Abstract
Background
Immunocompromised patients, particularly after lung transplantation, are at high risk to develop atypical forms of pulmonary infections including influenza A/H1N1. Acute Fibrinous and Organizing Pneumonia (AFOP) is a special histological pattern in acute respiratory failure with high mortality.
Case presentation
We describe a 66-year-old woman with double lung transplantation in August 2009 due to end stage pulmonary fibrosis. After prolonged weaning and subsequent promising course, she developed atypical pneumonia with diffuse pulmonary infiltrates in both lungs in January 2010. Infection with influenza A/H1N1 virus was verified. The patient rapidly suffered from respiratory insufficiency and died eight days after this diagnosis. The post-mortem revealed especially in the lower parts of the lungs the classical histological pattern of pure AFOP. Molecular analyses of lung tissue were positive for influenza A/H1N1.
Conclusion
To our knowledge we present the first case of AFOP triggered by viral infection, here proven to be influenza virus A/H1N1. Thus, also in the setting of viral infection the highly deadly differential diagnosis of AFOP must be considered.
Keywords: AFOP, Influenza A/H1N1, Acute lung failure, Lung transplantation, Viral infection
Background
Immunocompromised patients, especially after lung transplantation are at high risk to develop atypical pneumonias. Among viral infections, influenza A/H1N1 pneumonia can lead to severe respiratory conditions in this patient group often resulting in deadly respiratory insufficiency. Acute fibrinous and organizing pneumonia (AFOP) is a distinct reaction pattern in acute respiratory failure with high mortality rate of upto 90%. Although some causes of AFOP are known so far, its pure form without the presence of accompanying hyaline membranes typical for diffuse alveolar damage (DAD) has not been described to be linked to viral pneumonias.
Case presentation
Clinical course
The 66-year old caucasian female patient suffered from end-stage pulmonary fibrosis caused by usual interstitial pneumonia (UIP) first diagnosed in November 2004. According to the guidelines of the International Society for Heart and Lung Transplantation, the patient was submitted to double sided lung transplantation in August 2009. Immediate post-operative course was complicated by prolonged respiratory weaning with the necessity of percutaneous tracheotomy. The patient could not be immunized with influenza vaccination due to poor general condition in September and October 2009, the vaccination period for the seasonally expected influenza viruses. In November 2009, the patient was transferred to rehabilitation treatment. During the end of December 2009 and the beginning of January 2010, she complained about persistent cough. Because of aggravation of her general and especially respiratory condition, she was admitted to a local district hospital and, due to respiratory deterioration, subsequent intubation was needed. High-dose corticosteroid therapy was initiated to overcome acute organ rejection. As respiratory insufficiency further deteriorated, she was finally transferred to the intensive care unit of the medical department at the University Medical Center Freiburg.
Nasotracheal secretion was positive for viral influenza A/H1N1 RNA, proved by RT-PCR. Under the working diagnosis of influenza A/H1N1 pneumonia with bacterial superinfection, therapy with meropenem, clarithromycin and oseltamivir was initiated. Because a mutation causing resistance against Oseltamivir was detected.in further PCR work-up, oseltamivir was subsequently switched to zanamivir. Immunosuppressive therapy was continued with prednisolone and mycophenolate-mofetil, while tacrolimus was interrupted due to high serum levels.
Because of acute renal failure and suspected heparin-induced thrombocytopenia type II, continuous venous-venous hemofiltration was initiated. Despite extracorporeal interventional lung assist implantation, sufficient carbon dioxide elimination could not be reached and the patient died due to rapidly progressive respiratory failure four days after the diagnosis of influenza A/H1N1 infection of the lung (timeline of the clinical course see Table 1).
Table 1.
Timeline of the clinical course
| Date | Clinical course | Therapy |
|---|---|---|
| November 2004 |
Diagnosis of usual interstitial pneumonia (UIP) |
|
| August 2009 |
|
Double lung transplantation, complicated postoperative course |
| November 2009 |
Rehabilitation treatment |
|
| December 2009 - January 2010 |
Persistent cough |
Admission to general hospital |
| 3.1.2010 |
Aggravation of general/ respiratory condition |
Admission to intensive care unit |
| Respiratory insufficiency |
Intubation |
|
| 5.1.2010 |
Nasotracheal secretion positive for viral influenza A/H1N1 RNA (working diagnosis: H1N1 pneumonia with bacterial superinfection) |
Meropenem, clarithromycin and oseltamivir |
| 7.1.2010 |
Detection of viral influenza A/H1N1 RNA by real-time PCR (bronchoscopy) |
|
| Detection of mutation causing resistence against oseltamivir |
Switch from oseltamivir to zanamivir |
|
| Acute renal failure |
Continuous venous-venous hemofiltration |
|
| 8.1.2010 |
Respiratory insufficiency |
Extracorporeal interventional lung assist implantation |
| 11.1.2010 | Rapidly progressive respiratory failure | Death |
Bronchoscopy
After admission to the medical intensive care unit, bronchoscopy was performed to evaluate initial differential diagnoses of acute graft rejection and infectious pneumonia. Bronchial mucosa was edematous and reddened. Mucus was viscous but not extensive in amount. Surgical anastomoses were inconspicuous. No pus or signs of acute bacterial infection could be observed. Bronchioalveolar lavage (BAL) fluid and sputum were sent to microbiological examination.
Imaging
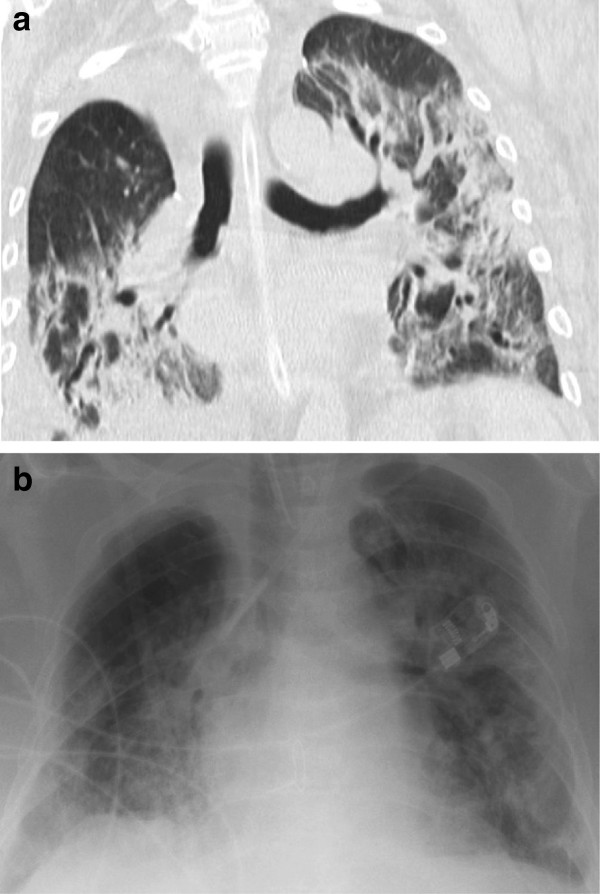
Chest radiography revealed increasing bilateral diffuse pulmonary infiltrates in both lungs with ground glass opacities. Additionally, bronchiectasis and consolidation in the right lower lobe were noticed (Figure 1).
Figure 1.
Radiographic findings: computer tomography shows bronchiectasis and consolidation especially in the right lower lobe (a) and increasing bilateral diffuse pulmonary infiltrates in both lungs with ground glass opacities seen in chest x-ray (b).
Infectious workup
In sputum and BAL samples, infection with influenza virus A/H1N1 could be verified four days prior to death by real-time RT-PCR [1]. Multiplex-PCR proved to be negative for other bacterial or viral infections. Accordingly, cytomegalovirus PCR, cell culture assays and bacterial and fungal cultures were negative during her last hospitalisation period at the University Medical Center Freiburg.
Post mortem
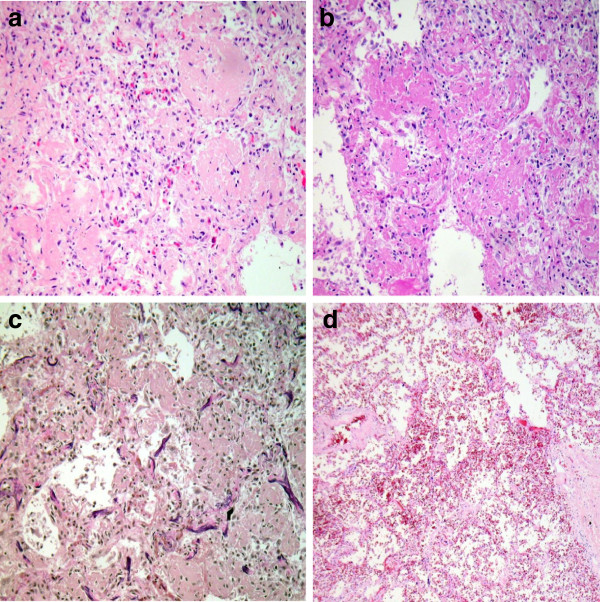
Thoracic situs with status post double sided lung transplantation was evident with sufficient anastomoses of the transplanted organs. The lungs were consolidated in consistency but patchy discolorization typical for bacterial pneumonic infections were not present. Especially the lower parts of the lungs showed classical microscopic features of AFOP with distinct patchy intraalveolar formation of fibrin balls, focal fibroblast foci and diffuse organizing pneumonia (Figure 2). Hyaline membranes were not detected in any histological specimens. Thus, pure AFOP not associated with other histological patterns of DAD was verified. No signs of graft rejection, bacterial or fungal superinfections or a relapse of UIP were seen.
Figure 2.
Postmortem histological findings in the lungs. a-c) AFOP: patchy involvment of lung parenchyma of the lower lobe with cubic intraalveolar fibrin deposits (so called fibrin balls) and formation of fresh fibroblast foci as sing for organizing pneumonia, hyaline membranes are absent (10×; H&E, PAS and EvG staining); d) unaffected lung parenchyma of the upper lobe (10×; H&E staining).
Molecular analysis of lung tissue revealed positive results for influenza A/H1N1by PCR. There was no detection of any bacterial or fungal infection in any analysed lung specimen, neither by microbiological nor by histological examination.
Discussion
Epidemic influenza A/H1N1 originally developed in the United States of America and Mexico in 2009 and finally spread worldwide to a pandemic disease [2,3]. The first case in Germany was registered in April 2009 [4]. Influenza virus infections may cause serious pulmonary complications with death rates of approximately 7% [5,6]. Although previously healthy patients may suffer from severe H1N1 infection, mortality proved to be particularly high in patients with predisposing conditions, i.e. obesity, pulmonary diseases, other than asthma or chronic obstructive pulmonary disease, pregnancy or pneumonia [6].
Patients with primary or secondary immunodeficiencies, particularly patients after lung transplantation are at especially high risk for development of severe pulmonary complications caused by influenza A/H1N1 infections. This, therefore, demonstrates a severe condition in lung transplanted patients [2,5,7].
AFOP was first described by Beasley et al. [8] in 2002 and represents a distinct histological pattern of lung injury related to DAD. Beasley described two forms of AFOP: one less aggressive with good response to corticosteroids, the other with poor clinical outcome and a high mortality rate of 53% [8]. AFOP presents with specific histological features, i.e. patchy intraalveolar fibrin deposits, diffuse organizing pneumonia and absence of hyaline membranes. In the current literature, causes of AFOP are idiopathic or in combination with collagen vascular diseases [9], bacterial infections [10] and after certain drug exposure (e.g. amiodarone, abacavir [8,11], statins [12] as well as exposure to inhalative agents (e.g. coal mining, construction, zoological work, hairspray) [8].
One of a newborn child is reported with acute respiratory distress syndrome and AFOP after respiratory syncytial virus pneumonitis [13]. However, histology revealed hyaline membranes in the vicinity of AFOP-patterns, thus classifying it as DAD with focal patterns of AFOP. In contrast, upon autopsy of our patient no hyaline membranes were detected in any tissue sample. Therefore, in the case presented here, pure AFOP in association with viral infection is to be diagnosed.
Conclusion
Concluding, for the first time proof is given that viral agents at least of the myxovirus family - here influenza A/H1N1 - can induce pure often fatal AFOP. Consequently, AFOP in the setting of viral pneumonia must be taken into account especially in immunocompromised patients as successful treatment with a combination of corticosteroids and mycophenolate-mofetil has recently been reported [14].
Consent
Informed and written consent for post-mortem investigation, scientific research and following publication as clinical image was given by the patient prior to death.
Competing interests
The authors declare that no competing interests exist.
Authors’ contributions
CO, GK: autopsy workup, preparation of the manuscript. DH: virological investigations, preparation of the manuscript. LK: radiological evaluation, preparation of the manuscript. CB, AH, SR, TP: clinical care, provision of clinical data, proof reading. MW: Histological evaluation, methodological support, proof reading. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Claudia Otto, Email: claudia.otto@uniklinik-freiburg.de.
Daniela Huzly, Email: daniela.huzly@uniklinik-freiburg.de.
Lars Kemna, Email: lars.kemna@uniklinik-freiburg.de.
Annegret Hüttel, Email: annegret.huettel@uniklinik-freiburg.de.
Christoph Benk, Email: christoph.benk@uniklinik-freiburg.de.
Siegbert Rieg, Email: siegbert.rieg@uniklinik-freiburg.de.
Till Ploenes, Email: till.ploenes@uniklinik-freiburg.de.
Martin Werner, Email: martin.werner@uniklinik-freiburg.de.
Gian Kayser, Email: gian.kayser@uniklinik-freiburg.de.
Acknowledgements
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding program Open Access Publishing.
References
- Panning M, Baumgarte S, Laue T, Bierbaum S, Raith S, Drexler JF, Helmer A, Falcone-Kapper V, Kochs G, Campe H. Singleplex real-time RT-PCR for detection of influenza A virus and simultaneous differentiation of A/H1N1v and evaluation of the RealStar influenza kit. J Clin Virol. 2011;50(2):171–174. doi: 10.1016/j.jcv.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger-Isakov LA, Husain S, Mooney ML, Hannan MM. The novel 2009 H1N1 influenza virus pandemic: unique considerations for programs in cardiothoracic transplantation. J Heart Lung Transplant. 2009;28(12):1341–1347. doi: 10.1016/j.healun.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang P, Seale H, Zhang Y, Deng Y, Pang X, He X, Wang Q. Estimates of the true number of cases of pandemic (H1N1) 2009, Beijing, China. Emerg Infect Dis. 2010;16(11):1786–1788. doi: 10.3201/eid1611.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggensee G, Gilsdorf A, Buda S, Eckmanns T, Claus H, Altmann D, Krause G, Haas W. The first wave of pandemic influenza (H1N1) 2009 in Germany: from initiation to acceleration. BMC Infect Dis. 2010;10:155. doi: 10.1186/1471-2334-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JW, Fowler RA. 2009 influenza A (H1N1): a clinical review. Hosp Pract. 2010;38(2):74–81. [PubMed] [Google Scholar]
- Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, Read RC, Taylor BL, Brett SJ, McMenamin J. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009) Thorax. 2010;65(7):645–651. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine. 2010;28(31):4895–4902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [DOI] [PubMed] [Google Scholar]
- Hariri LP, Unizony S, Stone J, Mino-Kenudson M, Sharma A, Matsubara O, Mark EJ. Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol Int. 2010;60(11):755–759. doi: 10.1111/j.1440-1827.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- Heo JY, Song JY, Noh JY, Yong HS, Cheong HJ, Kim WJ. Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology (Carlton, Vic. 2010;15(8):1259–1261. doi: 10.1111/j.1440-1843.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- Lee SM, Park JJ, Sung SH, Kim Y, Lee KE, Mun YC, Lee SN, Seong CM. Acute fibrinous and organizing pneumonia following hematopoietic stem cell transplantation. Korean J Intern Med. 2009;24(2):156–159. doi: 10.3904/kjim.2009.24.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro SG, Silvestri GA, Aqusti A. Clinical Respiratory Medicine. 4. Philadelphia: Elsevir Saunders; 2012. [Google Scholar]
- Cincotta DR, Sebire NJ, Lim E, Peters MJ. Fatal acute fibrinous and organizing pneumonia in an infant: the histopathologic variability of acute respiratory distress syndrome. Pediatr Crit Care Med. 2007;8(4):378–382. doi: 10.1097/01.PCC.0000269375.10806.60. [DOI] [PubMed] [Google Scholar]
- Bhatti S, Hakeem A, Torrealba J, McMahon JP, Meyer KC. Severe acute fibrinous and organizing pneumonia (AFOP) causing ventilatory failure: successful treatment with mycophenolate mofetil and corticosteroids. Respir Med. 2009;103(11):1764–1767. doi: 10.1016/j.rmed.2009.07.009. [DOI] [PubMed] [Google Scholar]