Abstract
BACKGROUND & AIMS
Although widely used, little information exists on the validity of using hospital administrative data to code acute pancreatitis (AP). We sought to determine if discharge diagnosis codes accurately identify patients whose clinical course met the standard for AP diagnosis.
METHODS
We analyzed data from 401 unique patients admitted through the emergency department who received a primary inpatient discharge diagnosis of AP at 2 University of Pittsburgh Medical Center hospitals in the years 2000, 2002, and 2005. Each patient was matched with a control patient who was admitted with abdominal pain and then discharged without a diagnosis of AP. Patients were matched based on demographics, testing for serum levels of pancreatic enzymes, year of visit to the emergency department, admission to the intensive care unit, and performance of abdominal computed tomography scan. The standard used to diagnose AP was the presence of 2 of 3 features (abdominal pain, ≥3-fold increase in serum levels of pancreatic enzymes, and positive results from imaging analysis).
RESULTS
The median age of AP cases was 53 years (interquartile range, 41.5–67 years); 47.1% were male, 85% were white. The most common etiologies were biliary (33.4%), alcohol-associated (16.2%), and idiopathic (24.2%). Serum levels of pancreatic enzymes were increased by any amount, and by ≥3-fold, in 95.3% and 68.6% of patients diagnosed with AP and in 16.2% and 2.2% of controls, respectively. The standard for diagnosis of AP was met in 80% of cases diagnosed with this disorder; they had no history of pancreatitis. The sensitivity, specificity, and positive and negative predictive values of the AP diagnosis code were 96%, 85%, 80%, and 98%, respectively.
CONCLUSIONS
Approximately 1 of 5 patients diagnosed with AP upon discharge from the hospital do not meet the guidelines for diagnosis of this disorder. Efforts should be made to more consistently use guidelines for AP diagnosis.
Keywords: Amylase, Lipase, Validation, Misdiagnosis
Acute pancreatitis (AP) typically presents with sudden onset of upper abdominal pain, which is often associated with nausea and vomiting. Practice guidelines from the gastroenterology societies recommend that a diagnosis of AP be made if 2 of the following 3 features are present: characteristic upper abdominal pain, elevation of serum pancreatic enzymes (amylase, lipase, or both) to 3 or more times the upper limit of normal, and imaging evidence of AP.1,2 However, it is unclear how often hospital billing codes for AP reflect patients who meet these criteria.
While imaging features of AP are specific, serum pancreatic enzymes are elevated in numerous nonpancreatic conditions,3 though usually less than 3 times the upper limit of normal. Conversely, these enzymes may be normal or only minimally elevated in the setting of true AP in cases with hypertriglyceridemia, delayed presentation, or acute exacerbation of established chronic pancreatitis (CP).3
Several recent epidemiologic studies,4-10 many using only administrative data, report 2 consistent observations: the increasing incidence and decreasing mortality rate of AP.9 Other studies have used administrative data to develop criteria for early prediction of disease severity.11,12 Unfortunately, few data confirm the validity of the billing codes used for AP, such as how often patients who receive a diagnosis of AP actually fulfill the established guidelines. We recently reported that the rate of serum pancreatic enzyme testing in the emergency department (ED) of 2 University of Pittsburgh Medical Center (UPMC) hospitals increased significantly over the period studied (1996 – 2005) and correlated highly with the number of unique patients who then received an inpatient primary discharge diagnosis of AP.13 Because we relied solely on hospital discharge diagnosis, we could not validate the diagnosis of AP, which led us to design the current study. Our aim was to determine if hospital discharge diagnosis accurately captured all patients whose clinical course met the gold standard for diagnosing AP. We purposely chose 2 hospital settings (university- and community-based) to determine whether the assigned discharge code is affected by practice settings and, based on the changes observed over a decade, 3 distinct years to identify any fluctuations over time.
Methods
Setting
This retrospective study was approved by the University of Pittsburgh Institutional Review Board and was conducted at 2 UPMC hospitals: UPMC-Presbyterian (PUH) and UPMC-St Margaret (SMH). PUH is an 800-bed, level 1, fully accredited regional trauma center, and SMH is a 250-bed, acute care community hospital in the UPMC system. While PUH, the primary teaching hospital of the University of Pittsburgh School of Medicine, trains residents and fellows in a wide variety of medical specialties and subspecialties, SMH trains only Family Practice residents.
Data Source
We used the Medical Archival Retrieval System (MARS; Medical Archival Retrieval System, Inc, Pittsburgh, PA), a repository for information derived from UPMC’s clinical, administrative, and financial computer databases. MARS captures all patient data from inpatient, outpatient, and ED visits, and the data are indexed on every word that is recorded.14 MARS allows identification of patients of interest using a single or combination of search criteria (eg, billing codes, diagnosis, laboratory abnormality, year, results of a test, etc). Data can be retrieved directly into a spreadsheet (eg, result of laboratory tests) or as text reports (eg, admission or discharge summaries, radiology, pathology reports) that can be reviewed to abstract the desired information. The data can be and were deidentified (using De-ID Software, University of Pittsburgh) and obtained with waiver of informed consent by an honest broker authorized by the Institutional Review Board.
Selection of AP Cases and Controls
As described previously,13 we used MARS to identify all patients who visited the ED at PUH or at SMH between years 1996 and 2005; who underwent serum pancreatic enzyme testing (amylase, lipase, or both enzymes) in the ED; and who were discharged following a hospital admission with a primary diagnosis of AP (based on the International Classification of Diseases, 9th revision [ICD-9] 577.0). To restrict our cohort to unique patients with their first inpatient admission for a primary diagnosis of AP, we excluded patients who had received a prior primary or secondary diagnosis code for AP or concurrent diagnosis code for CP (ICD-9 577.1). We further restricted the study cohort to those who received a discharge diagnosis in the years 2000, 2002, or 2005. We reviewed deidentified medical records from the index hospitalization for all patients to assess for documentation of a prior diagnosis of pancreatitis that may not have been captured by administrative data.
We chose controls from patients who were evaluated at the ED of PUH and SMH for chief complaint of abdominal pain in 2000, 2002, and 2005 who underwent serum pancreatic enzyme testing (amylase, lipase, or both) in the ED; who were admitted to the hospital and discharged without a primary or secondary diagnosis of AP or CP; and had no prior diagnosis of pancreatitis. For each patient, we matched the closest possible control between the 2 hospitals by age, sex, race, year of inpatient admission, intensive care unit (ICU) admission, and performance of an abdominal computed tomography (CT) scan during the relevant hospital admission.
Chart Review and Gold Standard for AP Diagnosis
Deidentified medical records for all cases and controls were reviewed to collect information on demographics, clinical presentation, laboratory tests, etiology (for AP cases) and final discharge diagnosis (for controls), imaging findings, ICU admission, length of hospital stay, and outcome. “Gold-standard” used for the AP diagnosis was the presence of 2 of 3 features—abdominal pain, ≥3 times the upper limit of normal elevation of serum pancreatic enzyme(s) or positive imaging evidence of pancreatitis (defined by presence of pancreatic enlargement, inflammatory changes in the pancreas/peripancreatic area, pancreatic necrosis, or presence of complications, ie, fluid collections, etc).
Data Analysis
Descriptive analyses are presented as proportions for categorical data, and as median and interquartile range (IQR) for continuous data. Univariate analysis for categorical data were performed using the χ2 or Fisher’s exact test and, for continuous variables, using Mann–Whitney U test. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for AP diagnosis code were calculated using the gold standard definition for AP. Data analysis was performed using SPSS software version 19 (SPSS, Inc, Chicago, IL). Two-tailed P values <.05 were considered significant.
Results
Demographics, Etiology, ICU Admission, and CT Scan
Of the 401 cases, 214 were admitted to the academic hospital (PUH) and 187 to the community hospital (SMH). As shown in Table 1, controls and AP cases were similar in demographics, need for ICU admission, whether an abdominal CT scan was performed during admission, and overall mortality. While there were no significant differences between pooled cases and controls, those from PUH were younger (median age 51 vs 58.5 years, P < .001) and less likely to be white (74.8% vs 96.6%, P < .001) compared with SMH, while the sex distribution was similar (47.4% vs 45.5%, P = .62) at the 2 hospitals. The most common etiologies for AP included gallstones, idiopathic, and alcohol. The proportion of patients who had gallstone (24.8% vs 43.3%, P < .001) or idiopathic AP (19.6% vs 29.4%, P = .02) was lower at PUH compared with SMH, while those with other etiologies (24.7% vs 2.1%, P < .001) were higher.
Table 1.
Demographics and Selected Characteristics of Acute Pancreatitis Cases and Controls
| Controls (n = 401) | Acute pancreatitis cases (n = 401) | |
|---|---|---|
| Hospital, n (%) | ||
| UPMC-PUH | 210 (52.4) | 214 (53.4) |
| UPMC-SMH | 191 (47.6) | 187 (46.6) |
| Year of admission, n (%) | ||
| 2000 | 94 (23.4) | 94 (23.4) |
| 2002 | 113 (28.2) | 116 (28.9) |
| 2005 | 194 (48.4) | 191 (47.6) |
| Age in years, median (IQR) | 55 (41–72.5) | 53 (41.5–67) |
| Male sex, n (%) | 184 (45.9) | 189 (47.1) |
| White, n (%) | 341 (85) | 341 (85) |
| Etiology, n (%) | See Supplementary Table 1 | |
| Biliary | 134 (33.4) | |
| Alcohol | 65 (16.2) | |
| Alcohol and gallstones | 5 (1.2) | |
| Idiopathic | 98 (24.4) | |
| Medications | 25 (6.2) | |
| Hypertriglyceridemia | 12 (3.0) | |
| Others | 62 (15.4) | |
| ICU admission, n (%) | 28 (7.0) | 24 (6.0) |
| CT scan of abdomen done, n (%) | 274 (68.3) | 264 (65.8) |
| Length of stay, median (IQR) | 5 (3–7)a | 4 (3–7) |
| In-hospital mortality, n (%) | 6 (1.5)b | 1 (0.2) |
| Prior history of pancreatitis, n (%) | — | 57 (14.2) |
NOTE. All comparisons were nonsignificant, except length of stay and mortality.
P = .02.
P = .06.
The majority of AP patients (355; 88.5%) underwent a CT scan (at PUH, 61.7%; SMH, 70.6%; P = .07) or ultrasound (PUH, 54.2%; SMH, 77.5%; P < .001) of the abdomen. Twenty-four patients (6%) and 28 controls (7%) were admitted to the ICU, and 17 patients (4.2%) experienced organ failure during the course of hospitalization. The median length of stay among AP patients was 4 days and only 1 patient died during the hospitalization.
On manual review of the medical records, a prior history of AP or CP was mentioned in 14.2% (57/401) cases (35 AP, 7 CP, 15 both AP and CP) with no difference between the 2 hospitals. Of these 57 patients, 50 were hospitalized for AP in the preceding year at a UPMC hospital, while the other 7 received pancreatitis diagnosis at other institutions. Our algorithm for patient selection did not identify these patients as they did not meet our inclusion criteria (ie, they were either admitted directly to the hospital as transfers or direct admits) or their pancreatic enzyme estimations were not performed in the ED.
Final diagnoses for controls were generally similar at the 2 hospitals (see Supplementary Table 1). Three control subjects were assigned a pancreas-related final diagnosis: pancreatic duct stone, pancreatic cancer, and pseudocyst.
Serum Pancreatic Enzyme Levels in Controls
Among controls, 36 (9%) had serum amylase only, 99 (24.7%) had lipase only, and 266 (66.3%) had both amylase and lipase measured. Elevation of any pancreatic enzyme was seen in 65 (16.2%) controls (Table 2); only 9 (13.8%) of these had levels ≥3 times the upper limit of normal. The probability of a control to have any elevation of pancreatic enzymes was significantly higher at PUH (23.3% vs 8.4% at SMH, P < .001) and in black patients (28.3% vs 14.1% in white patients; P = .01) but was similar by sex (19.6% in male vs 13.4% in female; P = .12).
Table 2.
Serum Pancreatic Enzyme Levels in Acute Pancreatitis Cases and Controls
| Normal | One to 2 times above normal | Two to <3 times above normal | Three or more times above normal | |
|---|---|---|---|---|
| Acute pancreatitis cases | ||||
| Both UPMC hospitals (n = 401) | 19 (4.7) | 61 (15.2) | 46 (11.5) | 275 (68.6) |
| PUH (n = 214) | 14 (6.5) | 30 (14) | 24 (11.2) | 146 (68.2) |
| SMH (n = 187) | 5 (2.7) | 31 (16.6) | 22 (11.8) | 129 (69.0) |
| Controls | ||||
| Both UPMC hospitals (n = 401) | 336 (83.8) | 48 (12.0) | 8 (2.0) | 9 (2.2) |
| PUH (n = 210) | 161 (76.7) | 35 (16.7) | 7 (3.3) | 7 (3.3) |
| SMH (n = 191) | 175 (91.6) | 13 (6.8) | 1 (0.5) | 2 (1.0) |
Serum Pancreatic Enzyme Levels and Imaging Findings in AP Cases
Among AP cases, 380/401 (94.7%) underwent measurement of both serum amylase and lipase. Overall, any elevation, mild elevation (ie, <3 times normal), and elevation ≥3 times the upper limit of normal for pancreatic enzymes was seen in 95.3%, 26.7%, and 68.6% cases, respectively (Table 2). Overall, the distribution of enzyme elevation did not differ by sex, race, or hospital. The proportion of AP cases who had pancreatic enzyme elevation ≥3 times the upper limit of normal was higher after excluding patients with any prior history of pancreatitis (73%).
Changes indicative of pancreatitis were seen in 43.9% (PUH 44.7%, SMH 43.2%, P = .83) of patients who had either a CT scan or ultrasound of the abdomen performed. In 1 patient, AP was documented on magnetic resonance imaging scan. Imaging evidence of AP in patients with normal, 1–3 times elevation, and ≥3 times elevation of pancreatic enzymes overall was 42.1%, 26.2%, and 43.6%, respectively; and among patients who underwent imaging studies was 61.5%, 32.9%, and 46.7%, respectively. Changes indicative of pancreatic necrosis, pancreatic/peripancreatic fluid collection, and pseudocysts were seen in 5.3%, 16.3%, and 12.5%, respectively, of patients who underwent a CT scan.
Validation of AP Diagnosis Code
Gold standard for AP diagnosis was fulfilled in 309 (77.1%) cases (PUH, 74.8%; SMH 79.7%; P = .28). In 88% of these cases, pancreatic enzyme levels were ≥3 times the upper limit of normal with or without imaging evidence of pancreatitis; in the remaining 12% of cases, there was imaging evidence of AP with normal or mildly elevated pancreatic enzyme levels. Distribution of enzyme elevation and fulfillment of gold standard definition is provided in Table 3. The proportion of patients who fulfilled gold standard definition for AP was higher in gallstone- and hypertriglyceridemia-related AP compared with other etiologies.
Table 3.
Serum Pancreatic Enzyme Elevation, Imaging Evidence of Pancreatitis, and Fulfillment of on Established Guidelines in Acute Pancreatitis Cases
| Etiology (n cases)a | Pancreatic enzymes ≥3 times above normal (%) | Imaging evidence of acute pancreatitis (%) | Fulfilled “gold standard” criteria (%) |
|---|---|---|---|
| All cases (401) | 68.6 | 39.2 | 77.8 |
| Biliary (134) | 82.1 | 40.3 | 88.8 |
| Alcohol (65) | 66.2 | 33.8 | 72.3 |
| Idiopathic (98) | 67.0 | 28.5 | 75.3 |
| Hypertriglyceridemia (12) | 58.3 | 75.0 | 83.3 |
| Medications (25) | 68.0 | 52.0 | 75.5 |
| Other (62) | 50.0 | 45.1 | 62.9 |
Patients with both alcohol and gallstones etiology (n = 5) not shown.
Table 4 displays the sensitivity, specificity, PPV, and NPV for the AP diagnosis code in patients with no prior diagnosis of pancreatitis. Overall, the sensitivity and NPV were excellent (>95%), while the specificity (81%) and PPV (77%) were in the good to excellent range. Although the overall predictive values were similar at the 2 hospitals, subset analysis showed a drop in the PPV and specificity for AP diagnosis code from 2000 to 2005 at SMH (PPV from 91% to 71%; specificity from 92% to 78%) but not at PUH. After excluding patients with a prior diagnosis of pancreatitis, the sensitivity and NPV remained excellent (>95%). An increase in the specificity (81% to 85%) and PPV (77% to 80%) was seen for all years as well as for individual years. The trend in specificity and PPV between the hospitals was similar, with a decrease from 2000 to 2005 in the specificity and PPV at SMH.
Table 4.
Validity of Primary Inpatient Discharge Diagnosis of Acute Pancreatitis in Patients With No Prior Pancreatitis History
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Both UPMC hospitals | ||||
| All years | 96 | 85 | 80 | 98 |
| 2000 | 99 | 88 | 84 | 99 |
| 2002 | 98 | 88 | 85 | 98 |
| 2005 | 95 | 82 | 74 | 96 |
| PUH | ||||
| All years | 95 | 85 | 79 | 97 |
| 2000 | 97 | 85 | 78 | 98 |
| 2002 | 98 | 85 | 80 | 99 |
| 2005 | 91 | 85 | 79 | 94 |
| SMH | ||||
| All years | 98 | 85 | 80 | 98 |
| 2000 | 100 | 92 | 91 | 100 |
| 2002 | 98 | 93 | 93 | 98 |
| 2005 | 97 | 80 | 71 | 98 |
NOTE. Gold standard for diagnosis requires 2 of the following 3 criteria: abdominal pain, serum enzyme elevation ≥3 times above normal, and/or positive imaging evidence. Numbers are rounded to whole numbers.
Characteristics of AP Patients Not Fulfilling the Gold Standard Diagnosis
Compared with patients who fulfilled the guidelines, those not fulfilling the guidelines were similar in demographics, were more likely to have no or atypical pain, but a similar duration of pain (when information was available). These patients were more likely to have a prior history of pancreatitis, milder disease, and different etiologic distribution (Table 5).
Table 5.
Characteristics of Acute Pancreatitis Patients Who Did and Did Not Meet the Guidelines for Diagnosis
| Met guidelines (n = 309) | Did not meet guidelines (n = 92) | P value | |
|---|---|---|---|
| Age (y)a | 55 (42–68) | 49.5 (40–66.5) | .39 |
| Male sex, n (%) | 147 (47.6) | 42 (45.7) | .81 |
| White, n (%) | 267 (86.4) | 74 (80.4) | .18 |
| Hospital, n (%) | .28 | ||
| PUH | 160 (58.7) | 54 (51.8) | |
| SMH | 149 (41.3) | 38 (48.2) | |
| Information on pain recorded, n (%) | 302 (97.7) | 89 (96.7) | .59 |
| Pain present, n/total (%)b | 296/302 (98)c | 78/89 (87.6) | <.01 |
| Typical pain, n/total (%)b | 256/285 (89.8) | 58/73 (79.5) | .02 |
| Information on duration of pain available, n/total (%)b | 277/296 (93.5) | 69/78 (88.5) | .16 |
| Duration of pain, n (%)b | .98 | ||
| <24 h | 152 (54.9) | 37 (53.6) | |
| 1–3 d | 63 (22.7) | 16 (23.2) | |
| >3 d | 62 (22.4) | 16 (23.2) | |
| Etiology, n (%) | .003 | ||
| Biliary | 118 (38.2) | 16 (17.4) | |
| Alcohol | 46 (14.9) | 19 (20.7) | |
| Alcohol and gallstonesd | 4 (1.3) | 1 (1.1) | |
| Idiopathic | 72 (23.3) | 25 (27.2) | |
| Medications | 19 (6.1) | 6 (6.5) | |
| Hypertriglyceridemia | 10 (3.2) | 2 (2.2) | |
| Others | 39 (12.6) | 23 (25) | |
| Prior pancreatitis, n (%) | 35 (11.3) | 22 (23.9) | .002 |
| CT scan of the abdomen done, n (%) | 292 (94.5) | 63 (68.5) | <.001 |
| ICU admission, n (%) | 22 (7.1) | 2 (2.2) | .09 |
| Organ failure, n (%) | 16 (5.2) | 1 (1.1) | .14 |
Median (interquartile range).
Proportions based on patients with available information.
Of the 6 patients with no pain: diagnosis confirmed by imaging and enzyme levels (1); patient with active alcohol/substance abuse presented with withdrawal and markedly elevated lipase (1); 3 patients had biliary pancreatitis with obstructive jaundice (2); common bile duct stones (1); and confusion (1); recent cholecystectomy complicated by development of biloma (1).
Combined with alcohol etiology for comparisons.
Discussion
In this large, retrospective, case-control study conducted at the university- and community-based hospitals of a major US healthcare center, we determined that approximately 1 of 5 patients who received a primary inpatient discharge diagnosis code of AP did not meet the recommended guidelines. We also confirmed that although serum pancreatic enzymes are often elevated in nonpancreatic conditions, significant elevation (ie, ≥3 times the upper limit of normal) is rare outside the setting of AP. Our data suggest that epidemiologic studies that rely solely on discharge diagnosis should consider that the incidence of AP may be inflated in these datasets due to nonfulfillment of recommended guidelines in a subset of patients. We discuss strategies to improve validity of AP diagnosis code in administrative datasets.
Guidelines for serum pancreatic enzyme elevations to diagnose AP are based on expert opinion recognizing that serum pancreatic enzymes are often elevated in nonpancreatic conditions; can be mildly elevated in AP especially when estimations are delayed, in hypertriglyceridemic AP or during exacerbation of CP; and, a cutoff of ≥3 times above normal in the setting of negative imaging best separates AP from nonpancreatic conditions.15-23 They are not perfect as patients with abdominal pain, and mild elevations in serum pancreatic enzyme may have pancreatic inflammation. While not formally validated, guidelines are used as a standard for AP diagnosis in clinical practice, for inclusion of patients into studies or validation of diagnosis.
Indeed, the incidence of AP appears to be increasing, based on epidemiologic studies.4-10 The number of hospital discharges from nonfederal US hospitals with a primary diagnosis of AP was calculated to be 225,600 in 2003, with associated direct medical costs of $2.2 billion.24 An accurate primary diagnosis is critical even after the hospitalization as patients with AP often undergo additional evaluation (eg, imaging) to determine etiology as well as surgical (eg, cholecystectomy) or endoscopic (eg, endoscopic retrograde cholangiopancreatography [ERCP]) procedures to prevent recurrence. Many patients may be hospitalized on more than 1 occasion25,26 or are subsequently diagnosed as CP.27-29
Administrative datasets are used in retrospective population studies to define trends and outcomes that would be difficult, time-consuming, and expensive to accomplish prospectively. However, those conducting this epidemiologic research must be confident about the validity of such data. Prior groups who relied on diagnostic codes in AP also conducted chart review to identify true cases for inclusion in their studies,7,30 but none included control subjects to validate AP diagnosis. In 2 smaller studies from the Netherlands and Denmark,31,32 and a recent study from Sweden,33 the PPV for AP diagnosis code was similar to our study (approximately 80%), and no significant change was noted over time.31 In another study evaluating the validity of the national registry in the Netherlands, Spanier et al reviewed the charts of all patients receiving any inpatient pancreas-related diagnosis (ICD-9 577.*) from 2002 to 2003 (284 unique patients out of 484 total admissions with 523 diagnoses).34 The PPV for AP diagnosis code was 78%, and using only the AP diagnosis code resulted in an underestimation of 16% AP episodes due to misclassification as other pancreas-related diagnoses. However, this study did not provide information on the validity of AP diagnosis code in unique patients or the number of unique patients with AP or CP.34
We found the sensitivity and NPV of AP diagnosis to be excellent (>90% and 95%), meaning that it is unlikely that patients with true AP will be missed. The specificity and PPV was in the range of 80% to 85%, indicating that about 1 in every 5 or 6 patients who receives a diagnosis of AP does not fulfill the established guidelines. Given our retrospective study design, we cannot know why a diagnosis of AP was assigned in these cases. We also do not have information on longitudinal follow-up with regard to readmission(s) or subsequent documentation of an alternative diagnosis. However, our study does provide insights into characteristics of patients who do not meet the recommended guidelines. Receiving a pancreatitis diagnosis also increases the likelihood of subsequent pancreatitis diagnosis. Most gastroenterologists may endorse consulting patients with frequent/chronic abdominal pain, mild elevations of pancreatic enzymes, and no imaging evidence who receive a diagnosis of AP and/or CP. Future research should focus on the reasons for assigning pancreatitis diagnosis in patients who do not fulfill the guidelines.
Determining the validity of AP diagnosis depends on a number of factors: definition used as “gold standard,” limiting the sample to unique patients, excluding patients with prior pancreatitis, the ability to link patient records longitudinally, and the practice setting. Using any enzyme elevation increases the specificity and PPV further (96% and 97%) but with a decrease in sensitivity (85%) and NPV (84%). In many publically available datasets in the United States, it is not possible to identify unique patients, exclude prior pancreatitis diagnosis, or link data files over time to evaluate longitudinal trends at the level of a unique patient. In our study, although we constructed an algorithm to exclude patients with prior pancreatitis, on chart review a small subset in the final cohort was noted to have such history. Using a lower cutoff (serum enzyme elevation to ≥2 times normal) in our study increases the specificity (87%) and PPV (86%) with only slight decrease in sensitivity (95%) or NPV (96%).
A schematic representation of the relationship between specificity and PPV with the criteria used to identify patients from administrative datasets is shown in Figure 1. The specificity and PPV can be improved by selecting unique patients (when the interest is to study individuals rather than episodes) with the primary diagnosis of AP during the index admission and excluding patients with prior history of AP (as in our study). The use of laboratory test results (eg, enzyme elevation to ≥3 times the upper limit of normal) will further increase specificity and PPV. This strategy, however, may miss patients who have mild elevations in enzymes but with positive imaging findings. Such patients can be identified using natural language processing of imaging reports.
Figure 1.
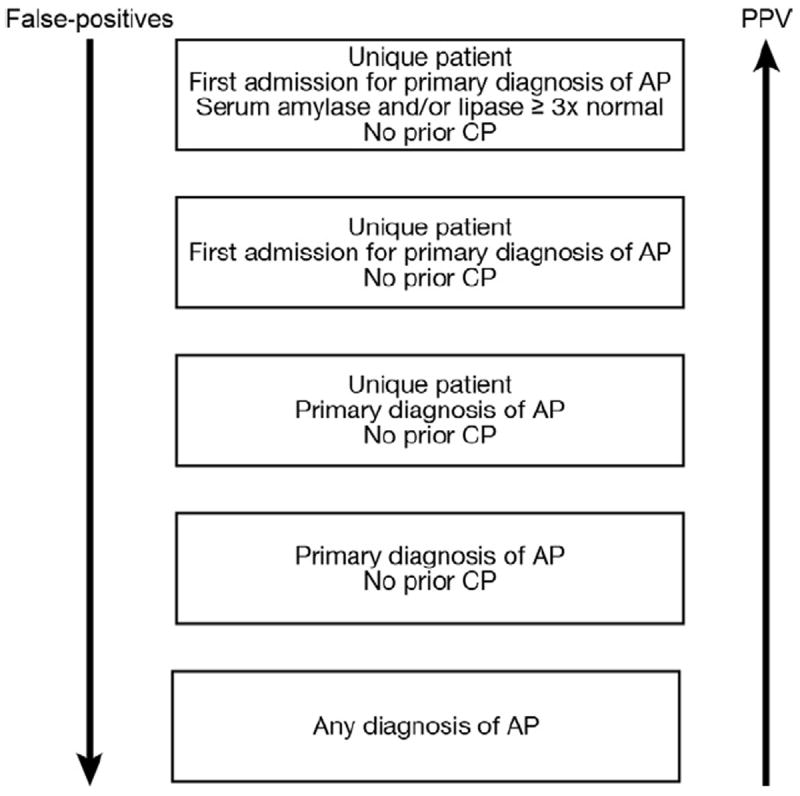
Relationship between specificity and PPV of the criteria used to identify patients with AP using administrative datasets. PPV can improve further if natural language processing for radiology reports is incorporated. Unique patients may not be chosen when the interest is to study episodes of AP. Exclusion of patients with AP or CP diagnosis will depend on study aims.
Our study additionally confirms prior reports16 that pancreatic enzymes are commonly elevated in nonpancreatic conditions (ie, control subjects in whom the final diagnosis was not AP), with most such elevations in the mild range. A cutoff of ≥3 times the upper limit of normal in conjunction with typical abdominal pain is therefore reasonable to separate the patients with pancreatitis from nonpancreatic conditions.
Our study has some limitations. We included only those patients who were admitted to the hospital through the ED (ie, no transfers, direct admits, following same-day surgery, etc). We purposefully chose this criterion to reflect the admission patterns for AP in most US hospitals. While the proportion of patients not admitted through the ED who receive a primary inpatient diagnosis of AP at the community hospitals is small (approximately 10% at SMH in 2005), such patients may form a significant proportion of all patients at the university hospitals (approximately 45% at PUH in 2005). It is conceivable that the validity of diagnosis is higher among patients transferred for the management of severe AP. Because our search algorithm aimed to exclude patients with a prior diagnosis of CP (a history of which on chart review was present only in a small subset of the final cohort), we cannot comment on the validity of the AP diagnosis in CP patients who are admitted for exacerbations. Similarly, validity of the AP diagnosis code for distinct etiologies (eg, drug reaction, diabetes, etc) can be answered only by a focused evaluation. Finally, although we analyzed data from both an urban academic and a community hospital, the findings of our study may not be generalizable to other institutions.
In conclusion, approximately 1 of 5 patients receiving a discharge code of AP does not meet the recommended diagnostic guidelines.1,2 Our study indicates that mild serum pancreatic enzyme elevation is common in nonpancreatic conditions, which could explain some of the excess use of AP discharge code. Efforts should be made to more consistently use guidelines for AP diagnosis. We identify strategies to improve the probability of identifying true AP patients in administrative datasets.
Supplementary Material
Acknowledgments
The authors thank Michelle L. Kienholz, Department of Medicine, University of Pittsburgh, for critical review and editorial assistance.
Funding
Dr Yadav is supported in part by the National Institutes of Health (RO1 NIDDK 077906).
Abbreviations used in this paper
- AP
acute pancreatitis
- CP
chronic pancreatitis
- CT
computed tomography
- ED
emergency department
- ICD-9
International Classification of Diseases, 9th revision
- ICU
intensive care unit
- IQR
interquartile range
- MARS
Medical Archival Retrieval System
- NPV
negative predictive value
- PPV
positive predictive value
- PUH
University of Pittsburgh Medical Center-Presbyterian
- SMH
University of Pittsburgh Medical Center-St Margaret
- UPMC
University of Pittsburgh Medical Center
Footnotes
These data were presented as a poster at the American College of Gastroenterology Annual Meeting in San Antonio, Texas, October 2010, and published in abstract form in the American Journal of Gastroenterology 2010.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2012.03.025.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Banks PA, Freeman ML. Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 2.Forsmark CE, Baillie J, et al. AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309–1318. doi: 10.1111/j.1572-0241.2002.05766.x. [DOI] [PubMed] [Google Scholar]
- 4.Fagenholz PJ, Castillo CF, Harris NS, et al. Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Frey CF, Zhou H, Harvey DJ, et al. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas. 2006;33:336–344. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- 6.O’Farrell A, Allwright S, Toomey D, et al. Hospital admission for acute pancreatitis in the Irish population, 1997-2004: could the increase be due to an increase in alcohol-related pancreatitis? J Public Health (Oxf) 2007;29:398–404. doi: 10.1093/pubmed/fdm069. [DOI] [PubMed] [Google Scholar]
- 7.Omdal T, Dale J, Lie SA, et al. Time trends in incidence, etiology, and case fatality rate of the first attack of acute pancreatitis. Scand J Gastroenterol. 2011;46:1389–1398. doi: 10.3109/00365521.2011.605464. [DOI] [PubMed] [Google Scholar]
- 8.Roberts SE, Williams JG, Meddings D, et al. Incidence and case fatality for acute pancreatitis in England: geographical variation, social deprivation, alcohol consumption and aetiology—a record linkage study. Aliment Pharmacol Ther. 2008;28:931–941. doi: 10.1111/j.1365-2036.2008.03809.x. [DOI] [PubMed] [Google Scholar]
- 9.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 10.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 11.Wu BU, Johannes RS, Sun X, et al. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129–135. doi: 10.1053/j.gastro.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698–1703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 13.Yadav D, Ng B, Saul M, et al. Relationship of serum pancreatic enzyme testing trends with the diagnosis of acute pancreatitis. Pancreas. 2011;40:383–389. doi: 10.1097/MPA.0b013e3182062970. [DOI] [PubMed] [Google Scholar]
- 14.Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Inf Process Manag. 1991;27:379–389. [Google Scholar]
- 15.Chase CW, Barker DE, Russell WL, et al. Serum amylase and lipase in the evaluation of acute abdominal pain. Am Surg. 1996;62:1028–1033. [PubMed] [Google Scholar]
- 16.Gumaste VV, Roditis N, Mehta D, et al. Serum lipase levels in nonpancreatic abdominal pain versus acute pancreatitis. Am J Gastroenterol. 1993;88:2051–2055. [PubMed] [Google Scholar]
- 17.Gwozdz GP, Steinberg WM, Werner M, et al. Comparative evaluation of the diagnosis of acute pancreatitis based on serum and urine enzyme assays. Clin Chim Acta. 1990;187:243–254. doi: 10.1016/0009-8981(90)90109-6. [DOI] [PubMed] [Google Scholar]
- 18.Keim V, Teich N, Fiedler F, et al. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas. 1998;16:45–49. doi: 10.1097/00006676-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kemppainen EA, Hedström JI, Puolakkainen PA, et al. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N Engl J Med. 1997;336:1788–1793. doi: 10.1056/NEJM199706193362504. [DOI] [PubMed] [Google Scholar]
- 20.Kylanpaa-Back ML, Kemppainen E, Puolakkainen P, et al. Comparison of urine trypsinogen-2 test strip with serum lipase in the diagnosis of acute pancreatitis. Hepato Gastroenterol. 2002;49:1130–1134. [PubMed] [Google Scholar]
- 21.Seno T, Harada H, Ochi K, et al. Serum levels of six pancreatic enzymes as related to the degree of renal dysfunction. Am J Gastroenterol. 1995;90:2002–2005. [PubMed] [Google Scholar]
- 22.Steinberg WM, Goldstein SS, Davis ND, et al. Diagnostic assays in acute pancreatitis. A study of sensitivity and specificity. Ann Intern Med. 1985;102:576–580. doi: 10.7326/0003-4819-102-5-576. [DOI] [PubMed] [Google Scholar]
- 23.Treacy J, Williams A, Bais R, et al. Evaluation of amylase and lipase in the diagnosis of acute pancreatitis. ANZ J Surg. 2001;71:577–582. doi: 10.1046/j.1445-2197.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- 24.Fagenholz PJ, Fernández-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302–307. doi: 10.1097/MPA.0b013e3180cac24b. [DOI] [PubMed] [Google Scholar]
- 25.Whitlock TL, Repas K, Tignor A, et al. Early readmission in acute pancreatitis: incidence and risk factors. Am J Gastroenterol. 2010;105:2492–2497. doi: 10.1038/ajg.2010.234. [DOI] [PubMed] [Google Scholar]
- 26.Pelli H, Sand J, Laippala P, et al. Long-term follow-up after the first episode of acute alcoholic pancreatitis: time course and risk factors for recurrence. Scand J Gastroenterol. 2000;35:552–555. doi: 10.1080/003655200750023840. [DOI] [PubMed] [Google Scholar]
- 27.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805. doi: 10.1038/ajg.2009.405. [DOI] [PubMed] [Google Scholar]
- 28.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7:S15–S17. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Nojgaard C, Becker U, Matzen P, et al. Progression from acute to chronic pancreatitis: prognostic factors, mortality, and natural course. Pancreas. 2011;40:1195–1200. doi: 10.1097/MPA.0b013e318221f569. [DOI] [PubMed] [Google Scholar]
- 30.Lindkvist B, Appelros S, Manjer J, et al. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol. 2004;2:831–837. doi: 10.1016/s1542-3565(04)00355-6. [DOI] [PubMed] [Google Scholar]
- 31.Eland IA, Sturkenboom MJ, Wilson JH, et al. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand J Gastroenterol. 2000;35:1110–1116. doi: 10.1080/003655200451261. [DOI] [PubMed] [Google Scholar]
- 32.Floyd A, Pedersen L, Nielsen GL, et al. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981–2000. Scand J Gastroenterol. 2002;37:1461–1465. doi: 10.1080/003655202762671369. [DOI] [PubMed] [Google Scholar]
- 33.Razavi D, Ljung R, Lu Y, et al. Reliability of acute pancreatitis diagnosis coding in a national patient register: a validation study in Sweden. Pancreatology. 2011;11:525–532. doi: 10.1159/000331773. [DOI] [PubMed] [Google Scholar]
- 34.Spanier BW, Schreuder D, Dijkgraaf MG, et al. Source validation of pancreatitis-related hospital discharge diagnoses notified to a national registry in the Netherlands. Pancreatology. 2008;8:498–503. doi: 10.1159/000151777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


