would be classified as very severe, compared to a much lower effect size of 0.11 – corresponding to a NNT of 16 – among patients characterized as having mild or moderate severity (8).
The factors reviewed above, among others, serve to limit the estimation of the true effectiveness of antidepressants for patients in real world settings. The most informative data regarding antidepressant effectiveness come from the large Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, which enrolled 4,041 adult outpatients with MDD (13,14). Following up to 12 weeks of treatment with the serotonin-selective reuptake inhibitor (SSRI) citalopram, the response and remission rates were 48.6% and 36.8%, respectively (14). Subsequent treatment steps involved several augmentation or switching strategies for patients who did not remit at the previous step and yielded response rates of 28.5%, 16.8% and 16.3% at the second, third and fourth treatment steps, respectively (14). Unfortunately no single treatment strategy appeared to possess a meaningful advantage over other options for patients who did not remit at a previous step (15,16).
In a follow up to the STAR*D study, the Combining Medications to Enhance Depression Outcomes (CO-MED) study was designed to compare 12-week response and remission rates between monotherapy with an SSRI and two antidepressant combinations (17). In this single-blind study, 665 outpatients with chronic or recurrent MDD were randomized to receive (1) escitalopram plus placebo, (2) bupropion SR plus escitalopram, or (3) venlafaxine XR plus mirtazapine. The authors found that response rates at 12 weeks varied between 51.6% and 52.4% and rates did not differ significantly between treatment groups. Remission rates were not different and ranged from 37.7%–38.9%.
Taken together, the body of evidence reviewed above indicates that currently available treatments for depression are modestly effective for many patients and are unfortunately not effective for a substantial proportion of patients. There are no clear guidelines to help clinicians select among first-line agents or to guide next-step treatment decisions for patients who do not response to an initial treatment trial.
The Antidepressant Pharmaceutical Pipeline: What’s New?
The limited effectiveness of current antidepressants – and their mechanistic similarity – underscores the urgent public health need for novel, more effective treatment for MDD. Are there new, innovative treatments for MDD on the horizon? Table 1 summarizes 22 compounds currently in development for MDD. These data were obtained from public sources, including the PhRMA Medicines in Development for Mental Illness 2012 Report (18). As expected, the majority of agents in development can be classified broadly as monoaminergic as their primary mechanism of action and include the so-called “triple-reuptake inhibitors.” Triple-reuptake inhibitors (TRIs) bind to and inhibit all three primary monoamine synaptic reuptake proteins: the serotonin transporter (SERT), the norepinephrine transporter (NET) and the dopamine transporter (DAT). Molecular entities in this category farthest along in development are EB-1010 by Euthymics Bioscience and BMS-820836 by Bristol-Myers Squibb. TRIs can be seen as the next logical step in an effort to broaden engagement of the monoamine systems, following the development of serotonin norepinephrine reuptake inhibitors (SNRIs). It was initially hoped that SNRIs would possess enhanced efficacy for depression compared to SSRIs, although there is unfortunately a paucity of data to support this conclusion (see above discussion). Whether or not the addition of dopaminergic modulation in the pharmacodynamic profile of this next generation of antidepressants confers enhanced efficacy compared to SSRI or SNRI agents remains to be seen. Other monoaminergic agents in Phase III development include the dual reuptake inhibitor (SERT, NET) levomilnacipran (Forest Laboratories), the selective NET inhibitor LY2216684 (Eli Lilly) and a D2-receptor partial agonist (OPC-34712, Lundbeck).
Table 1.
Select Compounds in Development for Major Depressive Disordera
| General Mechanism | Molecular Target | Compound | Pharmacodynamic Action | Sponsor | Development Phase |
|---|---|---|---|---|---|
| Monoaminergic | SERT, NET | Levomilnacipran | Reuptake Blockade | Forest Laboratories | Phase III |
| Monoaminergic | NET | LY2216684 | Reuptake Blockade | Eli Lilly | Phase III |
| Monoaminergic | D2 Receptor | OPC-34712 | Partial Agonist | Lundbeck | Phase III |
| Monoaminergic | SERT, Additional 5- HT Targets | Vortioxetine | Reuptake Blockade; Additional 5-HT Modulation | Lundbeck | Phase III |
| Monoaminergic | SERT, NET, DAT | EB-1010 | Reuptake Blockade | Euthymics Bioscience | Phase II/III |
| Monoaminergic | SERT, NET, DAT | BMS-820836 | Reuptake Blockade | Bristol-Myers Squibb | Phase II |
| Monoaminergic | SERT, Additional 5- HT Targets | TGBA01AD | Reuptake Blockade; Additional 5-HT Modulation | Fabre-Kramer Pharmaceuticals | Phase II |
| Monoaminergic | MAO-A | CX157 | Reversible Enzyme Inhibitor | CeNeRx Pharma | Phase I |
| Monoaminergic | SERT | DSP-1053 | Reuptake Blockade | Sunovion Pharmaceuticals | Phase I |
| Monoaminergic | SERT, NET, DAT | SEP-228432 | Reuptake Blockade | Sunovion Pharmaceuticals | Phase I |
| Monoaminergic | SERT, NET, DAT | Tedatioxetine | Reuptake Blockade | Lundbeck | Phase I |
| Cholinergic | Muscarinic M1 Receptor | BCI-224 | Partial Agonist | BrainCells | Phase I |
| Glutamatergic | NMDAR | AZD6765 | Antagonist | AstraZeneca | Phase II |
| Glutamatergic | NMDAR | GLYX-13 | Glycine-Site Partial Agonist | Naurex | Phase II |
| Glutamatergic | mGluR5 | RG7090 | Antagonist | Roche | Phase II |
| Glutamatergic | mGluR2/3R | BCI-632 | Antagonist | BrainCells | Phase I |
| HPA Axis Modulation | Glucocorticoid Receptor | Mifepristone | Antagonist | Corcept Therapeutics | Phase III |
| HPA Axis Modulation | Vasopressin- 1b Receptor | ABT-436 | Antagonist | Abbott Laboratories | Phase I |
| Neuropeptide | Galanin-3R | HT-2157 | Antagonist | Dart NeuroScience | Phase I/II |
| Inflammatory Modulation | Erythropoietin Receptor | ARA290 | Agonist | Araim Pharmaceuticals | Phase II |
| Neurotrophic | Hippocampal Neurogenesis | NSI-189 | Unknown/Not Reported | Neuralstem | Phase I |
| Melatonin Modulation | Melatonin-1, Melatonin-2 Receptors | Tasimelteon | Agonist | Vanda Pharmaceuticals | Phase I/II |
Examples of agents in the pharmaceutical development pipeline for major depressive disorder; Table organized by general mechanism and development phase. Information obtained from public sources, including the PhRMA Medicines in Development for Mental Illness Report 2012. DAT, dopamine transporter; HPA, hypothalamic–pituitary–adrenal; NET, norepinephrine transporter; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; MAOI, Monoamine oxidase inhibitor; RIMA, Reversible inhibitor of MAO-A; SERT, serotonin transporter
Although the majority of agents in development for depression do not represent marked departures from existing therapies, there are several notable exceptions. We identified four compounds that act on components of the glutamate system currently in Phase I or II development (Table 1). Two compounds directly target the glutamate NMDAR: AZD6765 (AstraZeneca) is an NMDAR antagonist and GLYX-13 (Naurex) is an NMDAR glycine-site partial agonist. Two compounds modulate glutamate metabotropic receptors (mGluR): RG7090 (Roche) is an mGluR5 antagonist and BCI-632 (BrainCells) is an mGluR2/3R antagonist. See (19) for a detailed discussion of glutamate receptor subtypes and their potential role in depression pathophysiology. In addition to glutamatergic agents, other examples of potential innovation in pharmaceutical development for depression include molecular entities targeting the hypothalamic–pituitary–adrenal (HPA) axis, the galanin neuropeptide system, the melatonin system, the inflammatory system and even hippocampal neurogenesis (Table 1). Here we focus on the glutamate system as a candidate for novel drug discovery.
Why target the glutamate system as a strategy for antidepressant development? As limitations of the monoamine theory of depression have become clearer (4,20,21), research focus has shifted to the glutamate system – among others – as an important component of the pathophysiology of depression (5,19,22,23). Glutamate is the ubiquitous excitatory neurotransmitter in the brain and is a critical mediator of neuroplasticity and learning and memory (24). Cortical and limbic brain circuits crucial for cognitive and emotion regulation largely utilize glutamate as the primary neurotransmitter and maladaptive alterations in synaptic structure and function observed in animal models of stress and depression are seen within glutamatergic pathways (25,26). The extensive plasticity of the glutamate synapse in response to environmental influences suggests a key physiological substrate for the well-known link between environmental stress and depression and the neurotoxic effects of abnormally high levels of glutamate – which may result from stress – is a candidate mechanism for regional reductions in brain volume observed in MDD (27). Perhaps most compelling from a clinical perspective is the observation that the glutamate NMDAR antagonist ketamine results in a rapid antidepressant effect in some patients – findings that we review in detail below. These and other data point towards the glutamate system as a principal candidate to target for therapeutic modulation in MDD.
In summary, the antidepressant development pipeline contains many so-called “me too” agents that primarily target the monoamine system and at the same time there are encouraging signs of innovation, particularly in the realm of the glutamate system. Monoaminergic drugs in development can be expected to have efficacy and tolerability profiles similar to currently available agents; in contrast, mechanistically novel molecular entities have the potential to offer much needed, more effective treatments for patients who do not have a favorable response to monoamine-based treatments. In the following section we will highlight the promise of the glutamate system as a novel target for drug development by describing research related to ketamine in depression.
The Example of Ketamine as a Novel, Rapidly Acting Antidepressant
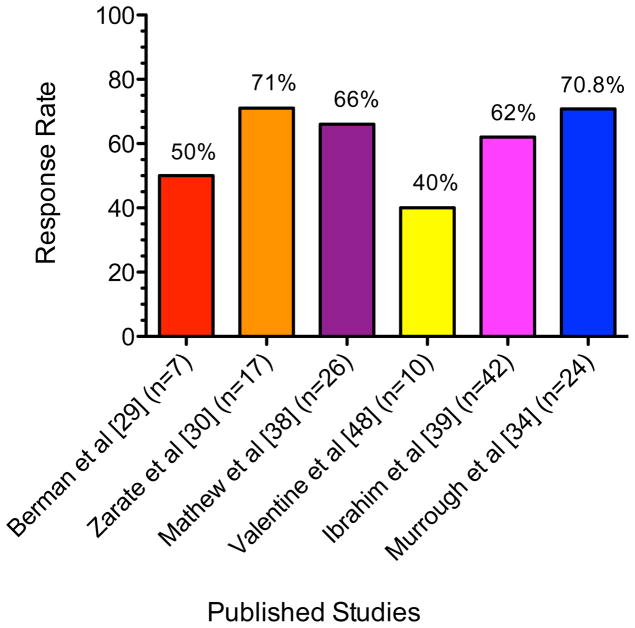
Ketamine is a high-affinity, non-competitive NMDAR antagonist that is currently FDA-approved as an anesthetic agent and is used off-label in the management of chronic pain (28). The potential antidepressant properties of ketamine were initially highlighted by a series of two small studies in which inpatients in a current depressive episode received a single low-dose of ketamine (0.5 mg/kg) intravenously (IV) or saline one week apart in a crossover design (29,30). In the first study, Berman et al reported a mean decrease of 14 points on the 25-item Hamilton Depression Rating Scale in the ketamine condition compared to a mean decrease of zero in the placebo condition at 72 hours post-treatment (29); the response rate was 25% at 24 hours and 50% at 72 hours (31). In the second study including 17 patients with treatment-resistant major depression (TRD), Zarate et al reported a large effect size for the drug-placebo difference (Cohen’s d = 1.46) and a response rate of 71% at 24 hours and 35% at one week (30). See Fig. 1 for a summary of acute antidepressant effects of ketamine in MDD.
Fig. 1. Peak Response Rates Following Acute Treatment with Ketamine in Major Depressive Disorder.
Figure summarizes acute response rates in major depressive disorder following a single intravenous infusion of ketamine across published studies. See text for details and full citations.
These initial studies suggested that ketamine could rapidly reduce core symptoms of depression within 24–72 hours of a single treatment. Two additional studies using a similar crossover design found support for a rapid antidepressant effect of ketamine in bipolar depression (32,33). Ketamine appears to be effective at reducing the range of depressive symptoms, including sadness, anhedonia, low energy, impaired concentration, negative cognitions and suicidal ideation (34). The rapid amelioration of suicidal ideation by ketamine may be particularly important since current antidepressants are slow to act and have been reported to potentially worsen suicidal ideation in the short-term (34–37). Ongoing research is investigating the potential anti-suicidal ideation effects of ketamine in patients admitted to an inpatient psychiatric hospital service (ClinicalTrials.gov Identifier: NCT01507181).
If ketamine can be effective in the short term for patients suffering from TRD, how can the response be maintained? Two RCTs have tested the glutamate-release inhibitor riluzole as a relapse prevention strategy following ketamine in TRD (38,39). In first study, 26 antidepressant-free patients with TRD received a single open-label infusion of ketamine and were randomized to riluzole up to 200 mg daily or placebo at 72 hours post-infusion if they met response criteria (38). The response rates were 65% and 54% at 24 and 72 hours, respectively and the responders at 72 hours (n=14) entered a 4-week riluzole-placebo RCT phase. The average time-to-relapse was 22–24.4 days and these values did not differ statistically between the riluzole and placebo groups; the overall cumulative probability of relapse during the 4-week period was 62%. In the second study, 42 subjects with TRD received a single infusion of ketamine followed by randomization on that day to riluzole up to 200 mg daily or placebo for 4 weeks (39). The investigators reported a significant effect of ketamine on depressive symptoms throughout the 28-day assessment period, although no difference between the riluzole and placebo groups. Overall the acute response rate was 62% and the cumulative probability of relapse among responders during the assessment period was 73%.
Another line of research has begun to investigate the effect of repeated administrations of ketamine in TRD (34,40). In an initial report, 9 out of 10 patients responded to a first IV dose of ketamine and proceeded to receive 5 additional doses on a three-times weekly schedule for 2 weeks (40). In that report, ketamine was found to be safe and well tolerated; all 9 participants maintained their response for the duration of the infusion period and for a variable time thereafter (40,41). In a follow up report that included the original 10 participants plus an additional 14 participants (total n=24), the response rate at both 24 hours and end of study was 70.8% (34). A rapid response was generally maintained throughout the study and was highly predictive of end of study response such that responder status at 4 hours following the first infusion was 94% sensitive and 71% specific for predicting end of study response. The median time to relapse among responders following the final ketamine infusion was 18 days. These data highlight the potential utility of ketamine in maintaining an antidepressant response, however also underscore the need to identify longer-term strategies to prevent relapse after a course of ketamine is discontinued.
How does ketamine achieve a rapid antidepressant effect? A series of recent basic science studies in animal models highlight alterations of synaptic structure and function as essential aspects of ketamine’s antidepressant mechanism of action (42–45). Low-dose ketamine appears to increase glutamate signaling in prefrontal cortical regions and initiate a cascade of molecular events resulting in synaptogensis and enhanced synaptic functioning (42,43). Importantly, glutamate signaling through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) may be enhanced by ketamine and several studies have shown that AMPA signaling is essential for the behavioral and molecular changes associated with ketamine (42–44,46). Signaling pathways linked to the action of ketamine include the brain-derived neurotrophic factor (BDNF)-tropomyosin-related kinase B (TrkB) pathway and the associated downstream phosphatidyl inositol-3 kinase (PI3K)-Akt and the Ras–mitogen-activated protein kinase (MAPK) pathways, glycogen synthase kinase-3 (GSK3) associated pathways and the mammalian target of rapamycin (mTOR) pathway. A recent study in depressed patients using magnetoencephalography found preliminary evidence for increased cortical excitability following ketamine that was specific to antidepressant responders, potentially consistent with synaptic potentiation resulting from AMPAR-mediated glutamatergic neurotransmission (47). A proton magnetic resonance spectroscopy ([1H]-MRS) study measured glutamate, gamma-amino butyric acid (GABA) and glutamine within the occipital cortex in patients with MDD in the context of a single blind one-week crossover design using ketamine and saline did not find an association between ketamine and changes in amino acid transmitters (48). Much more work is needed to establish the antidepressant mechanism of action of ketamine in humans.
Taken together, there is good preliminary data to support the potential utility of ketamine as a novel, rapid-acting antidepressant that may be effective even in cases of TRD. Caution is warranted, however, before a firm conclusion regarding the antidepressant potential of ketamine can be drawn; the largest controlled study of ketamine in MDD published to date included only 17 participants in a crossover design (30). The use of saline as a control condition in ketamine studies is also problematic since the sometimes-prominent acute psychoactive effects of ketamine will compromise the integrity of the study blind. A large National Institute of Health (NIH)-funded parallel-arm RCT of ketamine verse midazolam (a water-soluble benzodiazepine) is currently underway and is expected to provide a more definitive conclusion regarding the antidepressant efficacy of ketamine (ClinicalTrials.gov Identifier: NCT00768430).
Conclusions
Major depression is a primary public health problem and current treatments fall short of what is required to meet the challenge. Response rates may be less than 50% and continue to decrease as the level of treatment-resistance increases. Remission rates are lower still. The antidepressant pipeline continues to be dominated by monoaminergic drugs, which are unlikely to represent a major advance in treatment efficacy. Mechanistically innovative candidates, however, are in the pipeline with the largest number targeting components of the glutamate system. The glutamatergic agent with the most evidence to date for antidepressant efficacy is the NMDAR ketamine with observed response rates as high as 71% in small controlled studies conducted in treatment-resistant samples. Future studies will be necessary to establish the efficacy of ketamine in a rigorous design using a control condition with psychoactive properties.
Beyond establishing the efficacy of a single administration, future controlled studies will be required to establish the longer-term efficacy of a series of ketamine administrations in TRD and to identify maintenance strategies for patients who respond to a course of ketamine. While riluzole was not found to be superior to placebo in preventing relapse following ketamine, other pharmacotherapeutic strategies have not been systematically tested to date. The recent research implicating GSK3 in the mechanism of response to ketamine (45) might suggest the well-known GSK3 inhibitor lithium as a relapse prevention strategy. A schedule of repeated administrations of ketamine over a longer period of time, for example using a frequency taper schedule similar to ECT, will also be an important avenue for future research. The potential of ketamine to be a drug of abuse and concerns regarding neurotoxic effects of NMDAR antagonists in animals when given at high doses, however, warrants a cautious approach to the development of ketamine for TRD. Apart from ketamine, innovative drugs targeting the glutamate system currently in development hold substantial promise as urgently needed future treatments for MDD.
Footnotes
Disclosure: Dr. Murrough is supported by a Career Development Award from the National Institute of Mental Health (K23MH094707) and by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and the American Foundation for Suicide Prevention (Young Investigator Grant). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health or other funding agencies. In the past two years, Dr. Murrough has received research support from Evotec Neurosciences and Janssen Research & Development. Dr. Charney, Dean of Mount Sinai School of Medicine, has been named as an inventor on a use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr. Charney and Mount Sinai School of Medicine could benefit financially.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.World Health Organization. The global burden of disease: 2004 update. 2008. [Google Scholar]
- 2*.Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. This article provides an overview of the global burden of mental health and highlights depressive disorders as the more disabling brain-based disorders worldwide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manji HK, Quiroz JA, Sporn J, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 6.Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- 7.Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 8.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: Thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37:851–864. doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: A meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72:509–514. doi: 10.4088/JCP.09m05949blu. [DOI] [PubMed] [Google Scholar]
- 11.Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: An updated meta-analysis. Ann Intern Med. 2011;155:772–785. doi: 10.7326/0003-4819-155-11-201112060-00009. [DOI] [PubMed] [Google Scholar]
- 12.Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults III. Pharmacotherapy J Affect Disord. 2009;117:S26–43. doi: 10.1016/j.jad.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 14*.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. This is one of several landmark STAR*D reports, which summarizes response and remission rates across the sequential treatment steps. [DOI] [PubMed] [Google Scholar]
- 15.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): Acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 18.Pharmaceutical Research and Manufacturers of America (PhRMA) Medicines in development for mental illness report. 2012. [Google Scholar]
- 19.Sanacora G, Zarate CA, Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: A modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- 21.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 22.Paul IA, Skolnick P. Glutamate and depression: Clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 23.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 26**.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. This recent article provides an authoritative review of cellular mechanisms hypothesized to underlie the rapid antidepressant effects of ketamine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnone D, McIntosh AM, Ebmeier KP, et al. Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 28**.Mathew SJ, Shah A, Lapidus K, et al. Ketamine for treatment-resistant unipolar depression: Current evidence. CNS Drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. This recent article provides an authoritative review of the current evidence for the antidepressant efficacy of ketamine in human depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 30*.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. This article reports a landmark clinical trial of ketamine in treatment-resistant major depression. [DOI] [PubMed] [Google Scholar]
- 31.Aan Het Rot M, Zarate CA, Jr, Charney DS, et al. Ketamine for depression: Where do we go from here? Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.022. in press. This study demonstrated the safety and efficacy in the largest sample to date of three times weekly ketamine infusions over two weeks in patients with treatment-resistant major depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price RB, Nock MK, Charney DS, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 38.Mathew SJ, Murrough JW, aan het Rot M, et al. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: A pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: Results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Murrough JW, Perez AM, Mathew SJ, et al. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry. 2011;72:414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 42*.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. This report was among the first to demonstrate specific synaptic and intra-cellular messenger system alterations in association with a rapid antidepressant behavior effect in animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. This report provides important evidence for a role for BDNF in the rapid antidepressant response to ketamine in animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Cornwell BR, Salvadore G, Furey M, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentine GW, Mason GF, Gomez R, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]