Introduction
Posttraumatic osteoarthritis (PTOA) represents end-stage organ level failure of an injured joint, typically occurring after an intraarticular fracture (IAF). The initial impact to the cartilage, combined with the ensuing pathomechanical and pathobiological response of the cartilage, lead to PTOA. However, the relative contribution of acute mechanical damage (AMD) versus exposure to chronic abnormal loading leading to joint degeneration is unknown. It is plausible that AMD and chronic abnormal loading processes potentiate each other, with initial impact energy acting as a determinant of how poorly the cartilage tolerates long-term mechanical changes. Alternatively, under certain circumstances either AMD or chronic abnormal loading may predominate in the process of cartilage degeneration. This may be apparent as some joint injuries progress rapidly to PTOA. 1,2, whereas others slowly degenerate. 3,4,5
Current treatment for IAFs is primarily focused on restoring joint surface congruity to avoid chronically elevated articular surface contact stresses and/or instability. 4,6,7 Historically, the overwhelming emphasis placed on surgical restoration of articular surface congruity has led the majority of authorities to recommend invasive approaches to reduce and stabilize these injuries.4,6,8 Unfortunately, such aggressive operations, especially in acutely injured limbs, can cause devastating complications. This has led other authorities to recommend minimally invasive operations for intra-articular fractures. 9-12
Clinical decisions regarding interventions, including surgical approach, reduction strategies, internal fixation, and rehabilitation should be based on solid clinical evidence. Unfortunately, clinical evidence guiding treatment choices for IAFs is inconsistent. In fact, clinical evidence provides few rational answers and raises more questions in regard to treating IAFs. Questions such as to what degree does an articular surface need to be reduced, how can we accurately assess the amount of displacement after reduction and internal fixation, and how do you account for difficult-to-measure biologic differences between individuals with seemingly similar injuries remain unanswered.
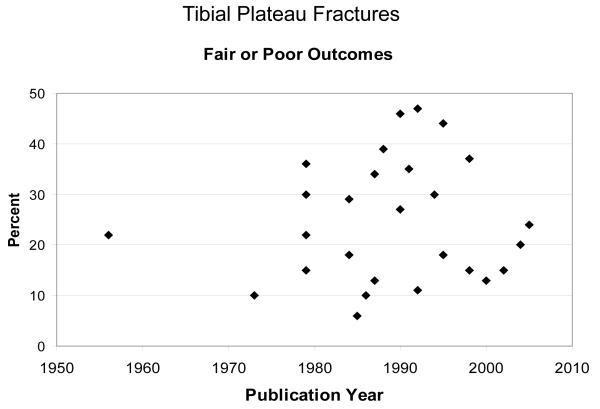
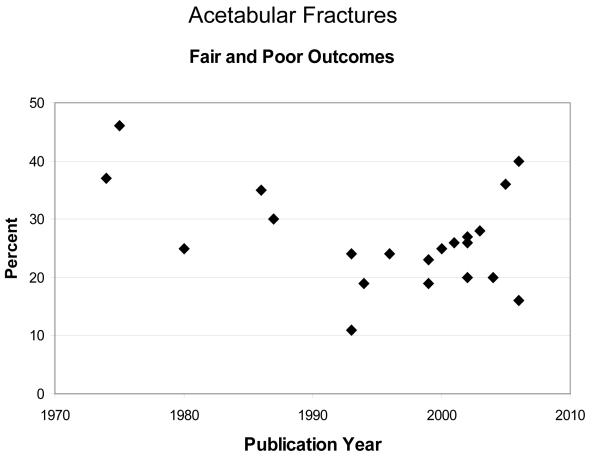
Joints and regions within the same joint respond differently to IAFs. 13,14,15,16 Clinical evidence shows that acetabular fracture outcomes are consistently correlated to accuracy of reduction. 4,8 Compared to the acetabulum, incongruity is well-tolerated in tibial plateau fractures. 17,18 There is little support for articular surface reduction to be within 2 mm. Authors have hypothesized that the relatively thick cartilage in this joint accounts for its tolerance of incongruity, but this is unproven.Because of clinical inconsistencies, optimal treatment of intraarticular fractures remains a hotly debated topic among orthopaedic trauma specialists. However, the arguments are based largely on anecdotal observation and intuition, rather than on rigorous scientific evidence. More importantly, the incidence of PTOA has changed very little over the past several decades, regardless of treatment specifics, suggesting the need for new treatment paradigms (Figure 1 and Figure 2).
Figure 1.
Percent fair and poor outcomes from tibial plateau fractures over approximately 50 years. Outcomes have changed little over a five decade span.
Figure 2.
Percent fair and poor outcomes from acetabular fractures over approximately 30 years. Outcomes have stagnated, especially over the past 20 years.
A large body of scientific evidence has emerged demonstrating that the effects of AMD (chondrocyte necrosis, chondrocyte apoptosis, chondrocyte biosynthetic dysfunction) play an important, and possibly a dominant role in the etiology of PTOA. 19-24 Therefore, it is possible that the most effective treatment to prevent PTOA would be to mitigate or arrest the injurious effects of AMD. Such a strategy is certainly not novel. In stark contrast to treatment of IAFs, clinical outcomes of acute myocardial infarction and ischemic stroke have improved dramatically during the last several decades, with evolution of treatments directed at the acute damage to myocardial or neuronal tissue in the acute setting. 25,26 Minimizing the acute injury at the tissue, cellular, and molecular level has been met with tremendous success in these patients.
Recent investigations have made significant progress in understanding PTOA by creating viable survival models of IAFs, understanding cellular level pathologic changes that occur after an impact injury, and by investigating promising interventions that prevent acute chondrocyte death after an impact. This manuscript summarizes recent basic scientific evidence presented at the 2009 Orthopaedic Trauma Association Basic Scientific Symposia on articular fractures.
IAF Models
An ideal model to study IAFs would be a survival IAF animal model created by a physiologic realistic impact delivered across the joint surfaces. Furthermore, the model would be conducive to surgical and pharmaceutical intervention. Historically, survival IAF models have not been created with a physiologic impact. 27,28 Recently, Backus and colleagues and Tochigi and colleagues have investigated chondrocyte death in IAFs created with realistic impulses in explanted whole joints from cows, pigs, and humans. 29,30 Both authors reported that the majority of chondrocyte death occurred in proximity to the fracture lines in all three species. Backus and colleagues devised methods to impact explanted bovine knees. 29 Specimens sustained an IAF if the load was offset from the midline of the knee through the lateral tibial plateau. In contrast, specimens with identical impact energy delivered into the center portion of the knee did not fracture. They showed that fractured specimens had significantly decreased chondrocyte viability compared to unfractured specimens impacted with the same amount of energy. The authors concluded that supraphysiologic cartilage strains associated with actual fracturing of the cartilage were responsible for the increased chondrocyte death. Tochigi and colleagues had essentially identical results in human ankle joints with chondrocyte death concentrated along fracture lines. 30
Furman and colleagues have created the first in vivo IAF model fractured using physiologic impact. 31 The authors have developed techniques to deliver a fracture-level impact through the knee creating tibial plateau fractures. The model serves as means of inducing PTOA in the pursuit of identifying the mechanisms by which arthritis progresses following articular fracture. By producing clinically relevant articular fractures without disrupting the capsule, the natural history of healing and progression of PTOA can be studied. Using their mouse IAF model, these investigators have investigated the role of fracture-associated inflammatory mediators in the development of cartilage degeneration after an IAF using mice with genetic variance. Following articular fracture, C57BL/6 mice showed significant signs of PTOA, including loss of bone density, increase in subchondral bone thickness, increased cartilage degeneration 31 and increased levels of biomarkers of cartilage turnover. 32 In contrast, the MRL/MpJ strain of mice demonstrated significantly decreased cartilage degeneration following fracture. 33 Interestingly, the MRL/MpJ strain has been shown to produce decreased levels of pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) following LPS challenge of peritoneal macrophages. 34,35
Chondrocyte Death after Cartilage Impact
AMD is one of the most significant factors leading to PTOA. Treatment modalities are limited and have not provided consistent long-term results. An early consequence of cartilage injury is chondrocyte death. 21,23,24,36 In the longer term, cell death is associated with matrix degradation in osteoarthritis. 37 Mechanistic links are highly likely, with matrix degradation resulting in loss of survival mechanisms and cell death contributing to matrix degradation 38 and calcification. 37 Researchers have consistently shown that chondrocyte death after impact is concentrated along cartilage matrix cracks and in the superficial layer of cartilage. 29,30,36
Impacted chondrocytes die by either necrosis or apoptosis. Understanding the mode of cell death is significant in developing treatments for AMD. Apoptosis, or programmed cell death, can be mitigated by several bioactive agents. A series of studies examined the potential of preventing apoptotic chondrocyte death as a means of mitigating PTOA after an impact injury. Several in vitro and in vivo models of acute cartilage injury established that chondrocytes undergo apoptosis in response to mechanical injury. 19,20,39,40,41 Apoptosis is mediated by a cascade of aspartate-specific cysteine proteases or caspases. 42 Caspase inhibition was effective in preventing apoptosis in vitro: in bovine and human cartilage explants and in chondrocytes. 41 These results were repeated in a live model of OA in which apoptosis was reduced in chondrocytes treated with caspase inhibition in anterior cruciate ligament transected knees. 39
Recently, Martin and colleagues have shown that chondrocytes in impacted bovine explants died primarily by necrosis. 22 Sixty-five percent of chondrocytes in these specimens died in the first twelve hours after impact. Minimal subsequent cell death occurred over the subsequent two days. In impacted specimens treated with N acetylcysteine, a potent scavenger of reactive oxidant species (ROS), within four hours of impact 48 hour chondrocyte viability increased to 70-80%. Specimens receiving treatment twelve hours after impact had no improvements in viability. The authors concluded that chondrocyte death occurred secondary to overproduction of ROS. Furthermore, they hypothesized that the chondrocyte mitochondria were the most likely source of the damaging acute impact-related ROS.
Subsequently, the investigators impacted bovine specimens treated with rotenone, an irreversible inhibitor of electron transport in the mitochondria. 43 The premise of the experiment was that impact-related mitochondrial ROS production could be mitigated by blocking electron transport. Specimens receiving rotenone had a 3X reduction in injury-induced ROS production compared to untreated specimens. Furthermore, chondrocyte viability in treated specimens was 80% compared to 35% in untreated specimens 48 hours after impact. These data clearly implicate chondrocyte mitochondria in impact-related chondrocyte necrosis after an impact injury.
Discussion
PTOA is a devastating complication of IAFs which is estimated to cost 12 billion dollars annually. 44 Seventy-six percent of IAFs occur in patients less than 45 years old, 45 and patients who developed PTOA were 9 to 14 years younger than patients who developed primary OA in the lower extremity. 44 Unfortunately, reconstructive options, including arthroplasty and arthrodesis, fare poorly in younger patients. Therefore, the specific patient population that is most affected by IAFs is the population that is most devastated by PTOA. The incidence of PTOA is substantial. In patients sustaining acetabular fractures, approximately 20-25% develop PTOA. 4,8 Studies have shown that between 23% and 44% of patients develop PTOA after IAFs of the knee. 3,5 Likewise, more than 50% of patients with fractures of the distal tibial articular surface develop PTOA. 1,2 Interestingly, while surgical techniques have improved, the basic surgical premise has remained unchanged, focusing on restoring joint surface congruity, with no attention directed toward treatment of the AMD sustained by the cartilage. Furthermore, the outcomes of IAFs have changed little over decades (Figures 1 and 2).
PTOA is an organ-level disease incited by impact-associated tissue, cellular, and molecular level damage. Therefore, to account for all levels of injury, it is necessary to understand the pathoetiology of PTOA at the organ level. This emphasizes the need to create a realistic survival intraarticular fracture model. The IAF mouse model described by Furman represents an important technical breakthrough in studying PTOA at the organ level. 31 This model accounts for injury effects for all intraarticular tissue. However, the major drawback to the mouse model is the size of the animal. It is difficult to apply standard surgical fixation to such an animal, making it difficult to study the effects of reduction and fixation on PTOA.
The findings of both D’Lima and colleagues and Martin and colleagues demonstrate that AMD causes chondrocyte death. 19,20,22,39,40,41 Two different modes of chondrocyte death were demonstrated with cells dying by both necrosis and apoptosis. Interestingly, mitochondrial dysfunction plays a central role in both apoptotic and necrotic cell death. Apoptotic cell death is enacted by a series of reactions culminating in activation of cytosolic caspases. However, the cytosolic caspase cascades leading to apoptosis are initiated by several mitochondrial-derived molecules including cytochrome c and apoptosis initiating factor. Injury leads to mitochondrial depolarization which allows cytochrome c and apoptosis initiating factor to leak into the cytoplasm and initiate apoptosis. These observations are supported by the work of Huser and Davies which demonstrated that impacting equine cartilage led to mitochondrial depolarization over a three to six hour period. 46 Subsequently, at 48 hours, impacted chondrocytes had increased apoptosis. Furthermore, they showed that blocking mitochondrial depolarization or by blocking caspase enzymes significantly improved chondrocyte viability. In the studies by Martin and colleagues, the data clearly implicate mitochondrial-originated ROS as the damaging molecules leading to necrotic chondrocyte death. 22 Stopping electron transport significantly reduced ROS production and increased chondrocyte survival. 43 These findings illustrate an exciting cellular level opportunity to prevent impact-related AMD from leading to PTOA. In summary, impact-related mitochondrial dysfunction appears to play a central role in both apoptotic and necrotic chondrocyte death.
In conclusion, mechanisms linking IAFs to PTOA are beginning to be understood at the cellular and molecular level. Organ-level survival models provide optimal opportunity to investigate pathoetiologic processes and therapeutic interventions for IAFs. Developing cellular and molecular level therapeutic interventions will be necessary to improve the current stagnant outcomes of IAFs.
Acknowledgements
This work has been funded by the following grants: NIAMS 1RC2AR058954-01; NIH P50 AR055533; NIH AR50245
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Todd O. McKinley, University of Iowa.
Joseph Borrelli, Jr., University of Texas Southwestern.
Darryl D. D’Lima, Scripps Research Institute.
Bridgette D. Furman, Duke University.
Peter V. Giannoudis, University of Leeds, UK.
References
- 1.Ovadia DN, Beals RK. Fractures of the tibial plafond. J Bone Joint Surg Am. 1986;68:543–551. [PubMed] [Google Scholar]
- 2.Teeny SM, Wiss DA. Open reduction and internal fixation of tibial plafond fractures. Variables contributing to poor results and complications. Clin Orthop Relat Res. 1993:108–117. [PubMed] [Google Scholar]
- 3.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9:273–277. doi: 10.1097/00005131-199509040-00001. [DOI] [PubMed] [Google Scholar]
- 4.Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78:1632–1645. [PubMed] [Google Scholar]
- 5.Volpin G, Dowd GS, Stein H, et al. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72:634–638. doi: 10.1302/0301-620X.72B4.2380219. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli J, Jr., Catalano L. Open reduction and internal fixation of pilon fractures. J Orthop Trauma. 1999;13:573–582. doi: 10.1097/00005131-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Borrelli J, Jr., Goldfarb C, Ricci W, et al. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16:73–81. doi: 10.1097/00005131-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980:81–106. [PubMed] [Google Scholar]
- 9.Marsh JL, Bonar S, Nepola JV, et al. Use of an articulated external fixator for fractures of the tibial plafond. J Bone Joint Surg Am. 1995;77:1498–1509. doi: 10.2106/00004623-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77:661–673. doi: 10.2106/00004623-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Starr AJ, Jones AL, Reinert CM, et al. Preliminary results and complications following limited open reduction and percutaneous screw fixation of displaced fractures of the acetabulum. Injury. 2001;32(Suppl 1):SA45–50. doi: 10.1016/s0020-1383(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 12.Starr AJ, Reinert CM, Jones AL. Percutaneous fixation of the columns of the acetabulum: a new technique. J Orthop Trauma. 1998;12:51–58. doi: 10.1097/00005131-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick DC, Otto JK, McKinley TO, et al. Kinematic and contact stress analysis of posterior malleolus fractures of the ankle. J Orthop Trauma. 2004;18:271–278. doi: 10.1097/00005131-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 14.McKinley TO, Bay BK. Trabecular bone strain changes associated with cartilage defects in the proximal and distal tibia. J Orthop Res. 2001;19:906–913. doi: 10.1016/S0736-0266(01)00011-0. [DOI] [PubMed] [Google Scholar]
- 15.McKinley TO, Rudert MJ, Tochigi Y, et al. Incongruity-dependent changes of contact stress rates in human cadaveric ankles. J Orthop Trauma. 2006;20:732–738. doi: 10.1097/01.bot.0000211150.00919.0e. [DOI] [PubMed] [Google Scholar]
- 16.Saterbak AM, Marsh JL, Nepola JV, et al. Clinical failure after posterior wall acetabular fractures: the influence of initial fracture patterns. J Orthop Trauma. 2000;14:230–237. doi: 10.1097/00005131-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Lansinger O, Bergman B, Korner L, et al. Tibial condylar fractures. A twenty-year follow-up. J Bone Joint Surg [Am] 1986;68:13–19. [PubMed] [Google Scholar]
- 18.Stevens DG, Beharry R, McKee MD, et al. The long-term functional outcome of operatively treated tibial plateau fractures. J Orthop Trauma. 2001;15:312–320. doi: 10.1097/00005131-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 19.D’Lima DD, Hashimoto S, Chen PC, et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 20.D’Lima DD, Hashimoto S, Chen PC, et al. Impact of mechanical trauma on matrix and cells. Clin Orthop Relat Res. 2001:S90–99. doi: 10.1097/00003086-200110001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 22.Martin JA, McCabe D, Walter M, et al. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91:1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morel V, Quinn TM. Short-term changes in cell and matrix damage following mechanical injury of articular cartilage explants and modelling of microphysical mediators. Biorheology. 2004;41:509–519. [PubMed] [Google Scholar]
- 24.Quinn TM, Allen RG, Schalet BJ, et al. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 25.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 26.Wardlaw JM, Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000213. CD000213. [DOI] [PubMed] [Google Scholar]
- 27.Llinas A, McKellop HA, Marshall GJ, et al. Healing and remodeling of articular incongruities in a rabbit fracture model. J Bone Joint Surg Am. 1993;75:1508–1523. doi: 10.2106/00004623-199310000-00012. [DOI] [PubMed] [Google Scholar]; caspase inhibitors and IGF-1. J Orthop Res. 2004;22:140–144. doi: 10.1016/S0736-0266(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell N, Shepard N. Healing of articular cartilage in intra-articular fractures in rabbits. J Bone Joint Surg Am. 1980;62:628–634. [PubMed] [Google Scholar]
- 29.Backus JD, Furman BF, DeFrate LE, et al. Chondrocyte Viability following Traumatic Joint Disruption in a Novel Closed Intraarticular Fracture Model of the Porcine Knee. Orthopaedic Trauma Association Annual Meeting Basic Science Focus Forum; San Diego. 2009. [Google Scholar]
- 30.Tochigi Y, Buckwalter JA, Zhang P, et al. Acute-phase aggravation of chondrocyte death in human ankle intraarticular fractures. Orthopaedic Trauma Association 25th Annual Meeting, Basic Science Focus Forum; San Diego. 2009. [Google Scholar]
- 31.Furman BD, Strand J, Hembree WC, et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 32.Seifer DR, Furman BD, Guilak F, et al. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:1532–1538. doi: 10.1016/j.joca.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward BD, Furman BD, Huebner JL, et al. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis and rheumatism. 2008;58:744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 34.Kench JA, Russell DM, Fadok VA, et al. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clin Immunol. 1999;92:300–310. doi: 10.1006/clim.1999.4754. [DOI] [PubMed] [Google Scholar]
- 35.Furman BD, Huebner JL, Seifer DR, et al. MRL/MpJ Mouse Shows Reduced IntraArticular and Systemic Inflammation Following Articular Fracture. Orthopaedic Research Society; Las Vegas: 2009. [Google Scholar]
- 36.Lewis JL, Deloria LB, Oyen-Tiesma M, et al. Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21:881–887. doi: 10.1016/S0736-0266(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto S, Takahashi K, Amiel D, et al. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis and rheumatism. 1998;41:1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Lo MY, Kim HT. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. J Orthop Res. 2004;22:140–144. doi: 10.1016/S0736-0266(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 39.D’Lima DD, Hashimoto S, Chen PC, et al. Prevention of chondrocyte apoptosis. J Bone Joint Surg Am. 2001;83-A(Suppl 2):25–26. doi: 10.2106/00004623-200100021-00006. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto S, Chen PC, et al. In vitro and in vivo models of cartilage injury. J Bone Joint Surg Am. 2001;83-A(Suppl 2):22–24. doi: 10.2106/00004623-200100021-00005. [DOI] [PubMed] [Google Scholar]
- 41.D’Lima DD, Hashimoto S, Chen PC, et al. Cartilage injury induces chondrocyte apoptosis. J Bone Joint Surg Am. 2001;83-A(Suppl 2):19–21. doi: 10.2106/00004623-200100021-00004. [DOI] [PubMed] [Google Scholar]
- 42.Rudel T. Caspase inhibitors in prevention of apoptosis. Herz. 1999;24:236–241. doi: 10.1007/BF03044967. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin W, McCabe D, Sauter E, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. doi: 10.1002/jor.21091. Epub Jan 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 45.Praemer AP, Furner S, Rice DP. Musculoskeletal Conditions in the United States. American Academy of Orthopaedic Surgeons; Park Ridge, Illinois: 1999. [Google Scholar]
- 46.Huser CA, Davies ME. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis and rheumatism. 2007;56:2322–2334. doi: 10.1002/art.22717. [DOI] [PubMed] [Google Scholar]