Abstract
Sleep is an important physiological process underlying maintenance of physical, mental and emotional health. Consequently, sleep deprivation (SD) is associated with adverse consequences and increases the risk for anxiety, immune, and cognitive disorders. SD is characterized by increased energy expenditure responses and sleep rebound upon recovery that are regulated by homeostatic processes, which in turn are influenced by stress. Since all previous studies on SD were conducted in a setting of social isolation, the impact of the social contextual setting is unknown. Therefore, we used a relatively stress-free SD paradigm in mice to assess the impact of social isolation on sleep, wakefulness and delta electroencephalogram (EEG) power during non-rapid eye movement (NREM) sleep. Paired or isolated C57BL/6J adult chronically-implanted male mice were exposed to SD for 6 hours and telemetric polygraphic recordings were conducted, including 18 hours recovery. Recovery from SD in the paired group showed a significant decrease in wake and significant increase in NREM sleep and rapid eye movement (REM), and a similar, albeit less robust response occurred in the isolated mice. Delta power during NREM sleep was increased in both groups immediately following SD, but paired mice exhibited significantly higher delta power throughout the dark period. The increase in body temperature and gross motor activity observed during the SD procedure was decreased during the dark period. In both open field and elevated plus maze tests, socially isolated mice showed significantly higher anxiety than paired mice. The homeostatic processes altered by SD are differentially affected in paired and isolated mice, suggesting that the social context of isolation stress may adversely affect the quantity and quality of sleep in mice.
Keywords: Sleep, Sleep deprivation, Social isolation stress, Delta power, Sleep homeostasis, Anxiety
1. Introduction
Sleep deprivation (SD) is a commonly used methodological strategy that enables differential evaluation of sleep homeostasis resulting in `sleep debt'. The slow wave activity (SWA) rebound after SD provides a reliable index of the sleep homeostatic response (Daan et al., 1984) and induces a predictable increase in EEG delta power in non-rapid eye movement (NREM) sleep during subsequent sleep (Dijk et al., 1990), which may be locally increased in a use-dependent manner (Vyazovskiy and Tobler, 2008). Many studies have shown that sleep loss has a significant effect on stress response (Martinez-Gonzalez et al., 2004), motivation (Kennedy, 2002), learning (Stickgold et al., 2001) and performance (Pilcher and Huffcutt, 1996). Stepanski (2002) observed that uninterrupted sleep for a minimum length of time is required for optimal daytime vigilance and neurocognitive function. Furthermore, the need for recovery of lost sleep, the homeostatic sleep response, is considered a universal phenomenon (Mignot, 2008), with the exception of post parturient marine mammals and migrating birds (Siegel, 2009).
The social environment in early life significantly influences not only the behavioral organization but also neurochemical and anatomical development of the brain. There is marked development and reorganization of the brain over adolescence in humans and in rats (Crews et al., 2007; de Graaf-Peters and Hadders-Algra, 2006). Many studies showed that social isolation induced behavioral abnormalities, such as increased aggressiveness, anxiety-related behaviors, cognitive deficits, and hyper locomotion (Chida et al., 2006; Oehler et al., 1986). Long-term isolation in mice increased locomotor activity (Bakshi and Geyer, 1999; Heidbreder et al., 2000; Hilakivi et al., 1989) and show high levels of fear-like behavior (Molina-Hernandez et al., 2001). Highly social animals, including rodents, birds, non-human primates and humans, show a better recovery from experiences of distress, when they are together. The effect of social interaction to reduce anxiety has been investigated extensively (for review see: Buwalda et al., 2005; Kikusui et al., 2006). The socially housed rats showed an anxiolytic effect following social defeat (de Jong et al., 2005; Haller et al., 2003; Ruis et al., 1999). Nakayasu and Ishii (2008) showed that rats subjected to social defeat followed by individual housing spent less time in open arms of an elevated plus maze compared to rats which were subjected to social defeat but housed with a paired companion rat, indicating more anxiety-like behavior than rats housed together.
Many studies have focused on the effects of stressful conditions, such as postnatal maternal separation and swim stress (Hattori et al., 2007; Kirby et al., 2007; Malberg and Duman, 2003; Millstein and Holmes, 2007). Social isolation can induce behavioral alterations, such as an increase in locomotor activity, anxiety, depression and aggression. However, how social isolation affects sleep, and how it affects the response to SD remains unexplored. Since mice are social animals, studies on social isolation could provide an interesting model to study the neurobiological effects of social support. A French-based epidemiological study has linked social isolation with increased risk of mortality (Berkman et al., 2004). Further, a study by Cole and colleagues (2007) showed that individuals who experience chronically high levels of subjective social isolation exhibited genome-wide transcriptional activity of impaired glucocorticoid response genes and increased activity of pro-inflammatory transcription control pathways.
Multiple methodological approaches, at varied time points, have been employed to restrict sleep, including the slow rotating wheel (Ramesh et al., 1999), disk over water (Rechtschaffen and Bergmann, 1995), small platform (Machado et al., 2005), treadmill (McKenna et al., 2007), gentle handling (Ramesh et al., 2007), shaking (Sinton et al., 2009), and motion detection (Newman et al., 2009). Most of these techniques are stress inducing, even though the stress levels may alleviate after long-term adaptations to such methodologies (Andersen et al., 2005). When subjected to long-term SD, the animals exhibit a well-defined physiologic sleep deprivation syndrome that markedly interferes with spontaneous behaviors. Moreover, the tethering cable used to record electroencephalogram (EEG) and electromyogram (EMG) may lead to persistent stress. Indeed, a recent study showed that cable weight and flexibility could affect amount and patterns of sleep in mice (Tang et al., 2004). Development of telemetry systems has provided not only a stress free environment but also an opportunity to study the effect of social interaction on sleep in mice (Ramesh et al., 2009). To examine whether social isolation differentially modulates natural sleep patterns and the `sleep pressure' responses to SD in mice, we implemented a novel SD instrument, wherein it employs intermittent tactile stimulation of freely behaving mice in a standard mouse laboratory cage (Ramesh et al., 2009). This method prevents the need for human contact and intervention, and minimizes physical activity during the entire SD procedure. Furthermore, the procedure was accurate and predictable.
Humans are social mammals and it is likely that most conditions associated with altered sleep or absent sleep would occur in social isolation, the latter being viewed as an intrinsic stress. Therefore, it is critically important to ascertain the impact of sleep manipulations in the social context in which they occur to enable extrapolation of disease states and their consequences, particularly in relation to the social contextual setting in which they develop. Restricted sleep and social isolation are frequent occurrences in humans, and have been both responsible for increased frequency and severity of multiple neurological and social disorders, suggesting the presence of interactive or cooperative mechanisms between them (Berkman et al., 2004; Cole et al., 2007). We therefore tested the hypothesis that the social contextual setting (isolation vs. social pairing) would differentially modulate natural sleep patterns and also alter the homeostatic sleep response to SD in mice. Since circadian distribution of vigilance states, body temperature (Tb) changes and gross motor activity (MAg) are tightly coupled, and play important roles in modulating arousal in rodents (McGinty et al., 2001), we also studied changes in Tb and MAg during the post SD recovery period in socially isolated and paired mice. Our findings suggest that social isolation-induced changes in sleep homeostasis is relevant to various clinical symptoms observed in neuropsychiatric disorders, including ADHD, schizophrenia and depression, and that socially isolated mice are a useful animal model to study the pathophysiology/ pathogenesis of these diseases.
2. Results
The main objectives of the present study were to assess changes in sleep architecture during and after SD under socially paired and isolated conditions (Fig. 1). The SD procedure achieved significantly high efficiency in preventing sleep.
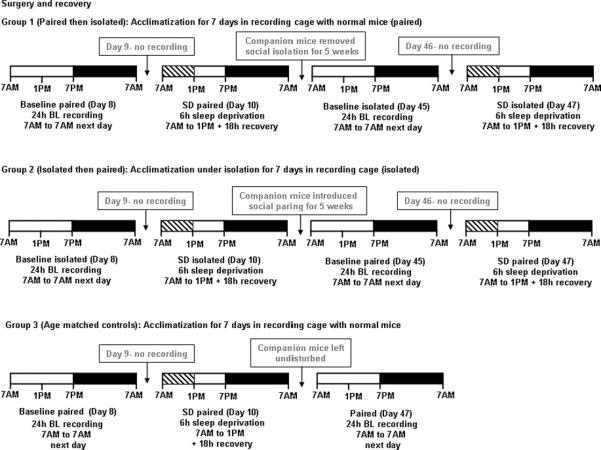
Figure 1. Experimental protocol diagram.
Open and dark portions of the bar represent light and dark periods of the 12:12-h light:dark cycle respectively. Hatched portion of the bar (within the light period) indicates the time of sleep deprivation.
2.1. Effect of SD on state
Multivariate analysis showed that behavioral state varied with time, state and condition, as reflected in a significant two-way interaction of time×state [F=4.05, p<0.0001] and condition×state [F=2.77, p<0.0001]. Furthermore, the significant three-way interaction of time×condition×state showed that the experimental manipulations did have an influence on state and across 24 h recordings [F=2.27, p<0.0001].
2.2. Sleep, wakefulness and EEG delta power in NREM sleep in socially paired mice
2.2.1. Sleep measures
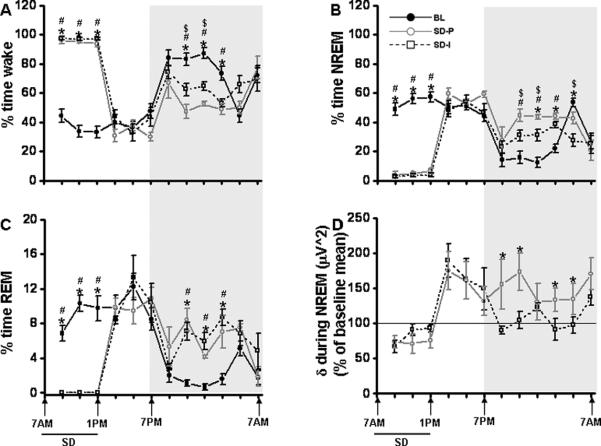
Overall analysis of the polygraphic data for a period of 24 h revealed significant changes between baseline and SD, [p<0.001], indicating that global sleep architecture influenced state (Fig. 2A, 2B, 2C). EEG monitoring during the first 6 h of light period (during SD procedure) showed a significant increase in wake in mice that underwent SD when compared to baseline (94.99 ±1.63% vs 37.44 ±3.90%; F=181.60, p<0.0001), decrease in NREM sleep (5.01 ±1.63% vs 55.32 ±3.93%; F=139.41, p<0.0001) and rapid eye movement (REM) sleep was totally abolished (0% vs 7.24 ±0.97%; F=55.43, p<0.0001). Total light period duration from 7.00 am to 7.00 pm, also showed an increase in wake (63.88 ±1.40% vs 36.86 ±3.40%; F=53.68, p<0.0001), decrease in NREM sleep (31.09 ±1.21% vs 54.45 ±3.42%; F=41.36, p<0.0001) when compared to the baseline. However, total light period REM sleep did not show significant difference (5.04 ±0.47% vs 8.70 ±1.39%; F=6.20, p<0.037) when compared to the baseline. While there were no significant differences between controls and SD animals for the 6 hours of the light period immediately following cessation of SD, SD-exposed mice showed a significant difference in wake, NREM sleep and REM sleep during the dark period (7.00 pm to 7.00 am) (Table 1). Total dark period duration showed a significant decrease in wake (57.10 ±1.13% vs 74.11 ±1.83%; F=62.48, p<0.0001). There was a robust NREM sleep (37.18 ±1.09% vs 24.02 ±1.93%; F=35.18, p<0.0001) and REM sleep (5.73 ±0.28% vs 1.85 ±1.58%; F=143.98, p<0.0001) rebound when compared to the baseline (Table 1).
Figure 2. Sleep, wakefulness and EEG delta power in NREM sleep in paired and socially isolated mice (paired then isolated) following SD.
All graphs are plotted per 2 h for a 24 h period. A. Percent time waking during baseline (Day 8 + day 45; black line), sleep deprivation – paired Day 10 (SD-P; gray line) and sleep deprivation – isolated Day 47 (SD-I; black dashed lines). B. Percent time in NREM sleep during baseline (Day 8 + day 45; black line), sleep deprivation – paired Day 10 (SD-P; gray line) and sleep deprivation – isolated Day 47 (SD-I; black dashed lines). C. Percent time in REM sleep during baseline (Day 8 + day 45; black line), sleep deprivation – paired Day 10 (SD-P; gray line) and sleep deprivation – isolated Day 47 (SD-I; black dashed lines). D. After the initial 6 hrs following SD, the socially isolated group (SD-I; black dashed lines) showed reduced EEG delta power in NREM sleep during the dark period (7.00 pm to 5 am). Shaded area represent dark period. The black line indicates SD period (7.00 am to 1.00 pm). BL, baseline; SD-P, sleep deprivation-paired; SD-I, sleep deprivation-isolated; SD, sleep deprivation. Baseline n=10, SD-P n=5 and SD-I n=5. p<0.05. * = comparison between baseline and SD-I; # = comparison between baseline and SD-P; $ = comparison between SD-P and SD-I.
Table 1.
The percentage time spent in wakefulness, non-rapid eye movement(NREM) sleep and rapid eye movement (REM) sleep for Group 1: paired followed by socially isolation and Group 2: isolation followed by social paring. Data are expressed mean ± SEM.
| Group 1: Social Isolation | Group 2: Social paring | ||||||
|---|---|---|---|---|---|---|---|
| Paired (Day 10) | Socially isolated (Day 47) | Significance | Isolated (Day 10) | Socially paired (Day 47) | Significance | ||
| State | Time of day | Percentage time spent | Percentage time spent | ||||
| Wakefulness | 7:00 AM – 9:00 AM | 96.30 ±2.50 | 97.34 ±0.89 | 95.30 ±3.76 | 96.34 ±0.83 | ||
| 9:00 AM – 11.00 AM | 95.08 ±0.91 | 96.86 ±0.83 | 97.08 ±0.97 | 97.86 ±0.96 | |||
| 11:00 AM – 1:00 PM | 93.60 ±1.95 | 97.06 ±0.89 | 94.6 ±2.38 | 97.76 ±1.74 | |||
| 1:00 PM – 3.00 PM | 30.70 ±4.07 | 43.62 ±4.72 | 32.7 ±2.37 | 45.62 ±5.3 | |||
| 3:00 PM – 5.00 PM | 37.68 ±3.74 | 32.80 ±6.02 | 35.68 ±6.38 | 42.8 ±5.12 | |||
| 5:00 PM – 7.00 PM | 29.92 ±3.11 | 43.32 ±6.39 | 31.92 ±5.5 | 43.32 ±9.45 | |||
| 7:00 PM – 9.00 PM | 67.06 ±10.92 | 74.30 ±4.49 | 64.06 ±8.23 | 76.3 ±6.61 | |||
| 9:00 PM – 11.00 PM | 47.10 ±4.87 | 62.24 ±4.35 | F=5.37, p<0.049 | 45.1 ±6.81 | 65.24 ±5.56 | F=6.47, p<0.03 | |
| 11:00 PM – 1.00 AM | 52.38 ±1.61 | 64.56 ±3.04 | F=12.48, p<0.008 | 50.38 ±2.74 | 66.56 ±4.17 | F=26.74, p<0.008 | |
| 1:00 AM – 3.00 AM | 48.78 ±3.62 | 52.94 ±2.76 | 49.78 ±7.92 | 62.94 ±3.91 | |||
| 3:00 AM – 5.00 AM | 49.94 ±4.33 | 65.86 ±5.63 | 50.94 ±4.64 | 55.86 ±3.44 | |||
| 5:00 AM – 7.00 AM | 77.34 ±7.91 | 70.14 ±8.81 | 75.34 ±5.3 | 70.14 ±4.35 | |||
| NREM sleep | 7:00 AM – 9.00 AM | 3.72 ±2.50 | 2.70 ±0.87 | 4.7 ±1.85 | 3.66 ±0.86 | ||
| 9:00 AM – 11.00 AM | 4.92 ±0.91 | 3.48 ±0.93 | 2.92 ±1.29 | 2.14 ±1.56 | |||
| 11:00 AM – 1.00 PM | 6.40 ±1.95 | 3.62 ±1.38 | 5.40 ±1.38 | 2.24 ±1.17 | |||
| 1:00 PM – 3.00 PM | 59.46 ±3.91 | 48.14 ±4.46 | 55.44 ±3.01 | 44.98 ±3.91 | |||
| 3:00 PM – 5.00 PM | 52.88 ±3.02 | 54.11 ±4.43 | 54.86 ±3.98 | 44.92 ±3.43 | |||
| 5:00 PM – 7.00 PM | 59.16 ±2.07 | 46.36 ±5.96 | 58.06 ±1.58 | 45.28 ±4.35 | |||
| 7:00 PM – 9.00 PM | 27.62 ±8.80 | 22.82 ±4.36 | 31.6 ±5.7 | 20.68 ±5.87 | |||
| 9:00 PM – 11.00 PM | 44.46 ±4.43 | 30.88 ±3.69 | F=5.60, p<0.045 | 46.98 ±3.37 | 28.64 ±8.71 | F=5.85, p<0.042 | |
| 11:00 PM – 1.00 AM | 43.58 ±1.52 | 31.08 ±4.00 | F=8.49, p<0.019 | 44.46 ±8.46 | 26.48 ±5.86 | F=7.49, p<0.019 | |
| 1:00 AM – 3.00 AM | 44.14 ±2.15 | 38.16 ±2.17 | 43.16 ±5.31 | 29.16 ±5.93 | F=5.90, p<0.041 | ||
| 3:00 AM – 5.00 AM | 42.54 ±4.02 | 27.24 ±4.25 | F=6.82, p<0.031 | 41.52 ±1.5 | 36.28 ±4.25 | ||
| 5:00 AM – 7.00 AM | 20.76 ±6.88 | 25.24 ±6.90 | 20.78 ±2.51 | 26.32 ±4.73 | |||
| REM sleep | 7:00 AM – 9.00 AM | 0 | 0 | 0 | 0 | ||
| 9:00 AM – 11.00 AM | 0 | 0 | 0 | 0 | |||
| 11:00 AM – 1.00 PM | 0 | 0 | 0 | 0 | |||
| 1:00 PM – 3.00 PM | 9.86 ±1.01 | 8.40 ±0.45 | 11.86 ±1.3 | 9.4 ±0.93 | |||
| 3:00 PM – 5.00 PM | 9.46 ±1.53 | 13.28 ±2.57 | 9.46 ±1.57 | 12.28 ±1.25 | |||
| 5:00 PM – 7.00 PM | 10.92 ±1.20 | 10.40 ±2.24 | 10.02 ±0.87 | 11.4 ±1.73 | |||
| 7:00 PM – 9.00 PM | 5.34 ±2.19 | 3.02 ±0.37 | 4.34 ±1.71 | 3.02 ±1.51 | |||
| 9:00 PM – 11.00 PM | 8.42 ±1.32 | 7.12 ±1.02 | 7.92 ±1.86 | 6.12 ±1.42 | |||
| 11:00 PM – 1.00 AM | 4.16 ±0.17 | 5.96 ±0.96 | 5.16 ±0.91 | 6.96 ±1.07 | |||
| 1:00 AM – 3.00 AM | 7.06 ±1.86 | 8.70 ±0.94 | 7.06 ±1.43 | 7.9 ±1.37 | |||
| 3:00 AM – 5.00 AM | 7.54 ±0.36 | 6.86 ±1.40 | 7.54 ±1.35 | 7.86 ±1.79 | |||
| 5:00 AM – 7.00 AM | 1.88 ±1.08 | 4.84 ±2.06 | 3.88 ±0.87 | 3.54 ±1.4 | |||
2.2.2. EEG delta power during NREM sleep
Overall analysis of the data for a period of 24 h showed significant changes between baseline and SD [p<0.001], indicating the experimental condition had significant effects on global EEG delta power in NREM sleep. SD animals showed significant increase in delta power immediately after SD procedure, 1.00 pm to 3.00 pm [F=30.04, p<0.008], 3.00 pm to 5.00 pm [F=16.75, p<0.008], 5.00 pm to 7.00 pm [F=19.83, p<0.05] and also during the dark period, 9.00 pm to 11.00 pm [F=16.69, p<0.04], 1.00 am to 3.00 am [F=17.975, p<0.05] (Fig. 2D and 3D).
Figure 3. Sleep, wakefulness and EEG delta power in NREM sleep in isolated and socially paired mice (isolated then paired) following SD.
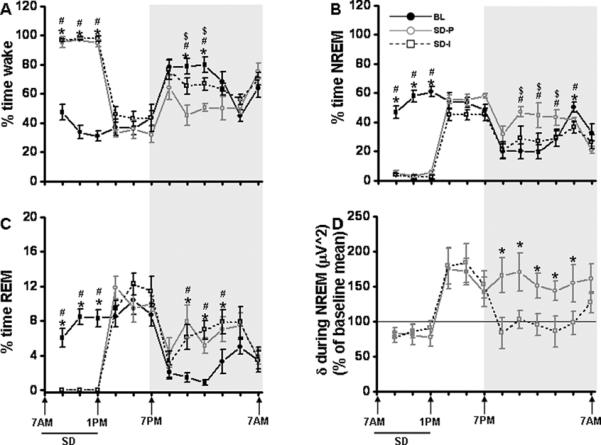
All graphs are plotted per 2 h for a 24 h period. A. Percent time waking during baseline (Day 8 + day 45; black line), sleep deprivation – isolated Day 10 (SD-I; black dashed lines) and sleep deprivation – paired Day 47 (SD-P; gray line). B. Percent time in NREM sleep during baseline (Day 8 + day 45; black line), sleep deprivation – isolated Day 10 (SD-I; black dashed lines) and sleep deprivation – paired Day 47 (SD-P; gray line). C. Percent time in REM sleep during baseline (Day 8 + day 45; black line), sleep deprivation – isolated Day 10 (SD-I; black dashed lines) and sleep deprivation – paired Day 47 (SD-P; gray line). D. After the initial 6 hrs following SD, the socially isolated group (SD-I; black dashed lines) showed reduced EEG delta power in NREM sleep during the dark period (7.00 pm to 5 am). Shaded area represent dark period. The black line indicates SD period (7.00 am to 1.00 pm). BL, baseline; SD-P, sleep deprivation-paired; SD-I, sleep deprivation-isolated; SD, sleep deprivation. Baseline n=10, SD-P n=5 and SD-I n=5. p<0.05. * = comparison between baseline and SD-I; # = comparison between baseline and SD-P; $ = comparison between SD-P and SD-I.
2.3. Sleep, wakefulness and EEG delta power in NREM sleep in socially isolated mice
2.3.1. Sleep Measures
Overall analysis of the polygraphic data for a period of 24 h revealed significant changes between baseline and SD, [p<0.001], indicating that global sleep architecture influenced state (Fig. 2A, 2B, 2C). EEG monitoring during the first 6 h of light period (during SD procedure) showed a significant increase in wake in mice that underwent SD when compared to baseline (97.08 ±0.47% vs 36.55 ±3.63%; F=273.47, p<0.0001), decrease in NREM sleep (3.26 ±0.63% vs 52.72 ±2.99%; F=260.45, p<0.0001) and REM sleep was totally abolished (0% vs 10.72 ±0.65%; F=267.48, p<0.0001). Total light period duration from 7.00 am to 7.00 pm, also showed an increase in wake (68.50 ±1.32% vs 41.35 ±3.56%; F=51.03, p<0.0001), decrease in NREM sleep (26.40 ±1.1.57% vs 48.18 ±2.95%; F=42.45, p<0.0001) and REM sleep (5.34 ±0.62% vs 10.47 ±0.71%; F=29.11, p<0.001) when compared to the baseline. While there were no significant differences between controls and SD animals for the 6 h of the light period immediately following cessation of SD, SD-exposed mice showed a significant difference only during REM sleep during the dark period (7.00 pm to 7.00 am) (6.08 ±0.39% vs 2.25 ±0.38%; F=47.92, p<0.0001) (Table 1). There was a trend level increase in wake and NREM sleep although not as robust as the response observed in paired group. Total dark period duration showed wake (65.00 ±1.97% vs 73.91 ±2.06%; F=9.726, p<0.14) and NREM sleep (29.22 ±1.75% vs 23.82 ±1.85%; F=4.47, p<0.067) when compared to the baseline (Table 1).
2.3.2. EEG delta power during NREM sleep
Overall analysis of the delta power for a period of 24h showed a significant change between treatments (control and SD) [p<0.001] (Fig. 2D), indicating the experimental condition had significant effects on global EEG delta power in NREM sleep. SD animals showed significant increase in delta power immediately after SD procedure, 1.00 pm to 3.00 pm [F=34.04, p<0.008], 3.00 pm to 5.00 pm [F=26.74, p<0.008], 5.00 pm to 7.00 pm [F=18.83, p<0.02], and also during the dark period, 11. 00 pm to 1.00 am [F=17.69, p<0.04], 5.00 am to 7.00 am [F=27.75, p<0.008]. We calculated the area under the curve for paired and socially isolated animals. The AUC was significantly higher in paired mice (881.4 %) when compared with isolated mice (368.2%; p<0.008 - two-tailed).
2.4. Comparison between socially paired and socially isolated groups (group 1: paired then isolated)
Two-way RM ANOVA between the groups showed a significant difference in wake (total 24h: F=15.459; p< 0.004; recovery following SD 18 h: F=16.753; p< 0.003). Similar trend was observed in NREM sleep (total 24h: F=14.861; p< 0.005; recovery following SD 18 h: F=15.32; p< 0.004). There was no significant difference during SD 6h procedure in both wake and NREM sleep (Fig. 2A, 2B). REM sleep did not show any significant differences between these groups (Fig. 2C). Although immediately following SD there was a significant rebound in the delta power in both groups for 6 hrs (from 1.00 pm to 7.00 pm), only the SD-P group showed elevated delta power during the dark period (7.00 pm to 9.00 pm, [F=5.176, p<0.036]), 9.00 pm to 11.00 pm, [F=8.208, p<0.012] and (1.00 am to 3.00 am, [F=5.245, p<0.035] and 3.00 am to 5.00 am, [F=5.273, p<0.049] (Fig. 2D).
2.5. Comparison between socially isolated and socially paired groups (group 2: isolated then paired)
Two-way RM ANOVA between the groups showed a significant difference in wake (total 24h: F=15.311; p< 0.004; recovery following SD 18 h: F=15.999; p< 0.003) (Fig. 3A). Similar trend was observed in NREM sleep (total 24h: F=15.222; P< 0.004; recovery following SD 18 h: F=16.799; P< 0.003) (Fig. 3B). There was no significant difference during SD 6h procedure in both wake and NREM sleep. REM sleep did not show any significant differences between these groups (Fig. 3C). Although immediately following SD there was a significant rebound in the delta power in both groups for 6 hrs (from 1.00 pm to 7.00 pm), only the SD-P group showed elevated delta power during the dark period (7.00 pm to 9.00 pm, [F=4.359, p<0.021]), 9.00 pm to 11.00 pm, [F=6.508, p<0.012], 11.00 pm to 1.00 am, [F=18.448, p<0.001], 1.00 am to 3.00 am, [F=23.993, p<0.001] and 3.00 am to 5.00 am, [F=4.614, p<0.046] (Fig. 3D).
2.6. Sleep, wakefulness and EEG delta power in NREM sleep in age-matched control groups
Overall analysis and pair wise comparisons showed there was no significant change in wake, NREM sleep, REM and EEG delta power in NREM sleep (except for 1.00 am to 3.00 am, [F=5.877, p<0.042 in age matched controls.
2.7. Delta power during NREM sleep is attenuated in socially isolated groups
2.7.1. Comparison between baselines
Overall data for the period of 24 h showed a significant reduction in delta power between baseline (paired) and baseline (socially isolated) mice [p<0.001]. During the light period, there was a significant decrease in delta power in socially isolated animals from 11.00 am to 3.00 pm, (11.00am to 1.00 pm, −31.3%, [F=7.12, p<0.028]; 1.00 pm to 3.00 pm, −42.1% [F=9.31, p<0.016]). During the dark period, significant decreases emerged from 11.00 pm to 3.00 am (11.00pm to 1.00 am, −28.2%, [F=6.08, p<0.039]; 1.00 am to 3.00 am, −14.6% [F=5.46, p<0.048]) (Fig. 4A).
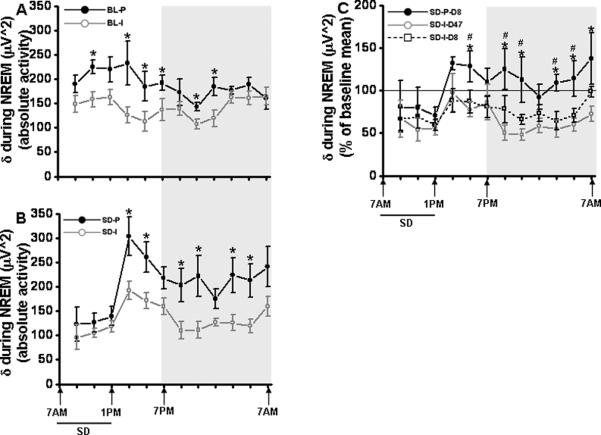
Figure 4. EEG delta power during NREM sleep is attenuated in socially isolated mice.
A. Baseline recording showed a decrease in EEG delta power in NREM sleep through out the 24 period in socially isolated mice (gray line) as compared to the paired mice. n=5. (black line). B. After sleep deprivation, the socially isolated mice showed a significant decrease in delta power through out the dark period. n=5. (gray line). C. Normalized data with the age matched controls also showed a significant decrease in delta power in socially isolated group. n=5. Shaded area represent dark period. The black line indicates SD period (7.00 am to 1.00 pm). BL-P, baseline- paired; BL-I; baseline-isolated; SD-P, sleep deprivation- paired; SD-I, sleep deprivation- isolated; SD, sleep deprivation. * p <0.05.
2.7.2. Comparisons between SD in paired and isolated conditions
Delta power for the period of 24 h showed a significant reduction between SD (paired) and SD (socially isolated) mice [p<0.001]. The SD (socially isolated) animals showed only mild homeostatic increases in delta power immediately following SD when compared to SD (paired) animals. There was a significant decrease in delta power in socially isolated animals during the light period following SD, (1.00 pm to 3.00 pm, (−36.54%, [F=6.47, p<0.03]; 3.00 pm to 5.00 pm, (−34.12%, [F=6.25, p<0.03]). However, delta power showed a significant decrease throughout the dark period (7.00 pm to 9.00 pm, −45.69%, [F=5.85, p<0.042]; 9.00 pm to 11.00 pm, −49.77%, [F=5.90, p<0.041]; 1.00 am to 3.00 am, −43.82% [F=6.15, p<0.038]; 3.00 am to 5.00 am, −43.80%, [F=6.34, p<0.03] (Fig. 4B).
2.7.3. Delta power comparisons between experimental group and age matched controls
Normalized data using the age matched control group showed a significant decrease in delta power in the socially isolated group. There was a significant decrease in delta power in socially isolated animals during the light period following SD, 3.00 pm to 5.00 pm, [F=3.378, p<0.087] and through out the dark period; 7.00 pm to 9.00 pm, [F=8.182, p<0.013]), 9.00 pm to 11.00 pm, [F=7.147, p<0.018]; 1.00 am to 3.00 am, [F=4.616, p<0.048]; 3.00 am to 5.00 am, [F=5.089, p<0.041] and 5.00 am to 7.00 am, [F=4.881, p<0.044] (Fig. 4C).
2.8. Temperature and activity
To determine the overall statistical significance of the effect of baseline, SD (paired) and SD (isolated) body temperature and gross motor activity, repeated measures ANOVA was performed. There was a significant effect on body temperature across the 24 h period between the experimental groups (F=18.79, p <0.001). Post hoc analyses comparing the baseline with SD (paired) and SD (isolated) for each 2 h bin showed a significant increase in body temperature during SD procedures (7.00 am to 9.00 am [F=5.91, p<0.026]; 9.00 am to 11.00 am [F=34.81, p<0.001]; 11.00 am to 1.00 pm [F=101.54, p<0.001]), and decrease during the dark period (7.00 pm to 9.00 pm [F=7.63, p<0.014]; 9.00 pm to 11.00 pm [F=10.79, p<0.005]; 1.00 am to 3.00 am [F=14.34, p<0.002] (Fig. 5A). Gross activity also showed a significant effect across the 24 h period between the experimental groups (F=18.16, p <0.001). Further, post-hoc analysis showed a significant increase in activity during SD procedures (7.00 am to 9.00 am [F=46.21, p<0.001]; 9.00 am to 11.00 am [F=49.77, p<0.001]; 11.00 am to 1.00 pm [F=69.1, p<0.001]), and decrease during the dark period (9.00 pm to 11.00 pm [F=16.34, p<0.001]; 11.00 pm to 1.00 pm [F=8.14, p<0.012];1.00 am to 3.00 am [F=8.76, p<0.01]) (Fig. 5B).
Figure 5. Body temperature and gross activity in paired and socially isolated mice.
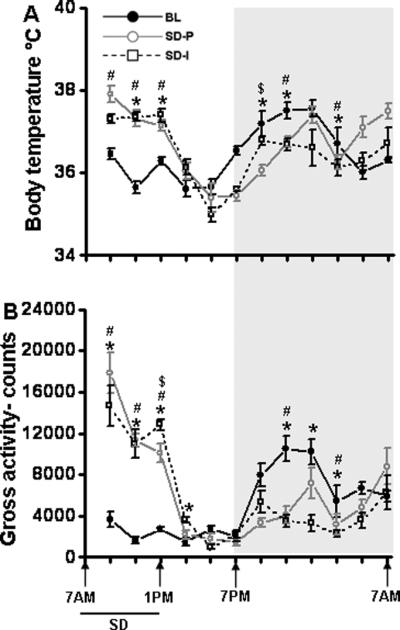
A. 6 h sleep deprivation significantly increased body temperature in both socially isolated mice (black dashed line) and paired mice (gray line) when compared to the baseline recording (black line). Post-SD period showed significant decrease in temperature in both isolated and paired animals. B. Similarly, 6 h sleep deprivation significantly increased gross activity in both socially isolated mice (black dashed line) and paired mice (gray line) when compared to the baseline recording (black line). Post-SD period showed significant decrease in activity in both isolated and paired animals. Shaded area represent dark period. The black line indicates SD period (7.00 am to 1.00 pm). BL, baseline; SD-P, sleep deprivation-paired; SD-I, sleep deprivation-isolated; SD, sleep deprivation. Baseline n=10, SD-P n=5 and SD-I n=5. p<0.05. * = comparison between baseline and SD-I; # = comparison between baseline and SD-P; $ = comparison between SD-P and SD-I.
2.9. Effect of isolation on anxious behavior in the elevated plus-maze test
In group 1, there was a significant difference between isolated and paired mice. The isolated male mice spent significantly less time in open arm (Fig. 6A) when compared with the paired mice [F=141.539; p< .001] and more time in closed arm [F=18.152; p< .002] (Fig. 6B). On the other hand, in group 2, the socially paired mice spent more time in open arm [F=26.363; p< .001] (Fig. 6D) and less time in closed arm [F=84.054; p< .001] (Fig. 6E), which was comparable to paired mice in group 1. In both the groups, there was no change in the time spent in center (Fig. 6C, 6F).
Figure 6. Effect of isolation on anxious behavior tested by the elevated plus-maze in mice.
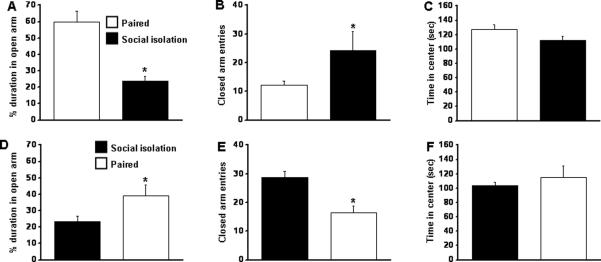
Figures A, B and C mice subjected to group 1 protocol (paired then isolated). Figures D, E and F mice subjected to group 2 protocol (isolated then paired). A and D represents percentage duration in open arms; B and E represents number of closed arm entries; C and F represents time spent in the center. n=12. * p <0.05.
2.10. Effect of isolation on locomotor activity in an open field test
In group 1, there was a significant difference between isolated and paired mice in locomotor activity. The isolated male mice showed significant higher locomotor activity when compared with the paired mice [F=22.234; p< .001] (Fig 7A) and the frequency of time visiting the center was significant lower [F=392.257; p< .001] (Fig. 7B). On the other hand, in group 2, the socially paired mice showed significantly lower locomotor activity [F=17.496; p< .002] (Fig. 7C) and the frequency of time visiting the center was significantly higher [F=23.975; p< .0001] (Fig. 7D), which was comparable to paired mice in group 1.
Figure 7. Effect of isolation on locomotor activity in open field in mice.
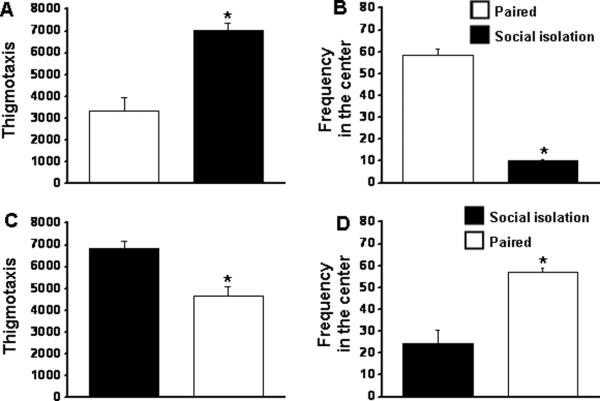
Figures A and B mice subjected to group 1 protocol (paired then isolated). Figures C and D mice subjected to group 2 protocol (isolated then paired). A and C represents thigmotaxis; B and D represents frequency in the center. n=12. * p <0.05.
3. Discussion
In this study, a relatively stress-free approach to elicit SD in mice was implemented that enabled animals to remain in their normal social contextual environment, thereby permitting assessment of the effects of social isolation. Many studies have shown that social isolation leads to depression and stress-induced anxiety disorders. In turn, augmented anxiety can induce sleep disorders (Reynolds et al., 1983). The behavioral, neurochemical and anatomical changes in socially isolated mice may be relevant to the clinical symptoms and pathophysiology in patients with neuropsychiatric disorders. Exposure to early social deprivation in humans has been shown to alter neuroendocrine and neurobiological systems and can cause structural and functional brain alterations (De Bellis et al., 2009; Heim and Nemeroff, 2002). This can lead to increased risk for the development of conduct disorders, personality disorders, major depression, posttraumatic stress disorder, schizophrenia, and anxiety disorders (Veenema, 2009). For instance, Wang et al., (2011) reported that rats isolated for 8–9 weeks showed structural and functional changes in the mesocorticolimbic dopaminergic system, exhibited hyperlocomotor activity and impaired sensorimotor gating compared to group-reared controls suggesting attributes related to schizophrenia and depression. Sleep deprivation per se can also induce stress and may lead to cardiovascular risk (Franzen et al., 2011), obesity (Cizza et al., 2011) and other morbidity.
Six hours of SD during the daylight cycle in both socially paired and socially isolated mice led to >95% of the time being spent in wakefulness (Fig. 2A). This indicates not only the effectiveness of the SD approach, but also its advantages as inherently not requiring human intervention, thereby maximizing reproducibility and minimizing error. Although both socially paired and isolated animals exhibited significant increases in NREM sleep after SD (Fig. 2B), delta spectral power analyses of the EEG revealed that socially isolated animals did not mount as robust homeostatic increases in delta power as compared to the socially paired group (Fig. 2D). These findings suggest that restorative sleep is improved when the normal murine social context is preserved, as indicated by higher sleep homeostatic responses or delta rebound following SD. It is well established that prior wake period directly influences the succeeding sleep bouts, and will manifest as a sleep rebound and an increase in EEG delta power during NREM sleep (Tobler and Borbely, 1986). However, with continuous or repeated arousal, desensitization may occur, which may attenuate or alter the neurotransmitter activity (Gainetdinov et al., 2004). Therefore, it would be important to understand how SD either potentiates or leads to desensitization of sleep recovery processes and EEG delta power during NREM sleep.
3.1. Methodological Considerations
One of the major limitations of existing methods aiming to induce sleep loss is that they are stressors per se, and as such, the intrinsic stress effect of sleep loss can not be ascertained. Sleep deprivation induced either by the `inverted flower pot' method, gentle manual handling, or by the forced locomotion method has been shown to increase levels of adrenocorticotropic hormone and corticosterone (CT) (Rechtschaffen and Bergmann, 1995; Ramesh et al., 2009). In addition, these methods are either labor intensive (e.g., manual handling) or impose forced locomotion (McKenna et al., 2007; Ramesh et al., 2007). In contrast, the automated SD approach used in the present study was not associated with global increases in CT after 6 hours, but CT serum concentrations may not be sensitive enough to detect organ specific stress hormone activity (Ramesh et al., 2009). Future studies are needed to determine whether how extended SD exposures affect the hypothalamic-pituitary-adrenal responses. Another surrogate indicator of stress is alterations in body temperature. Restraint and social stress has shown to increase Tb in mice (Bhatnagar et al., 2006; Gilmore et al., 2008). In the current study, the SD procedure increased body temperature (Fig. 5A), suggesting the increase in Tb may be due to the increased wakefulness activity observed during the same period (Fig. 5B). In addition, social isolation may also impose a direct modulatory influence on the stressor response associated with SD. In the present study, the SD procedure allowed for comparisons between the responses to SD in the context of social isolation and social pairing.
3.2. Social Isolation Affects Various Physiological Functions
Even though there was no change in the total time spent in sleep and wakefulness, socially isolated mice showed a dramatic decrease in EEG delta power in NREM sleep even during basal conditions (Fig. 4A). Following the SD procedure, both socially isolated and paired mice showed significant EEG delta power in NREM sleep rebound for the rest of the light period (6 hrs). However, only the socially paired mice showed increases in EEG delta power in NREM sleep in the dark period (Fig. 4B). Even when the delta power was normalized using an age-matched group, the isolated group showed significant reductions in delta power (Fig. 4C). This observation rules out the possibility that signal decay may occur in delta power during NREM sleep in the post-isolation condition (due to time difference and age). Reductions in delta power, without gross change in sleep-wake activity, were also reported when NF-κB activity was blocked by the inhibitor peptide, SN50 in rats, particularly during the first 2 h of recovery sleep following 6h SD (Ramesh et al., 2007). In addition to sleep, social isolation dramatically affects many physiological functions, including aggressiveness, anxiety-related behaviors, cognitive deficits, and hyperlocomotion (Machado et al., 2006; Oehler et al., 1986). Early social isolation in domestic pigs showed sustained changes in behavioral, neuroendocrine and immune regulation (Tuchscherer et al., 2009). In fact, a single social isolation in piglets caused age-dependent neuroendocrine and behavioral changes that indicate increased arousal and experienced distress (Tuchscherer et al., 2009). A recent study has shown that chronic immobilization stress can induce bimodal distribution of REM sleep, both stress-enhanced REM sleep and stress-reduced REM sleep (Hegde et al., 2011) and manual restraint reduced NREM sleep and REM sleep and increased delta power for the first 4h period (Tang et al., 2004).
In the present study, NREM sleep showed immediate rebounds in delta power in both paired and socially isolated animals, but only socially paired mice exhibited persistent increases in EEG delta power in NREM sleep throughout the dark period, irrespective of the order in which they were paired, indicating improved sleep depth and quality compared to the matched isolated condition. 18 h post deprivation recordings indicated that delta power did not return to baseline values within this time frame. We speculate that recovery from EEG delta power in NREM sleep following SD even for short periods such as 6 h will require more time. Although sleep rebound during the recovery period after acute SD is tightly regulated by homeostatic processes (Borbely, 1982), the present results indicate that social isolation may modulate the magnitude of this sleep rebound. In addition, the case may be advanced that the innate social nature of the mouse species may play a role in the prolonged elevated delta power following SD. Indeed, since mice are highly social animals, direct contact with a companion mouse would allow for the needed social bonding thereby reducing stress and fear, and thus, enabling improved quality sleep following perturbations such as SD. Acute stressors lead to a subsequent sleep rebound, while prolonged and/or chronic stress reduce the time spent in NREM sleep, thereby affecting sleep quality (McKenna et al., 2007; Tobler and Scherschlicht, 1990).
The immediate increase in REM sleep following SD in both groups could be related to a compensatory mechanism for the total REM deprivation seen during SD. Stress may affect REM sleep but the issue remains somewhat controversial. On the one hand, when restraint stress was used, REM sleep was suppressed (Tobler and Scherschlicht, 1990). Conversely, stress was associated with an increase in REM sleep in other studies (Wiberg and Grice, 1963). It is possible that different types of stressors and the underlying conditions in which the particular stressors are applied (i.e., time of day, intensity of stimulus applied and duration of stressor) may all account for the opposite effects of stress on REM sleep. Machado et al., (2006) showed in rats subjected to 6h SD using a modified multiple platform method, who had their bonding renovated everyday for the remainder 18 h, showed marked rebounds in REM sleep compared to socially isolated rats.
As anticipated, the effects of acute SD were more prominent than those following acute sleep fragmentation (SF) (Ramesh et al., 2009), and could be related to the differences between SD and SF in the amount of prior wake. In the paired group, the percent time spent in wake was 95% in SD vs 57.8% in SF, such that during SF procedures, mice successfully and gradually acclimatize to the repeated arousals and thus maintain reasonable amount of NREM sleep. This is opposed to the virtually absent (5%) NREM sleep during SD. Similar patterns and differences emerged for REM sleep among SF and SD-exposed mice.
3.3. Isolation-induced behavior changes in anxiety
The elevated plus-maze induces anxiety caused by fear of heights and open spaces (Pellow et al., 1985). Some reports show that social isolation causes anxiety in rats (Jankowska et al., 1991; Maisonnette et al., 1993; Morinan et al., 1992) while other studies showed no significant effect (Lapiz et al., 2001). In the present study, isolated mice, in both the groups, spent shorter duration in the open arms (Fig. 6A, 6D) and more entries on the closed arms (Fig. 6B, 6E) in the elevated plus-maze test, indicating increased anxiety.
We investigated the social-isolation induced anxiety-like behavior in mice, which is consistent with previous studies (Hellemans et al., 2004; Wei et al., 2007; Wright et al., 1991). It is possible that social isolation-induced hyperactivity may be attributable to the anxiolytic-like effect under a novel environment. In our study, socially isolated mice exhibited increase in locomotor activity (Fig. 7A, 7C) response to a novel environment compared with paired housing mice. Under such conditions, anxiety-like behaviors were demonstrated with socially-isolated mice. Social-isolated mice exhibited increased anxiety and social interactions in both the experiments.
In humans, social isolation predicts the onset of depression, as well as increased morbidity and mortality from cardiovascular and cerebrovascular diseases (Boden-Albala et al., 2005; Norman et al., 2010). Moreover, social isolation is a strong determinant of outcomes following experimental stroke and predisposes to ischemic damage (Karelina et al., 2009). Taken together, we can infer that chronic isolation could initiate early molecular changes leading to early neuronal damage, which in turn may lead to blunted homeostatic sleep and delta power responses in the socially isolated group. In summary, social interactions in the context of SD play an important role in modulating the quality of sleep and its recovery from SD, thereby emphasizing the need to incorporate the contextual social setting in future experiments aiming to determine the regulation of sleep homeostatic responses.
3.4. Isolation-induced hyperactivity
Many studies have reported that social isolation causes hyperactivity in male mice (Heidbreder et al., 2000; Hilakivi et al., 1989). Open field behavior in nonhuman animals represents a compromise between predator evasion tactics and the reinstatement of contact with in a group (Hofer, 2009). In the present study, similar results were also observed. Social isolation, in both the groups, caused higher accounts of locomotor activity (Fig. 7A, 7B) and lower exploratory activity towards the center (Fig. 7B, 7D) when compared to paired mice. The increased locomotor activity found among socially isolated mice is intriguing and establishes a potential causative link between social isolation and symptoms of hyperactivity. These findings are analogous to several studies that have documented socially isolated patients tend to have anxiety-related problems (Gazelle et al., 2010; Heinrich and Gullone, 2006; Modin et al., 2011).
4. Methods
4.1. Animals
Male C57BL/6J mice (20–25 g; age 10 weeks) were purchased from Jackson Laboratories, (Bar Harbor, Maine), were housed in a 12 hr light/dark cycle (light on 7:00 A.M. to 7:00 PM) at a constant temperature (26 ±1°C) and were allowed access to food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee and are in close agreement with the National Institutes of Health Guide in the Care and Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
4.2. Surgical procedure and implantation of telemetric transmitter and electrodes
All surgical procedures were performed under sterile conditions and general anesthesia (Induction: 3% isoflurane and 1 liter per min of O2. Maintenance: 2% isoflurane and ½ liter per min of O2) (Ramesh et al., 2009; Nair et al., 2011). A telemetric transmitter weighing 3.5 g, F20-EET (DSI, Minnesota, USA), which allows simultaneous monitoring of EEG and EMG, temperature and locomotor activity was chronically implanted (Ramesh et al., 2009; Nair et al., 2011). Following surgery, Ketoprofen, 3–5 mg/kg, s.c., every 24 h for 2 days was given for post-operative pain management.
4.3. Sleep deprivation
The device used to induce SD in rodents has been previously described (Ramesh et al., 2009 and Nair et al., 2011). Briefly, it employs intermittent tactile stimulation of freely behaving mice in a standard mouse laboratory cage, using a nearly-silent motorized mechanical device. Tactile stimulation is achieved with a horizontal bar sweeping just above the cage floor from one side to the other side of the mouse cage, the sweeper being powered by an electrical motor system in which the speed, torque, and interval of the intermittent/continuous functioning mode are controlled, eliminating error induced by human intervention. Indeed, many of the routine handling procedures may result in a stress response that does not readily habituate (Balcombe et al., 2004). The sweeper requires around 9 sec to sweep the floor of the cage one way, at which time, a relay engages the sweeper to move in the opposite direction and the cycle is repeated continuously. During sweeper motion, animals need to step over the sweeper and then resume their unrestrained behavior. The SD procedure was designed such as to minimize stress to the animals (although no technique can be totally stress free), while effectively eliciting SD. To verify this assumption, we determined the absence of significant increases in corticosterone (CT) plasma levels, as a surrogate indicator of stress (Ramesh et al., 2009).
4.4. Acclimatization, sleep recording and sleep deprivation
After recovery from surgery (7–10 days), the mice were transferred to the SD device for habituation to the cage and the sweeper for 7 days. During habituation, the device was switched on for 15 min (2 times per day) at random intervals during the light period. The recording cages were mounted on a DSI telemetry receiver (RPC-1), which was in turn connected to an acquisition computer through a data exchange matrix. After acclimatization in the cages, the magnetic switch of the transmitter was activated, and polygraphic recordings were begun at 7.00 AM. Physiological data were continuously acquired for 24h using Dataquest ART acquisition software (DSI, Minnesota, USA; version 3.1), at a sampling rate of 500 Hz. Data were first scored automatically using Sleepsign software (Kissei Comtec, Japan) (Espana et al., 2007; Ramesh et al., 2009; Nair et al., 2011), and records were visually confirmed or corrected as needed by an investigator who was blinded to the experimental condition.
Behavior was classified into 3 different states: wake, NREM sleep and REM sleep. EEG during wake had low-amplitude, high-frequency (desynchronized) waves. During wake, EMG records showed gross body movement artifacts and behaviorally, animals had grooming, scratching and orienting activity. The NREM sleep stage was characterized by low-frequency, high-amplitude (synchronized) EEG with a considerable reduction in EMG amplitude. REM sleep was characterized by desynchronized EEG, and a drastic reduction in EMG (muscle atonia). Sleep-related low frequency (delta) activity was also derived from the records using bandpass filtering of 1– 4.0 Hz. Delta power was computed offline by using SleepSign software by Fast Fourier Transform (FFT), which was based on 512 points corresponding to 10 sec epochs, at a sampling rate of 250 Hz with Hanning as the window filter of FFT. The software algorithm analysis was based on The Nyquist–Shannon sampling theorem, which states that perfect reconstruction of a signal is possible when the sampling frequency is greater than twice the maximum frequency of the signal being sampled. Those NREM sleep epochs that showed movement artifacts were excluded when computing delta power, since EEG signals are especially sensitive to movement, with the resulting artifact specifically enhancing signals in the delta band.
4.5. Temperature and activity
Body temperature and gross motor activity were acquired every 10 sec throughout all experiments. To increase the precision of recordings, the lower limit of temperature records was set at 34°C and the upper limit at 41°C, while in the activity record the lower limit was set at 0 counts (no gross activity) and upper limit was set at 3840 counts (a high level of activity) at the polling rate of 64 Hz. The transmitter underwent 3 point calibration at 35 °C, 37 °C and 39 °C.
4.6. Anxiety Behavior Test
Separate experiments, on two groups (n=12/group) were carried out for anxiety behavior tests. Group 1 (social isolation): Mice were paired with another male mouse with whom they had previously co-habited. On day 8, open field (OF) and elevated plus maze (EPM) tests were carried out. Following the above experiment, the companion mice were removed from the cages, and the experimental mice were placed in social isolation for 5 weeks. On day 45, OF and EPM were conducted. Group 2 (social paring): During the 7-day acclimatization period and prior to recordings, mice were isolated from their corresponding cage mate with whom they had previously co-habited. On day 8, open field (OF) and elevated plus maze (EPM) tests were carried out. Following the above experiment, the companion mice were introduced into the cages, and the experimental mice were allowed for social paring for 5 weeks. On day 45, OF and EPM were conducted.
4.7. Experimental design
The various phases of the experimental paradigm are illustrated in Fig. 1.
4.7.1. Group 1: paired then isolated
Part 1 :During the 7-day acclimatization period and prior to recordings, mice (n=5) were paired with another male mouse with whom they had previously co-habited. On day 8, baseline sleep recordings were carried out for 24h from 7.00 am to 7.00 am the next day (Fig. 1). The animals were left undisturbed on day 9. On day 10, animals were subjected to SD for 6 h during the light period from 7.00 am to 1.00 pm, and recovery sleep recordings were continued for the subsequent 18 h until 7.00 am next day.
Part 2: Following the above experiment, the companion mice were removed from the cages, and the experimental mice were placed in social isolation for 5 weeks. On day 45, baseline sleep recordings were conducted for 24h from 7.00 am to 7.00 am next day. The animals were left undisturbed on day 46. On day 47, the animals were subjected to SD for 6 h during the light period from 7.00 am to 1.00 pm, and recovery sleep recordings were continued for the subsequent 18 h until 7.00 am the next day.
4.7.2. Group 2: isolated then paired
Although the main purpose of this study was to understand the effect of social isolation in the same group of animals, we also conducted assessment of social paring using a paradigm aiming to exclude the possibility of an order effect.
Part 1: During the 7-day acclimatization period and prior to recordings, mice (n=5) were isolated from their corresponding cage mate with whom they had previously co-habited. On day 8, baseline sleep recordings were conducted for 24h from 7.00 am to 7.00 am the next day (Fig. 1). The animals were left undisturbed on day 9. On day 10, animals were subjected to SD for 6 h during the light period from 7.00 am to 1.00 pm, and recovery sleep recordings were continued for the subsequent 18 h until 7.00 am next day.
Part 2: Following the above experiment, the companion mice were introduced into the cages, and the experimental mice were allowed for social paring for 5 weeks. On day 45, baseline sleep recordings were conducted for 24h from 7.00 am to 7.00 am next day. The animals were left undisturbed on day 46. On day 47, the animals were subjected to SD for 6 h during the light period from 7.00 am to 1.00 pm, and recovery sleep recordings were continued for the subsequent 18 h until 7.00 am the next day.
4.7.3. Group 3: Age-matched controls
Part 1: During the 7-day acclimatization period and prior to recordings, implanted animals (n=5) were paired with another male mouse with which they had previously been housed. On day 8, baseline sleep recordings were carried out for 24h from 7.00 am to 7.00 am next day (Fig. 1). The animals were left undisturbed on day 9. On day 10, animals were subjected to SD for 6 h during the light period from 7.00 am to 1.00 pm, and recovery sleep recordings were continued for the subsequent 18 h until 7.00 am next day.
Part 2: Following the above experiment, the companion mice continued to stay in the cages. On day 47, baseline sleep recordings were conducted for 24h from 7.00 am to 7.00 am next day.
4.7.4. Elevated plus maze
The EPM was used to assess anxiety in 2 groups of mice, Group1: paired followed by social isolation and Group 2: isolation followed by social paring (n=12/group). The basic measure is the animal preference for dark, enclosed places over bright, exposed places (Montgomery, 1955; Nair et al., 2011). A 60 w light was placed above the apparatus and the test was recorded by an overhead camera using Ethovision software from Noldus. Mice were placed in the center of the maze facing a closed arm, and allowed to explore for 10 min in isolation. Each mouse received one recorded trial. Mice prefer to enter into closed arms compared to open arms. Time spent in the dark area is viewed as avoidance or anxiety-like behavior. The following parameters were scored: (a) Percent time spent in open and closed arms; (b) number of entries to closed arms; (c) Time spent in the center. An arm entry was defined as the entry of all four feet into either one of the closed arm. The maze was cleaned with 30 % ethanol between trials to remove any odor cues.
4.7.5. Open field
Locomotor activity was assessed in a black Plexiglas chamber (22 × 22 × 22 in.) in 2 groups of mice, Group1: paired followed by social isolation and Group 2: isolation followed by social paring (n=12/group). Activity was recorded by a video camera suspended above the open field (OF) and interfaced with a video tracking system (Noldus, Sterling, VA, U.S.A.). The mice were randomly selected animals from each group were acclimatized to the open field for 10 min before the start of the activity monitoring session, which lasted for 10 min. The chamber was thoroughly cleaned with 30% ethanol between trials to eliminate any confounding odor effects.
4.8. Data analysis
In all the experimental conditions, the sleep-wake data were divided into 10 sec epochs and scored. They were then divided into 2-h bins to observe the circadian variations, if any, between the groups. EEG delta power (1–4 Hz) during NREM sleep was calculated as percentage of each animal's baseline recording 2 days prior to SD. The baseline data on EEG delta power in NREM sleep is expressed relative to the mean total power in the NREM EEG. Data on EEG delta power in NREM sleep, during recovery sleep, is expressed as a percentage of the mean NREM EEG delta power value taken from the baseline recording 2 days prior to SD. EEG delta power (absolute activity) in NREM sleep was compared between the baseline (paired and isolated). We used multivariate MANOVA (SPSS 11) with the following model: Two hr time bins as within factors (12 time points) and Two between factors: (1) Condition (four levels): BL (paired), SD (paired), BL (socially isolation) and SD (socially isolation) (2) State (three levels): wakefulness, NREM sleep, and REM sleep. All F statistics are reported using Pillai's Trace. The interaction of three different factors, i.e., time, condition and state were determined using this mixed model repeated measures MANOVA. Further, we did a two-way RM ANOVA on factors, condition, (within-subjects with levels “paired” and “isolated”) and time (total 24h, during SD 6h and recovery 18h).
To further elucidate the nature of identified interactions for the paired and socially isolated conditions, all data were analyzed by one way RM ANOVA. The baseline data of paired (Day 8) and isolated mice (Day 45) were grouped together for statistical comparison. First, overall statistical significance was determined for the 24-h period between the treatment groups (baseline Paired and Isolated). In addition, statistical significance for 2 h bins for 24 h was assessed, followed by post-hoc Holm-Sidak analyses, as needed. For all comparisons, a p value <0.05 was considered to achieve statistical significance. To rule out the effect of signal decay on delta power during NREM sleep in post-isolation condition (due to time difference and age), the SD (paired) group was normalized with the day 8 of age control group and SD (socially isolated) group was normalized with day 47 of age control group (Fig. 4C).
Highlights
Recovery from sleep deprivation in paired group showed a significant decrease in wake and significant increase in slow wave sleep and rapid eye movement.
Paired mice exhibited significantly higher delta power throughout the dark period.
Socially isolated mice showed significantly higher anxiety than paired mice.
Social isolation stress may adversely affect the quantity and quality of sleep in mice.
Acknowledgments
Part of this work was conducted while authors were in the Department of Pediatrics, Kosair Children's Hospital Research Institute, University of Louisville School of Medicine, Louisville, KY 40202.
Financial Support: DG is supported by National Institutes of Health grants HL-065270 and HL-086662.
Abbreviations
- ANOVA
analysis of variance
- BL
baseline
- EEG
electroencephalogram
- EMG
electromyogram
- EPM
elevated plus maze
- FFT
Fast Fourier Transform
- MAg
gross motor activity
- MANOVA
multivariate analysis of variance
- NREM
non-rapid eye movement
- OF
open field
- REM
rapid eye movement
- SD
sleep deprivation
- SWA
slow wave activity
- Tb
body temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–83. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav. 1999;67:385–385. doi: 10.1016/s0031-9384(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–42. [PubMed] [Google Scholar]
- Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. Am J Epidemiol. 2004;159:167–167. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–13. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1888. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–83. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Mori J, Kubo C. Social isolation stress impairs passive avoidance learning in senescence-accelerated mouse (SAM) Brain Res. 2006;1067:201–201. doi: 10.1016/j.brainres.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Cizza G, Requena M, Galli G, de Jonge L. Chronic sleep deprivation and seasonality: implications for the obesity epidemic. J Endocrinol Invest. 2011 Jun 27; doi: 10.3275/7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–189. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc. 2009;15:868–868. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–257. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- de Jong JG, van der Vegt BJ, Buwalda B, Koolhaas JM. Social environment determines the long-term effects of social defeat. Physiol Behav. 2005;84:87–87. doi: 10.1016/j.physbeh.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Brunner DP, Beersma DG, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–430. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–1417. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, Buysse DJ. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–82. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–107. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gazelle H, Workman JO, Allan W. Anxious solitude and clinical disorder in middle childhood: bridging developmental and clinical approaches to childhood social anxiety. J Abnorm Child Psychol. 2010;38:1–1. doi: 10.1007/s10802-009-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AJ, Billing RL, Einstein R. The effects on heart rate and temperature of mice and vas deferens responses to noradrenaline when their cage mates are subjected to daily restraint stress. Lab Anim. 2008;42:140–140. doi: 10.1258/la.2007.06030e. [DOI] [PubMed] [Google Scholar]
- Haller J, Leveleki C, Baranyi J, Mikics E, Bakos N. Stress, social avoidance and anxiolytics: a potential model of stress-induced anxiety. Behav Pharmacol. 2003;14:439–439. doi: 10.1097/01.fbp.0000087735.21047.e7. [DOI] [PubMed] [Google Scholar]
- Hattori S, Hashimoto R, Miyakawa T, Yamanaka H, Maeno H, Wada K, Kunugi H. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav Brain Res. 2007;180:69–69. doi: 10.1016/j.bbr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Hegde P, Jayakrishnan HR, Chattarji S, Kutty BM, Laxmi TR. Chronic stress-induced changes in REM sleep on theta oscillations in the rat hippocampus and amygdala. Brain Res. 2011;1382:155–155. doi: 10.1016/j.brainres.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Heinrich LM, Gullone E. The clinical significance of loneliness: a literature review. Clin Psychol Rev. 2006;26:695–695. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies, Semin. Clin. Neuropsychiatry. 2002;7:147–147. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–103. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Ota M, Lister RG. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral 'despair'. Pharmacol Biochem Behav. 1989;33:371–371. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA. In Handbook of Neuroscience for the Behavioral Sciences. 2009. Developmental neuroscience. [Google Scholar]
- Jankowska E, Pucilowski O, Kostowski W. Chronic oral treatment with diltiazem or verapamil decreases isolation-induced activity impairment in elevated plus maze. Behav Brain Res. 1991;43:155–155. doi: 10.1016/s0166-4328(05)80065-8. [DOI] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106:5895–5895. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CH. Effects of REM sleep deprivation on a multiple schedule of appetitive reinforcement. Behav Brain Res. 2002;128:205–205. doi: 10.1016/s0166-4328(01)00322-9. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361:2215–2215. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–712. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Mateo Y, Durkin S, Parker T, Marsden CA. Effects of central noradrenaline depletion by the selective neurotoxin DSP-4 on the behaviour of the isolated rat in the elevated plus maze and water maze. Psychopharmacology (Berl) 2001;155:251–251. doi: 10.1007/s002130100702. [DOI] [PubMed] [Google Scholar]
- Machado RB, Suchecki D, Tufik S. Sleep homeostasis in rats assessed by a long-term intermittent paradoxical sleep deprivation protocol. Behav Brain Res. 2005;160:356–356. doi: 10.1016/j.bbr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Machado RB, Suchecki D, Tufik S. Comparison of the sleep pattern throughout a protocol of chronic sleep restriction induced by two methods of paradoxical sleep deprivation. Brain Res Bull. 2006;70:213–213. doi: 10.1016/j.brainresbull.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Maisonnette S, Morato S, Brandao ML. Role of resocialization and of 5-HT1A receptor activation on the anxiogenic effects induced by isolation in the elevated plus-maze test. Physiol Behav. 1993;54:753–753. doi: 10.1016/0031-9384(93)90087-v. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1562. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez D, Obermeyer W, Fahy JL, Riboh M, Kalin NH, Benca RM. REM sleep deprivation induces changes in coping responses that are not reversed by amphetamine. Sleep. 2004;27:609–609. [PubMed] [Google Scholar]
- McGinty D, Alam MN, Szymusiak R, Nakao M, Yamamoto M. Hypothalamic sleep-promoting mechanisms: coupling to thermoregulation. Arch Ital Biol. 2001;139:63–63. [PubMed] [Google Scholar]
- McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1462. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–3. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Modin B, Ostberg V, Almquist Y. Childhood peer status and adult susceptibility to anxiety and depression. A 30-year hospital follow-up. J Abnorm Child Psychol. 2011;39:187–187. doi: 10.1007/s10802-010-9462-6. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara P, Perez-Garcia J. Isolation rearing induced fear-like behavior without affecting learning abilities of Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1111–1111. doi: 10.1016/s0278-5846(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–254. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Morinan A, Parker V, Rich DA, Cariuk P, Horton RW. Social isolation does not alter brain regional benzodiazepine binding site numbers, affinity and coupling in the rat. Psychopharmacology (Berl) 1992;106:565–565. doi: 10.1007/BF02244831. [DOI] [PubMed] [Google Scholar]
- Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via Nnicotinamide Adenine Dinucleotide Phosphate Oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–1305. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu T, Ishii K. Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav Processes. 2008;78:477–477. doi: 10.1016/j.beproc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Newman SM, Paletz EM, Obermeyer WH, Benca RM. Sleep deprivation in pigeons and rats using motion detection. Sleep. 2009;32:1299–1299. doi: 10.1093/sleep/32.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci U S A. 2010;107:16342–16342. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler J, Jahkel M, Schmidt J. Altered neurobiological responses to acute immobilization in social-isolated mice. Pharmacol Biochem Behav. 1986;25:41–41. doi: 10.1016/0091-3057(86)90227-3. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–149. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–318. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Lakshmana MK, S B, Rao S, Raju TR, Kumar VM. Alterations in monoamine neurotransmitters and dendritic spine densities at the medial preoptic area after sleep deprivation. Sleep Res Online. 1999;2:49–49. [PubMed] [Google Scholar]
- Ramesh V, Thatte HS, McCarley RW, Basheer R. Adenosine and sleep deprivation promote NF-kappaB nuclear translocation in cholinergic basal forebrain. J Neurochem. 2007;100:1351–1351. doi: 10.1111/j.1471-4159.2006.04314.x. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Kaushal N, Gozal D. Sleep fragmentation in mice differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Sci. 2009;2:64–64. [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–55. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Shaw DH, Newton TF, Coble PA, Kupfer DJ. EEG sleep in outpatients with generalized anxiety: a preliminary comparison with depressed outpatients. Psychiatry Res. 1983;8:81–81. doi: 10.1016/0165-1781(83)90094-x. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–285. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10:747–53. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184:71–71. doi: 10.1016/j.jneumeth.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25:268–268. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1052. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Tang X, Orchard SM, Liu X, Sanford LD. Effect of varying recording cable weight and flexibility on activity and sleep in mice. Sleep. 2004;27:803–803. [PubMed] [Google Scholar]
- Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–74. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- Tobler I, Scherschlicht R. Sleep and EEG slow-wave activity in the domestic cat: effect of sleep deprivation. Behav Brain Res. 1990;37:109–109. doi: 10.1016/0166-4328(90)90086-t. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A, Viergutz T. Changes in endocrine and immune responses of neonatal pigs exposed to a psychosocial stressor. Res Vet Sci. 2009;87:380–380. doi: 10.1016/j.rvsc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–497. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–969. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0355-4. [Epub ahead of print], PubMed PMID: 22002740. [DOI] [PubMed] [Google Scholar]
- Wei XY, Yang JY, Dong YX, Wu CF. Anxiolytic-like effects of oleamide in group-housed and socially isolated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1189–1189. doi: 10.1016/j.pnpbp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Wiberg GS, Grice HC. Long-term isolation stress in rats. Science. 1963;142:507. doi: 10.1126/science.142.3591.507. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1129. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]