Abstract
BACKGROUND
Sigma receptors represent a unique structural class of proteins and they have become increasingly studied as viable medication development targets for neurological and psychiatric disorders, including drug abuse. Earlier studies have shown that cocaine and many other abused substances interact with sigma receptors and that antagonism of these proteins can mitigate their actions.
METHODS
In the present study, AC927 (1-(2-phenethyl)piperidine oxalate), a selective sigma receptor ligand, was tested against the behavioral and toxic effects of cocaine in laboratory animals.
RESULTS
Acute administration of AC927 in male, Swiss Webster mice significantly attenuated cocaine-induced convulsions, lethality, and locomotor activity, at doses that alone had no significant effects on behavior. Subchronic administration of AC927 also attenuated cocaine-induced conditioned place preference in mice, at doses that alone had no effects on place conditioning. In drug discrimination studies in male, Sprague Dawley rats, AC927 partially substituted for the discriminative stimulus effects of cocaine. When it was administered with cocaine, AC927 shifted the cocaine dose-response curve to the left, suggesting an enhancement of the discriminative stimulus effects of cocaine. In non-human primates, AC927 was self-administered, maintaining responding that was intermediate between contingent saline and a maintenance dose of cocaine.
CONCLUSION
The ability of AC927 to elicit some cocaine-like appetitive properties and to also reduce many cocaine-induced behaviors suggests that it is a promising lead for the development of a medication to treat cocaine abuse.
Keywords: Cocaine, Conditioned place preference, Drug discrimination, Locomotor activity, Self-administration, Sigma receptors
1. Introduction
In 1988, Kuhar and co-workers first reported the ability of cocaine to interact with sigma receptors at physiologically relevant concentrations (Sharkey et al., 1988). The distribution of sigma receptors in important target organs for cocaine, such as the brain, heart and lung (Gundlach et al., 1986; Kawamura et al., 2000; Novakova et al., 1995) suggested a role for these receptors in modulating the stimulant and toxic effects of cocaine. Within the brain, the presence of sigma receptors in limbic regions and dopamine-rich areas (Gundlach et al., 1986), together with their ability to influence dopamine uptake and release (Weatherspoon and Werling, 1999), further suggested sigma receptor involvement in the effects of cocaine. Subsequent studies confirmed that antagonism of sigma receptors, using small molecules or antisense oligonucleotides, reduced cocaine-induced behaviors and alterations in gene expression (Liu et al., 2005; Matsumoto et al., 2003; Menkel et al., 1991; Romieu et al., 2004), thereby providing a novel therapeutic strategy for mitigating the actions of cocaine.
There are two established subtypes of sigma receptors, sigma1 and sigma2, which can be distinguished from one another based on drug selectivity and anatomical localization patterns, as well as molecular biological profiles (Guitart et al., 2004; Matsumoto et al., 2003). The sigma1 receptor has been cloned with high homology and identity from several species and its sequence differs from other known proteins (Guitart et al., 2004; Su et al., 2010). Sigma1 receptors represent a unique structural class of proteins that possess chaperone-like functions (Hayashi and Su, 2007; Su et al., 2010; Tsai et al., 2009). They can translocate between different cellular compartments and appear to form protein-protein interactions to facilitate the modulation of ion channels, G protein coupled receptors, and signaling molecules (Aydar et al., 2002; Hayashi and Su, 2007; Su et al., 2010; Tsai et al., 2009).
In contrast, sigma2 receptors are slightly smaller in size than sigma1 receptors (Hellewell et al., 1990). They are enriched in lipid rafts, where they can affect calcium signaling through sphingolipid products (Crawford et al., 2002; Gebreselassie and Bowen, 2004). Sigma2 receptors have yet to be cloned, and although pharmacological agents with preferential affinity for these receptors have been identified, truly selective compounds which lack significant interactions with non-sigma sites are unavailable.
Cocaine directly binds to both sigma1 and sigma2 receptors, where it appears to act at least in part as a sigma receptor agonist (Matsumoto et al., 2003, 2009; Navarro et al., 2010). The functional relevance of these interactions is underscored by the ability of sigma receptor antagonists or antisense oligonucleotides to attenuate several acute effects of cocaine, including convulsions, lethality, and locomotor stimulation (Matsumoto et al., 2003, 2009; Menkel et al., 1991).
More limited investigations also demonstrate that sigma receptor antagonists can mitigate the subchronic effects of cocaine, including behavioral sensitization and conditioned place preference (Romieu et al., 2002, 2004; Ujike et al., 1996; Witkin et al., 1993; Xu et al., 2010). Accumulating data further indicate that neuroadaptations in sigma receptor expression occur upon exposure to cocaine, and that these changes have functional ramifications for responding to subsequent cocaine exposures (Liu et al., 2005; Liu and Matsumoto, 2008; Romieu et al., 2004). In behavioral sensitization studies, cocaine induces the up regulation of the immediate early gene fra-2, which is followed by a progressive increase in sigma1 receptor gene and protein expression in brain regions relevant to reward and addiction (Liu and Matsumoto, 2008). The magnitude of the up regulation of sigma1 receptors parallels the development of the behavioral sensitization, as measured as a progressive enhancement in the locomotor stimulatory response to repeated cocaine injections (Liu and Matsumoto, 2008). Pretreatment with the sigma1 receptor antagonist BD1063 attenuates the cocaine-induced changes at both the behavioral and molecular levels (Liu and Matsumoto, 2008). Similarly, the development of place conditioning to cocaine is accompanied by an enhancement in the expression of sigma receptors in brain regions relevant to reward (Romieu et al., 2004). Together, the body of data suggests that a better understanding of the role of sigma receptors in cocaine abuse could lead to the development of strategies that will improve treatment outcomes.
To further clarify the role of sigma receptors in the actions of cocaine and to elucidate the mechanisms through which antagonists attenuate its effects, there is a need to develop and utilize highly selective research tools. In this regard, 1-(2-phenethyl)piperidine oxalate, AC927 (Fig. 1), was identified as a selective sigma receptor ligand, which attenuates the behavioral stimulant and toxic effects of methamphetamine, another psychostimulant with significant affinity for sigma receptors (Matsumoto et al., 2008). AC927 exhibits moderate affinity for both subtypes of sigma receptors (Ki sigma1 30 ± 2 nM, sigma2 138 ± 18 nM; Matsumoto et al., 2008). Importantly, it exhibits preferential affinity for sigma receptors, as compared to 29 other non-sigma receptors, transporters and ion channels, including members of the following groupings: receptors – adenosine, adrenergic, dopamine, GABA, melatonin, muscarinic, NMDA, opioid, serotonin; transporters - dopamine, serotonin, norepinephrine; ion channels – calcium, chloride, potassium, sodium (Matsumoto et al., 2008). The preferential affinity of AC927 for sigma receptors vs. non-sigma sites is critical for attributing functional outcomes to sigma receptors, especially in complex systems, such as whole animal studies.
Fig. 1.
Structure of AC927.
Therefore, in the present study, AC927 was evaluated for its ability to mitigate a range of cocaine-related effects in vivo. The acute actions of AC927 were characterized in mice to determine its ability to attenuate cocaine-induced convulsions, lethality, and locomotor activity. In addition, the subchronic effects of AC927 were evaluated in place conditioning and drug discrimination studies in rodents to determine its effects alone and in combination with cocaine. Finally, AC927 was tested in self-administration studies in non-human primates to further assess its abuse liability. The present series of studies extends earlier efforts to evaluate the potential of sigma receptor ligands for the treatment of cocaine abuse by introducing a highly selective sigma receptor ligand, and then testing it in animal models in which the effects of sigma ligands are already well characterized (e.g., convulsions, locomotor activity, place conditioning), and to also evaluate it in drug discrimination and self-administration studies where selective sigma ligands have not been extensively studied in the context of cocaine abuse. Together, these investigations are intended to provide further insight into sigma ligands as potential pharmacotherapies to treat cocaine abuse.
2. Materials and methods
2.1. Subjects
All procedures involving animal subjects were performed as approved by the Institutional Animal Care and Use Committee at the institution where the studies were conducted. The rodents excluding those in the cocaine discrimination studies were housed in groups of 4–6 with a 12:12 h light/dark cycle and ad libitum food and water. Male, Swiss Webster mice (Charles River, Portage, MI; Harlan, Frederick, MD or Dublin, VA) were used for behavioral studies at the University of Oklahoma Health Sciences Center (Oklahoma City, OK). Male, Sprague Dawley rats (320–350 g, Taconic Farms, Germantown, NY) were used for the cocaine discrimination studies at the National Institutes of Health (Baltimore, MD) and were individually housed with unrestricted access to water, and maintained at approximately 325 g by daily feedings approximately 0.5 hr after experimental sessions. Four adult rhesus monkeys (Macaca mulatta) experienced with self-administration of MDMA (3,4-methylenedioxymethamphetamine), several congeners of MDMA, cocaine, and saline served as subjects for the self-administration studies at the University of Michigan (Ann Arbor, MI). The monkeys were housed individually in stainless steel cages. They were fed monkey chow, and water was available ad libitum. In addition, their diets were supplemented with chewable vitamins and/or fresh fruit and peanuts several days each week.
2.2. Drugs
AC927 was prepared as described previously (Maeda et al., 2002). Cocaine hydrochloride was obtained from Sigma (St. Louis, MO). The drugs were dissolved in saline for administration to the animals. For the behavioral studies in mice, the drugs were administered in a volume of 10 ml/kg. For the drug discrimination studies in rats, the injection volume was 1 ml/kg. For the self-administration studies in rhesus monkeys, the drugs were infused in a constant injection volume of 1.0 ml over a 5 second duration.
2.3. Cocaine-induced toxicity
To evaluate the effects of AC927 on cocaine-induced behavioral toxicity, male, Swiss Webster mice were pretreated with AC927 (0–20 mg/kg, i.p.) followed 15 minutes later with either a convulsive (60 mg/kg, i.p.) or lethal (125 mg/kg, i.p.) dose of cocaine. The convulsive and lethal doses of cocaine were selected based on earlier dose response studies (Matsumoto et al., 2001b; McCracken et al., 1999b) and represent the lowest dose that reliably produced these effects. Following injection with cocaine, the mice were observed for the next 30 minutes for the onset of convulsions, which were operationally defined as a loss of righting reflexes for at least 5 seconds together with clonic limb movements, or death. The data were expressed as the percentage of mice exhibiting cocaine-induced convulsions or death, and analyzed using Fisher’s exact tests.
2.4. Locomotor activity
Male, Swiss Webster mice were placed in the chambers of an automated activity monitoring device (San Diego Instruments, San Diego, CA) and habituated for 30 minutes before quantifying locomotor activity. To test the effects of AC927 alone, the mice received injections of saline or AC927 (0.1–10 mg/kg, i.p.) and horizontal locomotor activity was monitored for the next 30 minutes. To test the effects of AC927 on cocaine-induced locomotor activity, the mice were pretreated with saline or AC927 (0.1–10 mg/kg, i.p.), then challenged 15 minutes later with cocaine (10 mg/kg, i.p.); locomotor activity was measured immediately thereafter for 30 minutes. The dose of cocaine used in this study was based on an earlier dose response study and represents the dose that produced peak locomotor stimulant effects (McCracken et al., 1999a). The data were evaluated using one-way ANOVA, followed by Dunnett’s tests for post-hoc comparisons.
2.5. Conditioned place preference
The conditioning chambers were comprised of Plexiglas boxes (45 × 24 × 20.5 cm), painted gray on one half, and black with white vertical stripes on the other half. The floor on one half of the chamber was smooth Plexiglas, while the floor on the other half was a plastic mat with textured stripes at about 3 mm intervals. A pre-conditioning session, conditioning phase, and post-conditioning session were conducted.
During the pre-conditioning session, the mice were allowed to roam the entire chamber for 15 minutes and the time spent on each half of the chamber was measured to confirm that they did not have a preference for a particular side. The criterion for an unbiased response and to proceed to the conditioning phase was <2/3 of the time spent on a particular side of the chamber. Those mice that did not meet this criterion were excluded from further testing.
During the conditioning phase, the mice received a test drug and were subsequently confined to one half of the chamber for 30 minutes, while on alternate sessions the mice received saline and were confined to the other half of the chamber. The mice received a total of eight pairings during the conditioning phase (four drug, and four saline sessions). The side of the chamber in which the animals received their drug treatment was assigned randomly and counterbalanced. One-way ANOVA was conducted to confirm that the pre-conditioning scores did not differ significantly between the experimental groups. To determine the dose response for cocaine to induce a conditioned place preference, male, Swiss Webster mice were treated with cocaine (10, 20 or 30 mg/kg, i.p.) or saline. To determine whether AC927 alone induced conditioned place preference or aversion, mice were treated with a dose of AC927 (5 or 10 mg/kg, i.p.) or saline during the conditioning phase. To determine whether AC927 could prevent the place conditioning of cocaine, mice were treated with AC927 (5 or 10 mg/kg, i.p.) 15 minutes before cocaine (20 or 30 mg/kg, i.p.) during the conditioning phase. The mice received saline and were confined to the other side of the chamber during alternate (non-drug) sessions.
During the post-conditioning session, the mice were again allowed access to the entire chamber for 15 minutes and the time spent in each half was recorded. The conditioned score was calculated by subtracting the pre-conditioning time from the post-conditioning time on the side of the chamber in which the mice were trained to drug during the conditioning phase. The data from the conditioned scores were evaluated using one-way ANOVA, followed by Student-Newman-Keuls post-hoc tests.
2.6. Drug discrimination
Subjects were tested daily in two-lever operant-conditioning chambers (model ENV 007; Med Associates, St. Albans, VT) that were housed within light- and sound-attenuating enclosures. White noise was present throughout testing to mask extraneous sounds. Ambient light was provided by a lamp in the top center of the front panel (house light). Levers were set 17 cm apart, with pairs of lamps (light-emitting diodes; LEDs) above each of the levers, also on the front panel. A downward force on either lever of 0.4 N through about 1 mm was defined as a response and produced an audible click. Reinforced responses dispensed one 45 mg pellet (BioServe, Frenchtown, NJ) into a food tray centered between the levers on the front panel of the chamber. On-line experimental control and data collection were computer driven with Med Associates (St. Albans, VT) interface and operating software.
Rats were initially trained to press both levers under a 20 response fixed ratio schedule (FR20) for food reinforcement and to discriminate i.p. injections of cocaine (10 mg/kg) from saline. After cocaine injection, responses on only one lever were reinforced; after saline injection, responses on the other lever were reinforced. The assignment of cocaine- and saline-appropriate levers was counterbalanced across rats. Immediately after injection, rats were placed inside the experimental chambers. A 5-minute time-out period, during which the house light and LEDs were extinguished and responding had no scheduled consequences preceded the illumination of the house light and the LEDs. Only responses on the appropriate lever were reinforced, and responses on the inappropriate lever reset the FR response requirement. Each food presentation was followed by a 20 second time-out period during which all lights were off, and responding had no scheduled consequences. Sessions ended after 20 food presentations or 15 minutes, whichever occurred first. Training sessions with cocaine (C) and saline (S) injections were conducted daily 5–7 days per week and ordered in a double alternation sequence (e.g., …SCCS…).
Testing was initiated when performances reached criteria of at least 85% appropriate responding over the entire session, and during the first FR20 of the session over four consecutive sessions. Tests were conducted with different doses of cocaine, doses of AC927, or combinations of doses administered before sessions. After a test session, the rats were required to meet the abovementioned performance criteria over two consecutive (cocaine and saline) training sessions to be tested again. Repeated test sessions were conducted, with at least two training sessions between tests, until entire dose-effects were determined in each subject. Test sessions were identical to training sessions, with the exception that 20 consecutive responses on either lever were reinforced.
Mean values for the overall response rate and the percentage of responses occurring on the cocaine-appropriate lever were calculated. If less than one-half of the rats responded at a particular dose, the distribution of responses on the two levers was potentially not reliable, and no mean value was calculated for percentage of cocaine-appropriate responding at that dose. Each dose-effect curve was analyzed using ANOVA and linear regression techniques. The dose producing a half-maximal effect (50% drug-appropriate responding) was calculated (Snedecor and Cochran, 1967). For these analyses, points on the linear part of the ascending portions of the dose-effect curves were used. To assess the degree of change in the drug dose-effect curve produced by co-administration of AC927, data were also analyzed by standard parallel-line bioassay techniques (Finney, 1964). The relative potency value represents the dose of cocaine alone equal to 1 mg/kg cocaine in subjects coadministered AC927 (i.e., a relative potency value of 2 indicates a 2-fold shift to the left of the cocaine dose-effect curve in the presence of AC927), and is significant when the 95% confidence limits for the relative potency ratio exclude the value 1.0. For interaction studies, response rates were compared to response rates observed after cocaine alone using two-way repeated measure ANOVA with dose of AC927 and drug dose as factors.
2.7. Self-administration
Procedures were similar to those previously reported (Fantegrossi et al., 2004, 2005). Four adult rhesus monkeys were surgically prepared with indwelling silicone rubber catheters in either a jugular, femoral, or brachial vein using ketamine (10 mg/kg, i.m.) and xylazine (2 mg/kg, i.m.) as anesthetics. The catheters passed subcutaneously to the mid-scapular region, exited the body, and continued through a hollow restraining arm to the outside rear of the cage. During the studies, the monkeys wore teflon mesh jackets (Lomir, Quebec, Canada) connected to a flexible stainless steel spring arm attached to the rear of the cage.
Animals were individually housed in 83.3 × 76.2 × 91.4 cm deep stainless steel cages. A side-mounted panel was present in each cage, equipped with a row of three stimulus lamps (red-green-red) across the top, and two response levers (one mounted under each red light). Two, 60-minute experimental sessions were conducted each day: a morning session starting at 10:00 h and an afternoon session starting at 16:00 h. The onset of each session was signaled by illumination of a red stimulus light. When a monkey completed the fixed ratio requirement of 10 presses on that lever (FR10), a 5-s, 1-ml injection of drug solution or saline was delivered. During the 5-s infusion duration, the red stimulus light was extinguished, the center green light illuminated, and further lever presses had no programmed consequence. Immediately following the termination of each infusion, all stimulus lights were extinguished for a 1-minute timeout period during which lever presses had no programmed consequence. Each timeout period counted toward the total 60 minute testing session.
Under baseline conditions, subjects were maintained on a cocaine dose of 0.01 mg/kg/injection following the above outlined schedule requirements. This represents a standard cocaine maintenance dose under the self-administration schedule used herein (Fantegrossi et al., 2004, 2005). To ensure that responding was maintained by drug, saline was substituted for cocaine approximately every 3rd or 4th session, usually for two consecutive sessions which could occur on either the same or different days. Substitutions of AC927 occurred two to three times per week during morning sessions, and no substitutions were made on weekends. The reinforcing effects of AC927 were assessed over a dose range from 0.003 to 0.3 mg/kg/injection. AC927 substitutions followed an ascending dose order, and at least four recovery sessions occurred between AC927 substitution trials; the recovery sessions were comprised of either cocaine baseline or saline substitutions. The reinforcing effects of each dose of AC927 were assessed at least twice in each animal. The data were evaluated using one-way ANOVA, followed by Bonferroni multiple comparisons tests for post-hoc analysis.
3. Results
3.1. Cocaine-induced toxicity
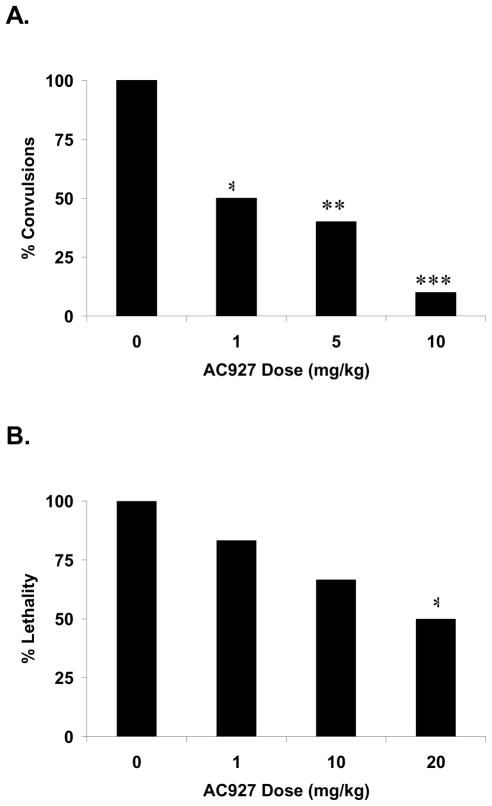
Similar to earlier studies, when 60 mg/kg of cocaine was administered in the absence of an antagonist, it produced convulsions in 100% of animals (Matsumoto et al., 2001a,b, 2002) and 125 mg/kg of cocaine produced death in all of the mice (Matsumoto et al., 2001b, 2002). In contrast, pretreatment with AC927 (1–20 mg/kg) produced a dose-dependent reduction in cocaine-induced convulsions and lethality in mice (Fig. 2). Fisher’s exact tests confirmed that the reductions were statistically significant for both the convulsive (p<0.01) and lethal (p<0.05) effects of cocaine.
Fig. 2.
Effects of AC927 on cocaine-induced convulsions and lethality in mice. Swiss Webster mice were pretreated with AC927 (0–20 mg/kg, i.p.) followed 15 min later with a convulsive (60 mg/kg, i.p.) or lethal (125 mg/kg, i.p.) dose of cocaine. (A) AC927 produced a dose-dependent reduction in the convulsive effects of cocaine. **P<0.01, ***P<0.001 Fisher’s exact test. (B) AC927 produced a dose-dependent reduction in the lethal effects of cocaine. *P<0.05 Fisher’s exact test.
3.2. Locomotor activity
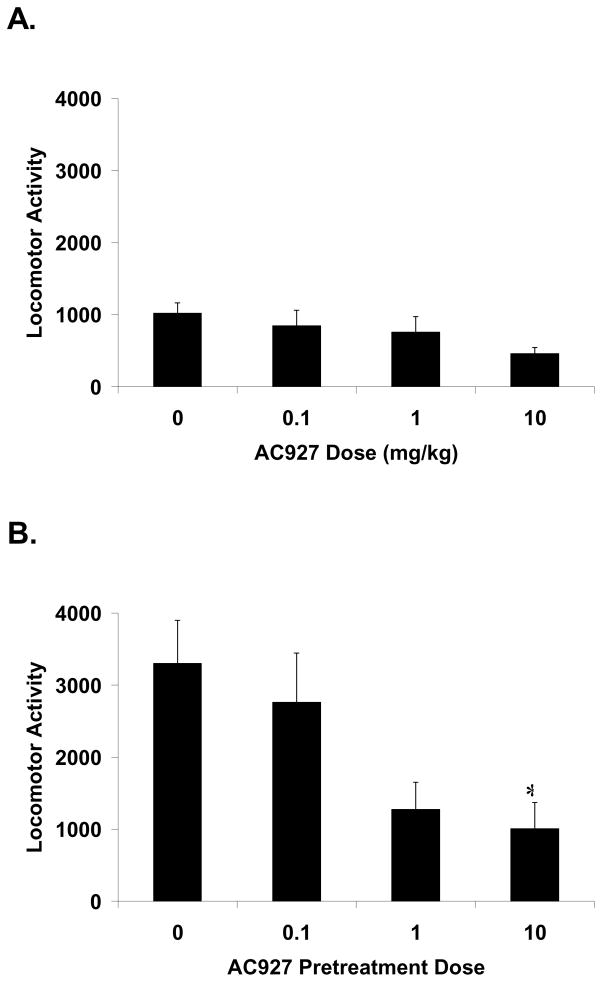
When administered alone, AC927 (0.1–10 mg/kg) had no significant effect on locomotor activity in mice (F[3,28]=1.68, n.s.). When administered in the absence of AC927, 10 mg/kg of cocaine produced robust locomotor stimulatory effects, comparable to those observed in earlier studies (Matsumoto et al., 2002; McCracken et al., 1999a,b). Pretreatment with AC927 significantly attenuated the locomotor stimulatory effects of cocaine in mice (Fig. 3; F[3,24]=3.20, p<0.05). Post-hoc Dunnett’s tests revealed that the reduction produced by pretreatment with 10 mg/kg of AC927 differed significantly from pretreatment with saline (q=2.57, p<0.05).
Fig. 3.
Effects of AC927 on basal and cocaine-induced locomotor activity in mice. Swiss Webster mice were treated with AC927 (0–10 mg/kg, i.p.) alone or followed 15 minutes later with a stimulatory dose of cocaine (10 mg/kg, i.p.). Horizontal locomotor activity was quantified as disruptions in a 4×4 photo beam grid for 30 min. (A) AC927 alone had no significant effect on locomotor activity. (B) Pretreatment with AC927 significantly attenuated the locomotor stimulatory effects of cocaine. *P<0.05 Bonferroni’s multiple comparisons post-hoc test.
3.3. Conditioned place preference
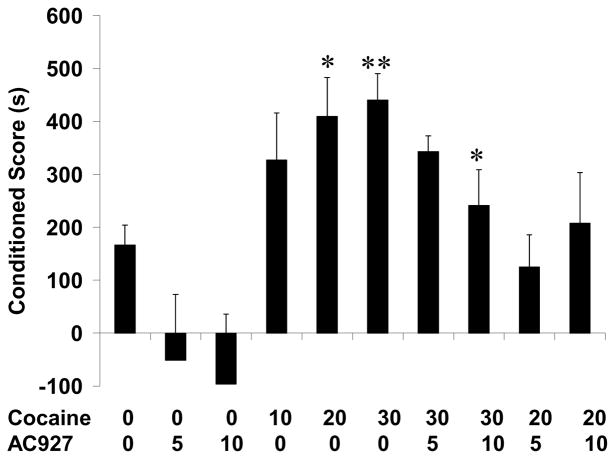
Cocaine (0–30 mg/kg, i.p.) produced conditioned place preference in a dose-dependent manner in mice (F[3,29]=4.86, p<0.01). Post-hoc tests confirmed that the effects of the 20 mg/kg (q=4.22, p<0.05) and 30 mg/kg (q=4.93, p<0.01) doses of cocaine differed significantly from saline.
The cocaine-induced conditioned place preference was attenuated by pretreatment with AC927 (Fig. 4). Post-hoc testing confirmed that reductions produced by the 10 mg/kg dose of AC927 against 30 mg/kg of cocaine were statistically significant (q=3.68, p<0.05). When administered alone, AC927 did not induce significant place conditioning (F[2,22]=0.14, n.s.).
Fig. 4.
Effects of AC927 on conditioned place preference in mice. Positive scores reflect rewarding effects, while negative scores suggest aversive effects. Alone, AC927 (5 or 10 mg/kg, i.p.) had no significant effect on conditioned place preference. Cocaine (10, 20, 30 mg/kg, i.p.) produced conditioned place preference in a dose-dependent manner. Pretreatment of the mice with AC927 (5 or 10 mg/kg, i.p.) significantly attenuated cocaine-induced conditioned place preference. *P<0.05, **P<0.01 Student-Newman-Keuls post-hoc test.
3.4. Drug discrimination
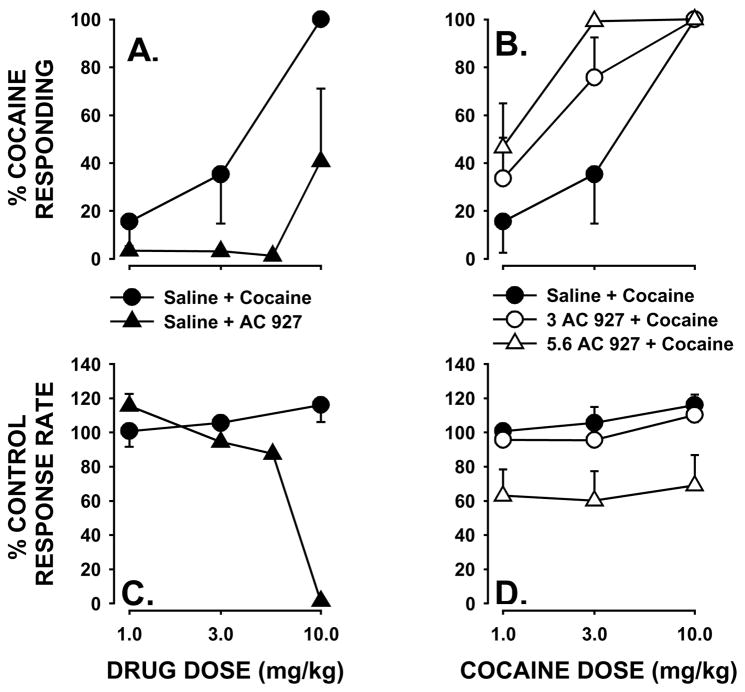
As in previous studies, all subjects readily acquired cocaine discrimination (Li et al., 2006). After cocaine injection during the baseline conditions, subjects responded almost exclusively on the cocaine-appropriate key, with at least 98.6 ± 1.19% of the responses on the cocaine-appropriate key. After saline injection, 97.43 ± 0.69% of the responses were emitted on the saline-appropriate key. During testing with different doses of cocaine, there was a dose related increase in the percentage of cocaine-appropriate responses as dose was increased from 1 to 10 mg/kg (Fig. 5A; circles). Cocaine produced a significant effect of dose, with an average ED50 value for its discriminative stimulus effects of 3.09 mg/kg. Cocaine did not produce a significant effect on rates of responding at the doses tested (Fig. 5C; circles).
Fig. 5.
Effects of AC927 in drug discrimination in rats. In the top panels, the percentage of responses on the cocaine-appropriate key are indicated. (A) Administration of either cocaine or AC927 dose dependently increased responding on the cocaine-appropriate key. (B) Pretreatment with AC927 shifted the dose response to cocaine upward. In the bottom panels, the rates at which responses were emitted (as a percentage of response rate after saline administration) are shown. (C) Cocaine did not alter rates of responding, although AC927 elicited a decrease in response rate with its highest dose tested. (D) Cocaine-induced rates of responding were significantly decreased by pretreatment with AC927.
When administered alone, AC927 produced partial substitution for the discriminative stimulus effects of cocaine (Fig. 5A; triangles). At the highest dose tested (10 mg/kg), AC927 produced an average of 40.7 ± 30.3% cocaine-appropriate responding, while the low doses of the compound did not engender cocaine-appropriate lever pressing beyond the level obtained after vehicle injections. AC927 also dose-dependently decreased response rates, particularly at the 10 mg/kg dose (Fig. 5C; triangles).
Co-administration of AC927 produced an enhancement of the discriminative-stimulus effects of cocaine (Fig. 5B). Both 3.0 (open circles) and 5.6 (triangles) mg/kg of AC927 significantly shifted the cocaine dose effect curve leftward, with the ED50 value for cocaine decreasing from 3.09 mg/kg (cocaine with vehicle) to 1.56 mg/kg and 0.822 mg/kg, respectively. Cocaine-induced response rates were also dose-dependently and significantly attenuated by the co-administration of AC927 (Fig. 5D; F[2,20]=7.52, p<0.05).
3.5. Self-administration
The maintenance dose of cocaine produced a level of self-administration that was comparable to that seen in earlier studies. Fig. 6 shows the mean dose-effect curve for AC927 self-administration in rhesus monkeys. All four animals self-administered AC927, although responding was intermediate between the low rates engendered by contingent saline and the high rates maintained at the 0.01 mg/kg/injection dose of cocaine. ANOVA confirmed a significant difference between the treatment groups (F[6,61]=123.65, p<0.0001), with AC927 generating an inverted U-shaped function across the dose range tested. Post-hoc analysis using Bonferroni’s multiple comparison tests revealed that AC927 doses of 0.01 mg/kg/injection (t=3.66, p<0.05) and 0.03 mg/kg/injection (t=8.09, p<0.001) maintained significantly more injections than saline. Responding for AC927 fell to saline-like levels when the available dose of AC927 was increased beyond 0.03 mg/kg/injection. No dose of AC927 maintained as many injections as 0.01 mg/kg/injection cocaine, and these differences were statistically significant as determined by post-hoc Bonferroni’s tests (t=10.45 to 17.47, p<0.001).
Fig. 6.
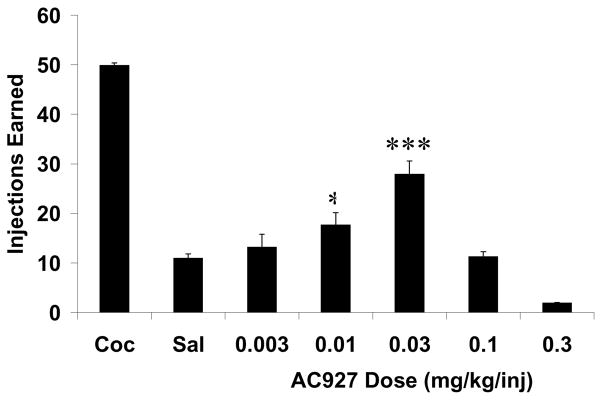
Effects of AC927 on self-administration in rhesus monkeys. AC927 generated an inverted U-shaped function. At its peak, all animals self-administered AC927, although responding was intermediate between that engendered by contingent saline and the maintenance dose of cocaine for all subjects. *P<0.05, ***P<0.001 Bonferroni’s multiple comparisons post-hoc test.
4. Discussion
Earlier studies demonstrated that AC927 exhibits a high degree of selectivity for sigma receptors, compared to 29 other binding sites, including transporters, receptors, and ion channels (Matsumoto et al., 2008). Although potential interactions with binding sites that were not tested cannot be ruled out, each of the major sites which have been problematic for previous sigma ligands were probed. Therefore, AC927 is known to be among the most selective sigma receptor ligands identified to date, and the effects produced by the drug herein are most likely mediated through these proteins.
The ability of AC927 to significantly attenuate the convulsive, lethal and locomotor stimulant effects of cocaine following acute administration is consistent with the actions of earlier described sigma receptor antagonists and antisense oligonucleotides (Matsumoto et al., 2001a,b, 2002, 2003; McCracken et al., 1999a,b). Since cocaine binds to sigma receptors at physiologically relevant concentrations (Sharkey et al., 1988), the data suggest that antagonism of sigma receptors mitigates the actions of cocaine by impeding the access of the drug to one of its target sites.
In addition to interfering with the ability of cocaine to interact with some of its binding sites, sigma receptor antagonists can negatively modulate other neurotransmitter systems that are involved in the effects of cocaine. For example, the convulsive effects of cocaine also involve NMDA receptors (Brackett et al., 2000; Lason, 2001; Witkin et al., 1999), and numerous studies have reported the ability of sigma receptor ligands to modulate NMDA-mediated activity in the brain (Martina et al., 2007; Monnet et al., 2003). The locomotor stimulatory effects of cocaine involve dopaminergic systems (e.g., Uhl et al., 2007), and sigma receptor ligands can affect motor behavior through the regulation of nigrostriatal dopamine pathways (Bastianetto et al., 1995; Goldstein et al., 1989; Gudelsky, 1995). In addition, a recent study has shown that sigma1 receptors heteromerize with dopamine D1 receptors resulting in modulation of cocaine-induced dopaminergic function in a sigma1-dependent, and dopamine transporter independent manner (Navarro et al., 2010). Therefore, through sigma-mediated modulation of other neuronal systems or through direct competition with cocaine for one of its binding sites, sigma receptor antagonists have the capability to mitigate the acute behavioral and toxic actions of cocaine.
The influence of sigma receptor ligands such as AC927 on the subchronic effects of cocaine is also noteworthy. In place conditioning studies, AC927 had no significant effects of its own, but significantly attenuated the development of cocaine-induced conditioned place preference. Earlier studies by Maurice and colleagues showed that selective antagonists and antisense oligonucleotides against sigma receptors likewise had no effects of their own on place conditioning, but prevented the development of cocaine-induced conditioned place preference (Romieu et al., 2000, 2002). In contrast, sigma receptor agonists enhanced cocaine-induced conditioned place preference, although these compounds did not elicit conditioned place preference on their own (Romieu et al., 2002, 2003). Similarly, a reactivation of place conditioning was obtained after its extinction by administration of cocaine or sigma receptor agonists, and the reactivation was blocked by the sigma receptor antagonist, BD1047 (Romieu et al., 2004). Together, these data suggest that sigma receptor ligands do not possess reinforcing effects of their own, but can modulate the reinforcing effects and drug-seeking behaviors evoked by other drug classes. This modulation may be facilitated by the altered expression of sigma receptors in the brain following repeated exposure to cocaine, since place conditioning to cocaine is accompanied by an increase in sigma receptor mRNA in the nucleus accumbens (Romieu et al., 2002). The ability of sigma receptor antagonists, in sufficient dosages, to reduce effects mediated through up regulated sigma receptors may therefore further contribute to the attenuation of an expanded range of cocaine-induced behaviors by this class of compounds.
In drug discrimination studies, AC927 partially substituted for the discriminative-stimulus effects of cocaine. When combined with cocaine, AC927 enhanced the discriminative-stimulus effects of cocaine. Together with the pattern of results in the self-administration studies which are discussed below, the data suggest that AC927 acts as a partial agonist at sigma receptors. Several points of comparison with data already in the literature supports such an interpretation, but direct testing of additional sigma ligands in this and other animal models are needed to validate such a mechanism (see Hiranita et al., 2010), as well as further characterization of AC927 across a range of functional studies. In earlier drug-discrimination studies, selective sigma receptor agonists, such as (+)-pentazocine, produced discriminative-stimulus effects when compared to saline (Steinfels et al., 1988). Similarly, the sigma-active neurosteroid pregnanolone produced discriminative-stimulus effects, which fully substituted for the sigma receptor agonist (+)-SKF 10,047 (Engel et al., 2001). The sigma receptor antagonist NE-100 has also been reported to attenuate the discriminative-stimulus effects of ketamine (Narita et al., 2001). The existing reports thus indicate that sigma receptor ligands can alter the discriminative stimulus properties of cocaine and other drugs. However, additional studies are needed to fully delineate the conditions under which such responses are elicited.
The differences in the effects of AC927 in the drug discrimination vs. place conditioning studies deserve further comment. Different rodent species were used in the two studies, which could contribute to some variations in response. However, the highly conserved nature of the sigma receptor across species suggests that such differences would be relatively minor. A more likely influence may stem from the drug histories of the animals. In the drug-discrimination experiments, as well as the self-administration studies described below, the animals receiving AC927 had been previously exposed to cocaine. In the place-conditioning studies, the animals were drug-naïve prior to treatment. The data, considered together, suggest reduced cocaine-like effects of AC927, with functional attributes influenced by drug history. Those animals with prior exposure to cocaine (e.g., drug discrimination and self administration studies) respond to AC927 as being more like cocaine than subjects without that history (e.g., place conditioning studies). However, the effects of AC927 were not equivalent to those of cocaine, even in subjects previously exposed to cocaine, as it did not fully substitute in subjects discriminating cocaine, and did not maintain rates of responding in self administration that were comparable to those maintained by cocaine. Thus, pharmacological history may modulate the response to AC927.
For the self-administration studies, AC927 was presented to primates who were experienced with self-administering a variety of abused substances including cocaine and MDMA. In these animals, AC927 was self-administered and engendered responding that was intermediate between that maintained by saline or cocaine. In earlier investigations, sigma receptor agonists such as (+)-SKF 10,047 and (+)-cyclazocine, were self-administered in primates to a level comparable to cocaine (Slifer and Balster, 1983). Also, the sigma receptor agonists DTG and PRE-084 engendered rates of responding similar to cocaine in rats trained to self administer cocaine (Hiranita et al., 2010). Well established sigma receptor antagonists such as BD1047 and BD1063, on the other hand, were not self administered (Hiranita et al., 2010). These findings are consistent with a partial agonist effect of AC927 at sigma receptors, which would explain the ability of AC927 to produce low efficacy reinforcing effects and partial substitution in cocaine discrimination, as well as a potentiation (leftward shift) of the cocaine discriminative effect, and still exhibit apparent antagonist actions under other conditions. Potential partial agonist actions of AC927 would however, have to be mediated through sigma1 receptors because in vitro dose response characterizations indicate that AC927 is an antagonist at sigma2 receptors (W.D. Bowen, personal communication).
Together, the pattern of in vivo pharmacological responses indicates that AC927, most likely acting as a partial agonist at sigma receptors, attenuates a number of cocaine-induced behaviors. In addition, the weak reinforcing and discriminative stimulus effects of AC927 suggest its potential as a pharmacotherapy for cocaine abuse. Thus, the present data along with previous results with sigma receptor antagonists suggest these compounds as promising leads for the development of new medications to treat cocaine abuse, and further investigations of their mechanisms and effects should be pursued.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Rouqier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between σ sites and nigro-striatal dopaminergic pathways. Neuropharmacology. 1995;34:281–287. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- Brackett RL, Pouw B, Blyden JF, Nour M, Matsumoto RR. Prevention of cocaine-induced convulsions and lethality in mice: effectiveness of targeting different sites on the NMDA receptor complex. Neuropharmacology. 2000;39:407–418. doi: 10.1016/s0028-3908(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Coop A, Bowen WD. σ2 Receptors up regulate changes in sphingolipid levels in breast tumor cells. Eur J Pharmacol. 2002;443:207–209. doi: 10.1016/s0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- Fantegrossi WE, Winger G, Wood JH, Woolverton WL, Coop A. Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend. 2005;77:161–168. doi: 10.1016/j.drugalcdep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Statistical Method in Biological Assay. 2. Hafner; New York: 1964. [Google Scholar]
- Gebreselassie D, Bowen WD. σ2 Receptors are specially localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Matsumoto RR, Thompson TL, Patrick RL, Bowen WD, Walker JM. Motor effects of two sigma ligands mediated by nigrostriatal dopamine neurons. Synapse. 1989;4:254–258. doi: 10.1002/syn.890040311. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Effects of σ receptor ligands on the extracellular concentration of dopamine in the striatum and prefrontal cortex of the rat. Eur J Pharmacol. 1995;286:223–228. doi: 10.1016/0014-2999(95)00415-8. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benxomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of σ-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Ishiwata K, Tajima H, Ishii S, Matsuno K, Homma Y, Senda M. In vivo evaluation of [11C]SA4503 as a PET ligand for mapping CNS sigma1 receptors. Nucl Med Biol. 2000;27:255–261. doi: 10.1016/s0969-8051(00)00081-0. [DOI] [PubMed] [Google Scholar]
- Lason W. Neurochemical and pharmacological aspects of cocaine-induced seizures. Pol J Pharmacol. 2001;53:57–60. [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates fra-2 and σ-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. Alterations in fra-2 and σ1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- Maeda DY, Williams W, Kim WE, Thatcher LN, Bowen WD, Coop A. N-Arylalkylpiperidines as high affinity sigma-1 and sigma-2 receptor ligands: phenylpropylamines as potential leads for sigma-2 agents. Bioorg Med Chem Lett. 2002;12:497–500. doi: 10.1016/s0960-894x(01)00788-0. [DOI] [PubMed] [Google Scholar]
- Martina M, Turcotte M-EB, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Exp Rev Clin Pharmacol. 2009;2:351–358. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, Newman AH. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001a;41:878–886. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, de Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotides targeting σ1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001b;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through antagonism of sigma receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, Matsumoto RR. Novel σ ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol. 1999a;365:35–38. doi: 10.1016/s0014-2999(98)00876-0. [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma ligands, BD1047 and LR172, attenuate cocaine-induced convulsions and locomotor activity. Eur J Pharmacol. 1999b;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective σ ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Morin-Surin MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by σ1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshizawa K, Aoki K, Takagi M, Miyatake M, Suzuki T. A putative sigma1 receptor antagonist NE-100 attenuates the discriminative stimulus effects of ketamine in rats. Addict Biol. 2001;6:373–376. doi: 10.1080/13556210020077091. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortes A, Casado V, Canela EI, Ortiz J, Fuxe K, Lluis C, Ferre S, Franco R. Direct involvement of σ-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M, Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Iontropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur J Pharmacol. 1995;286:19–30. doi: 10.1016/0014-2999(95)00424-j. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Bowen WD, Maurice T. Sigma1 (σ1) receptor-related neuroactive steroids modulate cocaine-induced reward. J Neurosci. 2003;23:3572–3576. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the σ1 receptor in the cocaine-induced conditioned place preference. NeuroReport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. The sigma1 (σ1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology. 2004;175:154–162. doi: 10.1007/s00213-004-1814-x. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at σ receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Slifer BL, Balster RL. Reinforcing properties of stereoisomers of the putative sigma agonists N-allylnormetazocine and cyclazocine in rhesus monkeys. J Pharmacol Exp Ther. 1983;225:522–528. [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. 6. Iowa State University Press; Ames, IA: 1967. pp. 135–171. [Google Scholar]
- Steinfels GF, Alberici GP, Tam SW, Cook L. Biochemical, behavioral, and electrophysiological actions of the selective sigma receptor ligand (+)-pentazocine. Neuropsychopharmacology. 1988;1:321–327. [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9:184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Ujike H, Kuroda S, Otsuke S. σ Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- Weatherspoon JK, Werling LL. Modulation of amphetamine-stimulated [3H]dopamine release from rat pheochromocytoma (PC12) cells by sigma type 2 receptors. J Pharmacol Exp Ther. 1999;289:278–284. [PubMed] [Google Scholar]
- Witkin JM, Gasior M, Heifets B, Tortella FC. Anticonvulsant efficacy of N-methyl-D-aspartate antagonists against convulsions induced by cocaine. J Pharmacol Exp Ther. 1999;289:703–711. [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy)-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–382. [PubMed] [Google Scholar]
- Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, Matsumoto RR. A novel substituted piperazine, 3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione (CM156), attenuate the stimulant and toxic effects of cocaine in mice. J Pharmacol Exp Ther. 2010;333:491–500. doi: 10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]