Abstract
Recent data suggests that psychotic major depression (PMD) may be a discrete disorder distinguishable from nonpsychotic major depression (NPMD), and that patients with PMD may be more similar to individuals with schizophrenia than individuals with NPMD. The insula is a brain region in which morphometric changes have been associated with psychotic symptom severity in schizophrenia and affective psychosis. It was hypothesized that insular volumes would be reduced in PMD compared to NPMD and controls, and insular volumes would correlate with psychosis but not depression severity. Insular gray matter volumes were measured in PMD and NPMD patients and matched healthy controls using magnetic resonance images and manual morphometry. Clinical measures of illness severity were obtained to determine their relationship with insular volume. Posterior insular volumes were significantly reduced in PMD compared to HC. There were also significant group-by-gender interactions for total, anterior and posterior insular volumes. Using Pearson product-moment correlations, anterior insular volumes did not correlate with depression severity. Left anterior insular volume was significantly correlated with total and positive symptom psychosis severity in the PMD group. Atypical insular morphometry may be related to the inability to distinguish between internally- and externally-generated sensory inputs characteristic of psychosis.
Keywords: psychosis, major depression, insula, morphometry, cortex
Introduction
Recent data suggest that psychotic major depression (PMD) may be a discrete disorder distinguishable from nonpsychotic major depression (NPMD) (Schatzberg et al., 2000; 1992). Various cognitive measures have been used to show that patients with PMD have some clinical similarities to patients with schizophrenia, and both groups are different from patients with NPMD (Jeste et al., 1996; Nelson et al., 1998; Kim et al., 1999). In addition to the similarities on cognitive measures, PMD and schizophrenic patients both express psychotic symptoms. Insular cortex is an important sensory-limbic integrative processing region, and this function may have a role in psychotic feature manifestation. Previous neuroimaging studies utilizing voxel-based morphometry, positron emission tomography (PET), and functional magnetic resonance imaging (fMRI) have found insular involvement in various psychopathological diseases, including mood disorders and schizophrenia (Chen et al., 2009). Goldstein et al. (1999) and Crespo-Facorro et al. (2000) both found total insular volume reductions in schizophrenic patients, while the latter study also reported a significant correlation between total insular volumes and overall severity of psychosis. Several studies involving affective psychosis also reported insular volume reductions (Morgan et al., 2007; Kubicki et al., 2002). A positron emission computed tomography study found that patients with major depression with psychotic features had a focus of decreased regional cerebral blood flow in the right inferior frontal cortex compared to nonpsychotic major depressed patients, with the voxel of maximal significance in the insula (Skaf et al., 2002). Furthermore, when patients with schizophrenia reported experiencing auditory verbal hallucinations, increased activation of the right anterior insula was observed (Sommer et al., 2008).
The current study was designed to examine unipolar major depression with and without psychotic features to determine if the morphology of insular cortex could distinguish between these two sub-populations. Morphometric measures were compared across groups of patients with PMD, NPMD and matched healthy controls; and the relationships of structure volumes to clinical measures were explored through correlation analyses. Insular mophometry was assessed using an established native-space methodology that quantifies anterior and posterior connectivity-based sub-regional volumes in addition to total insular volume (Cohen et al., 2009). Based upon findings from other populations with psychotic features, it was hypothesized that insular volumes would be reduced in PMD compared to both NPMD and healthy controls (HC). It was also expected that insular volumes would correlate with psychotic symptom severity but not depressive symptom severity. While total insular volumes were correlated with overall psychosis severity, total insular volumes did not correlate with positive symptom severity in previous studies (Crespo-Facorro et al., 2000; Pressler et al., 2005). The current study analyzed insular sub-regions directly, which allowed for more functionally specific relationships between volume and overall psychosis, as well as positive, symptom severity. Given the sensory-limbic integrative function of the anterior insula, the regional cerebral blood flow abnormalities previously seen in this region (Skaf et al., 2002; Sommer et al., 2008), and the focus on anterior insular function in psychopathology (Menon and Uddin, 2010); the previous data suggested that group differences in the anterior insula would be more anomalous than posterior insula. However, it is important to emphasize that the method in the current study aimed to better differentiate between anterior and posterior insular involvement in psychopathology. In fact, unexpected posterior insular volume reductions were found in the past (Cohen et al., 2009; 2011), and, therefore, it was possible that posterior insular volumes would also be reduced in PMD compared to both NPMD and HC. Based on the functional abnormalities seen in the anterior insula during auditory hallucinations, the anterior insula was a specific region-of-interest in terms of its relationship with psychotic symptoms, but not depressive symptoms. Ultimately, the goal of this study was to examine the manner in which anterior and posterior insular regions work together in psychosis.
Materials and Methods
Subjects
Patients were either recruited from outpatient facilities at Stanford University or self-referred via online and in response to print advertisements. Twenty patients with psychotic major depression (PMD), 19 patients with non-psychotic major depression (NPMD), and 22 healthy control subjects participated in the study. Inclusion and exclusion criteria, including all screening procedures, for the three groups were reported previously in Keller et al., 2008. The psychiatric medication distribution of PMD and NPMD was also previously reported in Keller et al. (2008) and was not repeated here. Subjects with major depression with and without psychosis were diagnosed with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997). Participants with major depression with psychosis had diagnoses confirmed by consultation with the subjects’ treating psychiatrists.
Psychometric assessment of participants pertinent to this study included clinical measures of depression (i.e the 21-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960)) and psychosis (i.e the total and positive symptom subscale of the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962)). The 21-item Hamilton Depression Rating Scale (HAM-D) was used to quantify depression severity in depressed patients with and without psychosis. The Brief Psychiatric Rating Scale (BPRS) was used to quantify overall psychosis severity, and the positive symptom subscale of the BPRS, which consists of four items: conceptual disorganization, suspiciousness, hallucinations, and unusual thought content, was used to quantify positive symptom severity. These metrics were used to correlate symptom severity with anterior insular volume measurements.
Control subjects were required to have a score less than 6 on the Hamilton Depression Rating Scale (HAM-D) and have no psychotic symptoms.
After complete description of the study was given, written informed consent was obtained. Eligibility screening procedures were followed according to Keller et al. (2008). All eligible participants underwent structural magnetic resonance imaging (MRI). In addition, all eligible subjects participated in neuropsychological testing and psychological interviews (these findings are reported elsewhere [Gomez et al., 2006; Keller et al., 2006]). The human subjects review board at Stanford University School of Medicine, Stanford, CA, approved all protocols.
MRI Acquisition and Image Processing
Structural images were acquired on a 3T GE Signa scanner, using a standard GE whole-head coil. The scanner runs on an LX platform, with gradients in “Mini-CRM” configuration (35 mT/m, SR 190 mT/m/second), and has a Magnex 3T 80-cm magnet. The following three dimensional, volumetric radio frequency, spoiled-gradient echo parameters were used: TR=35 msec, echo time=6 msec, flip angle=45°. The field of view was 240×240 mm2, and the acquisition matrix size was 256×192. One hundred twenty-four scan locations were acquired at 1.5-mm slice thickness. MRI scans were imported into BrainImage software for semiautomated whole-brain segmentation, including bias field correction, and quantification in the coronal plane (Kates et al., 1999; Reiss et al., 1998). Total brain volume used in analyses was computed as the sum of gray matter, white matter and cerebrospinal fluid (CSF).
The insula was manually delineated using the sagittal and coronal planes for each participant according to previously described methods (Cohen et al., 2009), using grayscale volume stacks derived from whole-brain analysis. These data sets were standardized in their orientation, paralellel to the axial plane passing through the anterior and posterior commissures, using a six-parameter rigid-body transform. Insular regions-of interest (ROI) were traced on the non-segmented grayscale images, and the resulting gray matter volumes were quantified by projecting the ROIs onto the segmented gray matter fraction stack for each subject. The reliability of the insular ROIs was established by achieving intraclass correlation coefficient greater than 0.93.
The superior and inferior circular sulci separate the insula from the fronto-parietal and temporal opercula, respectively. Since the inferior circular sulcus does not extend rostral to the limen of the insula, there is no well-defined boundary between the anterior insula and the orbital frontal cortex. The orbitoinsular sulcus is considered the topographic boundary between the anterior insula and adjacent orbitofrontal cortex. The superior and inferior circular sulci fuse to form the posterior pole of the insula. The medial boundary of the insula is a band of white matter called the extreme capsule. The anterior and posterior boundaries of the insula were viewed best from the sagittal plane. The coronal plane was utilized to define the superior, inferior and medial boundaries of the superior sulcus, inferior sulcus, and extreme capsule respectively.
Statistical Analyses
A two-tailed alpha of 0.05 was chosen as the threshold for statistical significance for all statistical tests. Univariate analysis of variance (age, education, total brain volume) and chi square (gender) analyses were used to assess possible demographic differences among the three groups. A repeated measures analysis of covariance (ANCOVA) was used to detect group differences in total insular volume, where hemisphere (Left, Right) was considered the repeated measure, gender and group were independent factors, and total brain volume and age were covariates. To test anterior and posterior insular differences, a MANCOVA with repeated measures was used in which anterior and posterior volumes were dependent variables, hemisphere was the repeated measure, group and gender were independent variables,, and total brain volume and age were covariates. It should be emphasized that each insular region (total, anterior, posterior) in each hemisphere (left, right) was included in all statistical analyses to ensure that each of the regions from each subject were represented individually in the full statistical models. However, for graphical display purposes, since no hemispheric differences were found (i.e. no significant asymmetry), insular volumes were graphically represented by averaging across hemispheres [(Left+Right)/2] in order to show a single mean for each group. Finally, significant differences at the (M)ANCOVA level were assessed by pair-wise (M)ANCOVA comparisons.
Pearson product-moment correlations were used to examine relationships between anterior insular volumes and clinical assessment scores. HAM-D scores from both PMD and NPMD groups were used in the anterior insular volume correlation analysis. To be clear regarding depressive symptoms and anterior insular volume, PMD and NPMD groups were combined as both groups have significant depressive symptoms. HAM-D scores were also compared to anterior insular volumes in PMD and NPMD groups separately to thoroughly verify that depressive symptoms do not correlate with anterior insular volume. As the NPMD group is, by definition, lacking psychotic symptoms, only total and positive symptom subscale BPRS scores from the PMD group were used in the insular volume correlation analyses. Correlations were not corrected for multiple comparisons.
Results
Demographics and total brain volume
The objective of this study was to examine potential insular morphometry differences among patients with major depression with and without psychosis and matched healthy controls. The groups were well matched (Table 1); there were no significant differences in age (F(2,55) = 1.853, p=0.166), education (F(2,55) = 0.328, p=0.722), total brain volume (F(2,55) = 1.854, p=0.166), or gender (χ2(2, N = 61, p=0.303). While gender did not significantly differ across groups, gender was included as an independent variable for all insular analyses to determine if insular volume differences could be related to gender differences by group. While there was no overall significant difference in age across groups, age differences for men and women were also examined separately across groups. There were no significant group differences in age for either men (F(2,25) = 0.747, p = 0.484) or women (F(2,30) = 2.566, p = 0.094) (Table 1). Nevertheless, since age has been shown to affect insular volumes (Foundas et al., 1996), age was added as a covariate along with total brain volume to all insular analyses. Finally, there was no hemisphere by group differences for any insular ROI (F’s(2,51) < 1.182, p’s > .315).
Table 1.
Subject Demographics
| Group | |||||
|---|---|---|---|---|---|
| PMD | NPMD | Controls | Significancea | ||
| Subjects | Men | 11 | 6 | 11 | |
| Women | 9 | 13 | 11 | ||
| Total | 20 | 19 | 22 | p = 0.410 | |
| Age (years) | Men | 37.09 (15.08) | 45.00 (12.20) | 38.09 (11.84) | p = 0.484 |
| Women | 35.56 (11.84) | 33.77 (9.98) | 26.36 (7.88) | p = 0.094 | |
| Total | 36.40 (13.39) | 37.32 (11.68) | 32.23 (11.51) | p = 0.166 | |
| Education (years) | Men | 16.00 (3.63) | 15.50 (1.98) | 16.27 (2.10) | |
| Women | 15.50 (3.46) | 14.54 (2.30) | 14.64 (2.06) | ||
| Total | 15.79 (3.47) | 14.84 (2.19) | 15.45 (2.20) | p = 0.722 | |
| Total Brain Volume (cm3) | Men | 1599.08 (137.23) | 1628.25 (112.12) | 1474.68 (149.39) | |
| Women | 1299.99 (105.04) | 1317.20 (88.37) | 1320.65 (143.24) | ||
| Total | 1464.49 (194.58) | 1415.43 (175.40) | 1397.66 (163.13) | p = 0.166 | |
Table lists means (standard deviations) for participants’ age, education, and total brain volume. Data organized by total group means and group by gender means.
p-values indicate comparisons across PMD, NPMD, and controls.
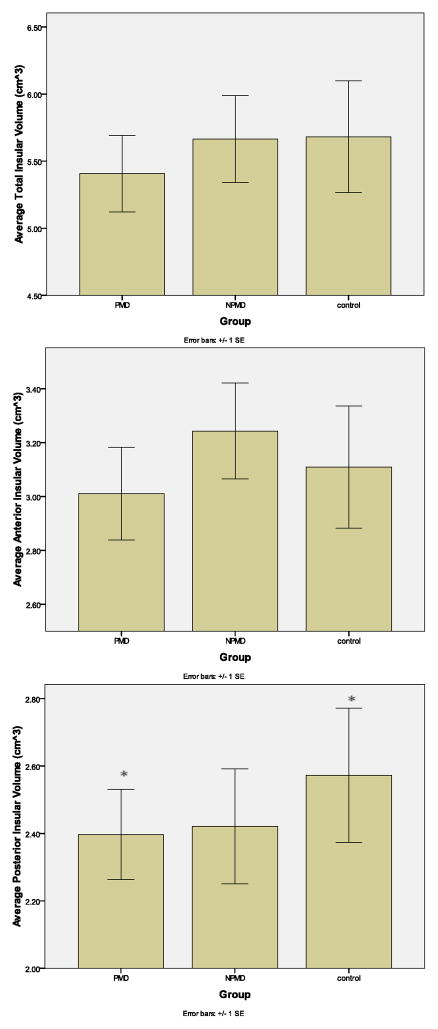
Posterior, but not Total or Anterior, Insular Volume Reduction in PMD
For insular ROI volumes by region, group and gender see Table 2. Repeated measures ANCOVA (total) and MANCOVA (anterior and posterior) were used to test for differences in insular volumes across groups (Figure 1). No significant difference of total insular volume was found (F(2,51) = 2.24, p = 0.116) using ANCOVA, though PMD volumes did appear to be smaller than both NPMD and HC. This result was inconsistent with previous data that suggested total and anterior insular volumes would be reduced in PMD. The repeated measures MANCOVA revealed a significant group difference in posterior insular volumes (F(2,51) = 3.32, p = 0.044), but not anterior (F(2,51) = 1.17, p = 0.319) as originally predicted by previous research. PMD had significantly smaller posterior insular volumes than HC (F(1,35) = 6.91, p-uncorrected = 0.013; p-corrected = 0.038) (p is corrected for multiple comparisons; See Methods). The two depressed groups did not significantly differ in posterior insular volumes (F(1,32) = 0.45, p-corrected = 0.879) nor did NPMD and HC (F(1,34) = 1.15, p-corrected = 0.645), although NPMD posterior insular volumes appeared smaller than HC. Hemisphere (Left, Right) was included as a repeated measure in all analyses to test for asymmetry differences across groups. There were no significant hemispheric differences in any insular ROI with regard to group (p’s > 0.435) or gender (p’s > 0.765). Because there were no hemispheric differences in any insular ROI across groups, the average [(Left+Right)/2] for each insular ROI in each group was used for graphical representation of insular volumes in Figures 1 and 2 (see Methods).
Table 2.
Interhemispheric Average Insular Volumes (cm3)by Group and Gender
| Group | ||||
|---|---|---|---|---|
| PMD | NPMD | Controls | ||
| Subjects | Men | 11 | 6 | 11 |
| Women | 9 | 13 | 11 | |
| Total | 20 | 19 | 22 | |
| Anterior Volume | Men | 3.097 (0.856) | 3.412 (0.744) | 3.630 (1.090) |
| Women | 2.904 (0.684) | 3.162 (0.808) | 2.590 (0.770) | |
| Total | 3.011 (0.770) | 3.243 (0.777) | 3.110 (1.064) | |
| Posterior Volume | Men | 2.393 (0.467) | 2.707 (0.733) | 2.957 (1.066) |
| Women | 2.402 (0.764) | 2.290 (0.738) | 2.188 (0.613) | |
| Total | 2.397 (0.600) | 2.421 (0.743) | 2.572 (0.936) | |
| Total Volume | Men | 5.491 (1.223) | 6.126 (1.418) | 6.587 (2.107) |
| Women | 5.306 (1.400) | 5.451 (1.418) | 5.187 (1.372) | |
| Total | 5.407 (1.273) | 5.665 (1.415) | 5.682 (1.952) | |
The table depicts the mean hemispheric averaged insular volumes [(left + right hemisphere)/2] (cm3) for total, anterior and posterior insula adjusted for total brain volume and age, and organized by group and gender.
FIGURE 1. Group Differences in Total and Posterior Insula.
The graphs depict the mean hemispheric averaged insular volumes [(left + right hemisphere)/2] (cm3) for total, anterior and posterior insula adjusted for total brain volume and age, and organized by group. *Indicates significant group difference.
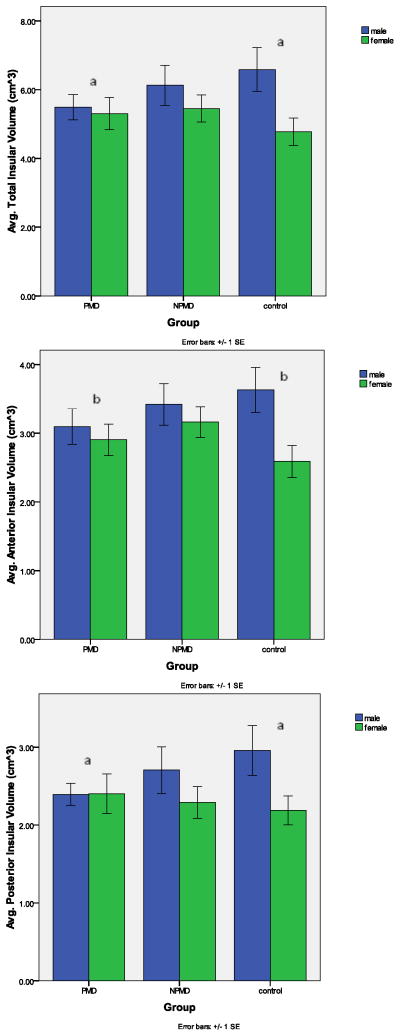
FIGURE 2. Gender Dependence of Insular Volume.
The graphs depict the mean hemispheric averaged insular volumes [(left + right hemisphere)/2] (cm3) for total, anterior and posterior insula adjusted for total brain volume and age, and organized by group. Men (blue) and women (green) are indicated by the different colors. Significant interaction between PMD and HC for total (ap-corrected <0.01), anterior (bp-corrected <0.05), and posterior (ap-corrected <0.01). The group-by-gender interaction was not significant for the total (p-corrected =0.867), anterior (p-corrected =0.997), or posterior (p-corrected =0.561) insula between the depressed groups. The group-by-gender interaction was not significant for the total insula (p-corrected=0.148), anterior (p-corrected = 0.059) or posterior insula (p-corrected =0.461) between the NPMD patients and controls.
Interaction of Gender and Group
In addition to the reduced posterior insular volumes in PMD as reported above, there was a significant group-by-gender interaction among the 3 groups within each insular ROI. This interaction is clearly recognized by noting that the ratio of male to female volumes is different across the three groups (Figure 2; Table 2). The group-by-gender interaction (Figure 2) was significant across groups for total (F(2,51) = 5.61, p = 0.006), anterior (F(2,51) = 5.21, p = 0.009), and posterior (F(2,51) = 4.99, p = 0.010) insular regions (Figure 2). In all insular regions, PMD men and women had significantly more similar insular volumes compared to HC men and women (Total: F(1,35) = 10.37, p-corrected =.009; Anterior: F(1,35) = 8.31, p-uncorrected = 0.007; p-corrected = 0.021; Posterior: F(1,35) = 10.56, p-uncorrected = 0.003; p-corrected = 0.009) (Figure 2). The group-by-gender interaction was not significant in any insular region between NPMD and HC (Total: F(1,34) = 4.07, p-corrected = 0.148; Anterior: F(1,34) = 5.95, p-corrected = 0.059; Posterior: F(1,34) = 1.83, p-corrected = 0.461) or between the depressed groups (Total: F(1,32) = 0.49, p-corrected = 0.867; Anterior: F(1,32) = 0.03, p-corrected = .998; Posterior: F(1,32) = 1.43, p-corrected = 0.561). Specifically, the significant interaction was between HC and PMD. Whereas HC men and women had divergent insular volumes (M>F; Total, Anterior, Posterior), PMD men and women failed to show the same M>F distribution in all insular regions (Total, Anterior, Posterior). Finally, the NPMD volumes generally appeared more like HC than PMD (i.e. M>F) in all insular regions (Total, Anterior, Posterior).
Correlations between Psychotic Symptoms and Left Anterior Insular Volumes
All participants were evaluated for overall depression by the HAM-D, and psychosis by the total BPRS and positive symptom subscale of the BPRS. There were significant group differences in total HAM-D (F(2,58) = 519.82, p<0.001) and BPRS (F(2,58) = 209.10, p<0.001) scores (Table 3). As expected, participants with PMD had significantly higher scores relative to NPMD patients, and NPMD patients had higher scores relative to healthy controls on both measures. It is important to note that NPMD had significantly higher scores on the BPRS than HC because the BPRS is also sensitive to depressive symptoms despite being primarily focused on recording the presence of psychotic symptoms. There were also significant group differences in BPRS positive symptom subscale scores (F(2,58) = 95.19, p<0.001), with PMD patients scoring significantly higher than both NPMD patients and controls, as expected. As anticipated, the NPMD patients and controls did not differ on this metric.
Table 3.
Clinical Measures
| Group | |||||
|---|---|---|---|---|---|
| PMD | NPMD | Controls | Significance | ||
| HAMD-21 | Men | 28.09 (4.35) | 25.00 (4.34) | 0.73 (1.01) | |
| Women | 32.22 (3.46) | 23.08 (2.47) | 0.45 (0.52) | ||
| Total | 29.95 (4.41) | 23.68 (3.18) | 0.59 (0.80) | PMD>NPMDa NPMD>HCa PMD>HCa |
|
| BPRS | Men | 28.64 (7.90) | 15.00 (1.41) | 0.45 (0.82) | |
| Women | 33.89 (7.61) | 15.38 (3.28) | 0.09 (0.30) | ||
| Total | 31.00 (8.03) | 15.26 (2.79) | 0.27 (0.63) | PMD>NPMDa NPMD>HCa PMD>HCa |
|
| Positive Symptoms | Men | 7.73 (3.61) | 0.17 (0.41) | 0.00 (0.00) | |
| Women | 11.00 (4.44) | 0.08 (0.28) | 0.00 (0.00) | ||
| Total | 9.20 (4.24) | 0.11 (0.32) | 0.00 (0.00) | PMD>NPMDa PMD>HCa |
|
Table lists means (standard deviations) for participants’ clinical evaluations for Hamilton Depression Rating Scale 21-item (HAMD-21), the Brief Psychiatric Rating Scale (BPRS), and the positive symptom subscale of the BPRS. Data is organized by total group means and group by gender means.
p < 0.001.
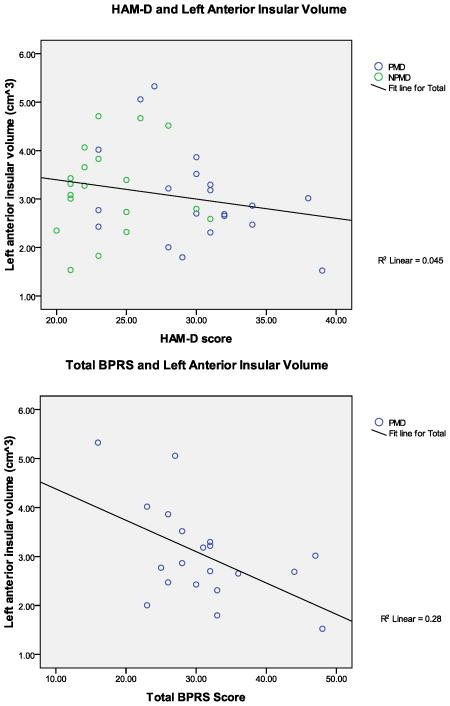
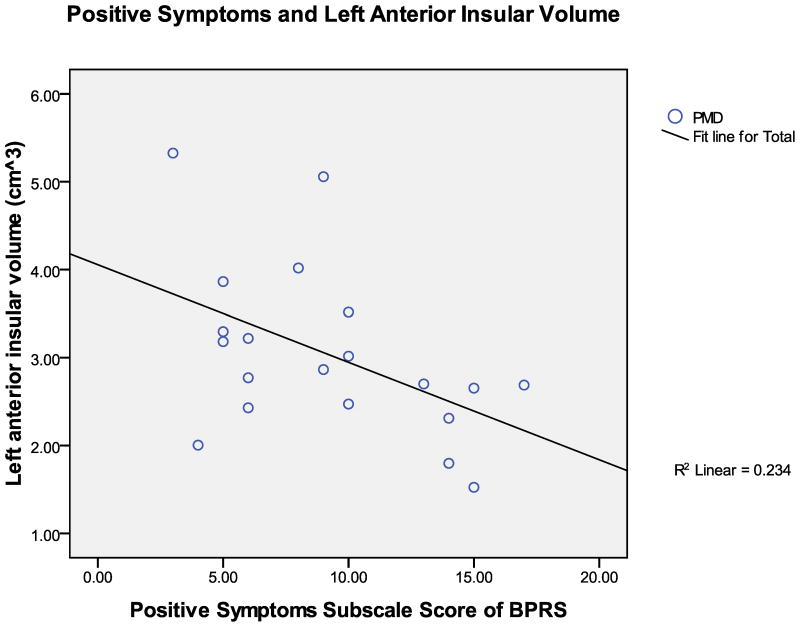
Correlations between psychometric measures (Figure 3) and insular volumes (adjusted to account for total brain volume and age) were examined in the combined sample of depressed participants (i.e., PMD+NPMD; HAM-D), and in the PMD group alone (BPRS and positive symptoms). As stated above, and evident in Table 3, PMD had significantly higher HAM-D scores than NPMD. However, there were no significant correlations between depression severity and left or right anterior insular volumes (r’s(37) < −0.212, p’s>0.195). The depressed groups were combined for this analysis since they both qualify for major depression. To further assess this aspect, correlations between HAM-D and insular volumes were examined within PMD and NPMD groups independently. Depression severity was not correlated with left or right anterior insula in the PMD group (r’s(18) < −0.385, p’s > 0.094), or in the NPMD group (r’s(17) < 0.333, p’s > 0.163). On the other hand, there was a significant negative correlation between the total BPRS score and the left anterior insular volume in the PMD group (r(18) = −0.529, p = 0.016) (Figure 3). Interestingly, contrary to expectation, total BPRS score was not correlated with the right anterior insula (r(18) = −0.440, p = 0.052), although there was a strong negative trend relationship. Overall, as general psychotic symptoms increased, anterior insular volumes (although primarily left anterior) decreased. Furthermore, there was also a significant negative correlation between the positive symptom sub-scale of the BPRS and the left anterior insular volume in the PMD group (r(18) = −0.483, p=0.031) (Figure 3), but again not with the right anterior insula (r(18) = −0.374, p=0.104). That is, PMD patients with higher BPRS and positive symptom scores had smaller left anterior insular volumes. Interestingly, the right anterior insula only had a trend relationship with total BPRS and was not correlated with the positive symptom sub-scale of the BPRS. This suggests that the anterior insula is indeed involved in both overall and positive psychotic symptom manifestation, and that the left anterior insula has a more pronounced role in those symptoms than the right anterior insula.
FIGURE 3. Correlation scatterplot of Psychotic Symptoms and Insular Volumes.
The graphs show scatterplots and trendlines for the relationships between left anterior insular volumes in cm3 (adjusted for total brain volume and age) and clinical measures of symptom severity (HAM-D, BPRS, positive symptom subscale of BPRS). The top graph shows HAM-D scores vs. left anterior insular volumes for all depressed patients (PMD + NPMD) since both groups show depressive symptoms. Correlations were also computed for PMD and NPMD groups separately, but graphical representations of those non-significant relationships were not shown here.
Discussion
The objective of the current study was to examine unipolar depression with and without psychotic features to determine if insular anatomy delineates these sub-populations. The study produced three major findings. First, posterior insular volumes were found to be significantly reduced in PMD compared to HC. There was also a significant interaction of group and gender for all insular regions in which PMD men and women failed to significantly show the M>F volume distribution seen in HC men and women. Lastly, left anterior insular volume was found to correlate with both overall psychotic symptom severity and positive symptom severity in PMD as scored by the BPRS. It is also worth noting that while insular volumes were not statistically different at the group level in PMD versus NPMD, more subtle differences in the correlation of left anterior insular volume to total and positive psychotic symptoms, but not depressive symptoms, show a neural correlate related to specific clinical symptoms that distinguishes the groups.
No statistically significant differences in total or anterior insular volume were found among the three groups. This is noteworthy as previous research suggested the hypothesis that total and anterior insular volumes would be reduced in PMD compared to NPMD and HC. The data here did not support the previous model of exclusively anterior insular involvement in psychosis, but in fact added to the model suggesting that posterior insula contributes as well. A pair-wise comparison of PMD and HC did reveal a significant reduction in total insular volume in PMD compared to HC, but this result did not hold statistically after correcting for multiple comparisons. Specifically, and noteworthy, only PMD, and not NPMD, had significantly reduced posterior insular volumes relative to HC. The lack of posterior insular volume reduction in NPMD is emphasized by a similar finding by Takahashi et al. (2010). Unexpected by previous models of psychopathology, the method used here shows that posterior insular morphometry distinguishes between PMD and NPMD. Furthermore, while the PMD group had only a trend volume reduction of total insula compared to HC, it may be that this effect was driven by the significant volume reduction of the posterior insula. This suggests that although posterior insula is volumetrically smaller than anterior insula, the posterior insular region is of vital importance, especially considering its functional role in somato- and vestibular sensation. In fact, stroke lesions of posterior insular cortex have been linked to anosognosia, a disorder in which patients either believe their limbs function normally after a stroke despite obvious motor deficits or actually perceive paralyzed limbs as belonging to a stranger (Karnath et al., 2005). Based on positron emission tomography results, it is believed that damage to posterior insular cortex could disturb the feeling of being involved versus not being involved in a movement and may even disturb the subject’s beliefs about ownership and function of body parts (Karnath et al., 2005; Farrer et al., 2003). Therefore, the reduced posterior volumes in PMD may reflect (a structural lesion leading to) a bodily disconnect that helps drive an enhanced deficiency in monitoring internal versus external stimuli. This may clinically manifest as the physical component of hallucinatory and/or delusional psychotic symptoms, such as feeling physical sensations that aren’t there, believing something unusual is happening in their body, or believing their body is being controlled by someone else. The posterior insula is also involved in secondary auditory processing and abnormalities in this region may contribute to auditory hallucinations, which may be misinterpretations of internal thoughts as coming from an external source. This region tends to be overlooked in the literature in favor of its larger counterpart (i.e. anterior insula) because of that regions’ role in higher order functions of interoception and self-awareness. In fact, insular models of psychopathology tend to focus solely on the anterior insula and neglect the posterior insula (Sommer et al., 2010; Chen et al., 2009). The results here indicate that the posterior insula should not be ignored in depressive phenotypes, and that methodological protocols similar to the one here should be used when investigating the insula to begin to better understand the roles of the posterior insula in normal and abnormal function.
The lack of simple group difference in total and anterior insular volume was notable. One important factor to consider is the use of psychiatric medication (i.e. antipsychotics and antidepressants) among patient groups and the possible impact those medications have on brain volume. Previous studies investigating effects of antipsychotic drug use in schizophrenia found relationships between length of exposure to antipsychotics and decreases in brain volume, though the primary volume reductions were in the frontal lobe (Lewis, 2011; Moncrieff and Leo, 2010). Another study that showed brain volume reductions with antipsychotic drug exposure noted that it was difficult to dissociate the effects of the disease process in schizophrenia from the effect of the antipsychotic drugs (Lieberman et al., 2005). If the use of antipsychotic drugs impacted insular cortex in the same manner as antipsychotic drugs impacted frontal cortices in schizophrenia, it is more likely that significant volume reductions would have been seen in every insular region in PMD compared to HC and NPMD. However, this was not the case. In fact the use of antipsychotic drug therapy by the PMD patients may have limited overall insular volume reductions as a previous study of insular morphometry suggested that prolonged use of antipsychotic medication over time may increase insular volume (Pressler et al., 2005). Furthermore, no differences in insular volume were found in depressed patients taking versus not taking antidepressant drugs (Takahashi et al., 2010). While previous evidence suggests that neither antipsychotics nor antidepressants result in insular volume reductions, future studies of the insula in individuals with PMD should take this factor into account. It is important to note that while no statistical difference in gender distribution across groups was found, NPMD did have twice as many women as men compared to equal men and women in PMD and HC. The (expected) imbalance of gender in NPMD, and greater variability among the fewer NPMD men compared to NPMD women, could have negated potential simple group differences between PMD and NPMD; particularly in the total insular region. Also of note here was the fact that anterior insular volumes were not reduced in NPMD compared to HC as was reported in Takahashi et al. (2010). In fact, NPMD volumes were larger than HC, which was driven predominantly by NPMD women having larger volumes. The increase in female NPMD anterior insular volumes is notable as most of the subjects in the study by Takahashi et al. (2010) were women. The contrast in results between the current study and Takahashi et al. (2010) in the anterior insula may be due in part by the methods used. The current method is functionally based (i.e. bisecting the insula based on its functional compartmentalization) whereas Takahashi used a structurally based method (i.e. bisected the insula based on the insular central sulcus). Using the central sulcus to divide the insula neglects function and therefore may be less valid and less useful for understanding the functional roles of anterior and posterior insulas. The two methods create distinctively different “anterior” and “posterior” regions.
There was also a significant group-by-gender interaction for the total, anterior and posterior insular regions. The significant interaction suggests that gender influences insular volume differently across groups. After controlling for age and total brain volume, total insular volumes in men were significantly larger than women in the HC group, while men and women had more similar volumes in both depressed groups. It should be noted that NPMD men and women were more similar to HC than PMD; and that low NPMD male representation, along with higher variability among NPMD men than NPMD women, could have contributed to the lack of gender difference (men vs. women) in NPMD that is seen in HC. The same pattern in volume difference between men and women across groups was found for both the anterior and posterior insula, with PMD men and women having particularly equal posterior insular volumes.
In contrast to a negative correlation between left anterior insula and Beck depression inventory reported by Takahashi et al. (2010), HAM-D scores were not correlated with left or right anterior insular volume. Most notably, left anterior insular volumes were significantly correlated with total and positive symptom severity BPRS scores for psychoses exclusively in the PMD group. Right anterior insula was not found to be correlated with these psychosis severity measures indicating a hemispheric or lateralized affect between anterior insula and psychosis severity, though the relationship approached statistical significance. Previous reports of insular volume correlations with psychotic symptom severity are mixed, but are dependent on the specific clinical measure used to rate symptom severity and whether the patients are first-episode or not (Crespo-Facorro et al., 2000; Kasai et al., 2003; Pressler et al., 2005). Furthermore, while previous studies have failed to find significant correlations between positive symptoms and total insular volume (Crespo-Facorro et al., 2000; Pressler et al., 2005), the findings presented here indicate that a more specific measure of insular sub-regions (i.e., anterior and posterior) may be required to elucidate the relationship between brain structure and specific symptom characteristics. The method utilized (Cohen et al., 2009) here yielded a significant correlation between both total and positive symptoms BPRS scores and the left anterior insular sub-region. These results are in contrast to total insular volume being related to total psychotic symptoms only by Crespo-Facorro et al. (2000). The results also indicate a possible functional asymmetry in which changes in left anterior insula are more involved in psychotic symptom manifestation, particularly positive symptoms. Overall, the findings support the importance of the current morphometric method and its delineation of insular sub-regions, as opposed to only total insular approaches; and suggest that studies of insular morphometry need to examine the insula at the sub-regional level. Of course, the manner in which insular sub-regions are designated is also important. The current method is based on functional compartmentalization via connectivity with other brain regions (Mesulam and Mufson, 1982). On the other hand, using the central sulcus to divide the insula structurally into anterior and posterior lobes neglects insular functional compartmentalization and creates two functionally heterogeneous regions.
The insula is important in the interoceptive system which monitors and helps regulate the physiological state of the body (Craig, 2003). This allows the insula the unique ability to integrate incoming external sensory information with the internal physiological response related to emotional state. Insular cortex has been associated with the integration of sensory phenomena and mediates temporally defined auditory-visual interaction at an early stage of cortical processing (Bushara et al., 2001; Calvert, 2001) as well as the misinterpretation of internal and external perceptions (Brebion et al., 1998; Frith and Dolan, 1997). This is interesting considering the physical sensory integrative function of the posterior insula and posterior insular volume reduction in PMD reported here. Notably, the posterior region is the first to receive sensory information from the body, and strong connections from posterior insula send this information to anterior insula. Considering this flow of information, the possibility emerges that posterior insular volume reductions in PMD may in fact be driving more subtle changes in anterior insular volumes, which then correlate with severity of psychotic symptoms that are specifically assessed by clinical evaluations. Perhaps it is that the more rudimentary physical sensory processing changes attributable to posterior insular volume changes are not assessed by clinical questionnaires. Changes in insular morphometry and consistent correlations between insular volume and psychotic symptom severity across disorders with psychotic features, and now a correlation with positive symptoms specifically, suggest that anterior and posterior insula significantly interact to contribute to psychotic symptom manifestation.
The current study was the first to examine insular morphometry in only unipolar depression with and without psychotic features. Changes in insular morphometry have been found in schizophrenia (Crespo-Facorro et al., 2000; Pressler et al., 2005; Goldstein et al., 1999), affective psychosis (Morgan et al., 2007; Kubicki et al., 2002; Kasai et al., 2003), and now PMD. Despite differences in the overall symptom complexes of these disorders, the common component of each disorder is psychosis. The current data significantly adds to the already existing literature concerning insular involvement in psychotic symptoms. Specifically, the current data shows unique anterior and posterior insular anomaly in PMD with posterior insular volume reduction, and a correlation between total and positive symptom severity and anterior insular volume. Further investigation into how changes in anterior and posterior insular regions interact to contribute to specific psychotic symptoms is warranted in both men and women across different populations with psychosis.
Highlights.
Insular sub-regional volumes were measured in psychotic major depression.
Posterior insular volumes were reduced in PMD compared to healthy controls.
Depressive symptoms did not correlate with any insular region-of-interest.
Positive and overall psychotic symptoms correlated with left anterior insula.
Changes in anterior and posterior insular morphometry distinguish PMD from non PMD.
Acknowledgments
This study was performed at Stanford University School of Medicine, Stanford, California with the following grant support from the National Institutes of Health: NIH 5 T32 MH019908 and R01MH050604. This study was also supported by grants from the Pritzker Foundation, National Alliance for Research on Schizophrenia and Depression, and grant RR-00070 from the NIH General Clinical Research Center Program.
Footnotes
Financial Disclosure
Jeremy Cohen, Taylor Nichols and Allan Reiss do not have any conflicts of interest pertaining to this study. Dr. Cohen received funding from NIH 5 T32 MH019908. Drs. Schatzberg and Reiss received funding from R01MH050604 (AFS PI). This study was also supported by grants from the Pritzker Foundation, NARSAD, and grant RR-00070 from the NIH General Clinical Research Center Program. These sponsors have no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Although the current study has no treatment component, the study has implications for treatment that can pose as a conflict of interest. The following conflicts of interest for authors for the original study include: Alan F. Schatzberg, M.D. is a cofounder and owns shares in pharmacology company Corcept Therapeutics. Jennifer Keller, Ph.D. and Rowena G. Gomez, Ph.D have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: The University of Iowa; 1984. [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, Amador X, Malaspina D, Gorman JM. Word recognition, discrimination accuracy, and decision bias in schizophrenia: association with positive symptomatology and depressive symptomatology. J Nerv Ment Dis. 1998;186:604–609. doi: 10.1097/00005053-199810000-00003. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Grafman J, Hallett M. Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci. 2001;21:300–304. doi: 10.1523/JNEUROSCI.21-01-00300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cerebral Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Chen S, Li L, Xu B, Liu J. Insular cortex involvement in declarative memory deficits in patients with post-traumatic stress disorder. BMC Psychiatry. 2009;9:39. doi: 10.1186/1471-244X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Mock JR, Nichols T, Zadina J, Corey DM, Lemen L, Bellugi U, Gallaburda A, Reiss AL, Foundas AL. Morphometry of human insular cortex and insular volume reduction in Williams syndrome. J Psychiatry Res. 2009;44:81–89. doi: 10.1016/j.jpsychires.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL. Insular volume reduction in fragile X syndrome. Int J Devl Neuroscience. 2011;29:489–494. doi: 10.1016/j.ijdevneu.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. NeuroImage. 2003;18:324–333. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York: New York State Psychiatric Institute, Biometrics Research; 1997. Patient Edition (SCID-I/P) [Google Scholar]
- Foundas AL, Eure KF, Seltzer B. Conventional MRI volumetric measures of parietal and insular cortex in Alzheimer’s disease. Prog Neuropsychopharm Biol Psychiatry. 1996;20:1131–1144. doi: 10.1016/s0278-5846(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Frith C, Dolan RJ. Brain mechanisms associated with top-down processes in perception. Philos Trans R Soc Lond B Biol Sci. 1997;352:1221–1230. doi: 10.1098/rstb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, Solvason B, Schatzberg AF. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry. 2006;60:472–478. doi: 10.1016/j.biopsych.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153:490–496. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the Functioning of One’s Own Limbs Mediated by the Insular Cortex? J Neurosci. 2005;25(31):7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelin-Todd D, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, et al. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss AL, Schatzberg AF. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165:872–880. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim BL, Sohn SE, Lim SW, Na DG, Paik CH, et al. Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharm Biol Psychiatry. 1999;23:793–807. doi: 10.1016/s0278-5846(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. NeuroImage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Antipsychotic Medications and Brain Volume Do We Have Cause for Concern? Arch Gen Psychiatry. 2011;68(2):126–127. doi: 10.1001/archgenpsychiatry.2010.187. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RSE, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic Drug Effects on Brain Morphology in First-Episode Psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;40(9):1409–1422. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Orr KG, Hutchinson G, Chitnis X, Suckling J, et al. Grey matter abnormalities in first-episode schizophrenia and affective psychosis. Brit J Psychiatry Suppl. 2007;51:s111–116. doi: 10.1192/bjp.191.51.s111. [DOI] [PubMed] [Google Scholar]
- Nelson EB, Sax KW, Strakowski SM. Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry. 1998;155:137–139. doi: 10.1176/ajp.155.1.137. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DE. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pressler M, Nopoulos P, Ho BC, Andreasen NC. Insular cortex abnormalities in schizophrenia: Relationship to symptoms and typical neuroleptic exposure. Biol Psychiatry. 2005;57:394–398. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, et al. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry. 2000;157:1095–1100. doi: 10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Rothschild AJ. Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry. 1992;149:733–745. doi: 10.1176/ajp.149.6.733. [DOI] [PubMed] [Google Scholar]
- Skaf CR, Yamada A, Garrido GE, Buchpiguel CA, Akamine S, Castro CC, et al. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. J Affect Disord. 2002;68:295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Diederen KM, Blom JD, Willems A, Kushan L, Slotema K, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;13(12):3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yucel M, Lorenzetti V, Tanino R, Whittle S, Suzuki M, Walterfang M, Pantelis C, Allen NB. Volumetric MRI study of the insular cortex in individuals with current and past major depression. J Affect Disord. 2010;121(3):231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Thase ME, Hersen M, Bellack AS, Himmelhoch JM, Kupfer DJ. Validation of a Hamilton subscale for endogenomorphic depression. J Affect Disord. 1983;5:267–278. doi: 10.1016/0165-0327(83)90050-2. [DOI] [PubMed] [Google Scholar]