Abstract
Background
Our aim was to determine whether a low plasma adiponectin level is an independent predictor of coronary heart disease (CHD).
Methods and Results
We measured adiponectin levels in frozen plasma samples (−80°C) in a total of 3,188 male and female participants in cycle 6 of the Framingham Offspring Study, using a novel full-automated assay. CHD cases at baseline were excluded, and participants were followed for a mean of 7.5 years (mean age was 57 years in both men and women, and mean BMI 28.5 kg/m2 in men and 27.3 kg/m2 in women). Plasma adiponectin levels (median [25percentile, 75percentile]) were significantly higher in female than male controls (14.8 [10.7, 20.5] μg/mL versus 9.0 [7.0, 12.2] μg/mL, p< 0.001). After adjustment for age, body mass index, smoking status, systolic blood pressure, treatment for hypertension, diabetes status, use of cholesterol-lowering medication, total cholesterol level, and high-density lipoprotein cholesterol level. A decreased plasma adiponectin level was a highly significant predictor of future CHD events (n =117) in men, with a hazards ratio of 0.4728 (p=0.0024). A bottom-quartile value of < 7.0 μg/mL doubled the risk of CHD in men. The identical trend was observed in women; however, the statistical significance of these associations disappeared after multivariate adjustments, possibly due to a low number of female CHD cases (n=60).
Conclusion
An adiponectin level of < 7.0 μg/mL is a powerful independent predictor of CHD in men in the United States.
Keywords: adiponectin, coronary heart disease, risk factor, Framingham Offspring Study, obesity
Introduction
Adiponectin is one of the major proteins produced in adipose tissues and has been found to be pathologically anti-atherogenic molecules (1–3). Adiponectin is also a strong anti-inflammatory compound acting through the nuclear factor kappa beta (NFkB) pathway (1). Adiponectin has been shown to down-regulate adhesion molecule expression in endothelial cells (2). In apo E-deficient mice, administration of adiponectin has been shown to protect against diet-induced atherosclerosis (3).
In contrast to the consensus in pathology, there is debate whether adiponectin is a significant independent risk factor for coronary heart disease (CHD) in epidemiological fields (4–10). In the Health Professional Study a doubling of baseline adiponectin was reported to be associated with a significant 20% reduction in risk of myocardial infarction, a multivariate analysis after adjustment for many risk factors (6). However, other studies including a recent meta-analysis have not supported these observations (8). These studies included the British Regional Health Services follow-up study, Strong Heart Study, as well as the British Women’s Heart Health Study (8,11,12). The difference between these studies and the Health Professionals Study is that they were all carried out in the United Kingdom as opposed to the United States, and their mean body mass indexes were significantly lower. In most studies, plasma adiponectin levels were measured by enzyme-linked immunosorbent assay (ELISA). A novel full-automated assay has been developed for measuring plasma adiponectin levels. The coefficients of variation (CV) within run and between runs of this assay are less than 2%, which are much less than those of ELISA. Using the new assay, we evaluated the hypothesis that plasma adiponectin levels are an independent predictor for CHD coronary heart disease in the Framingham Offspring Study.
Methods
Subjects
The participants in the Framingham Offspring Study (FOS), a long-term community-based prospective observational study of risk factors for coronary heart disease are the offspring and their spouses of the original Framingham Heart Study (FHS) cohort (13,14). During cycle 6 of FOS (1995–1998) participants had a standardized medical history, physical examination, and fasting lipid measurements. Exclusion criteria for controls were any evidence of heart or vascular disease, hepatic or kidney disease, thyroid dysfunction, and drug or alcohol abuse. Selection criteria for the CHD cases included a history of documented myocardial infarction, coronary artery bypass, percutaneous coronary angioplasty, or documented coronary disease on angiography. We performed our analyses on all available plasma samples from male and female participants in cycle 6, which comprised 173 male heart disease cases and 1335 male controls and 74 female cases and 1606 female controls.
Laboratory Measurements
Fasting plasma samples stored at −80 degrees were used. Plasma concentrations of standard lipids and glucose levels had been previously measured. Adiponectin was measured by a latex turbidometric immunoassay with kits obtained from Otsuka Pharmaceutical and Mitsubishi Chemical Medience, Tokyo, Japan. Briefly, agglutination with adiponectin is generated by rabbit anti-human adiponectin polyclonal antibodies immobilized on latex beads by antigen-antibody reaction (15, 16). This assay was performed on a Hitachi 911 automated analyzer, and the coefficients of variation (CV) within and between run assays were 0.6% and 1.3%, respectively. Insulin levels were measured with an automated immunoassay distributed from Kamiya Biomedical, Seattle, WA, and within and between run coefficients of variation were 3.1 and 3.4%, respectively.
Statistical Analysis
Data are expressed as means ± standard deviations for continuous variables or proportions for categorical variables. The distribution of the variables was compared between subjects with or without type 2 diabetes, using non-paired T tests for continuous variables and Chi-square tests for categorical variables. P values <0.05 were considered as statistically significant.
In the prospective analysis, subjects with established coronary heart disease at baseline were excluded and the rest of the participants (1,331 men and 1,603 women) were followed up for a mean of 7.5 years. Hazard ratios for the analysis of CHD outcome were calculated with the use of the Cox proportional-hazards regression model, which was adjusted for body mass index and the Framingham risk factors such as age, smoking, systolic blood pressure, diabetes status, plasma total cholesterol level, plasma high-density lipoprotein cholesterol level, the use of anti-hypertensives, and the use of cholesterol-lowering medications at the 6th visit of FOS.
Results
Characteristics of healthy men and women without CHD or diabetes, not on cholesterol lowering medication, and women not on hormonal replacement therapy at baseline in exam 6 are shown in Table 1. As can be seen, women had significantly higher levels of adiponectin than did men. They also had significantly lower body mass index (BMI), waist size, systolic and diastolic blood pressure, treatment for hypertension, aspirin treatment, use of alcohol, fasting triglyceride, glucose and insulin levels, and significantly higher levels of HDL cholesterol than men. Data on healthy premenopausal and postmenopausal women without CHD or diabetes, and not taking cholesterol lowering medication or hormonal replacement therapy are shown in Table 2. Postmenopausal women had significantly higher age, waist size, systolic blood pressure, antihypertensive treatment rate, aspirin use, and fasting levels of triglyceride, LDL cholesterol, glucose, insulin, and adiponectin than premenopausal women.
Table 1.
Characteristics, disease prevalence, and plasma glucose, insulin, adiponectin, and lipid levels in men and women participating in the Framingham Offspring Study (Exam 6) without coronary heart disease, diabetes mellitus, or receiving hormone replacement therapy
| Variable | Men (n = 1080) | Women (n = 1012) | p value for differences |
|---|---|---|---|
|
|
|||
| Age (years) | 57.1 ± 9.7 | 57.4 ± 10.5 | 0.4851 |
| Body Mass Index (kg/m2) | 28.2 ± 4.3 | 27.1 ± 5.6 | <0.0001 |
| Body Mass Index > 30 (%) | 27.2 | 24.2 | *0.1146 |
| Waist (cm) | 100.4 ± 10.7 | 93.1 ± 14.7 | <0.0001 |
| Waist > 102cm (%) | 39.8 | 23.6 | *<0.0001 |
| Systolic Blood Pressure (mmHg) | 128.3 ± 16.5 | 124.8 ± 19.3 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 77.8 ± 9.3 | 73.7 ± 9.3 | <0.0001 |
| Hypertension (%) | 36.1 | 30.7 | *0.0091 |
| Hypertensive Treatment (%) | 21.2 | 17.9 | *0.0561 |
| Taking Aspirin Regularly (%) | 26.1 | 17.6 | *<0.0001 |
| Cigarette Smokers (%) | 15.1 | 16.2 | *0.4832 |
| > One drink alcohol/week (%) | 55.9 | 30.9 | *<0.0001 |
| TC (mg/dl) | 201.4 ± 35.7 | 211.8 ± 39.5 | <0.0001 |
| TG (mg/dl) | 114 [81, 160] | 100 [73, 142] | <0.0001 |
| Log (TG) | 4.73 ± 0.51 | 4.64 ± 0.48 | <0.0001 |
| HDL-cholesterol (mg/dl) | 44.9 ± 12.3 | 57.1 ± 15.2 | <0.0001 |
| LDL-cholesterol (mg/dl) | 130.7 ± 31.6 | 131.3 ± 35.5 | 0.7218 |
| Fasting Glucose (mg/dl) | 99.4 ± 9.3 | 95.2 ± 10.1 | <0.0001 |
| Insulin (μU/ml) | 10.8 [8.4,14.6] | 10.2 [8.0,13.4] | 0.0001 |
| Log (Insulin) | 2.43 ± 0.42 | 2.36 ± 0.40 | <0.0001 |
| Adiponectin (μg/ml) | 9.0 [7.0,12.2] | 14.8 [10.7,20.5] | <0.0001 |
| Log (Adiponectin) | 2.23 ± 0.44 | 2.68 ± 0.47 | <0.0001 |
Values are expressed as mean ± SD, median [25th percentile, 75th percentile], or percentage.
p-value obtained by Chi-Square test
Table 2.
Characteristics, disease prevalence, and plasma glucose, insulin, adiponectin, and lipid levels in premenopausal and postmenopausal women participating in the Framingham Offspring Study (Exam 6) without coronary heart disease, diabetes mellitus, nor hormone replacement therapies
| Variable | Premenopausal (n = 313) | Postmenopausal (n = 698) | p value for differences |
|---|---|---|---|
|
|
|||
| Age (years) | 46.4 ± 5.0 | 62.4 ± 8.3 | <0.0001 |
| Body Mass Index (kg/m2) | 26.7 ± 5.5 | 27.2 ± 5.7 | 0.1622 |
| Body Mass Index > 30 (%) | 23.8 | 24.4 | *0.8383 |
| Waist (cm) | 89.4 ± 13.6 | 94.7 ± 14.8 | <0.0001 |
| Waist > 102cm (%) | 16.6 | 26.7 | *0.0005 |
| Systolic Blood Pressure (mmHg) | 115.3 ± 15.5 | 129.1 ± 19.3 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 72.9 ± 9.2 | 74.0 ± 9.3 | 0.0787 |
| Hypertension (%) | 15.1 | 37.7 | *<0.0001 |
| Hypertensive Treatment (%) | 9.0 | 22.0 | *<0.0001 |
| Taking Aspirin Regularly (%) | 9.3 | 21.4 | *<0.0001 |
| Cigarette Smokers (%) | 16.3 | 16.2 | *0.9741 |
| > One drink alcohol/week (%) | 34.8 | 29.1 | *0.0715 |
| TC (mg/dl) | 194.4 ± 36.4 | 219.5 ± 38.4 | <0.0001 |
| TG (mg/dl) | 83 [64,120] | 106 [79,153] | <0.0001 |
| TG (mg/dl) | 4.49 ± 0.45 | 4.71 ± 0.48 | <0.0001 |
| HDL-cholesterol (mg/dl) | 56.8 ± 15.1 | 57.3 ± 15.2 | 0.6906 |
| LDL-cholesterol (mg/dl) | 117.8 ± 34.1 | 137.3 ± 34.4 | <0.0001 |
| Fasting Glucose (mg/dl) | 91.7 ± 8.3 | 96.7 ± 10.5 | <0.0001 |
| Insulin (μU/ml) | 9.2 [7.6,12.2] | 10.6 [8.2,14.0] | #0.0002 |
| Log (Insulin) | 2.29 ± 0.38 | 2.40 ± 0.40 | <0.0001 |
| Adiponectin (μg/ml) | 13.0 [9.6,17.3] | 15.8 [11.3,22.3] | <0.0001 |
| Log (Adiponectin) | 2.54 ± 0.45 | 2.75 ± 0.47 | <0.0001 |
Values are expressed as mean ± SD, median [25th percentile, 75th percentile], or percentage.
p-value obtained by Chi-Square test
p-value obtained by using log-transformed values
Normal ranges for fasting levels of adiponectin, glucose, and insulin are presented in Table 3 for the same subjects as selected for tables 1 and 2 (i.e. no CHD, diabetes, cholesterol lowering medication, or hormonal replacement therapy). The 25th percentile value for adiponectin for men was 7.0 micrograms per mL, while for premenopausal women this value was 9.6 micrograms per mL, and for postmenopausal women it was 11.3 micrograms per mL.
Table 3.
Means and selected percentiles of plasma glucose, insulin and adiponectin levels in normal population, by gender and menopausal status for women
| N | Mean ± SD | Percentiles
|
|||||
|---|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 75 | 90 | |||
|
| |||||||
| Glucose (mg/dl) | |||||||
| Men | 1080 | 99.4 ± 9.3 | 88.0 | 93.0 | 99.0 | 105.0 | 112.0 |
| Women | 1012 | 95.2 ± 10.1 | 83.0 | 88.0 | 94.0 | 100.0 | 109.0 |
| Premenopausal Women | 313 | 91.7 ± 8.3 | 82.0 | 86.0 | 91.0 | 97.0 | 102.0 |
| Postmenopausal Women | 698 | 96.7 ± 10.5 | 84.0 | 89.0 | 95.0 | 103.0 | 112.0 |
| Insulin (μU/ml) | |||||||
| Men | 1080 | 12.6 ± 6.3 | 6.9 | 8.4 | 10.8 | 14.6 | 20.5 |
| Women | 1012 | 11.6 ± 5.3 | 6.6 | 8.0 | 10.2 | 13.4 | 18.5 |
| Premenopausal Women | 313 | 10.7 ± 4.8 | 6.3 | 7.6 | 9.2 | 12.2 | 16.7 |
| Postmenopausal Women | 698 | 12.0 ± 5.5 | 6.8 | 8.2 | 10.6 | 14.0 | 19.0 |
| Adiponectin (μg/ml) | |||||||
| Men | 1080 | 10.3 ± 5.2 | 5.6 | 7.0 | 9.0 | 12.2 | 16.2 |
| Women | 1012 | 16.3 ± 7.7 | 7.9 | 10.7 | 14.8 | 20.5 | 26.7 |
| Premenopausal Women | 313 | 14.0 ± 6.1 | 7.0 | 9.6 | 13.0 | 17.3 | 21.9 |
| Postmenopausal Women | 698 | 17.3 ± 8.1 | 8.4 | 11.3 | 15.8 | 22.3 | 28.4 |
Data comparing heart disease cases and controls at cycle 6 are presented in Table 4 and 5. As can clearly be seen, adiponectin levels were not significantly different between CHD cases and controls at the baseline examination in men; however, they were significantly lower in women (p=0.0121). Men with heart disease had significantly higher age, diastolic blood pressure, prevalence of diabetes, use of antihypertensive medication, beta blockers, oral glucose lowering agents, insulin, and cholesterol lowering medication, than men without CHD (See Table 4). Male CHD cases also had significantly higher fasting levels of triglyceride, glucose, and insulin, and significantly lower levels of LDL cholesterol and HDL cholesterol than male non-cases (see Table 4). Women with heart disease had significantly higher age, body mass index, waist size, systolic blood pressure, prevalence of diabetes, use of antihypertensive medication, beta blockers, oral glucose lowering agents, insulin, and cholesterol lowering medication, fasting levels of triglyceride, glucose, and insulin, and significantly lower levels of LDL cholesterol and HDL cholesterol as compared to women without CHD (see Table 5).
Table 4.
Characteristics and plasma glucose, insulin, adiponectin, and lipid levels in male subjects with and without coronary heart disease participating in the Framingham Offspring Study (Exam 6)
| Variable | Coronary Heart Disease | ||
|---|---|---|---|
| NO (n = 1335) | YES (n = 173) | p value for differences | |
|
| |||
| Age (years) | 58.0 ± 9.7 | 65.3 ± 7.9 | <0.0001 |
| Body Mass Index (kg/m2) | 28.5 ± 4.4 | 28.7 ± 4.3 | 0.6249 |
| Body Mass Index > 30 (%) | 30.1 | 31.8 | *0.6542 |
| Waist (cm) | 101.3 ± 10.9 | 102.4 ± 10.9 | 0.1922 |
| Waist > 102cm (%) | 42.6 | 46.2 | *0.3669 |
| Systolic Blood Pressure (mmHg) | 129.6 ± 17.1 | 129.4 ± 17.6 | 0.8633 |
| Diastolic Blood Pressure (mmHg) | 77.7 ± 9.3 | 73.4 ± 9.5 | <0.0001 |
| Hypertension (%) | 41.9 | 66.5 | *<0.0001 |
| Hypertensive Treatment (%) | 27.7 | 58.5 | *<0.0001 |
| Taking Aspirin Regularly (%) | 31.1 | 78.0 | *<0.0001 |
| Diabetes Mellitus (%) | 10.1 | 26.6 | *<0.0001 |
| Oral Glycemic Control Drug Users (%) | 4.4 | 12.2 | *<0.0001 |
| On Insulin Treatment (%) | 1.4 | 4.6 | *0.0029 |
| β-blocker Users (%) | 9.4 | 54.9 | *<0.0001 |
| Cholesterol Lowering Drug Users (%) | 11.4 | 46.8 | *<0.0001 |
| Cigarette Smokers (%) | 14.5 | 12.7 | *0.5190 |
| > One drink alcohol/week (%) | 54.5 | 46.8 | *0.0563 |
| TC (mg/dl) | 199.7 ± 35.9 | 186.0 ± 36.7 | <0.0001 |
| TG (mg/dl) | 118 [83,166] | 127 [93,187] | #0.0127 |
| Log (TG) | 4.77 ± 0.52 | 4.88 ± 0.51 | 0.0127 |
| HDL-cholesterol (mg/dl) | 44.1 ± 12.3 | 40.2 ± 11.0 | <0.0001 |
| LDL-cholesterol (mg/dl) | 128.5 ± 31.7 | 115.4 ± 30.2 | <0.0001 |
| Fasting Glucose (mg/dl) | 106.2 ± 26.8 | 116.2 ± 34.1 | 0.0003 |
| Insulin (μU/ml) | 11.3 [8.6,15.5] | 12.3 [8.8,18.1] | #0.0404 |
| Log (Insulin) | 2.48 ± 0.45 | 2.57 ± 0.53 | 0.0404 |
| Adiponectin (μg/ml) | 8.9 [6.7,11.9 ] | 8.4 [6.1,12.5] | #0.2761 |
| Log (Adiponectin) | 2.20 ± 0.45 | 2.16 ± 0.51 | 0.2761 |
Values are expressed as mean ± SD, median [25th percentile, 75th percentile], or percentage.
p-value obtained by Chi-Square test
p-value obtained by using log-transformed values
Table 5.
Characteristics and plasma glucose, insulin, adiponectin, and lipid levels in female subjects with and without coronary heart disease participating in the Framingham Offspring Study (Exam 6)
| Variable | Coronary Heart Disease | ||
|---|---|---|---|
| NO (n = 1606) | YES (n = 74) | p value for differences | |
|
| |||
| Age (years) | 58.1 ± 9.6 | 66.1 ± 8.0 | <0.0001 |
| Body Mass Index (kg/m2) | 27.3 ± 5.7 | 29.1 ± 5.4 | 0.0082 |
| Body Mass Index > 30 (%) | 25.8 | 35.1 | *0.0757 |
| Waist (cm) | 93.9 ± 14.8 | 101.1 ± 13.3 | <0.0001 |
| Waist > 102cm (%) | 26.3 | 39.7 | *0.0110 |
| Systolic Blood Pressure (mmHg) | 126.5 ± 19.6 | 140.4 ± 24.6 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 73.9 ± 9.1 | 73.6 ± 11.6 | 0.8502 |
| Hypertension (%) | 35.8 | 83.8 | *<0.0001 |
| Hypertensive Treatment (%) | 22.9 | 73.0 | *<0.0001 |
| Taking Aspirin Regularly (%) | 19.8 | 62.2 | *<0.0001 |
| Diabetes Mellitus (%) | 6.9 | 25.0 | *<0.0001 |
| Oral Glycemic Control Drug Users (%) | 2.5 | 10.8 | *<0.0001 |
| On Insulin Treatment (%) | 1.2 | 9.5 | *<0.0001 |
| β-blocker Users (%) | 8.8 | 47.3 | *<0.0001 |
| Cholesterol Lowering Drug Users (%) | 8.8 | 35.1 | *<0.0001 |
| On Estrogen Therapy (%) | 26.3 | 23.0 | *0.5307 |
| Postmenopause (%) | 76.5 | 94.6 | *0.0003 |
| Cigarette Smokers (%) | 15.5 | 18.9 | *0.4322 |
| > One drink alcohol/week (%) | 30.5 | 21.6 | *0.1035 |
| TC (mg/dl) | 211.9 ± 38.3 | 215.8 ± 39.3 | 0.3887 |
| TG (mg/dl) | 111.0 [78,163] | 135.5 [95,182] | #0.0030 |
| Log (TG) | 4.74 ± 0.51 | 4.92 ± 0.56 | 0.0030 |
| HDL-cholesterol (mg/dl) | 57.4 ± 16.0 | 54.3 ± 16.1 | 0.0725 |
| LDL-cholesterol (mg/dl) | 128.2 ± 34.6 | 128.6 ± 31.8 | 0.9241 |
| Fasting Glucose (mg/dl) | 99.8 ± 25.6 | 117.9 ± 44.6 | 0.0009 |
| Insulin (μU/ml) | 10.2 [8.0,13.6] | 13.6 [9.7,18.7] | #<0.0001 |
| Log (Insulin) | 2.37 ± 0.43 | 2.67 ± 0.54 | <0.0001 |
| Adiponectin (μg/ml) | 14.5 [10.4,19.9] | 12.4 [8.6,17.6] | #0.0121 |
| Log (Adiponectin) | 2.65 ± 0.47 | 2.51 ± 0.48 | 0.0121 |
Values are expressed as mean ± SD, median [25th percentile, 75th percentile], or percentage.
p-value obtained by Chi-Square test
p-value obtained by using log-transformed values
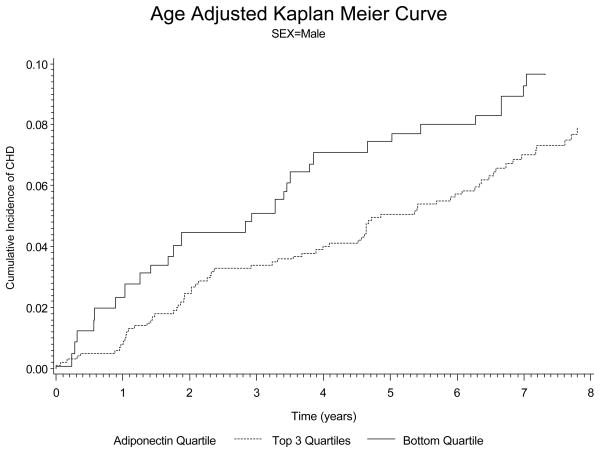
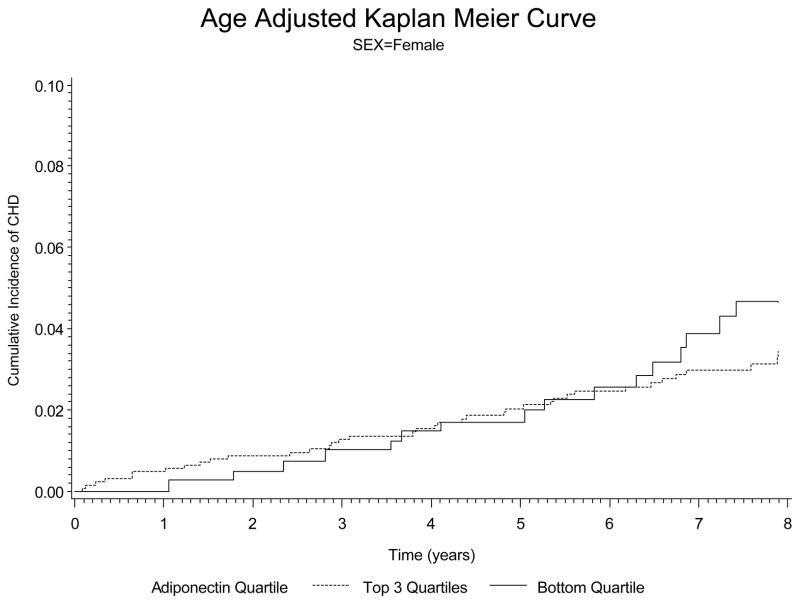
With regard to prospective analysis of the data, there were 117 new CHD cases in men and 60 new cases of CHD in the women over a mean of 7.5 years of follow-up. Data on the hazards ratio for adiponectin after various adjustments is shown in Table 6. In men The hazards ratio for low adiponectin was 0.4141 (p<0.001) in men after adjustment for age and smoking. The hazards ratio for low adiponectin in men remained significant at 0.4728 (p=0.0024) after adjustment for all risk factors (age, body mass index, smoking, diabetes, blood pressure, total cholesterol, and HDL cholesterol) and use of cholesterol lowering and antihypertensive therapy (see Table 6). In women, a low adiponectin was also a significant risk factor for CHD after adjustment for age and smoking with a hazards ratio of 0.4278 (p=0.0017). However after adjustment for all risk factors and medication use, adiponectin was no longer significant (hazards ratio 0.7101, p=0.3083). In figure 1 and 2 we show the plots based on incidence of coronary heart disease. These data clearly indicate that lower levels of adiponectin markedly increase the risk of CHD in men (figure 1). In the women we see a similar trend, but our ability to detect significance in women may have been affected by a smaller number of new CHD cases than in the men (figure 2).
Table 6.
Hazard ratio for coronary heart disease of adiponectin after adjusting conventional risk factors in male and female subjects. (mean follow-up period was 7.5 years) *
| Male | Female | |||
|---|---|---|---|---|
| Hazard Ratio | P value | Hazard Ratio | P value | |
|
|
||||
| Model 1 | 0.4141 | <0.0001 | 0.4278 | 0.0017 |
| Model 2 | 0.4731 | 0.0005 | 0.5693 | 0.0488 |
| Model 3 | 0.4610 | 0.0004 | 0.5945 | 0.0735 |
| Model 4 | 0.4870 | 0.0033 | 0.8124 | 0.5229 |
| Model 5 | 0.4728 | 0.0024 | 0.7107 | 0.3083 |
Covariates: Model 1; age and smoking status,
Model 2; age, smoking status, systolic blood pressure, hypertensive treatment, and diabetes status,
Model 3; age, smoking status, systolic blood pressure, hypertensive treatment, diabetes status, and cholesterol lowering medication,
Model 4; age, smoking status, systolic blood pressure, hypertensive treatment, diabetes status, cholesterol lowering medication, total cholesterol level, and high-density lipoprotein cholesterol level.
Model 5; age, smoking status, systolic blood pressure, hypertensive treatment, diabetes status, cholesterol lowering medication, total cholesterol level, high-density lipoprotein cholesterol level, and body mass index.
There were 117 new coronary heart disease cases in men out of 1332 participants, and 60 cases in women out of 1605 participants.
Figure 1.
Kaplan-Meyer curves for the baseline plasma adiponectin levels and the cause of cardiovascular diseases in men after adjusted for age. The bottom quartile of plasma adiponectin levels had significantly higher event rates than the top 3 quartiles.
Figure 2.
Kaplan-Meyer curves for the baseline plasma adiponectin levels and the cause of cardiovascular diseases in women after adjusted for age. The bottom quartile of plasma adiponectin levels had significantly higher event rates than the top 3 quartiles.
Discussion
Our data clearly indicate that adiponectin is a significant independent predictor of heart disease in men, with a similar trend being observed in women. The major difference between our population and those of other investigators was that the mean body mass index (BMI) in our population was considerably higher, and our data may be more relevant to US populations. Even in normal subjects, the mean BMI was 27.0 kg/m2 in women, which was higher than the subjects in other observations (3–10). However, the results showed that the hazard ratio did not change after adjusting BMI, which indicated that obesity did not effect on the relationship between adiponectin levels and the cause of CHD events. Regarding obesity and/or insulin resistance, a parameter using the combination of plasma adiponectin levels and the homeostasis model assessment (HOMA) was also proposed to provide a highly discriminatory parameter for CHD risk assessment (17). We calculated the HOMA and the HOMA/adiponectin ratio, but unfortunately they were not better marker than plasma adiponectin level itself in the FOS. These findings indicated that adiponectin is a unique marker and probably different from other markers for insulin resistance.
There was significant difference in plasma adiponectin levels between men and women, consistent with previous studies (5,7,9,10). In addition, postmenopausal women had significantly higher levels of plasma adiponectin than premenopausal women. For determining reference range for plasma adiponectin level, the gender difference and menopausal status should be taken into consideration.
At baseline, plasma adiponectin levels were not significantly lower in male CHD cases than in male controls, however the prospective analysis showed they were an independent predictor for future CHD risks. These findings indicate that an adiponectin level of < 7.0 μg/mL is a powerful independent predictor of heart disease in men. Practically a high plasma adiponectin level may not be problem but a low adiponectin level is surely problem in men.
It has been shown weight loss and exercise can increase plasma adiponectin levels (18,19). In addition some methods have been shown to be effective. Thiazolidinediones, metformin and niacin have all been reported to increase plasma adiponectin levels significantly (20–24). Among 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA)-reductase inhibitors (statins), pravastatin has been reported to increase plasma adiponectin levels significantly (25.26). Other statins have not been reported to have a significant effect on plasma adiponectin levels. On the other hand, some drugs might have considerable effects on adiponectin levels, which we do not know very well yet. In this study, the 90 percentile of plasma adiponectin levels in healthy postmenopausal women was 28.4μg/ml. Some studies reported that the mean plasma adiponectin levels in the top quartile was over 40μg/ml and their conclusions were high adiponectin levels increase the risk for coronary events and/or all mortality. Even though the methods for the adiponectin measurement were different, we could speculate that some factors (e.g. medication) increase plasma adiponectin levels, causing discrepant results in population studies.
Our measurements of plasma adiponectin levels were done using assay kits that could be run on an automated analyzer and the CVs were excellent. Other assays for measuring plasma high-molecular-weight adiponectin levels have been developed, but they are not automated, are more labor-intensive, and have higher coefficients of variation (27).
In summary, our data in the Framingham Offspring Study indicate that low adiponectin is a significant independent CHD risk factor in men. For middle age individuals, low plasma adiponectin is a considerable risk for CHD. Any intervention for raising adiponectin levels >7.0μg/ml could be helpful for preventing CHD.
Abbreviations
- CHD
Coronary Heart Disease
- FOS
Framingham Offspring Study
- TC
Total Cholesterol
- TG
Triglyceride
- HDL-C
High Density Lipoprotein Cholesterol
- LDL
low density lipoprotein
References
- 1.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 4.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 5.Wolk R, Berger P, Lennon RJ, Brilakis ES, Davison DE, Somers VK. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28:292–8. doi: 10.1093/eurheartj/ehl361. [DOI] [PubMed] [Google Scholar]
- 6.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 7.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and Coronary Heart Disease; a Procpective Study and Meta-Analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 9.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and Risk of Coronary Heart Disease in Older Men and Women. J Clin Endocrinol Metab. 2008;93:3357–64. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CDA, Snijder MB, Bouter LM, Matsuzawa Y, Shimomura I, Heine RJ. Prognostic Value of Adiponectin for Cardiovascular Disease and Mortality. J Clin Endocrinol Metab. 2008;93:1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay RS, Resnick HE, Zhu J, Tun ML, Howard BV, Zhang Y, Yeh J, Best LG. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–e16. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–7. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2006;371:163–8. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CDA, Nijpels G, Funahashi T, Matsuzawa Y, Shimomura I, Dekker JM. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: The Hoorn Study. Diabetes Care. 2006;29:2498–2503. doi: 10.2337/dc06-0952. [DOI] [PubMed] [Google Scholar]
- 17.Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Funahashi T, Matsuzawa Y. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007;77:151–4. doi: 10.1016/j.diabres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 19.Yatagai T, Nishida Y, Nagasaka S, Nakamura T, Tokuyama K, Shindo M, Tanaka H, Ishibashi S. Relationship between exercise training-induced increase in insulin sensitivity and adiponectinemia in healthy men. Endocr J. 2003;50:233–8. doi: 10.1507/endocrj.50.233. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–9. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 21.Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, Wang JP, Chen CL, Tai TY, Chuang LM. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–380. doi: 10.2337/diacare.25.2.376. [DOI] [PubMed] [Google Scholar]
- 22.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, Baxi S, Mudaliar SR, Henry RR. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–74. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- 23.Westphal S, Borucki K, Taneva E, Makarova R, Luley C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis. 2007;193:361–5. doi: 10.1016/j.atherosclerosis.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Extended-Release Niacin Alters the Metabolism of Plasma Apolipoprotein (Apo) A-I-and ApoB-Containing Lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–8. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama S, Fukushima H, Kugiyama K, Maruyoshi H, Kojima S, Funahashi T, Sakamoto T, Horibata Y, Watanabe K, Koga H, Sugamura K, Otsuka F, Shimomura I, Ogawa H. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis. 2007;194:e43–51. doi: 10.1016/j.atherosclerosis.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto K, Sakamoto T, Ogawa H Kumamoto Joint Research on Hypercholesterolemia Investigators. The effect of 6 months of treatment with pravastatin on serum adiponection concentrations in Japanese patients with coronary artery disease and hypercholesterolemia: a pilot study. Clin Ther. 2006;28:1012–21. doi: 10.1016/j.clinthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Nakano Y, Tajima S, Yoshimi A, Akiyama H, Tsushima M, Tanioka T, Negoro T, Tomita M, Tobe T. A novel enzyme-linked immunosorbent assay specific for high-molecular-weight adiponectin. J Lipid Res. 2006;47:1572–82. doi: 10.1194/jlr.D600010-JLR200. [DOI] [PubMed] [Google Scholar]