The authors report that a laparoscopic technique could facilitate orthotopic kidney transplantation in selected patients.
Keywords: Laparoscopic surgery, Orthotopic kidney transplant, Pig model
Abstract
Background and Objectives:
Laparoscopic surgery has rapidly expanded in surgical practice with well-accepted benefits of minimal incision, less analgesia, better cosmetics, and quick recovery. The surgical technique for kidney transplantation has remained unchanged since the first successful kidney transplant in the 1950s. Over the past decade, there were only a few case reports of kidney transplantation by laparoscopic or robotic surgery. Therefore, the aim of this study is to develop a laparoscopic technique for kidney transplantation at the region of the native kidney.
Methods:
After initial development of the laparoscopic technique for kidney transplant in cadaveric pigs, 5 live pigs (Sus scrofa, weighing 45–50 kg) underwent laparoscopic kidney transplant under general anesthesia. First, laparoscopic donor nephrectomy was performed, and then the kidney was perfused and preserved with cold Ross solution. The orthotopic auto-transplant was subsequently performed using the laparoscopic technique. The blood flow of the kidney graft was assessed using Doppler ultrasonography, and urine output was monitored.
Results:
The laparoscopic kidney transplant was successful in 4 live pigs. Immediate urine output was observed in 3 pigs. The blood flow in the kidney was adequate, as determined using Doppler ultrasonography.
Conclusion:
It has been shown that laparoscopic kidney orthotopic transplant is feasible and safe in the pig model. Immediate kidney graft function can be achieved. A further study will be considered to identify the potential surgical morbidity and mortality after recovery in a pig model before translating the technique to clinical human kidney transplantation.
INTRODUCTION
The use of laparoscopic surgery has been widely expanded in clinical surgical practice. In the kidney transplant field, it is well accepted that laparoscopic donor nephrectomy has equal kidney graft function with minimal incision, less analgesia, better cosmetic appearance, and quick recovery.1–5 The surgical technique for kidney transplantation has remained unchanged since the first successful kidney transplant in the 1950s.6 The kidney is routinely placed in the iliac fossa with the renal artery being anastomosed to the iliac artery and the renal vein end being anastomosed to the side of the external iliac vein. The disadvantage of this technique is that the kidney graft is vulnerable to injury or trauma because it is exposed to the front of the pelvis. It also becomes very challenging when a third or fourth subsequent kidney transplant is required because of adhesions around the iliac vessels from previous transplant surgery. Therefore, the aim of this study is to develop a laparoscopic technique for orthotopic kidney transplant. We predict that laparoscopic kidney transplant will have the advantages of equal graft function, a smaller incision, less pain, a better cosmetic appearance, a quicker recovery, and a shorter hospital stay compared with conventional open surgery.
METHODS
The study was approved by the animal ethics committee of the university. The study consists of 2 parts. In part 1 the laparoscopic technique for kidney transplant was developed on 10 cadaveric pigs that were used for other studies. The technique development included the justification of port position and vessel anastomosis. In part 2 the laparoscopic kidney transplant was performed in live pigs.
Animals
Ten cadaveric pigs were used after a study conducted by the department of respiratory medicine. The lungs of the pigs were removed by the respiratory medicine team for histopathology. Approval was obtained from the animal ethics committee of the university for this secondary study. Five live pigs (Sus scrofa, 2 male and 3 female pigs), weighing 45 to 50 kg, were transported to the Large Animal Facility of the university 2 weeks before surgery for acclimatization. The pigs were housed in a pen above the floor. They were fed a maintenance diet (Grower; Westfeeds Pty Ltd, Bentley, Australia) and allowed free access to tap water. Daily care was provided by the staff of the Large Animal Facility. The pigs were euthanized by intravenous injection of pentobarbital (160 mg/kg) at the end of the study.
Anesthesia
Food was withheld for 12 hours before anesthesia and surgery, but the pigs were allowed free access to water. Anesthesia was induced with a combination of Zoletil (4.4 mg/kg) and xylazine (2.2 mg/kg) by intramuscular injection in the neck. An auricular vein was cannulated, and propofol (1 mg/kg) was administered intravenously to achieve an adequate depth of anesthesia for tracheal intubation. The trachea was intubated with a cuffed endotracheal tube (Portex Soft Seal Cuff, 8.0-mm inner diameter; SIMS Portex Ltd, Hythe, United Kingdom). Anesthesia was maintained with isoflurane delivered in 100% oxygen. Mechanical ventilation was commenced immediately after oral intubation of the trachea with a tidal volume of 10 to 15 mL/kg and peak inspiratory pressure <25 cm of water. The respiratory rate was adjusted to achieve normocapnia (end-tidal carbon dioxide level, 35–45 mm Hg). The depth of anesthesia was assessed subjectively by a veterinary anesthetist throughout the procedure and altered accordingly. The auricular artery was also cannulated for direct blood pressure measurement. In addition, a central line catheter was inserted into the left jugular vein (7-French, 2-lumen catheter; Arrow International, Reading, Pennsylvania) for measurement of central venous pressure and delivery of intravenous fluid therapy (Hartmann solution and Gelofusin). Pancuronium was administered (0.1 mg/kg intravenously) for neuromuscular blockade. The observations were continuous including oxyhemoglobin saturation, end-tidal carbon dioxide level, invasive blood pressure, central venous pressure, pharyngeal temperature, and electrocardiogram. The parameters were recorded every 5 minutes.
Surgical Procedure
After general anesthesia, the pig was placed in the right decubitus position. The camera port was inserted via a small open incision (1.5 cm) at a site to the left of midline and above the umbilicus. The other ports were placed under vision; the sites of the ports are shown in Figure 1. The left kidney was identified and the bowel dissected medially. The renal artery and vein and ureter were exposed and dissected. The renal artery was dissected to the origin of the aorta and the renal vein to the origin of the vena cava to obtain the best possible vessel exposure. This facilitated the subsequent auto-transplant. The left kidney was mobilized free from its attachments. The ureter was divided first at the level of the lower pole. At this point, 1,500 IU of heparin was given intravenously. A small low midline incision (6 cm) in the abdomen was created by longitudinally cutting the skin and fascia at the midline. Then, the muscular layer was opened through the left para-midline (Figure 2). The peritoneal layer remained intact at this stage. The renal artery and vein were clamped with an endoscopic Bulldog device (B. Braun Melsungen AG, Melsungen, Germany) and divided. The kidney was then delivered by opening the peritoneum via the incision and perfused on the back table immediately with cold (4°C) Ross perfusion fluid (Orion Laboratories, Balcatta, Australia) (1 L of Ross solution plus 10,000 IU of heparin). The kidney graft was prepared on the back table. No. 6-0 Prolene stitches (Ethicon, Somerville, New Jersey) were placed at the upper and lower corners of the renal vein as marking stitches, thus facilitating the manipulation of the vessel during the anastomosis. The kidney was preserved in the cold perfusion fluid until implantation.
Figure 1.
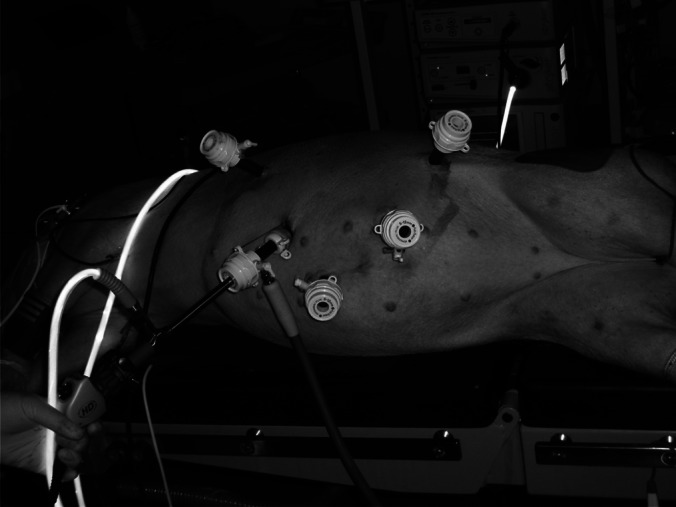
Positions of ports.
Figure 2.
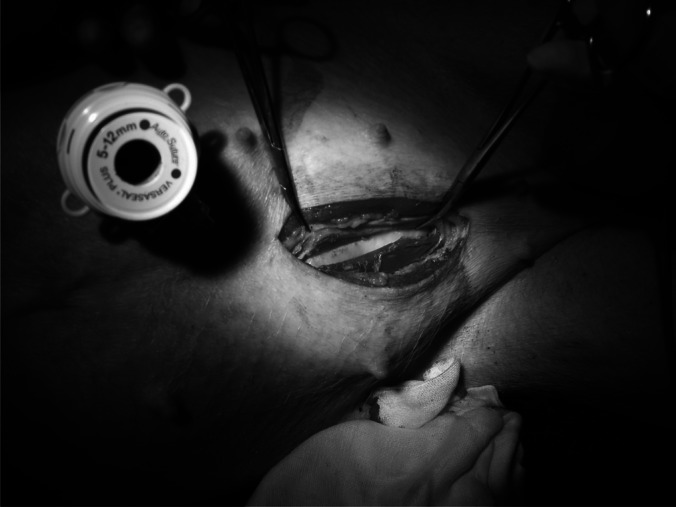
Small midline incision for delivery of kidney graft.
The kidney was then implanted at the orthotopic location by a laparoscopic technique. The kidney graft was continuously flushed with cold (4°C) normal saline solution via an extra 5-mm port during the period of vessel anastomosis. The renal artery was anastomosed end to end to the renal artery stump and the renal vein was anastomosed end to end to the renal vein stump by No. 6-0 Prolene running sutures (Figures 3, 4, and 5) in the first 4 pigs and by interrupted stitches in the fifth pig (Figure 6). The venous running suture was tied with a growth factor margin allowing distension of the venous anastomosis after reperfusion. The kidney graft was reperfused by removing the venous Bulldog device, followed by the arterial Bulldog device. Forty milligrams of furosemide was given intravenously after kidney reperfusion. The ureteral catheter that was inserted into the ureter was brought out via the laparoscopic port for observation of urine output.
Figure 3.

Renal artery end-to-end anastomosis.
Figure 4.

Renal vein end-to-end anastomosis.
Figure 5.
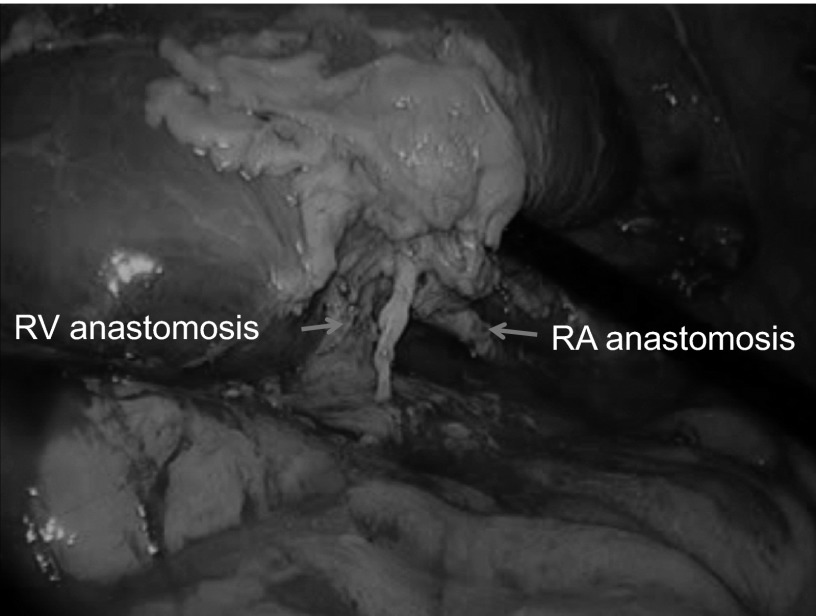
Renal graft reperfused rapidly and uniformly. The arrows show renal artery (RA) and renal vein (RV) anastomosis by running suture.
Figure 6.
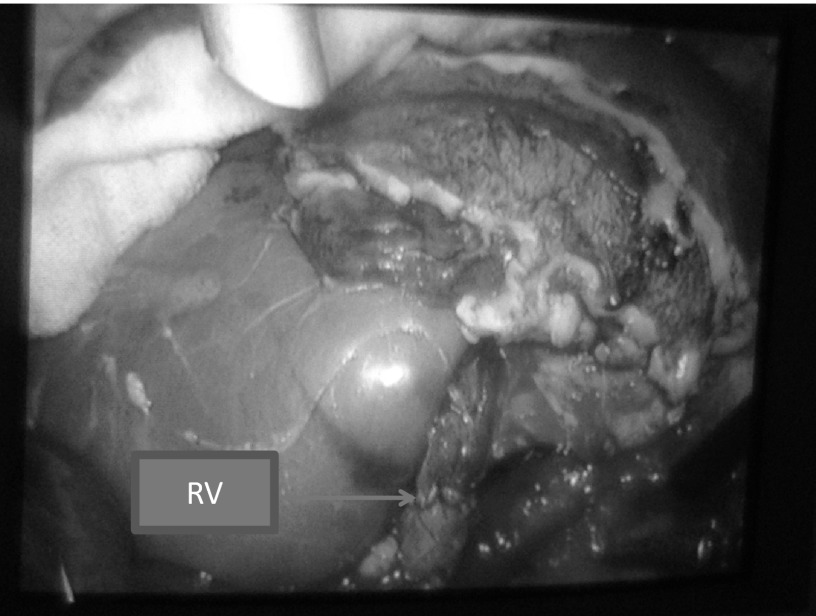
Renal graft reperfused rapidly and uniformly. The arrow shows renal vein (RV) interrupted stitches.
Postoperative Course
The pig remained under anesthesia during the period of observation. The vital signs were recorded every 5 minutes. The urine output was monitored. Doppler ultrasonography was performed to check the blood flow in the kidney graft. Graft nephrectomy was performed by a laparoscopic technique at the end of the observation period. The pig was then euthanized according to the study protocol. The vessel anastomoses were examined on the bench table.
RESULTS
The vital signs of all pigs were stable during the surgery and postoperative observation period. The blood pressure was stable from 100 mm Hg/60 mm Hg to 140 mm Hg/80 mm Hg. There were no intraoperative complications and no conversion to open surgery. There was minimum blood loss in 4 pigs, and 1 pig had more blood oozing during the anastomosis. The operative time for laparoscopic donor nephrectomy ranged from 1 hour 20 minutes to 1 hour 50 minutes (mean, 1 hour 30 minutes). The time for renal artery anastomosis ranged from 30 to 40 minutes, whereas the time for renal vein anastomosis ranged from 25 to 35 minutes. The graft warm ischemic time ranged from 3 to 5 minutes. The graft cold ischemic time ranged from 1 hour 50 minutes to 2 hours 20 minutes. Four of five grafts were reperfused rapidly and uniformly (Figures 5 and 6). Urine output was immediately seen in 3 pigs. The blood flow in the kidney grafts was satisfactory on Doppler ultrasonography; the arterial waveforms in the kidney parenchyma were normal, as was venous flow. On postmortem examination, the vessel anastomosis was shown to be widely patent in 3 pigs (Figure 7), whereas there was narrowing at the anastomoses of the renal artery and vein in the other 2 pigs.
Figure 7.

Removed kidney graft after transplantation. The renal artery and vein anastomoses are patent.
DISCUSSION
The use of laparoscopic surgery has expanded rapidly in clinical surgical practice, replacing conventional open surgery. A laparoscopic technique has also been successfully used for vessel anastomosis with satisfactory results and significant benefits to patients.7,8 However, the surgical technique for kidney transplant has remained unchanged since the first successful kidney transplant in the 1950s.6 Basically, an open incision is needed at the lower abdomen and the kidney graft is placed at the iliac fossa in a heterotopic position. In this open surgery, the incision is approximately 15 to 20 cm long and cutting of the muscular layers is unavoidable. Recently, there have been few reports of heterotopic kidney transplant by laparoscopic/robotic surgery, which could minimize the incision for placement of the kidney graft.9–14 However, the disadvantage of these techniques is that the kidney graft is located at the pelvis, where it is relatively superficial and susceptible to injury during contact sports or trauma. Moreover, a third or fourth kidney transplant can also be very difficult because of the formation of adhesions surrounding the iliac vessels from previous transplant surgery. Open surgery for orthotopic kidney transplant has been reported in the literature as an effective alternative for those recipients in whom a heterotopic transplant was inappropriate, such as patients with a retained iliac fossa from a former graft, thrombosis of the iliac vein, or severe arthromatosis on the iliac artery.15–18 The long-term patient and graft survival rates are comparable with those for conventional heterotopic kidney transplant.18 However, open orthotopic kidney transplant has not been the favored approach because it is more difficult when compared with conventional heterotopic kidney transplant. It usually requires a longer midline incision or use of a posterolateral lumbotomy, in which massive muscles will be cut.17,18 As a result, more analgesia is required and the recovery takes longer. This study has shown that orthotopic kidney transplant can be performed safely by a laparoscopic approach, in which only a small midline (6-cm) incision is needed. The application of the laparoscopic technique for human orthotopic kidney transplant could facilitate the consideration in selected recipients. In particular, it could be useful for younger recipients who wish to enjoy active sports and those who have an unsuitable pelvic condition for heterotopic kidney transplant.
To our knowledge, this is the first report of laparoscopic kidney orthotopic transplant in a pig model. Obviously, in this experiment, laparoscopic kidney transplant only requires a small incision (6 cm) in the lower midline for delivery of the kidney graft in and out. The incision was created by opening the skin and fascia at the midline and opening the muscular layer in the para-midline, which makes the incision function like a valve to effectively reduce gas leak. This incision is time and cost saving because it does not need to be closed or sealed to prevent gas leak, and it thus minimizes the warm ischemic time of the graft. In this study, we have learned that the laparoscopic vessel anastomosis for kidney transplant is safe and feasible. The quality of vessel anastomosis by the laparoscopic technique is reliable on postmortem examination. Nevertheless, the anastomotic stenosis tends to occur in the fashion of a continuous anastomosis because the renal vein wall is very thin and the renal artery is very small in caliber. This phenomenon was also reported in open kidney orthotopic transplant in a pig model.19 The interrupted stitch for vessel anastomosis could be considered in a future study when the laparoscopic suture technique is improved (Figure 6). This could prevent the possibility of anastomotic stenosis in this model. Alternatively, the sutures can be tied after the Bulldog device has been repositioned, allowing the blood flow through the anastomosis in the renal artery and the growth factor created at the renal vein anastomosis to prevent the anastomotic stenosis. It was noticed that the vessel anastomotic time was longer by the laparoscopic technique. However, this is a fairly new technique. The laparoscopic instruments used in this experiment were not fine enough because the renal artery was usually smaller in caliber (∼2–3 mm in diameter) than those in the human kidney. We believe the laparoscopic vessel anastomotic time will be reduced dramatically with further practice over time and modification of the laparoscopic instruments. Robotic surgery may have advantages in vessel anastomosis because of its 3-D view of the system compared with laparoscopic surgery.11,13,14 It may shorten the vessel anastomotic time, improve the quality of vessel anastomosis, and minimize the ischemic-reperfusion injury to the graft. Further studies will also be of value in determining the technique for endoscopic vessel anastomosis.
Regarding animal welfare concerns, this model of laparoscopic kidney transplant could be applied to other research projects in which the kidney transplant model is involved, such as ischemic-reperfusion injury with benefits of smaller incision and less requirement for analgesia. This model could also provide an opportunity for surgeons who wish to perform human laparoscopic kidney transplant to obtain the skill.
A limitation of this study is that the observation of graft function was performed for only up to 4 hours after kidney transplant. Further studies have been approved by our animal ethics committee to investigate the impact of laparoscopic kidney transplant on morbidity and mortality in a survival pig model. The pigs and kidney graft function will be observed for 4 weeks after transplant using the laparoscopic technique. The graft function will be monitored by regular testing of the serum creatinine level after laparoscopic kidney transplant. Further information on safety and feasibility will be provided before application to human clinical kidney transplant with the laparoscopic technique. Nevertheless, our initial experience suggests that the use of the laparoscopic technique could facilitate orthotopic kidney transplant in selected recipients.
Contributor Information
Bulang He, Liver and Kidney Transplant Unit, Sir Charles Gairdner Hospital, Nedlands, Australia..
Gabby C. Musk, Animal Care Service, The University of Western Australia, Perth, Australia.
Lingjun Mou, Liver and Kidney Transplant Unit, Sir Charles Gairdner Hospital, Nedlands, Australia..
Gerald L. Waneck, Transplant Immunology Laboratory, The University of Western Australia, Perth, Australia.
Luc Delriviere, Liver and Kidney Transplant Unit, Sir Charles Gairdner Hospital, Nedlands, Australia..
References:
- 1. Tooher R, Boult M, Maddern GJ, Rao MM. Final report from the ASERNIP-S audit of laparoscopic live-donor nephrectomy. ANZ J Surg. 2004;74(11):961–963 [DOI] [PubMed] [Google Scholar]
- 2. Merlin TL, Scott DF, Rao MM, et al. The safety and efficacy of laparoscopic live donor nephrectomy: a systematic review. Transplantation. 2000;70(12):1659–1666 [DOI] [PubMed] [Google Scholar]
- 3. Melcher ML, Carter JT, Posselt A, et al. More than 500 consecutive laparoscopic donor nephrectomies without conversion or repeated surgery. Arch Surg. 2005;140(9):835–839; discussion 839–840 [DOI] [PubMed] [Google Scholar]
- 4. Hadjianastassiou VG, Johnson RJ, Rudge CJ, Mamode N. 2509 living donor nephrectomies, morbidity and mortality, including the UK introduction of laparoscopic donor surgery. Am J Transplant. 2007;7(11):2532–2537 [DOI] [PubMed] [Google Scholar]
- 5. He B, Mitchell A, Delriviere L, et al. Laparoscopic donor nephrectomy. ANZ J Surg. 2011;81(3):159–163 [DOI] [PubMed] [Google Scholar]
- 6. Murray JE, Merrill JP, Harrison JH. Kidney transplantation between seven pairs of identical twins. Ann Surg. 1958;148(3):343–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung BI, Gill IS. Laparoscopic splenorenal venous bypass for nutcracker syndrome. J Vasc Surg. 2009;49(5):1319–1323 [DOI] [PubMed] [Google Scholar]
- 8. Bruls S, Quaniers J, Tromme P, Lavigne JP, Van Damme H, Defraigne JO. Comparison of laparoscopic and open aortobifemoral bypass in the treatment of aortoiliac disease. Results of a contemporary series (2003–2009). Acta Chir Belg. 2012;112(1):51–58 [DOI] [PubMed] [Google Scholar]
- 9. Hoznek A, Zaki SK, Samadi DB, et al. Robotic assisted kidney transplantation: an initial experience. J Urol. 2002;167(4):1604–1606 [PubMed] [Google Scholar]
- 10. Rosales A, Salvador JT, Urdaneta G, et al. Laparoscopic kidney transplantation. Eur Urol. 2010;57(1):164–167 [DOI] [PubMed] [Google Scholar]
- 11. Giulianotti P, Gorodner V, Sbrana F, et al. Robotic transabdominal kidney transplantation in a morbidly obese patient. Am J Transplant. 2010;10(6):1478–1482 [DOI] [PubMed] [Google Scholar]
- 12. Modi P, Rizvi J, Pal B, et al. Laparoscopic kidney transplantation: an initial experience. Am J Transplant. 2011;11(6):1320–1324 [DOI] [PubMed] [Google Scholar]
- 13. Boggi U, Vistoli F, Signori S, et al. Robotic renal transplantation: first European case. Transpl Int. 2011;24(2):213–218 [DOI] [PubMed] [Google Scholar]
- 14. Benedetti E. Robotic intra-peritoneal kidney transplant in obese recipients. Presented at: American Transplant Congress; April 30-May 4, 2011; Philadelphia, PA [Google Scholar]
- 15. Ferri M, Russell JD, Whelan JP. Orthotopic renal transplantation in a patient with a massive pelvic arteriovenous malformation. J Urol. 2000;163(3):899. [PubMed] [Google Scholar]
- 16. Rodrigues P, D'Imperio M, Campagnari M, Azevedo LA, Campagnari JC, van Bellen B. Alternative grafting technique for patients unsuited to heterotopic transplantation due to diseased pelvic conditions. Urol Int. 2004;73(4):316–319 [DOI] [PubMed] [Google Scholar]
- 17. Paduch DA, Barry JM, Arsanjani A, Lemmers MJ. Indication, surgical technique and outcome of orthotopic renal transplantation. J Urol. 2001;166(5):1647–1650 [PubMed] [Google Scholar]
- 18. Musquera M, Peri LL, Alvarez-Vijande R, Oppenheimer F, Gil-Vernet JM, Alcaraz A. Orthotopic kidney transplantation: an alternative surgical technique in selected patients. Eur Urol. 2010;58(6):927–933 [DOI] [PubMed] [Google Scholar]
- 19. Jochmans I, Lerut E, Heedfeld V, Wylin T, Pirenne J, Monbaliu D. Reproducible model for kidney autotransplantation in pigs. Transplant Proc. 2009;41(8):3417–3421 [DOI] [PubMed] [Google Scholar]