Abstract
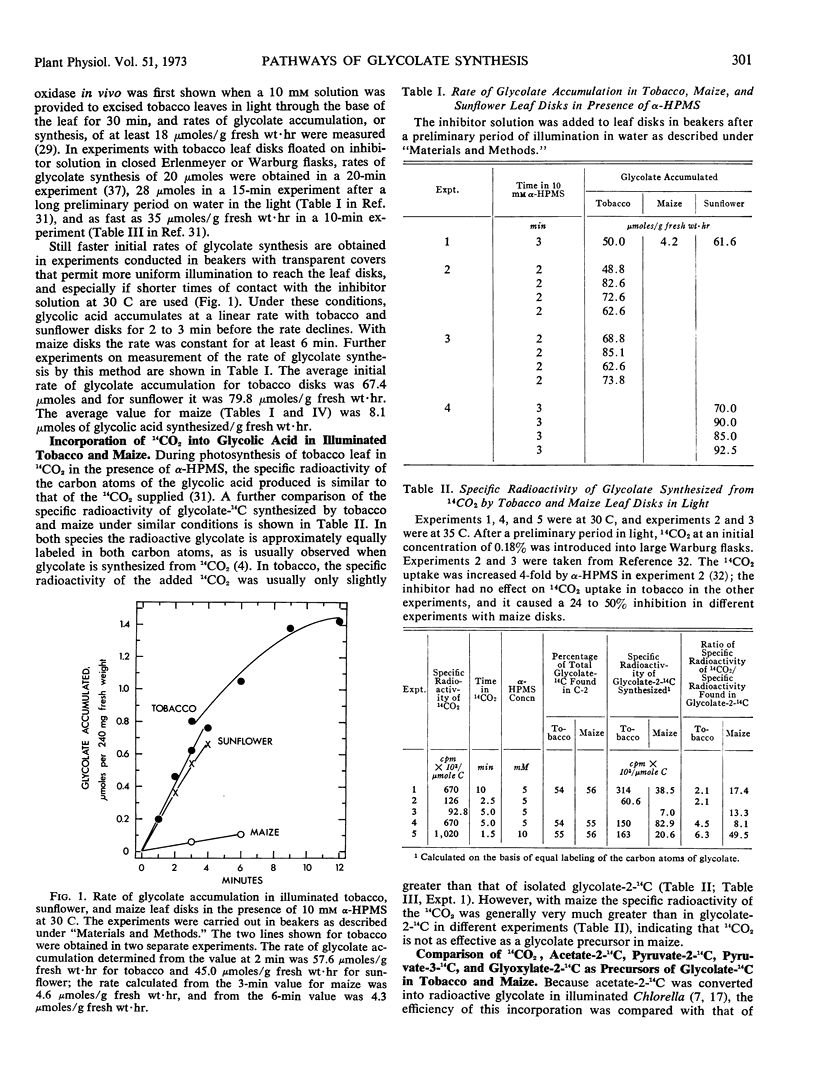
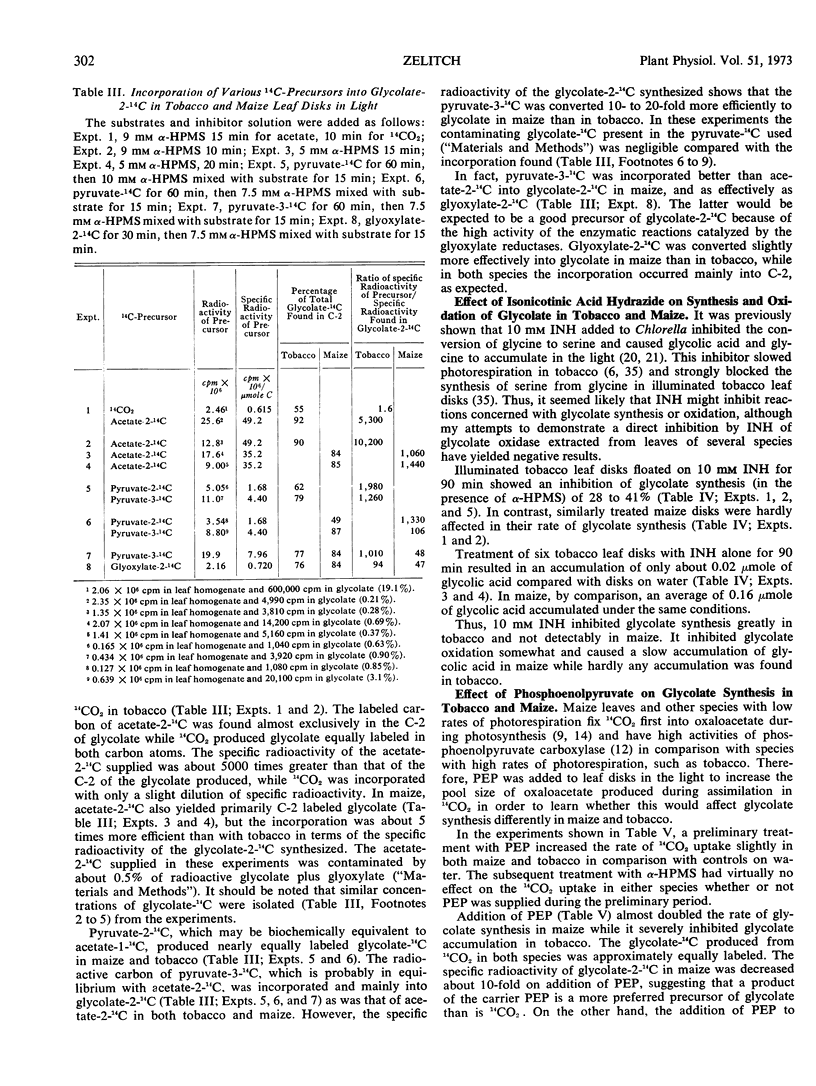
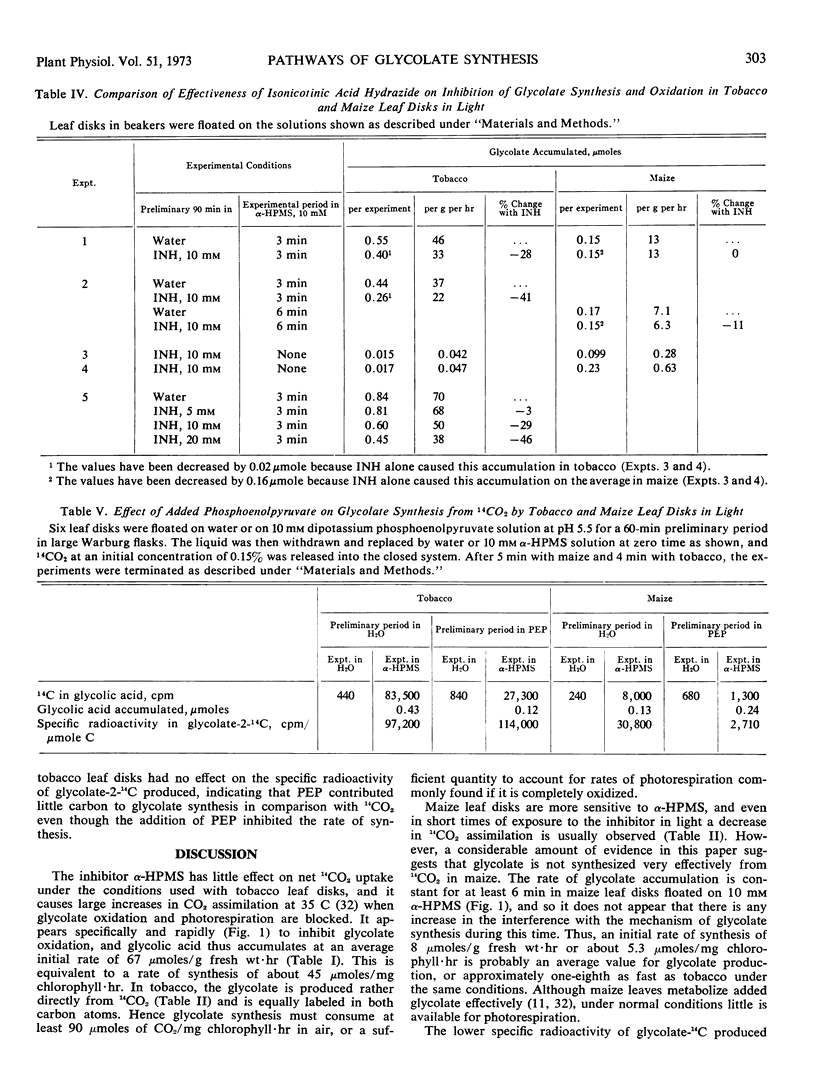
After a preliminary period in light, leaf disks floated on 10 mm α-hydroxy-2-pyridinemethanesulfonic acid to inhibit glycolate oxidase accumulate glycolate at average initial rates of 67 micromoles in tobacco and 8 micromoles per gram fresh weight per hour in maize under optimal conditions in air. In the presence of 14CO2, the glycolate synthesized has a high specific radioactivity in illuminated tobacco and a low one in maize. Isonicotinic acid hydrazide also inhibits glycolate oxidation and causes a slow accumulation of glycolate in maize but not in tobacco, while it inhibits glycolate synthesis in tobacco but not in maize. Radioactive carbon in acetate-2-14C and especially pyruvate-3-14C is incorporated predominantly into the C-2 of glycolate in both species, but the specific radioactivity is much greater in maize. Glyoxylate-2-14C is readily converted to glycolate-2-14C in both species. The addition of phosphoenolpyruvate stimulated glycolate formation in maize and inhibited its synthesis in tobacco, and in the presence of 14CO2 the specific radioactivity in glycolate-14C was decreased greatly by the added phosphoenolpyruvate only in maize.
Thus, unsymmetrically labeled glycolate is mainly synthesized from pyruvate-3-14C by a slow pathway in maize. Tobacco possesses an additional rapid pathway that produces equally labeled glycolate more directly from fixed CO2 during photosynthesis. Glycolate is believed to be the primary substrate of photorespiration, and sufficiently rapid rates of glycolate synthesis have been observed in tobacco to account for this function. Hence the high rates of photorespiration observed in tobacco leaves compared with maize result partly from differences between these species in the pathway of glycolate synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Incorporation of molecular oxygen into glycine and serine during photorespiration in spinach leaves. Biochemistry. 1971 Dec 7;10(25):4777–4782. doi: 10.1021/bi00801a027. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- CALVIN M., MASSINI P. The path of carbon in photosynthesis. XX. The steady state. Experientia. 1952 Dec 15;8(12):445–457. doi: 10.1007/BF02139287. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1968 Jan;106(1):141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. The C 4 -pathway of photosynthesis. Evidence for an intermediate pool of carbon dioxide and the identity of the donor C 4 -dicarboxylic acid. Biochem J. 1971 Nov;125(2):425–432. doi: 10.1042/bj1250425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heichel G. H. Postillumination respiration of maize in relation to oxygen concentration and glycolic Acid metabolism. Plant Physiol. 1972 Apr;49(4):490–496. doi: 10.1104/pp.49.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Warren W. A., Carroll W. R. The structural properties of spinach leaf glyoxylic acid reductase. J Biol Chem. 1970 Aug 10;245(15):3821–3830. [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. Y., Black C. C., Jr Glycolate metabolism in mesophyll cells and bundle sheath cells isolated from crabgrass, Digitaria sanguinalis (L.) Scop., leaves. Arch Biochem Biophys. 1972 Mar;149(1):269–280. doi: 10.1016/0003-9861(72)90322-0. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- Plamondon J. E., Bassham J. A. Glycolic Acid Labeling During Photosynthesis with CO(2) and Tritiated Water. Plant Physiol. 1966 Oct;41(8):1272–1275. doi: 10.1104/pp.41.8.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Intracellular localisation of phosphoglycollate phosphatase and glyoxalate reductase. Biochim Biophys Acta. 1967;143(3):642–644. doi: 10.1016/0005-2728(67)90074-6. [DOI] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G. [Glycolic acid formation in Chlorella]. Z Naturforsch B. 1960 Mar;15B:197–199. [PubMed] [Google Scholar]
- Yamamoto Y., Beevers H. Malate Synthetase in Higher Plants. Plant Physiol. 1960 Jan;35(1):102–108. doi: 10.1104/pp.35.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]
- Zelitch I. Comparison of the effectiveness of glycolic Acid and glycine as substrates for photorespiration. Plant Physiol. 1972 Jul;50(1):109–113. doi: 10.1104/pp.50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. The photooxidation of glyoxylate by envelope-free spinach chloroplasts and its relation to photorespiration. Arch Biochem Biophys. 1972 Jun;150(2):698–707. doi: 10.1016/0003-9861(72)90088-4. [DOI] [PubMed] [Google Scholar]
- Zelitch I., Walker D. A. The Role of Glycolic Acid Metabolism in Opening of Leaf Stomata. Plant Physiol. 1964 Sep;39(5):856–862. doi: 10.1104/pp.39.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]



