Abstract
Background: Vemurafenib, a selective BRAF inhibitor that has antineoplastic activity in patients with unresectable or metastatic malignant melanoma whose tumor harbors a BRAF V600E mutation, has multiple drug-associated cutaneous adverse effects. Purpose: To provide a detailed and comprehensive review of reported changing or new pigmented lesions in oncology patients who have been treated with vemurafenib. Methods: The new appearance of melanocytic nevi on normal-appearing skin after initiating treatment with vemurafenib is described in two men with metastatic malignant melanoma whose tumors demonstrated a BRAF V600E mutation. Using the PubMed database, an extensive literature search was performed for the following topics: vermurafenib, nevus, nevi, melanoma, pigmented lesion, cutaneous, adverse effect, side effect. The results of the search were used to secure all reports of new or changing pigmented lesions after initiating treatment with vemurafenib. Results: Vemurafenib is associated with both changes in existing pigmented lesions (including involution, alteration of color and size, and progression to melanoma) and the onset of new melanocytic lesions—nevi (in 5 patients) and primary melanomas (in 2 patients). Visual examination, dermoscopic evaluation, and reflectance confocal microscopy have been used to document the changes in existing or new melanocytic lesions subsequent to initiating treatment with vermurafenib. Histopathology analysis has shown these lesions to usually be either dysplastic nevi or new primary melanomas. Conclusion: Vemurafenib-treated patients can develop new pigmented lesions (such as nevi) and/or morphological changes in their existing melanocytic lesions (such as involution, increase in size, or alternation of color). In addition, they can develop new primary malignant melanomas that either occur de novo on normal-appearing skin or develop in pre-existing melanocytic lesions. Therefore, total body skin examination should be considered prior to initiating treatment with vemurafenib. Regularly scheduled follow-up skin examinations are also recommended for patients while they are receiving this drug. In addition, for patients who are being treated with vemurafenib, either dermoscopic or photographic or visual modalities should be used to evaluate new or changing pigmented lesions. Also, biopsy for histopathology should be considered for vemurafenib-treated patients who develop new pigmented lesions or whose existing melanocytic lesions have morphological changes in size or color.
Vemurafinib is a selective BRAF inhibitor that was approved by the United States Food and Drug Administration (FDA) on August 17, 2011, as a first-line single agent for the treatment of individuals with unresectable or metastatic malignant melanoma whose tumors demonstrated a BRAF V600E mutation as detected by an FDA-approved test.1-4 Clinical trials have demonstrated improved survival in patients with either previously untreated or treated BRAF V600E mutant metastatic malignant melanoma.5,6 The authors describe two men with metastatic malignant melanoma for which their tumor genotype demonstrated BRAF V600E mutation who experience the new onset of nevi after initiating treatment with vemurafenib and discuss changing or new pigmented lesions in patients with metastatic malignant melanoma after starting this molecularly targeted therapy.
CASE SERIES
Case 1. A 39-year-old Caucasian man with Fitzpatrick skin type II presented with a pigmented lesion of two years duration on the posterior aspect of his right thigh that had changed in appearance and would intermittently bleed after being traumatized. An excisional biopsy on October 5, 2010, showed a 6mm, Clark level IV, ulcerated, nodular melanoma with 5 mitotic figures/mm2, vascular invasion, and microscopic satellitosis. He underwent a wide local excision and sentinel lymph node biopsy that identified metastatic melanoma in 3 of 3 lymph nodes. Subsequently, a right inguinal femoral lymph node dissection discovered three additional lymph nodes positive for melanoma. Staging studies were negative for additional metastatic melanoma and the patient declined adjuvant treatment with interferon.
The patient noted enlargement of the lymph nodes on the left side of his neck in October 2011; biopsy showed recurrent melanoma that was harboring a BRAF V600E mutation. Additional work-up revealed a nodule in the lower lobe of his left lung with an accompanying pleural effusion, enlargement of left axillae, mediastinal, abdomen, and right pelvis lymph nodes and a normal lactate dehydrogenase (LDH) level. On December 30, 2011, he began vemurafenib (960mg twice daily) for his stage IV M1b metastatic malignant melanoma.
After four weeks of vermurafenib, he had developed migratory arthralgias and pruritus. Total body skin examination on January 27, 2012, showed diminished size of his left neck lymphadenopathy and the dermal cutaneous melanoma metastases on his right thigh. Diffuse xerosis and papules on the right (7 papules) and left (2 papules) arm were also noted. Moisturizing cream was prescribed for his dry skin and the papules were treated with liquid nitrogen cryotherapy.
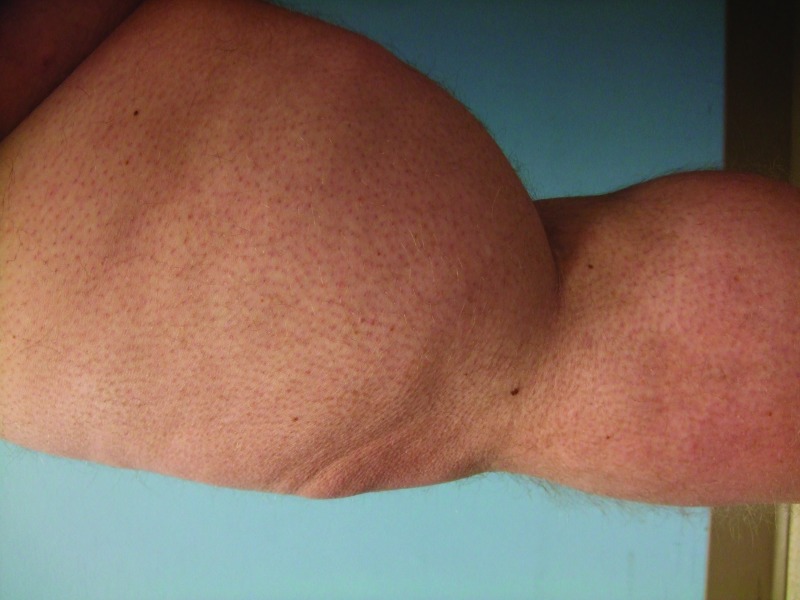
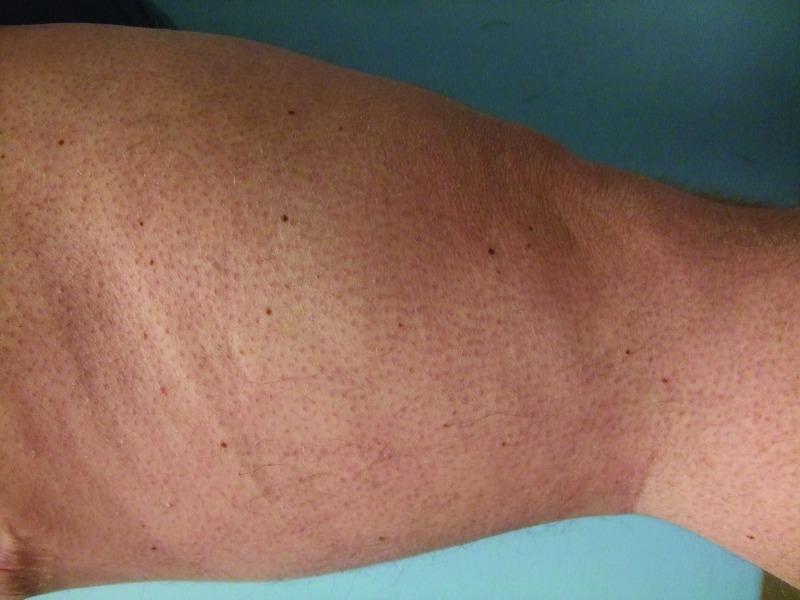
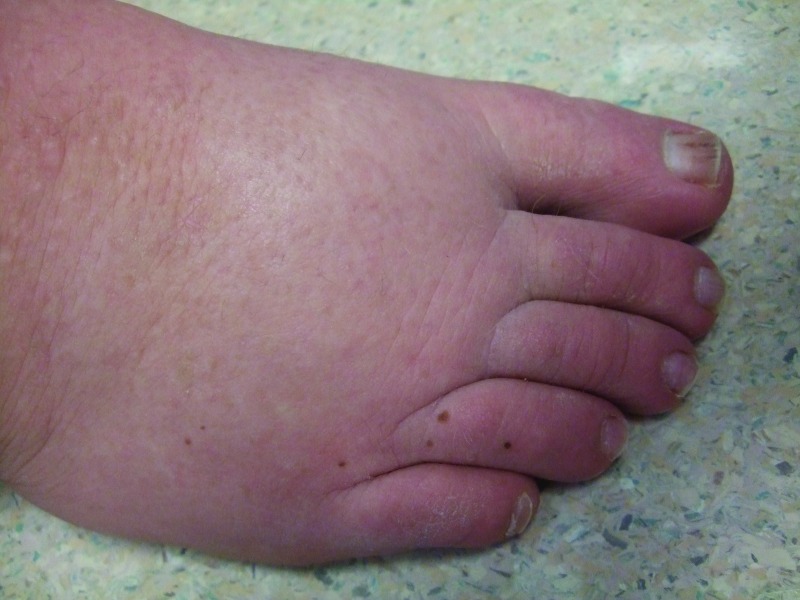
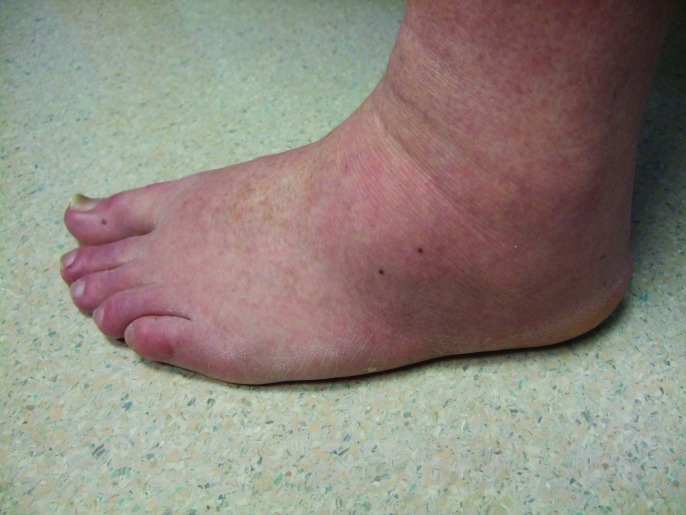
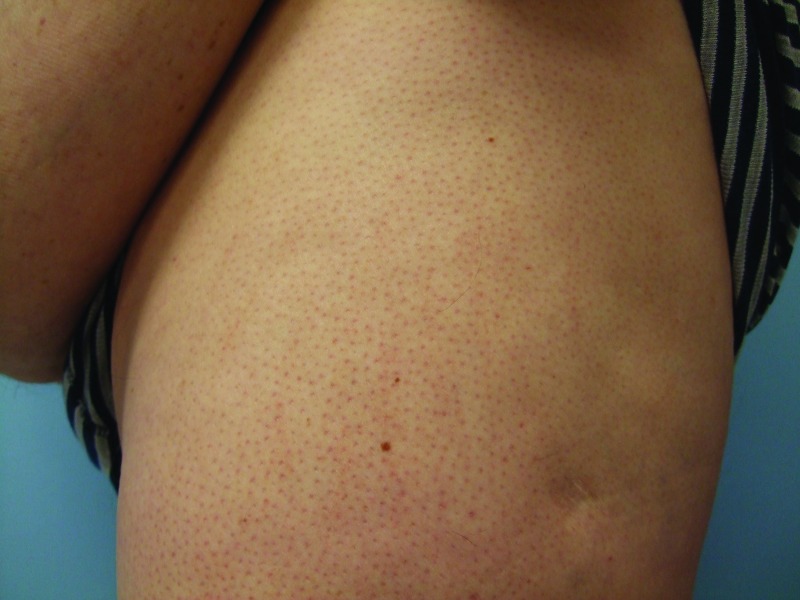
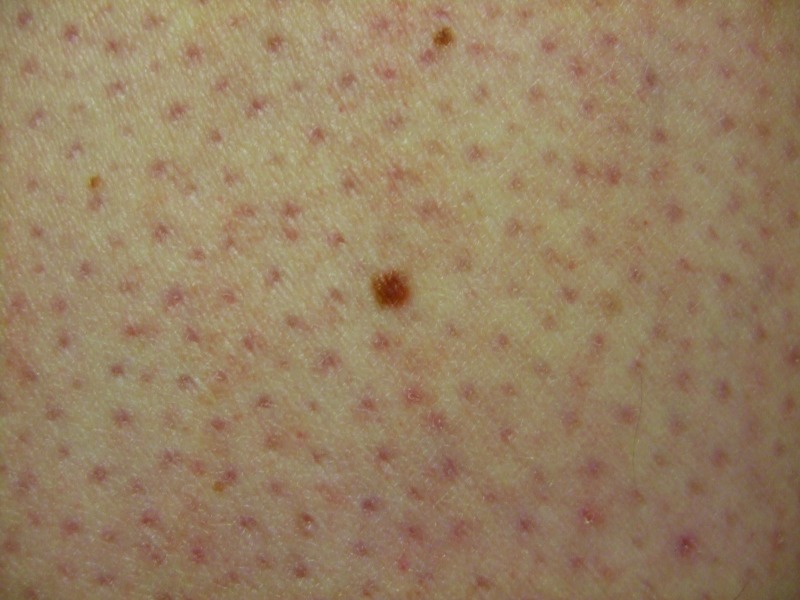
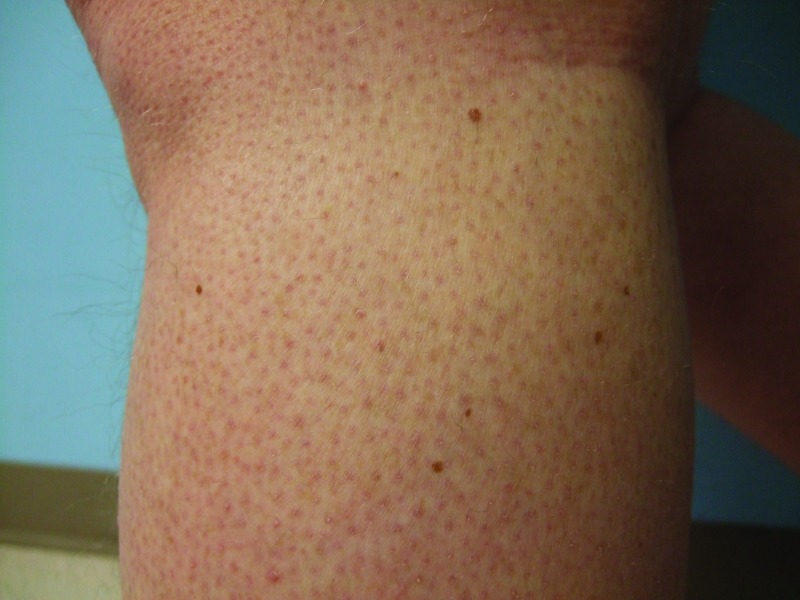
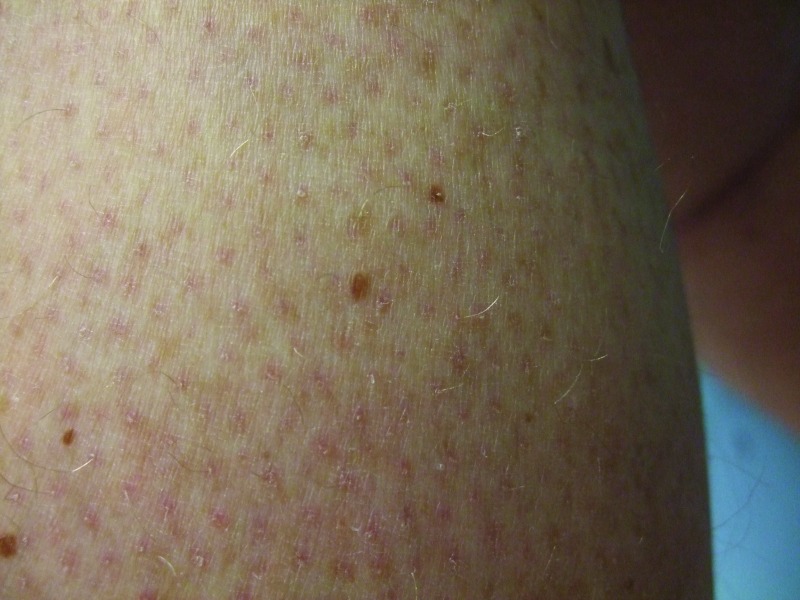
Follow-up cutaneous examination 12 weeks after starting vemurafenib was remarkable for a keratosis pilaris-like eruption on his arms and legs and the appearance of at least 23 new benign-appearing, tan-brown macules, ranging in size from 1 to 3mm in diameter, on previously normal-appearing skin. The pigmented lesions were predominantly located on his right thigh; however, approximately five lesions were on his left leg (Figure 1). Additional new pigmented macules were also noted on the right (Figure 2) and left (Figure 3) dorsal feet. Cryotherapy was used to treat eight new papular lesions on his back and upper extremities; the previously treated lesions had all resolved without persistence or recurrence.
Figures 1A and 1B.
Posterior-lateral view of left (A) and right (B) proximal legs of a 39-year-old man with a BRAF V600E mutation metastatic malignant melanoma show new pigmented lesions after initiating treatment with vemurafenib.
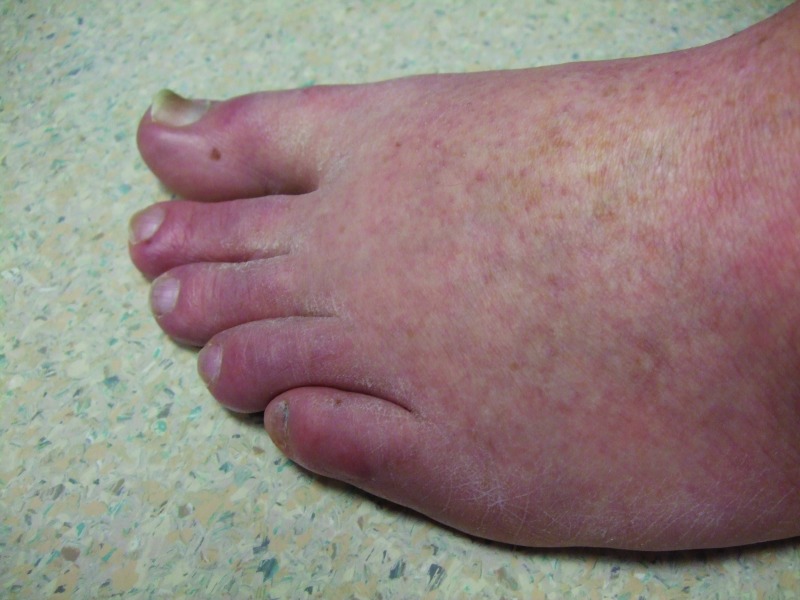
Figure 2.
The right dorsal foot shows new melanocytic lesions 12 weeks after starting vemurafenib—three nevi on the fourth toe and three nevi on the dorsal foot proximal to his toes.
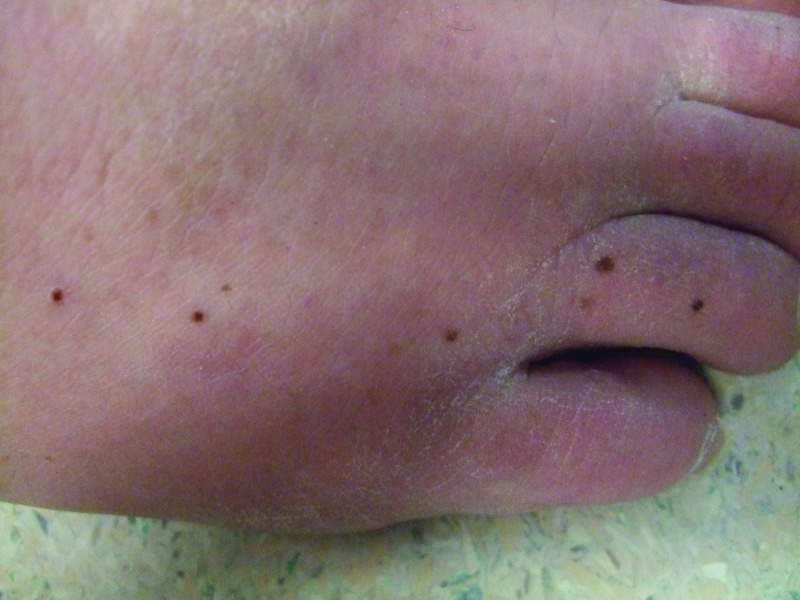
Figures 3A and 3B.
Distant (A) and closer (B) views of the lateral left foot show new pigmented lesions on the left heel (1), proximal dorso-lateral foot (2), great toe (1), and fifth toe (1) that appeared three months after vemurafenib therapy was begun.
One month later, one additional new tan macule was noted on each of his distal feet; some of the earlier-appearing new pigmented lesions on his dorsal feet also had increased in size (Figure 4). His pruritus and xerosis had improved with the topical moisturizing cream and there were no new skin papules. Although his new pigmented lesions clinically appeared benign, the authors were unaware of any other patients developing new melanocytic lesions after starting vemurafenib treatment; therefore, punch biopsy of two of his new pigmented lesions, located on the left thigh and the left calf, was performed (Figure 5); microscopic examination of the tissue specimens both showed similar findings—a dysplastic nevus characterized by a compound melanocytic nevus with moderate architectural disorder and moderate cytologic atypia.
Figure 4.
Another new melanocytic lesion (proximally located) has appeared on the right dorsal foot at follow-up examination four months after beginning vemurafenib treatment; in addition, some of the earlier appearing new pigmented lesions have increased in size.
Figures 5A, 5B, 5C, and 5D.
Distant (A and C) and closer (B and D) views of the left lateral thigh (A and B) and left calf (C and D) show the vemurafenib-associated new melanocytic nevi that were biopsied; these were the inferior and larger brown lesion on the thigh (B) and the superior and lateral brown lesion on the calf. Pathology analysis of each pigmented lesions showed a dysplastic nevus characterized by a compound melanocytic nevus with moderate architectural disorder and moderate cytologic atypia. His keratosis pilaris-like eruption is also prominent.
The patient continued to develop new nevi. Cutaneous examination, 5.5 months after starting vemurafenib, noted two small, tan macules located on his left ventral wrist and his proximal right thigh; his other pigmented lesions remained unchanged. He also had a new papule on the left ventral arm distal to his antecubital fossa (which was treated using liquid nitrogen cryotherapy), persistence of his keratosis pilaris-like eruption on his proximal upper and lower extremities, and diffuse spiny follicular hyperkeratosis.
Imaging studies on November 18, 2012, revealed progressive metastatic disease involving his chest, abdomen, and pelvis lymph nodes. Vemurafenib was discontinued; his joint pain had decreased and his skin was less dry at follow-up examination one week later. He was referred to a phase I program to be considered for possible additional treatment.
Case 2. A 33-year-old Caucasian man with Fitzpatrick skin type II noted a lump in his right axillae in June 2010. The mass increased in size and became tender following empiric treatment with oral antibiotics. Computerized tomography scan of the chest showed a nodule; an excisional biopsy revealed metastatic melanoma (with a tumor size of 4.5x4.0cm) in the fibroadipose tissue.
There was no history of cutaneous melanoma. A right axillary lymphadenectomy showed metastatic melanoma present in the soft tissue and 9 of 34 lymph nodes. Genotype of the melanoma demonstrated a BRAF V600E mutation.
Staging studies for additional metastatic disease were negative. Initial management for his stage IIIC metastatic malignant melanoma with an unknown primary included radiation therapy to the right axillae (30 Gray in 5 fractions of 600 cGy per fraction); this was followed by high-dose intravenous interferon (for 1 month) and subcutaneous interferon (20 million units on Monday, Wednesday, and Friday). In February 2011, the interferon was stopped because of severe drug-associated symptomatic side effects.
Four months later, bilateral adrenal metastases were discovered on staging evaluation. For his stage IV (M1c) metastatic melanoma, the patient began a phase II study from August to October of ipilimumab (infusion every 3 weeks) and temozolomide (400mg orally x 4 days each cycle). He only completed three cycles of therapy secondary to developing pancreatitis and progressive disease.
He started vemurafenib (960mg twice daily) on December 28, 2011, and was seen in dermatology clinic every 3 to 4 weeks. He developed dry skin and migratory joint pain by his follow-up visit two weeks later. After one month of therapy, his legs had keratosis pilaris-like lesions and there were two keratotic papules on his back; numerous pre-existing pigmented lesions showed no change.
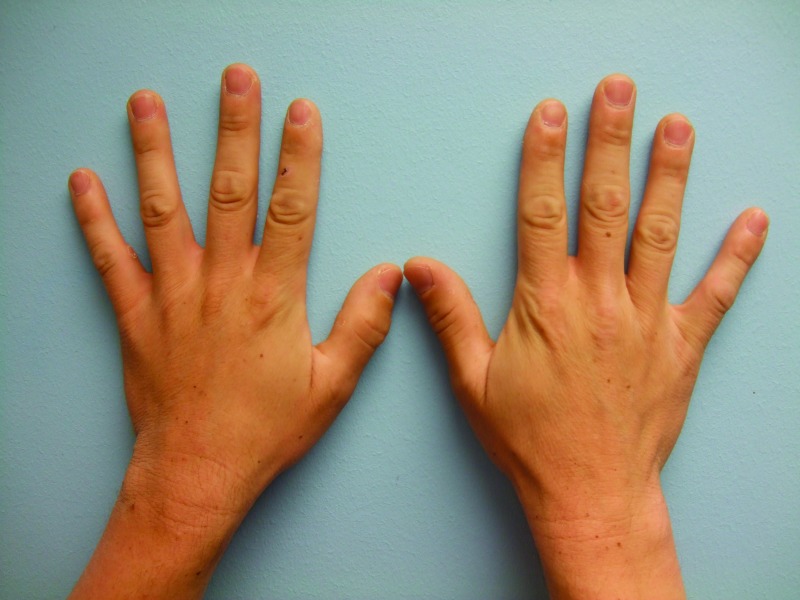
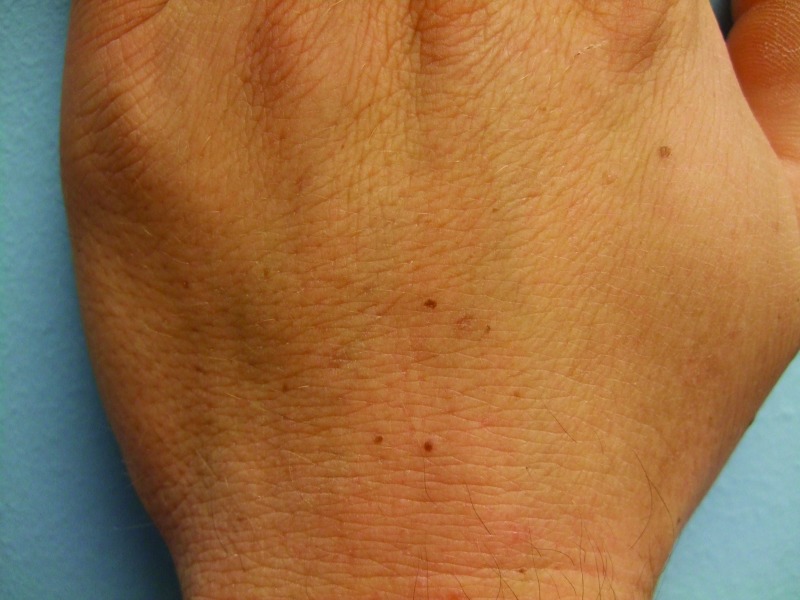
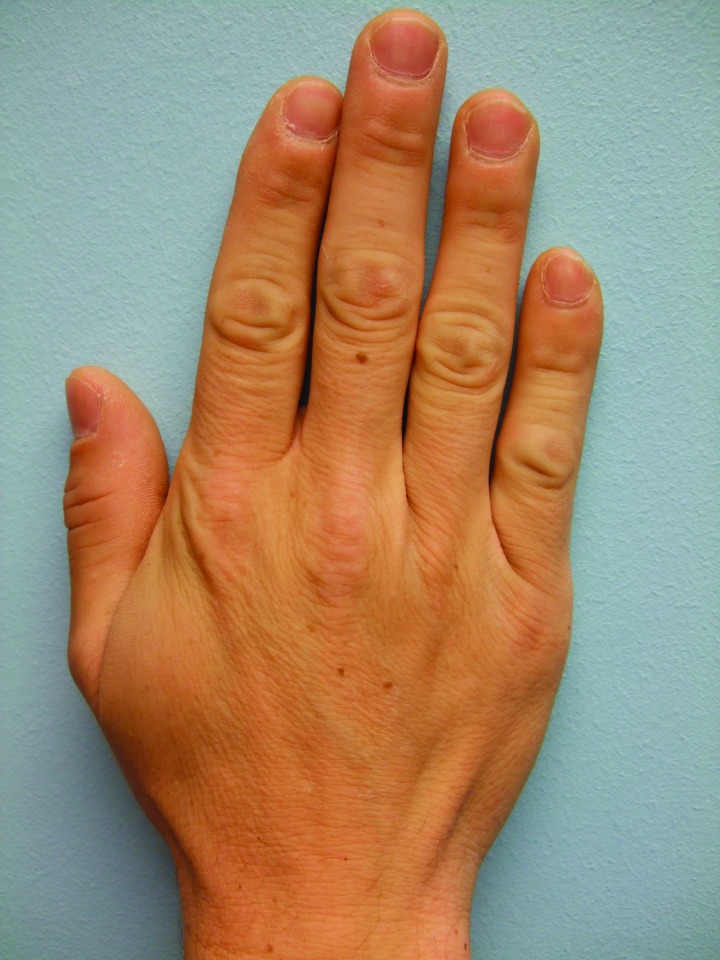
After two months of vemurafenib therapy, he noted new pigmented lesions on his dorsal hands at sites of previously normal-appearing skin. Cutaneous examination four months after starting vemurafenib showed tan, 1 to 3mm macules on the central areas of his right (2) and left (5) dorsal mid hands. In addition, there was a small tan macule on the right dorsal third digit between the metacarpal-phalangeal and proximal interphalangeal joints (Figure 6). An additional benign-appearing pigmented lesion was subsequently noted two weeks later on his left dorsal hand.
Figures 6A, 6B, and 6C.
Distant (A) and closer (B and C) views of the left (B) and right (C) dorsal hands of a 33-year-old man with metastatic malignant melanoma that harbors a BRAF V600E mutation show new melanocytic nevi that appeared two months after initiating vemurafenib treatment—five on the left hand (B) and three on the right hand and third finger.
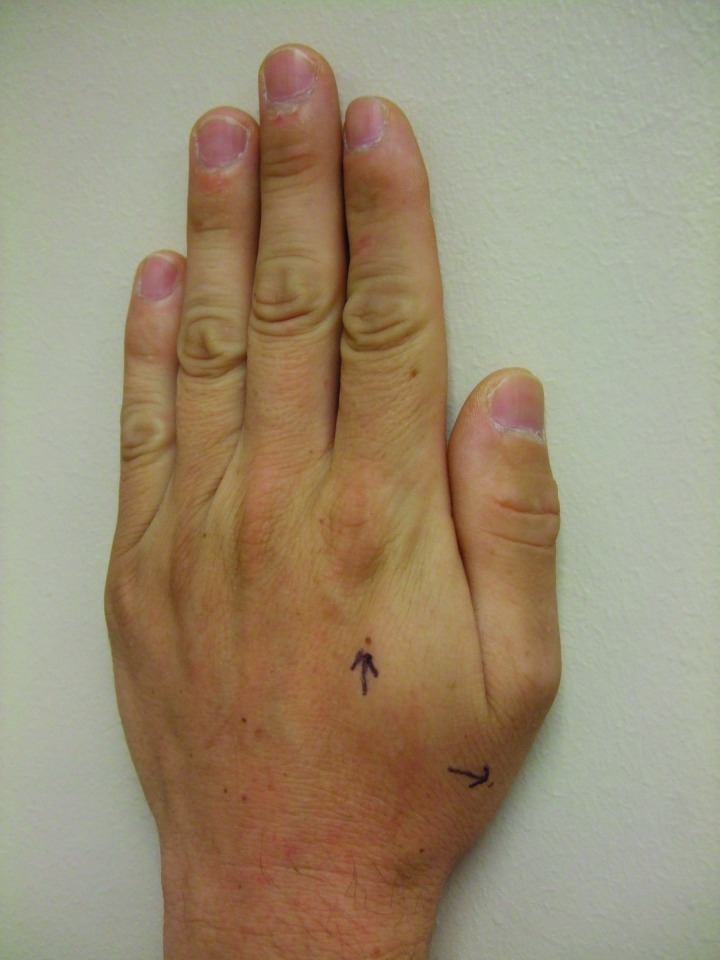
Although his new pigmented lesions clinically appeared benign, the authors were only aware of their previous patient (Case 1) who had developed new melanocytic lesions after starting vemurafenib treatment. On May 5, 2012, punch biopsies of two benign-appearing new pigmented lesions were performed; the pigmented lesions were located on the left dorsal hand proximal to thumb and proximal to the index finger (Figure 7). Microscopic examination of the tissue specimen from the lesion proximal to the thumb showed a benign compound melanocytic nevus. The second specimen, from the lesion proximal to the index finger, showed a dysplastic nevus characterized by a junctional melanocytic nevus with moderate architectural disorder and moderate cytologic atypia. Incidentally, spongiosis and focal acatholytic dyskeratosis were also noted.
Figure 7.
Newly appearing pigmented lesions—after beginning therapy with vemurafenib—on the left hand that were biopsied (arrows). Pathologic analysis of the lesion proximal to the thumb showed a benign compound melanocytic nevus. Pathologic analysis of the lesion proximal to the index finger showed a dysplastic nevus characterized by a junctional melanocytic nevus with moderate architectural disorder and moderate cytologic atypia; incidentally, spongiosis and focal acantholytic dyskeratosis were also noted.
The patient also developed several other vemurafenib-associated cutaneous adverse effects. These included photosensitivity, papular keratotic lesions, an acneiform perioral eruption, keratosis pilaris-like eruption, xerosis, and an erosive dermatosis of the nipples. The presentation and therapeutic interventions are summarized.
He experienced severe photosensitivity. This always occurred when he was driving and was exposed to the sun through the car windows. He was only able to prevent subsequent flares by wearing clothing that covered all exposed areas of his body.
He would develop between 20 to 40 or more papular keratotic lesions between visits. These measured between 1 to 4mm in greatest dimension and were predominantly on his arms and back. They were successfully treated with liquid nitrogen cryotherapy at each visit.
After starting vemurafenib, an acneiform perioral facial eruption appeared. Because of the medication-associated phototoxicity, oral doxycycline was not prescribed. The lesions did not improve with either topical clindamycin 1% gel or oral zithromycin at a 250mg daily dose. However, some resolution occurred with daily topical application of azeleic acid 15% gel.
A keratosis pilaris-like eruption appeared on his arms and legs. In addition, he had widespread dry skin. He also developed tenderness and superficial erosions of his nipples—particularly when they would rub against his shirt. The latter problem improved with the placement of self-adhesive covers (pasties) over his areola.
In August 2012, monthly high-dose bolus interleukin-2 infusion was initiated while continuing daily vemurafenib between infusions. As of November 2012, he has received four courses of interleukin-2.
DISCUSSION
The RAS-RAF-MEK-ERK mitogen-activated protein kinase (MAPK) pathway mediates the growth signals that promote cancer development and progression. Multiple mutations are expressed in cutaneous malignant melanoma; however, the most frequently mutated protein kinase (40-60% of tumor) is BRAF and 75 to 90 percent of these mutations result from valine (V) being substituted by glutamic acid (E) at codon 600 (which is referred to as V600E). The kinase activity of BRAF V600E is substantially elevated as compared to the nonmutated (wild-type) BRAF; therefore, melanomas that are BRAF V600E-positive not only bypass RAS activation, but also result in deregulated downstream signaling of the MAPK pathway, which causes apoptosis prevention, excessive cell proliferation, and prolonged cell survival.1-4
Treatment with vemurafenib, an adenosine triphosphate (ATP)-competitive and reversible oral kinase inhibitor that selectively targets, most specifically, BRAF V600E, has been demonstrated to have antineoplastic activity in patients whose melanoma harbor a BRAF V600E mutation as compared to individuals with wild-type BRAF melanomas.1-4 However, the drug is associated with numerous mucocutaneous side effects, including the development of keratoacanthomas and squamous cell carcinomas (Table 1).7-12 In addition, changes in existing melanocytic nevi or development of new pigmented lesions occur in some of the patients who receive vemurafinib (Table 2).13-23
TABLE 1.
Mucocutaneous adverse effects of vemurafenib
| ACNEIFORM FACIAL ERUPTIONS |
| Cystic lesions |
| Follicular plugging |
| Milia-like lesions |
| Perioral eruption |
| BREAST |
| Dermatosis (erosive) of the nipple |
| Hyperkeratosis of the areola |
| DERMATITIS |
| Asteototic (xerosis) |
| Post-ipilimumab “sensitivity skin reaction”a |
| Seborrheic |
| FOLLICULAR ERUPTIONS |
| Diffuse spiny follicular hyperkeratosisb |
| Keratosis pilaris-like eruption |
| Prominent follicular plugging |
| GROVER’S DISEASE (TRANSIENT ACANTHOLYTIC DERMATOSIS) |
| HAIR CHANGES |
| Alopecia |
| Alteration of hair structure |
| HAND-FOOT SKIN REACTION |
| Hyperkeratotic tender palmar and plantar papules and plaques |
| Palmoplantar erythrodysaesthesia |
| MELANOCYTIC LESIONS |
| Alteration (of color and of size) of existing nevi |
| Involution of existing nevi |
| New lesions: nevi and melanomas |
| PANNICULITIS (ERYTHEMA NODOSUM-LIKE) |
| PAPULES |
| Keratotic |
| Verrucous |
| PHOTOSENSITIVITY (ULTRAVIOLET A INDUCED) |
| TUMORS |
| Keratoacanthomas |
| Melanomas |
| Squamous cell carcinomas |
The onset of this dermatitis occurs between 6 to 8 days after starting vemurafenib; most of these patients had their ipilimumab discontinued within the previous four weeks. Lesions begin on the face and chest, then they spread to the back, trunk, and extremities. Pathology shows a spongiotic perivascular dermatitis with eosinophils. Many of the patients had to stop vemurafenib for up to 11 days before restating treatment at a lower dose, which is then well tolerated.
This is characterized by prominent spicules protruding only from the follicular orifices.
TABLE 2.
Observations in pigmented lesions in patients treated with vemurafenib
CHANGES IN EXISTING MELANOCYTIC NEVI
Involution of nevi. Involution of nevi was observed by Haenssle et al.14 In August 2012, they reported a 56-year-old woman with atypical mole syndrome, Fitzpatrick skin type II, and BRAF V600E-positive metastatic melanoma whose melanocytic lesions were sequentially monitored using digital dermoscopy before vemurafenib treatment and after three months of therapy. The investigators commented that she had “a partial response of her metastatic melanoma lesions and, in comparison with baseline images, dynamic changes within pre-existing melanocytic nevi were noticed.”14 Most of her compound nevi (which had a papillomatous center) involuted. In addition to their clinical observation, rapid involution or senescence without clear signs of an immunological regression was noted on dermoscopic analysis.14 Haenssle et al14 speculated that, similar to her melanoma, these benign-appearing nevi also harbored BRAF V600E mutation and therefore were targeted by vemurafenib.
Dalle et al,13 in a letter regarding second primary melanomas under vemurafenib, also commented that many changes, including regression, are occurring on pigmented lesions under vemurafenib. Their October 2012 publication did not cite the earlier paper by Haenssle et al.14 Hence, although their statement regarding regression of existing pigmented lesions may be a personal observation, the authors did not provide any additional details.
Alteration of nevi color and size. Several researchers have described color differences or size alterations or both in some of the patient’s pigmented lesions that were present prior to initiating treatment with vemurafenib. The 56-year-old patient described by Haenssle et al14 also had two flat pre-existing nevi (which had a reticular pretreatment dermoscopic pattern) that increased in pigmentation. One of the lesions, which had developed an unusual central dark-purple color and had also increased in size, was excised. Microscopic examination showed cytologic dysplasia, melanophages, and lymphohistiocytic inflammation in the dermis, but sharply demarcated with only minimal asymmetry and without mitoses in the dermal component; the nevus showed wild-type BRAF.14
Dalle et al20 reported five patients who had BRAF V600E mutation-positive metastatic melanomas; systematic total body-surface monitoring of skin with a dermatoscope was performed. Between Week 4 and Week 12, the investigators observed and removed six small atypical lesions from 4 of the 5 patients who were otherwise having a response to treatment. Based on subsequent papers from these investigators,13,19 it is assumed that these lesions were present prior to the patients receiving vemurafenib. All of the lesions were BRAF wild-type; one was a dysplastic nevus and the other five were early primary melanomas.20
Zimmer et al15 reported on 19 patients with BRAF V600E mutant metastatic malignant melanoma who were undergoing treatment with a selective BRAF inhibitor. In these patients, 22 cutaneous melanocytic lesions were excised; the lesions were clinically suspect to be malignant melanoma and they had either newly developed (at least 2 lesions) or changed in morphology (at least 19 lesions) after starting therapy. Ten pre-existing lesions (in 8 patients) showed either dysplastic nevi (9 lesions) or a “common” nevus (1 lesion); none of the 10 lesions carried a detectable BRAF V600E mutation. The other 12 lesions (in 11 patients) were new primary melanomas.
Cutaneous effects of BRAF inhibition therapy was described in 33 of 53 treated patients who met exclusion/inclusion criteria; however, the investigators did not report whether BRAF V600E mutation was present or absent in the patient’s tumor.18 “Changing nevus” (as per the column heading on the table summarizing cutaneous findings) was observed in two women with Fitzpatrick skin type II whose melanoma was treated with vemurafenib— one nevus in a 50 year old (which also developed drug-associated photosensitivity) and between 2 to 5 nevi in a 26 year old. Interestingly, Mattei et al18 also observed a single changing nevus in a 65-year-old man whose melanoma was treated with combination therapy that included a different selective BRAF inhibitor (GSK2118436 = dabrafenib)4 and a MEK (mitogen-activated protein kinase) inhibitor (GSK1120212 = trametinib).
Chu et al14 described the diverse cutaneous side effects associated with BRAF inhibitor therapy in 14 individuals whose tumors harbored BRAF V600E mutation. The patients were either treated with vemurafenib (11 people), dabrafenib (1 person),4 dabrafenib and trametinib (a MEK inhibitor) (1 person), or dabrafenib and trametinib, followed by vemurafenib (1 patient). Color change of preexisting nevi were observed in a 57-year-old Chinese man with metastatic papillary thyroid cancer whose tumor genotyping revealed BRAF V600E mutation after eight weeks of treatment with vemurafenib. Based on pretreatment and post-treatment evaluations, both the patient and the clinician noted marked darkening of multiple evenly pigmented nevi scattered on his trunk and arms after starting the medication. A dark nevus on the trunk was biopsied; microscopic examination showed a junctional dysplastic nevus with moderate atypia. In addition, the man developed two verrucous keratoses (on the nose and left cheek), focal palmoplantar hyperkeratosis (hand-foot skin reaction), and three new pigmented macules on acral sites.
Dermatoscopic evaluation of dysplastic nevi was described by Germani et al17 in a 55-year-old Caucasian man with vemurafenib-treated metastatic melanoma (whose tumor genotyping was not stated). Visual and dermatoscopy examination was performed prior to initiating vermurafenib and after five weeks of treatment. Baseline dermatoscopic cutaneous evaluation noted more than 100 nevi, ranging in size between 5 and 8mm, which were darkly pigmented and had a regular reticulated network. A number of his nevi showed marked changes at follow-up dermatoscopic examination—hyperkeratosis overlying a central area with negative pigment network, prominent globules, and peripheral distorted reticulated network, raising concern for the possibility of a new primary melanoma. Biopsy of five nevi all showed severe dysplasia. Genetic typing of three lesions each revealed the pigmented lesions to be BRAF wild-type. In addition, the patient also developed more than 20 squamous cell carcinomas.
Debarbieux et al19 recently described pre-existing benign-appearing nevi that demonstrated clinical changes, dermatoscopic alterations, and/or reflectance confocal microscopy abnormalities in patients with BRAF V600E mutant metastatic malignant melanoma after treatment with vemurafenib. Reflectance confocal microscopy was used to evaluate 42 lesions, three only before vemurafenib treatment, 10 before and after vemurafenib treatment, and 29 only after vemurafenib treatment. Atypia was observed under reflectance confocal microscopy in 23 of the 42 lesions and they were excised. Four of the excised lesions were melanomas.
In summary, several patients developed dermatoscopic and/or clinical changes in their size and/or color of their preexisting melanocytic nevi after initiating treatment with vemurafenib. The morphological changes were noted as early as two weeks and as late as 42 weeks after starting vermurafenib therapy.15 In all of the lesions that were evaluated, genotyping of the biopsied nevi demonstrated wild-type BRAF.
Some of the investigators have postulated that there either has been transactivation of wild-type BRAF17 or a paradoxical activation of the MAPK pathway in these individuals.14,15,20 Specifically, Zimmer et al15 speculate that one mechanism whereby selective BRAF inhibitors may promote the growth of pre-existing melanocytic nevi may be activation of MEK-ERK signaling. However, they also hypothesize that a potential factor resulting in morphological and histological changes of baseline pigmented lesions may be attributed to an upregulation of other signaling pathways, such as PI3K/AKT.
Development of nevi into melanoma. Additional primary melanomas have been discovered in patients with BRAF V600E mutant metastatic malignant melanoma after starting vemurafenib.13 Some of the reports do not clearly specify whether the melanoma developed de novo or from a pre-existing nevi.20,21 However, an additional publication from one of the groups13 suggests that a precursor pigmented lesion was present at the site of the subsequent melanoma.20
Dalle et al20 reported that early BRAF wild-type primary melanomas were diagnosed from six atypical melanocytic lesions that were removed from 4 of the 5 patients with metastatic malignant melanoma with V600E mutation that developed between 4 to 12 weeks after starting vemurafenib therapy. In response to these findings, Chapman et al21 commented that only five superficial melanoma—in addition to the five primary melanomas described by Dalle et al20—have been reported among the other 464 patients treated by other investigators in the phase 2 and 3 trials. Zimmer et al15 observed 12 new primary melanomas in 11 patients who had BRAF V600E mutant metastatic malignant melanoma; at least 9 of the new tumors (in 8 patients) occurred in a pre-existing nevus and all of the new cancers were wild-type BRAF.
Debarbieux et al19 were able to evaluate, using reflectance confocal microscopy, 10 pigmented lesions from two BRAF V600E mutant metastatic malignant melanoma patients both prior to and after receiving three months of treatment with vemurafenib. The selected melanocytic nevi were clinically and dermoscopically benign before beginning vemurafenib. Reflectance confocal microscopy showing changes of newly appearing atypias were observed in only 1 of 6 lesions from the first patient and 3 of 4 lesions from the second patient after three months of therapy. The four lesions were excised and showed that all of these previously benign-appearing nevi had developed into new primary melanomas.
ONSET OF NEW MELANOCYTIC LESIONS
Melanoma. Vemurafenib-associated new primary melanomas were discovered in at least two patients with BRAF V600E metastatic malignant melanoma in whom the new pigmented malignant lesions developed on normal-appearing skin between 6 to 19 weeks after the commencement of vemurafenib treatment.15 One patient was a 40-year-old woman with a new 0.45mm melanoma on her scalp. The other patient was a 65-year-old man with a new 0.65mm melanoma on his scalp.
Fearfield et al23 comment that as additional, larger, vemurafenib studies with longer follow-up are presented, a more precise estimate of the actual incidence of new primary melanoma in BRAF-inhibitor-treated patients will be important for assessing the ratio of risk versus benefit of BRAF inhibitors in this setting. Essentially all investigators concur that baseline and regular total body skin examination should be performed in patients who are being treated with vemurafenib or another BRAF inhibitor. The recommended method of cutaneous surveillance for the patient varied and include several, not mutually exclusive, modalities including visual inspection, dermoscopy examination, and reflectance confocal microscopy evaluation.
Nevus. Common acquired nevi are melanocytic proliferations that are typically acquired during the first three decades of life on areas of sun-exposed skin.24 Indeed, they have their greatest increase in number per unit of skin surface during childhood and prior to adolescence.25 Also, the combined effects of vulnerability to solar radiation and local sun exposure influence the number of nevi acquired on a specific area of skin.26
Eruptive melanocytic nevi often present on previously uninvolved sun-exposed skin as a simultaneous appearance of hundreds of melanocytic nevi.27 They have been observed to occur in patients who are immunosuppressed secondary to autoimmune bullous dermatoses, cytotoxic drugs and other medication, HIV infection, and/or organ transplant.28 For example, an eruptive onset of multiple new pigmented lesions were documented in a 20-year-old man 2 to 3 months after completing three years of induction and maintenance chemotherapy for acute lymphocytic leukemia.29 Also, eruptive melanocytic nevi have occurred in patients with inflammatory bowel disease (Crohn’s disease28 or ulcerative colitis30) who are receiving either azathioprine,28 a combination of azathioprine and infliximab,28 or 6-mercaptopurine.30
Eruptive melanocytic nevi have also been observed in oncology patients who were treated with sorafenib.31-34 In addition, sorafenib treatment has also been associated with several cutaneous adverse effects including the development of keratoacanthomas and squamous cell carcinomas.35-37 Similar to vermurafenib, sorafenib also inhibits RAF; however, vemurafenib is a selective and highly potent BRAF inhibitor in contrast to sorafenib that is a pan-RAF inhibitor. In addition, sorafenib also blocks FLT3 (fms-like tyrosine kinase 3), KIT, platelet-derived growth factor receptor-beta, VEGFR (VEGFR receptors)-2, and VEGFR-3.38
Including the authors’ patients, new melanocytic nevi after beginning vemurafenib occurred in five oncology patients (Table 3).14,16,22 Four of the individuals (3 men and 1 woman) had BRAF V600E mutant metastatic malignant melanoma, whereas one man had metastatic papillary thyroid carcinoma that harbored a BRAF V600E mutation. The patients ranged in age from 33 to 57 years (median, 51 years). The nevi appeared within 8 to 14 weeks (median, 12 weeks) after starting vemurafenib. The number of nevi ranged from three to more than 30; 60 percent of the patients had less than 10 new nevi. The new nevi were acral in location (hands, feet, or both) in at least three of the patients.
TABLE 3.
Characteristics of vemurafenib-treated patients who develop new nevia
| C | ARS | FITZ | CANCER | 1°MM DEPTH TYPE SITE | WEEKS ON VEM | NUMBER OF NEVI | LOCATION OF NEVI | EVAL | BIOPSY OF NEVUS (RESULT) | BRAFb | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 WM | II | MMM | Unknown primaryc | 8 | 9 | Right and left dorsal hands | V | CNx1 DNx1d | NP | CR (Case 2) |
| 2 | 39 WM | II | MMM | 6mm Nodular Right thigh | 12 | >30 | Right and left legs Left ventral wriste | V | DNx2f | NP | CR (Case 1) |
| 3 | 51 WM | II | MMM | 8mm Nodular Scalp | 14 | Many | “On previously unaffected skin” | V | NP | NP | 22 |
| 4 | 56 WF | II | MMM | Not stated | 12 | >3 | Right buttock | D,V | DNx1g | Wild-type | 14 |
| 5 | 57 Ch M | III | PTC | N/A | 8 | 3 | Right palm (2) Foot (1)h | V | NP | NP | 16 |
Abbreviations: A=age (years) at time of diagnosis of cancer; BRAF=a human gene referred to as either v-Raf murine sarcoma viral oncogene homolog B1 or proto-oncogene B-Raf; CN=compound nevus; CR=current report; D=dermoscopy; DN=dysplastic nevus; Eval=evaluation method, female; Fitz=Fitzpatrick skin type; II=skin type II (very sun sensitive, burns easily, tans minimally; example: fair skinned, fair haired Caucasians); III=skin type III (sun sensitive skin, sometimes burns, slowly tans to light brown; example: darker Caucasians); M=man; mm=millimeters; MM=malignant melanoma; MMM=metastatic malignant melanoma; N/A=not applicable; NP=not performed; PTC=papillary thyroid carcinoma (metastatic); R=race; Ref=reference; S=sex; V=visual; VEM=vemurafenib; W=white; 1º=primary
The BRAF molecular genotype mutational status of the nevus
The patient’s metastatic melanoma presented as an axillary soft tissue mass with no cutaneous primary melanocytic lesion
The pigmented lesion located on the left dorsal hand proximal to the thumb showed a compound melanocytic nevus. The pigmented lesion located on the left dorsal hand proximal to the index finger showed a dysplastic nevus characterized by a junctional melanocytic nevus with moderate architectural disorder and moderate cytologic atypia; spongiosis and acantholytic dyskeratosis were also observed
Most of the lesions were on the right leg (the thigh had more lesions than below the knee). Fewer lesions (at least four) were on the left leg above and below the knee. Six or five lesions initially appeared on the dorsal right and left foot, respectively. Two lesions subsequently appeared on the left ventral wrist
Pigmented lesions from the left hip and the left calf both showed dysplastic nevi characterized by a compound nevus with moderate architectural disorder and moderate cytologic atypia of melanocytes
The histopathologic analysis revealed a melanocytic nevus with cytologic dysplasia, melanophages, and lymphohistiocytic inflammation in the dermis, but sharply demarcated with only minimal asymmetry and without mitoses in the dermal component. It also showed a suprabasal spreading in the central part as well as fibroplasia in the papillary dermis and was therefore difficult to distinguish from initial melanoma. Immunohistochemical stainings for phosphohistone H3 (PH3) and Ki-67 antigen (MIB-1 antibody) showed no evidence for an increased mitotic activity in both lesions.
The new brown macule on the plantar aspect of his foot appeared on a background of new focal palmoplantar hyperkeratosis.
All of the nevi were evaluated visually. Dermatoscopic examination was performed for one patient. Many of the new nevi morphologically appeared to be benign and were followed clinically. Both of the authors’ patients had clinically benign-appearing nevi for which pathology showed moderate dysplasia. A third patient had both visual and dermoscopic examination of a new atypical-appearing nevus on her right buttock, which prompted removal of the lesion. Histopathology analysis of her lesion showed a compound nevus with cytologic dysplasia; however, molecular profiling demonstrated it to be BRAF wild-type.
Most of the patients with vemurafenib-associated new nevi had other drug-induced cutaneous side effects. Ma et al’s22 patient, a 51-year-old Caucasian man with metastatic malignant melanoma, also had vemurafenib-related keratosis pilaris-like eruption and hidradenitis suppurativa; however, the latter condition is not commonly reported in patients receiving vemurafenib. Chu et al’s16 patient—a 57-year-old Chinese man with metastatic papillary thyroid carcinoma—had not only flesh-colored and slightly erythematous verrucous papules on the nose and left cheek, but also new hand-foot skin reaction presenting as focal palmoplantar hyperkeratosis. One of the authors’ patients also had severe photosensitivity, an acneiform perioral eruption, and an erosive dermatosis of his nipples, whereas the other patient had pruritus and diffuse spiny follicular hyperkeratosis. Both of the patients described herein had liquid nitrogen cryotherapy-responsive keratotic papules predominantly on their back and upper extremities, diffuse xerosis, and keratosis pilaris-like eruption on their arms and legs.
CONCLUSION
Vemurafenib is a targeted therapy approved for patients with metastatic malignant melanoma that has a BRAF V600E mutation. In addition to several drug-associated mucocutaneous side effects, vemurafenib-treated patients can develop new pigmented lesions (such as nevi) and/or morphological changes in their existing nevi (such as involution, increase in size, or alternation of color). In addition, vemurafenib treatment may be associated with not only the appearance of keratoacanthomas and squamous cell carcinomas, but also new primary malignant melanomas that either occur de novo on normal-appearing skin or develop in pre-existing nevi. Therefore, total body skin examination should be considered prior to initiating treatment with vemurafenib. Also, regularly scheduled follow-up skin examinations are recommended for patients while they are receiving this drug. In addition, for patients who are being treated with vemurafenib, either dermatoscopic or photographic or visual modalities should be used to evaluate new or changing pigmented lesions. Also, biopsy for histopathology analysis should be considered for vemurafenib-treated patients who develop new melanocytic lesions or whose existing nevi have morphological changes in size or color.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Jordan EJ, Kelly CM. Vemurafenib for the treatment of melanoma. Expert Opin Pharmacother. 2012;13:2533–2543. doi: 10.1517/14656566.2012.737780. [DOI] [PubMed] [Google Scholar]
- 2.Ravnan MC, Matalka MS. Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma. Clin Ther. 2012;34:1474–1486. doi: 10.1016/j.clinthera.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Fisher R, Larkin J. Vemurafenib: a new treatment for BRAF-V600 mutated advanced melanoma. Cancer Management and Research. 2012;4:243–252. doi: 10.2147/CMAR.S25284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT. BRAF inhibitors and melanoma. Cancer J. 2011;17:505–511. doi: 10.1097/PPO.0b013e31823e5357. [DOI] [PubMed] [Google Scholar]
- 5.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, et al. (BRIM-3 Study Group). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang V, Hepper D, Anadkat M, Cornelius L. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2102;148:628–633. doi: 10.1001/archdermatol.2012.125. [DOI] [PubMed] [Google Scholar]
- 8.Boyd KP, Vincent B, Andea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375–1379. doi: 10.1016/j.jaad.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Zimmer L, Vaubel JV, Livingstone E, Schadendorf D. Side effects of systemic oncological therapies in dermatology. J Dtsch Dermatol Ges. 2012;10:475–486. doi: 10.1111/j.1610-0387.2012.07942.x. [DOI] [PubMed] [Google Scholar]
- 10.Drummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy [letter] N Engl J Med. 2012;366:480–481. doi: 10.1056/NEJMc1113752. [DOI] [PubMed] [Google Scholar]
- 11.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab [letter] N Engl J Med. 2012;366:866–868. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 12.Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br J Dermatol. 2012;167:987–994. doi: 10.1111/bjd.12010. [DOI] [PubMed] [Google Scholar]
- 13.Dalle S, Poulalhon N, Debarbieux S, Thomas L. Second primary melanomas under vemurafenib [letter[ Br J Dermatol. doi: 10.1111/bjd.12093. 2012 Oct 15 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Haenssle HA, Kraus SL, Brehmer F, et al. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib. Importance of sequential dermatoscopy. Arch Dermatol. 2012;148:1183–1185. doi: 10.1001/archdermatol.2012.2649. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375–2383. doi: 10.1200/JCO.2011.41.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265–1267. doi: 10.1016/j.jaad.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerami P, Sorrell J, Martini M. Dermatoscopic evolution of dysplastic nevi showing high-grade dysplasia in a metastatic melanoma patient on vemurafenib [letter] J Am Acad Dermatol. 2012;67:e275–e276. doi: 10.1016/j.jaad.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Mattei PL, Alora-Palli MB, Kraft S, et al. Cutaneous effects of BRAF inhibitor therapy: a case series. Ann Oncol. 2013;24:530–537. doi: 10.1093/annonc/mds292. [DOI] [PubMed] [Google Scholar]
- 19.Debarbieux S, Dalle S, Depaepe L, et al. Secondary primary melanomas under BRAF blockers: study by reflectance confocal microscopy. Br J Dermatol. doi: 10.1111/bjd.12210. 2013 Jan 10 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Dalle S, Poulalhon N, Thomas L. Vemurafenib in melanoma with BRAF V600E mutation [letter] N Engl J Med. 2011;365:1448–1449. doi: 10.1056/NEJMc1108651. [DOI] [PubMed] [Google Scholar]
- 21.Chapman PB, Hauschild A, McArthur GA. Vemurafenib in melanoma with BRAF V600E mutation. The authors reply [letter] N Engl J Med. 2011;365:1450. doi: 10.1056/NEJMc1108651. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Dominguez AR, Collins GR, et al. Hidradenitis suppurativa, eruptive melanocytic nevi, and keratosis pilaris-like eruption in a patient treated with vemurafenib. Arch Dermatol. 2012;148:1428–1429. doi: 10.1001/2013.jamadermatol.23. [DOI] [PubMed] [Google Scholar]
- 23.Fearfield L, Newton-Bishop JA, Sinha R, et al. Second primary melanomas under vemurafenib—reply from the author [letter] Br J Dermatol. doi: 10.1111/bjd.12094. 2012 Oct 15 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Mackie RM, English J, Aitchison TC, et al. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol. 1985;113:167–174. doi: 10.1111/j.1365-2133.1985.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 25.English DR, Armstrong BK. Melanocytic nevi in children. I. Anatomic sites and demographic and host factors. Am J Epidemiol. 1994;139:390–401. doi: 10.1093/oxfordjournals.aje.a117011. [DOI] [PubMed] [Google Scholar]
- 26.Autier P, Boniol M, Severi G. for the Epidemiology, Prevention and Genetics Sub-group of the European Organization for Research and Treatment of Cancer (EORTC) Melanoma Co-operative Group (EPIMEL). The body site distribution of melanocytic naevi in 6-7 year old European children. Melanoma Res. 2001;11:123–131. doi: 10.1097/00008390-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 27.John JK, Smalley KSM. Identification of BRAF mutations in eruptive melanocytic nevi: new insights into melanomagenesis? Expert Rev Anticancer Ther. 2011;11:711–714. doi: 10.1586/era.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bovenschen HJ, Vermaat TH, de Hoop D, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biological. Br J Dermatol. 2006;154:880–884. doi: 10.1111/j.1365-2133.2006.07189.x. [DOI] [PubMed] [Google Scholar]
- 29.Reutter JC, Long EM, Morrell DS, et al. Eruptive post-chemotherapy in situ melanomas and dysplastic nevi. Pediatr Dermatol. 2007;24:13–137. doi: 10.1111/j.1525-1470.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 30.Sekulic A, Colgan MB, Davis MDP, et al. Activating BRAF mutations in eruptive melanocytic naevi. Br J Dermatol. 2010;163:1095–1098. doi: 10.1111/j.1365-2133.2010.09989.x. [DOI] [PubMed] [Google Scholar]
- 31.Kong HH, Sibaud V, Turner MLC, et al. Sorafenib-induced eruptive melanocytic lesions. Arch Dermatol. 2008;144:820–822. doi: 10.1001/archderm.144.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennani-Lahlou M, Mateus C, Escudier B, et al. Eruptive naevi associated with sorafenib treatment. Ann Dermatol Venereol. 2008;135:672–674. doi: 10.1016/j.annder.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Sibaud V. Cutaneous side effects of angiogenesis inhibitors in cancer patients. Oncologie. 2009;11:291–297. [Google Scholar]
- 34.Sibaud V, Delord J-P, Chevreau C, et al. Dermatologic adverse events of the new targeted anticancer therapies used in oncodermatology. Annales de Chirurgie Plastique Esthetique. 2012;57:106–113. doi: 10.1016/j.anplas.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Robert C, Mateus C, Spatz A, et al. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Arnault JP, Weschsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib [letter] J Clin Oncol. 2009;27:e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 37.Williams VL, Cohen PR, Stewart DJ. Sorafenib-induced premalignant and malignant skin lesions. Int J Dermatol. 2011;50:396–402. doi: 10.1111/j.1365-4632.2010.04822.x. [DOI] [PubMed] [Google Scholar]
- 38.Arnault JP, Mateus C, Escudier B, et al. Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway activation and oncogenic mutations of HRAS TP53, and TGFBR1. Clin Cancer Res. 2011;18:263–272. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]