Abstract
Background
To evaluate the prevalence of the accessory left hepatic artery (ALHA; defined as a vessel arising from the left gastric artery, which, together with a typical left hepatic artery, supplies blood to the left lobe of the liver) and its short-term clinical implications in patients undergoing radical gastrectomy for gastric cancer.
Methods
Clinical data of 1173 patients with gastric cancer who underwent laparoscopy-assisted radical gastrectomy were retrospectively analyzed. Groups of patients with and without ALHA were compared to identify differences in intraoperative and postoperative variables and changes in liver function.
Results
Of the 1173 patients, 135 (11.5%) had an ALHA and 1038 (88.5%) did not. There were no significant between-group differences in clinicopathological and intraoperative characteristics, postoperative recovery, and morbidity and mortality rates (P>0.05 each). None of the patients had postoperative symptoms associated with impaired liver function. Glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) and total bilirubin (TBIL) concentrations were similar preoperatively. TBIL concentrations on postoperative days 1, 3, and 7 were similar (P>0.05), while GOT and GPT activities were higher in the ALHA than in the non-ALHA group on days 1 and 7 (P<0.05), with all three markers similar in the two groups on day 14. In patients without chronic liver disease (CLD), GOT, GPT and TBIL concentrations were similar in patients with and without ALHA; whereas, in patients with CLD, GOT and GPT concentrations on days 1 and 3 and GOT on day 7 were higher in patients with than without ALHA.
Conclusion
ALHA is a common anomaly that was found in 11.5% of patients. It can be safely severed during radical gastrectomy in patients without CLD, but should be left intact in patients with CLD to prevent liver dysfunction. If severed in the latter, the patient should be monitored and liver-protecting therapy may be necessary.
Background
Japanese guidelines for the treatment of gastric cancer indicate the need for complete removal of the gastrohepatic ligament during radical gastrectomy for gastric cancer [1]. Vascular variations, however, are frequently encountered [2], including an accessory left hepatic artery (ALHA) and accessory left gastric artery, with the ALHA showing the highest incidence [3], [4]. Although variations in the hepatic artery have been assessed by medical imaging and anatomic methods, as well as in patients undergoing liver transplantation or transcatheter arterial chemoembolization (TACE)[5]–[9], few large studies have evaluated the prevalence of ALHA and its impact on patients undergoing radical gastrectomy for gastric cancer. We therefore retrospectively evaluated clinical data of 1173 gastric cancer patients who underwent laparoscopy-assisted radical gastrectomy to determine the prevalence of ALHA and its short-term clinical implications in these patients.
Materials and Methods
Patients
Between May 2007 and February 2012, 1173 patients underwent laparoscopy-assisted radical gastrectomy for gastric cancer at Fujian Medical University Union Hospital, with all operations performed by the same group of gastric surgeons. All data were collected from a “clinical data mining system for gastric cancer surgery”[10] and a video data system.
Before surgery, all patients were examined by multidetector computed tomography (MDCT) to evaluate the tumors and their relationship with peripheral vascular structures.The presence or absence of an ALHA was assessed intraoperatively, and patients were divided into groups, with and without ALHA. Intraoperative and postoperative variables and changes in liver function were compared in the two groups. Patients with chronic liver disease (CLD) were defined as those with[11]: (1) at least two abnormal results on tests of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) or total bilirubin (TBIL), at least 6 months apart; (2) imaging results showing radiological signs of cirrhosis and portal hypertension, or a hepatic mass, and evidence of CLD; (3) a liver biopsy consistent with CLD; or (4) a previous diagnostic clinical event (e.g. variceal bleed, spontaneous bacterial peritonitis, or ascites). All patients were stratified by the presence or absence of CLD. Patients were staged according to the tumor, node, metastasis (TNM) classifications of the 7th edition of the Union for International Cancer Control (UICC) [12]. Liver function was assessed by measuring changes over time in GOT, GPT, and TBIL concentrations. Though meticulous preoperative preparation all our patients before surgery are A level according to Child-Pugh classification.
All surgical procedures were performed after obtaining informed consent from each patient. Patients were included if they had histologically confirmed adenocarcinoma of the stomach; no evidence of distant metastasis (e.g., in the liver or lungs) or para-aortic lymph node involvement during preoperative examination; and had undergone R0 radical gastrectomy, as assessed postoperatively. Patients were excluded if they had confirmed stage T4b tumors or intraoperative evidence of peritoneal disseminated or distant metastasis; or incomplete clinicopathological data.
Ethics Statement
Ethics committee of Fujian union hospital approved this retrospective study (Approval number: 20070428). Written consent was given by the patients for their information to be stored in the hospital database and used for research.
Surgical procedure
The type of surgical resection (i.e. distal subtotal gastrectomy, proximal subtotal gastrectomy or total gastrectomy) was selected based on tumor location. Lymphadenectomy was performed in all patients according to the Japanese Gastric Cancer Association [1]. During surgery, all of the tissues around the common hepatic, proper hepatic, celiac axis and splenic arteries were meticulously cleared to remove perigastric lymph nodes, to expose the root of the left gastric artery. If any vessels were encountered during complete resection of the hepatogastric ligament along the inferior border of the liver, fat and lymphoid tissue above and below the vessel was dissected to bare the vessel. If identified, the vessel was dissected along its entire course (up to the hepatic parenchyma and down to its origin from the left gastric artery). Subsequently, the stomach was lifted upwards (towards the head). Fatty connective tissue and lymph nodes along the left gastric artery were thoroughly removed from its root to its access in the stomach to bare the artery. During these procedures, we attempted, whenever possible, to preserve the main vessels and structures and their anatomical relationships to each other. The origin of the ALHA and its course were identified intraoperatively and on preoperative CTA images (Fig. 1), which were carefully recorded onto our video data system by the surgeons. After firmly clipping the ALHA, it was severed at the inferior border of the liver. Finally, the left gastric artery was divided at its origin with double clips to completely remove the lymph nodes (group 7).
Figure 1. Schematic drawing of the accessory left hepatic artery (ALHA).
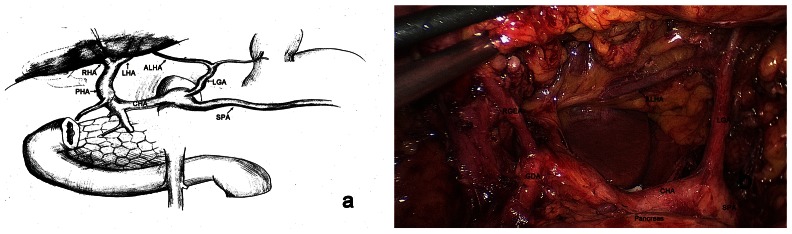
(a). Intraoperative photograph showing an ALHA arising from the left hepatic artery and entering the left liver. (b). LGA, left gastric artery; SPA, splenic artery; CHA, common hepatic artery; PHA, proper hepatic artery; GDA, gastroduodenal artery; RGEA, right gastroepiploic artery; LHA, left hepatic artery; RHA, right hepatic artery.
Statistical analysis
All statistical analyses were performed using the statistical program SPSS 18.0. Data were reported as mean ±SD and compared using the chi-square test or unpaired Student'st-test, as appropriate. P<0.05 was considered statistically significant.
Results
1. Anatomy and prevalence of the ALHA
The ALHA has been defined as a vessel arising from the left gastric artery, which, in combination with a typical left hepatic artery, supplies blood to the left lobe of the liver [4]. Surgery allows a comprehensive evaluation of the ALHA owing to the meticulous dissection required to trace its origin and terminus, as well as good visualization after the stomach is lifted upwards (Fig. 2). Combining intraoperational finding with CTA images (Fig. 3) enabled a more complete description of the anatomy of the ALHA. The ALHA originates from the left gastric artery, branching off from this artery at its highest or turning point before it runs down toward the lesser curvature of the stomach. The course of the left gastric artery continues toward the upper-left, finally dividing into several branches near the cardia to supply the cardia and the fundus of the stomach. The part of the ALHA outside the liver is short. Within the gastrohepatic ligament, the ALHA extends towards the right or upper-right in a straight or slightly tortuous course to enter the hepatic parenchyma through the left sagittal groove, anterior to the caudate lobe. It extends to the left, dividing into several branches, with the diameters of segmental or subsegmental arteries, to supply the left lateral lobe of the liver. We found that severing a relatively large ALHA intraoperatively resulted in hepatic ischemia, but that the ischemic parenchyma was limited and had clear boundaries separating ischemic from normal parenchyma (Fig. 4).
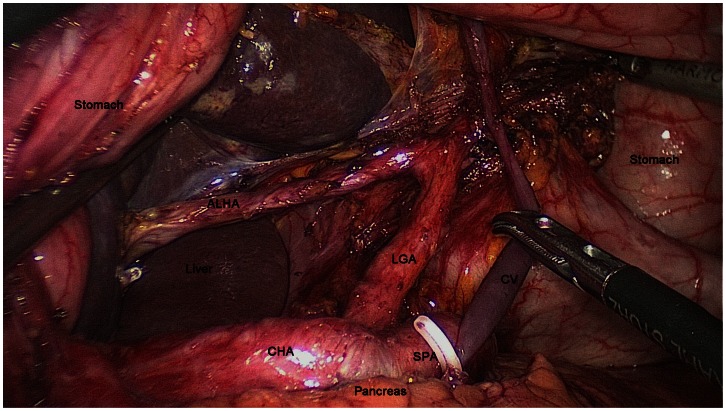
Figure 2. Intraoperative exposure of the origin and terminus of the accessory left hepatic artery (ALHA) though meticulous dissection.
LGA, left gastric artery; SPA, splenic artery; CHA, common hepatic artery; CV, coronary vein.
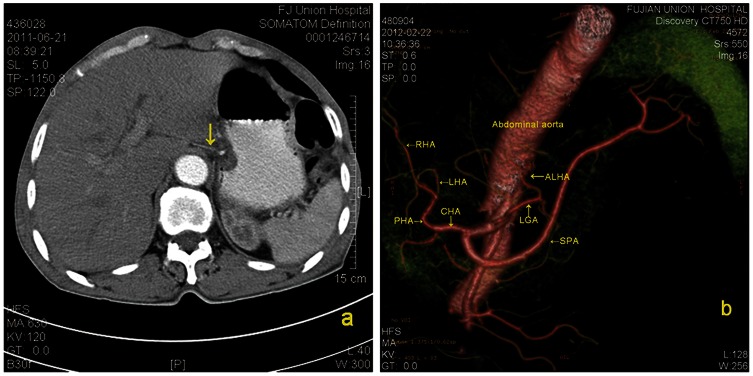
Figure 3. Preoperative enhanced transverse CT image showing a fine accessory left hepatic artery (ALHA) in the gastrohepatic ligament.
(a). Three-dimensional CT reconstruction,showing an ALHA originating from the left gastric artery. (b). LGA, left gastric artery; SPA, splenic artery; CHA, common hepatic artery; PHA, proper hepatic artery; LHA, left hepatic artery; RHA, right hepatic artery.
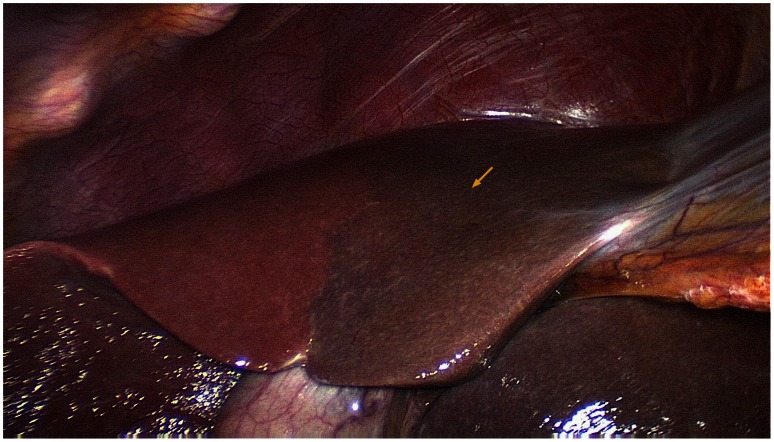
Figure 4. Image showing impairment of the blood supply to the left hepatic parenchyma after a relatively large accessory left hepatic artery (ALHA) was severed (arrows).
Of our 1173 patients who underwent radical gastrectomy for gastric cancer 135 (11.5%) had an ALHA, including 107 of the 979 (10.9%) patients without CLD and 28 of the 194 (14.4%) with CLD, a difference that was not statistically significant(P = 0.162).
2. Patient clinicopathologic characteristics
The clinicopathologic characteristics of the patients are presented in Table 1. The 1173 patients included 891 men and 282 women, of mean age 60.9 years (range 12 to 101 years). Age, gender, tumor size, body mass index (BMI), location of neoplasm, tumor depth, total number of harvested lymph nodes, lymph node status (N stage), TNM stage, histologic type, resection extent, and gastrointestinal reconstruction type did not differ between the groups of patients with and without an ALHA (P>0.05 each).
Table 1. Comparison of clinicopathological characteristics in groups of patients with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Characteristics | ALHA group(n = 135) | non-ALHA group(n = 1038) | P value |
| Sex | 0.831 | ||
| Female | 31 | 251 | |
| Male | 104 | 787 | |
| Age(years) | 60.6±12.4 | 61.0±11.5 | 0.721 |
| Tumor size(cm) | 5.1±2.8 | 4.9±2.7 | 0.507 |
| BMI(kg/m2) | 21.8±2.9 | 22.2±3.4 | 0.175 |
| Tumor location | 0.641 | ||
| Upper | 34 | 264 | |
| Middle | 33 | 290 | |
| Lower | 68 | 484 | |
| Tumor depth | 0.559 | ||
| T1 | 28 | 247 | |
| T2 | 34 | 228 | |
| T3 | 31 | 254 | |
| T4a | 42 | 309 | |
| Total retrieved lymph nodes | 30.7±10.8 | 32.4±10.7 | 0.104 |
| N stage | 0.405 | ||
| N0 | 46 | 384 | |
| N1 | 25 | 153 | |
| N2 | 18 | 180 | |
| N3 | 46 | 321 | |
| TNM stage | 0.608 | ||
| IA | 23 | 209 | |
| IB | 16 | 83 | |
| IIA | 12 | 99 | |
| IIB | 13 | 135 | |
| IIIA | 17 | 119 | |
| IIIB | 25 | 200 | |
| IIIC | 29 | 193 | |
| Histology | 0.285 | ||
| Differentiated | 21 | 210 | |
| Undifferentiated | 114 | 825 | |
| Resection extent | 0.942 | ||
| TG | 70 | 531 | |
| PG | 3 | 28 | |
| DG | 62 | 479 | |
| Reconstruction | 0.924 | ||
| BillrothI | 56 | 420 | |
| BillrothII | 6 | 59 | |
| Roux-en-Y | 70 | 531 | |
| EGP | 3 | 28 |
BMI, Body mass index; TG, total gastrectomy; PG, proximal subtotal gastrectomy; DG, distal subtotal gastrectomy; EGP, esophagogastrostomy. P-values are for comparison of the ALHA and non-AHLA groups.
3. Intraoperative and postoperative characteristics
Operation time, estimated blood loss, volumes transfused, first ambulation time, bowel function recovery time and duration of hospital stay were similar in patients with and without an ALHA (P>0.05each; Table 2).
Table 2. Comparison of intraoperative and postoperative characteristics in groups of patients with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Variables | AHLA group(n = 135) | non-AHLA group(n = 1038) | P value |
| Operation time(minutes) time(minutes)time(minutes) | 204.0±379.8 | 185.3±45.6 | 0.567 |
| Blood loss(ml) | 83.7±87.4 | 81.64±108.7 | 0.829 |
| Transfused patients | 1 | 36 | 0.113 |
| Time to first ambulation(d) | 2.5±1.0 | 2.5±1.1 | 0.387 |
| Time to first flatus(d) | 3.9±1.7 | 3.7±1.3 | 0.251 |
| Time to fluid die(d) | 4.6±2.0 | 4.6±1.8 | 0.959 |
| Time to soft die(d) | 8.4±2.7 | 8.7±3.1 | 0.148 |
| Hospital stay(d) | 13.7±7.1 | 13.6±8.4 | 0.853 |
d represents postoperative days. P-values are for comparison of the ALHA and non-AHLA groups.
4. Morbidity and mortality
The overall postoperative morbidity and mortality rates among all patients were 11.0% and 0.6%, respectively. Postoperative complication rates (11.1% vs. 10.9%, P>0.05) and mortality rates (0.7% vs. 0.6%, P>0.05) did not differ between the ALHA and non-AHLA groups (P>0.05; Table 3). None of the patients in either group died of liver failure.
Table 3. Comparison of morbidity and mortality in groups of patients with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Variables | AHLA group(n = 135) | non-ALHA group(n = 1038) | P value |
| Surgical | 10 | 69 | 0.740 |
| Duodenal stump fistula | 1 | 4 | |
| Anastomotic leakage | 2 | 12 | |
| Pancreatic fistula | 1 | 8 | |
| Lymphatic fistula | 1 | 10 | |
| Abdominal infection | 2 | 10 | |
| Gastric stasis | 2 | 13 | |
| Anastomotic bleeding | 1 | 9 | |
| Anastomotic stenosis | 0 | 3 | |
| Medical | 5 | 45 | 0.733 |
| Pneumonia | 4 | 35 | |
| Angiocardiopathy | 0 | 8 | |
| DIC | 1 | 2 | |
| Mortality | 1 | 6 | 0.859 |
DIC, disseminated intravascular coagulation. P-values are for comparison of the ALHA and non-ALHA groups.
5. Changes in Liver Function
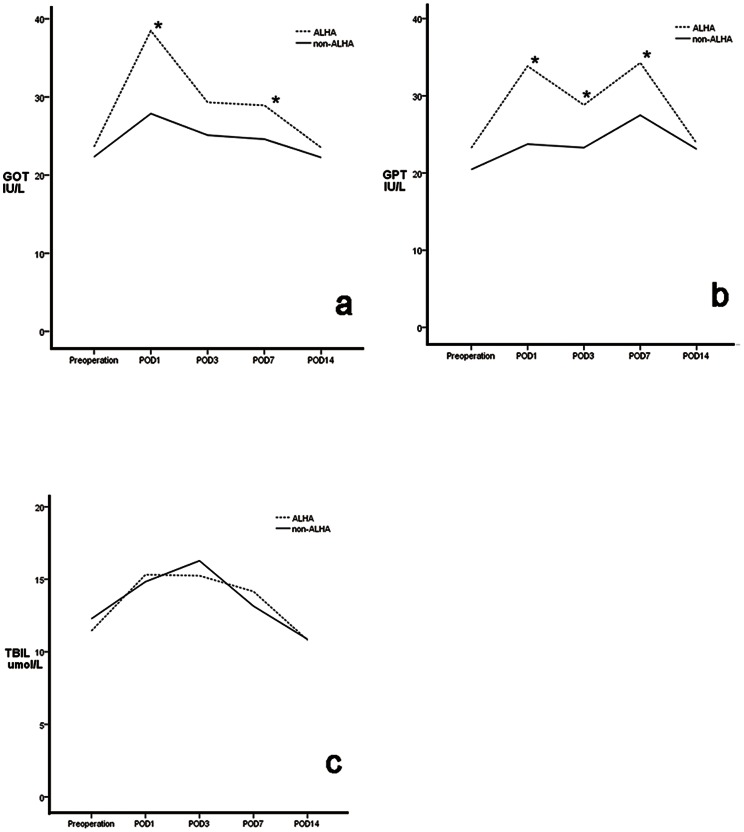
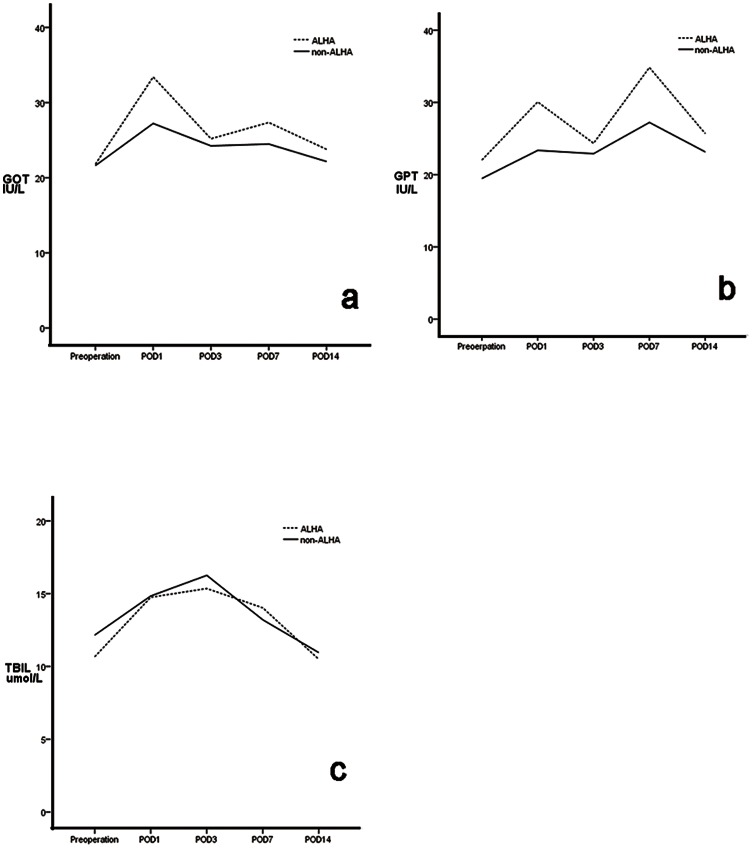
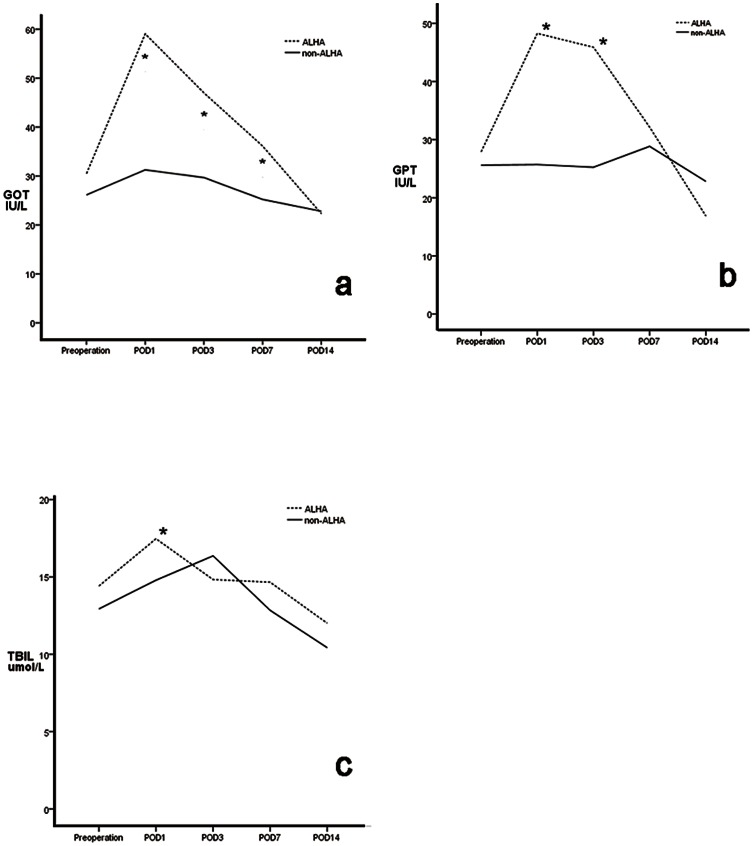
We observed postoperative liver related complication of patients within 1 month. None of the patients in either group had postoperative symptoms, such as jaundice or pruritus, related to liver dysfunction. Preoperative GOT, GPT and TBIL concentrations were similar in the two groups, as were TBIL concentrations on postoperative days 1,3, and 7 (P>0.05 each). However, GOT and GPT activities were higher in the ALHA than in the non-ALHA group on days 1 and 7 (P<0.05 each). None of these three parameters differed significantly on day 14 (Fig. 5). The proportion of patients whose liver function was still impaired at postoperative 1 week were significantly lower in the non-ALHA group (33 of the 135 (24.4%) in the ALHA group vs.174 of the 1038 (16.8%) in the non-ALHA group) (P = 0.028) (Table 4). Stratified analysis showed that preoperative GOT, GPT and TBIL concentrations in patients with and without ALHA did not differ significantly between patients with and without CLD. Furthermore, in patients without CLD, these three parameters were similar on postoperative days 1, 3, 7, and 14 in patients with and without ALHA(P>0.05 each; Fig. 6, Table 5)). Among patients with CLD, GOT and GPT concentrations on days 1 and 3 and GOT concentrations on day7 were significantly higher in patients with than without an ALHA, but returned to normal on day 14, with no significant difference between patients with and without an ALHA (Fig. 7, Table 6)). Stratified analysis also showed that the proportion of patients whose liver function was still impaired at postoperative 1 week did not differ between the ALHA group and non-ALHA group both in patients without CLD (22.4% vs. 16.4%, P = 0.118) and in patients with CLD (32.1% vs. 18.7%, P = 0.103).
Figure 5. Mean GOT (a), GPT (b), and TBIL (c) concentrations in groups of patients with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
Each value represents the mean.* P<0.05.
Table 4. Mean GOT, GPT, and TBIL concentrations in groups of patients with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Group | No. | GOT | P value | GPT | P value | TBIL | P value | |
| Preoperation | Non- ALHA | 1038 | 22.3±11.3 | 0.322 | 20.5±16.9 | 0.232 | 12.3±7.4 | 0.212 |
| ALHA | 135 | 23.6±14.5 | 23.3±26.4 | 11.5±6.7 | ||||
| POD1 | Non -ALHA | 1038 | 27.9±21.7 | 0.002 | 23.7±19.0 | 0.002 | 14.8±5.4 | 0.33 |
| ALHA | 135 | 38.5±39.0 | 33.9±36.4 | 15.3±5.6 | ||||
| POD3 | Non -ALHA | 1038 | 25.1±24.7 | 0.056 | 23.3±19.8 | 0.002 | 16.3±7.6 | 0.128 |
| ALHA | 135 | 29.3±17.4 | 28.8±15.5 | 15.2±5.8 | ||||
| POD7 | Non -ALHA | 1038 | 24.6±22.6 | 0.035 | 27.5±29.1 | 0.039 | 13.1±10.1 | 0.269 |
| ALHA | 135 | 28.9±22.2 | 34.3±36.4 | 14.2±8.4 | ||||
| POD14 | Non -ALHA | 1038 | 22.3±15.9 | 0.377 | 23.1±19.3 | 0.665 | 10.9±8.3 | 0.934 |
| ALHA | 135 | 23.5±13.1 | 23.9±19.5 | 10.8±4.3 |
POD, postoperative day; GOT, Glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; TBIL, total bilirubin; P-values are for comparison of the ALHA and non-AHLA groups.
Figure 6. Mean GOT (a), GPT (b), and TBIL (c) concentrations in groups of patients without chronic liver diseases (CLD), with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
Each value represents the mean.
Table 5. Mean GOT, GPT, and TBIL concentrations in groups of patients without chronic liver diseases (CLD), with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Group | No. | GOT | P value | GPT | P value | TBIL | P value | |
| Preoperation | Non- ALHA | 872 | 21.6±9.7 | 0.846 | 19.5±15.4 | 0.143 | 12.2±7.4 | 0.065 |
| ALHA | 107 | 21.8±9.6 | 22.0±27.2 | 11.3±5.1 | ||||
| POD1 | Non -ALHA | 872 | 27.2±13.1 | 0.106 | 23.4±17.8 | 0.073 | 14.8±5.5 | 0.877 |
| ALHA | 107 | 33.4±39.0 | 30.1±37.8 | 14.8±5.2 | ||||
| POD3 | Non -ALHA | 872 | 24.2±23.0 | 0.671 | 22.9±20.5 | 0.480 | 16.3±7.6 | 0.235 |
| ALHA | 107 | 25.2±11.0 | 24.4±12.7 | 15.4±6.0 | ||||
| POD7 | Non -ALHA | 872 | 24.5±22.8 | 0.236 | 27.2±30.1 | 0.061 | 13.2±10.6 | 0.443 |
| ALHA | 107 | 27.4±23.7 | 34.8±40.3 | 14.0±9.3 | ||||
| POD14 | Non -ALHA | 872 | 22.2. ±11.7 | 0.188 | 23.2±17.7 | 0.238 | 11.0±8.7 | 0.592 |
| ALHA | 107 | 23.8±14.5 | 25.7±21.3 | 10.5±4.4 |
POD, postoperative day; GOT, Glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; TBIL, total bilirubin; P-values are for comparison of the ALHA and non-AHLA groups.
Figure 7. Mean GOT (a), GPT (b), and TBIL (c) concentrations in groups of patients with chronic liver diseases (CLD), with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA.
Each value represents the mean. *P<0.05.
Table 6. Mean GOT, GPT, and TBIL concentrations in groups of patients with chronic liver diseases (CLD), with (ALHA group) and without (non-ALHA group) an accessory left hepatic artery (ALHA).
| Group | No. | GOT | P value | GPT | P value | TBIL | P value | |
| Preoperation | Non- ALHA | 166 | 26.2±16.9 | 0.369 | 25.6±22.7 | 0.626 | 12.9±7.1 | 0.340 |
| ALHA | 28 | 30.6±24.9 | 27.9±22.9 | 14.4±9.8 | ||||
| POD1 | Non -ALHA | 166 | 31.3±45.2 | 0.003 | 25.7±24.4 | 0.001 | 14.8±5.0 | 0.049 |
| ALHA | 28 | 57.8±33.0 | 48.3±26.5 | 17.5±6.6 | ||||
| POD3 | Non -ALHA | 166 | 29.7±32.1 | 0.017 | 25.3±15.5* | 0.001 | 16.4±7.6 | 0.302 |
| ALHA | 28 | 45.1±26.3 | 45.9±13.1 | 14.8±4.9 | ||||
| POD7 | Non -ALHA | 166 | 25.2±21.2 | 0.021 | 28.8±23.6 | 0.472 | 12.8±7.6 | 0.215 |
| ALHA | 28 | 34.9±14.4 | 32.1±13.8 | 14.7±3.7 | ||||
| POD14 | Non -ALHA | 166 | 22.8±29.4 | 0.961 | 22.8±26.2 | 0.234 | 10.4±5.5 | 0.141 |
| ALHA | 28 | 22.5±5.6 | 16.9±6.5 | 12.0±3.6 |
POD, postoperative day; GOT, Glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; TBIL, total bilirubin; P-values are for comparison of the ALHA and non-AHLA groups.
Discussion
Radical gastrectomy can improve the disease-free survival rate in patients with gastric carcinoma [13]. Surgeons performing these operations should be well acquainted with the normal perigastric vascular anatomy and its variations, because failure to recognize the presence of a variant vessel can result in bleeding and other complications[14], [15]. Although an ALHA is an anomaly frequently encountered within the gastrohepatic ligament, its prevalence has been found to vary widely, with most such studies involving cadavers or angiographic data of patients undergoing hepatobiliary surgery or liver transplantation. For example, an analysis of 200 cadavers found that 16 (8%) had ALHAs arising from the left gastric artery [4]. A study of 701 patients undergoing hepatobiliary surgery or liver transplantation reported that the left hepatic artery branched from the proper hepatic artery in 89% of patients, but showed an anatomical variationin 11% [16].
Few studies, however, have assessed the prevalence of ALHA through surgical dissection. We therefore retrospectively reviewed the clinical data of 1173 patients who underwent radical gastrectomy for gastric cancer. This method, in which the hepatogastric ligament was completely separated and the vessels within it were elaborately dissected during gastric cancer surgery, accurately determined the prevalence of ALHA as well as clearly showing its anatomical features. Therefore, these results may be more accurate than those obtained at autopsy. We found that the incidence of ALHA was 11.5%, suggesting that this anomaly is quite common. Embryonic research has shown a close original relationship between the liver and stomach, which simultaneously evolve from the foregut terminal [17]. Blood is supplied to the fetal liver from the common hepatic artery, the right hepatic artery originating from the superior mesenteric artery, and the left hepatic artery originating from the left gastric artery. During embryonic development, these arteries undergo constant differentiation, growth, branching and distribution to the mature organ. The ALHA corresponds to the partial or complete persistence of the fetal pattern of the left hepatic artery, making its incidence relatively high [18]–[21]. Without knowledge of its presence, general surgeons using anultrasonic scalpel or electrotome may inadvertently sever the ALHA, increasing the risks of intraoperative and postoperative hemorrhage.
It is unclear, however, whether the ALHA should be severed during radical gastrectomy. Complications have been reported following intentional or accidental division of the ALHA, including abscess formation, cholangitis, liver failure, and even liver lobe necrosis [22]–[24]. Thus in the presence of an ALHA, some authors [24] even suggested performing a prophylactic resection of the left liver lobe when extended dissection of the lesser omentum is required in gastric or esophageal resection for malignancy.
We found, however, that our groups of patients with and without an ALHA had similar intraoperative and postoperative recovery characteristics and morbidity and mortality rates. Although our findings suggest that patients with an ALHA had poorer liver function on postoperative day 7 than those without an ALHA, stratified analysis showed no significant differences in liver function in patients without CLD between patients with a severed ALHA and those without an ALHA. In patients with CLD, however, those with a severed ALHA had significantly higher liver function indices than those without an ALHA. Angiographic examinations before and after therapeutic ligation of the hepatic artery as well as corrosion-cast studies [25]–[27] have shown collateralization between the intrahepatic and adjacent arteries. Consistent arterio-arterial anastomoses have been observed between the inferior phrenic arteries and branches of the main hepatic artery, making it possible to fill the entire arterial system of the liver by injecting into the inferior phrenicartery. Three anastomotic pathways are present from the right to the left hepatic artery, through portal(hilar) anastomoses, translobar vessels, and capsular arteries. These collaterals are observed no later than 10 h after arterial ligation [28], and neither hepatic necrosis nor death from hepatic ischemia was observed following hepatic artery ligation [26]. However, since infiltration of inflammatory cells, extensive hepatocyte necrosis and proliferation of fibrous tissue reduce liver reserve function in patients with CLD, including chronic hepatitis and cirrhosis [29]–[31], these patients are less tolerable of ischemia and hypoxia than patients without CLD. Thus, severing of the ALHA can easily induce liver dysfunction in patients with CLD. Whenever possible, therefore, the ALHA should be left intact in patients with CLD undergoing radical gastrectomy, by, for example, dividing the left gastric artery distal to the origin of the ALHA. If the ALHA is severed, however, these patients should be intensively monitored and may require liver-protecting therapy.
In conclusion, ALHA is a common anomaly that was found in 11.5% of patients. It can be safely severed during radical gastrectomy in patients without CLD, but should be left intact in patients with CLD to prevent liver dysfunction. If severed in the latter, the patient should be monitored and liver-protecting therapy may be necessary. However, because this study was nonrandomized and was based on a retrospective clinical analysis, our conclusion must be confirmed by a prospective randomized study.
Funding Statement
The authors have no support or funding to report.
Refrences
- 1. Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14: 113–123. [DOI] [PubMed] [Google Scholar]
- 2. Weiglein AH (1996) Variations and topography of the arteries in the lesser omentum in humans. Clin Anat 9: 143–150. [DOI] [PubMed] [Google Scholar]
- 3.Adachi B, Hasebe K, Daigaku TT (1928) Das arteriensystem der Japaner: Kaiserlich-Japanischen Universität zu Kyoto.
- 4. Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112: 337–347. [DOI] [PubMed] [Google Scholar]
- 5. Abdullah SS, Mabrut JY, Garbit V, De La Roche E, Olagne E, et al. (2006) Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat 28: 468–473. [DOI] [PubMed] [Google Scholar]
- 6. Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT (2002) Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 224: 542–547. [DOI] [PubMed] [Google Scholar]
- 7. Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220: 50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr JC, Nemcek AA Jr, Abecassis M, Blei A, Clarke L, et al. (2003) Preoperative evaluation of the entire hepatic vasculature in living liver donors with use of contrast-enhanced MR angiography and true fast imaging with steady-state precession. J Vasc Interv Radiol 14: 441–449. [DOI] [PubMed] [Google Scholar]
- 9. Paprottka PM, Jakobs TF, Reiser MF, Hoffmann RT (2012) Practical vascular anatomy in the preparation of radioembolization. Cardiovasc Intervent Radiol 35: 454–462. [DOI] [PubMed] [Google Scholar]
- 10. Hu YF, Yu J, Zhang C, Wang YN, Cheng X, et al. (2010) [Development and implementation of a clinical data mining system for gastric cancer surgery]. Zhonghua Wei Chang Wai Ke Za Zhi 13: 510–515. [PubMed] [Google Scholar]
- 11.Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, et al. (2008) The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol 103: : 2727–2736; quiz 2737. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM Classification of Malignant Tumours: New York, Wiley.
- 13.Munson JL, O'Mahony R (2005) Radical gastrectomy for cancer of the stomach. Surg Clin North Am 85: : 1021–1032, vii. [DOI] [PubMed] [Google Scholar]
- 14. Sano T, Martin IG (1996) Lymphadenectomy and pancreatico-splenectomy in gastric cancer surgery. Lancet 348: 195–196. [DOI] [PubMed] [Google Scholar]
- 15. Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, et al. (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19: 532–536. [DOI] [PubMed] [Google Scholar]
- 16. Gruttadauria S, Foglieni CS, Doria C, Luca A, Lauro A, et al. (2001) The hepatic artery in liver transplantation and surgery: vascular anomalies in 701 cases. Clin Transplant 15: 359–363. [DOI] [PubMed] [Google Scholar]
- 17.Williams PL (1995) Gray&s anatomy: Churchill livingstone London.
- 18. Polguj M, Gabryniak T, Topol M (2010) The right accessory hepatic artery; a case report and review of the literature. Surg Radiol Anat 32: 175–179. [DOI] [PubMed] [Google Scholar]
- 19.Chevrel JP, Bonnel F, Bossy J (1991) Anatomie clinique: Springer.
- 20. Cavdar S, Sehirli U, Pekin B (1997) Celiacomesenteric trunk. Clin Anat 10: 231–234. [DOI] [PubMed] [Google Scholar]
- 21. Osawa T, Feng XY, Sasaki N, Nagato S, Matsumoto Y, et al. (2004) Rare case of the inferior mesenteric artery and the common hepatic artery arising from the superior mesenteric artery. Clin Anat 17: 518–521. [DOI] [PubMed] [Google Scholar]
- 22. Friesen SR (1957) The significance of the anomalous origin of the left hepatic artery from the left gastric artery in operations upon the stomach and esophagus. Am Surg 23: 1103–1108. [PubMed] [Google Scholar]
- 23. Hemming AW, Finley RJ, Evans KG, Nelems B, Fradet G (1992) Esophagogastrectomy and the variant left hepatic artery. Ann Thorac Surg 54: 166–168. [DOI] [PubMed] [Google Scholar]
- 24. Lurie AS (1987) The significance of the variant left accessory hepatic artery in surgery for proximal gastric cancer. Arch Surg 122: 725–728. [DOI] [PubMed] [Google Scholar]
- 25. Plengvanit U, Chearanai O, Sindhvananda K, Dambrongsak D, Tuchinda S, et al. (1972) Collateral arterial blood supply of the liver after hepatic artery ligation, angiographic study of twenty patients. Ann Surg 175: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koehler RE, Korobkin M, Lewis F (1975) Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology 117: 49–54. [DOI] [PubMed] [Google Scholar]
- 27. Reimann B, Lierse W, Schreiber HW (1983) [Anastomoses between the segmental arteries of the liver and phrenicohepatic arterio-arterial anastomoses]. Langenbecks Arch Chir 359: 81–92. [DOI] [PubMed] [Google Scholar]
- 28. Mays ET, Wheeler CS (1974) Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med 290: 993–996. [DOI] [PubMed] [Google Scholar]
- 29. Pinzani M, Rosselli M, Zuckermann M (2011) Liver cirrhosis. Best Pract Res Clin Gastroenterol 25: 281–290. [DOI] [PubMed] [Google Scholar]
- 30. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, et al. (1978) The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol 31: 395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gall EA (1960) Posthepatitic, postnecrotic, and nutritional cirrhosis: a pathologic analysis. Am J Pathol 36: 241–271. [PMC free article] [PubMed] [Google Scholar]