Robotic liver surgery was found to offer advantages not inherent in conventional laparoscopic liver resection.
Keywords: Robotic liver resection, Minimally invasive liver surgery, Robotic hepatectomy, SILS, Single port, Single access
Abstract
Background and Objective:
Robotic-assisted surgery offers a solution to fundamental limitations of conventional laparoscopic surgery, and its use is gaining wide popularity. However, the application of this technology has yet to be established in hepatic surgery.
Methods:
A retrospective analysis of our prospectively collected liver surgery database was performed. Over a 6-month period, all consecutive patients who underwent robotic-assisted hepatic resection for a liver neoplasm were included. Demographics, operative time, and morbidity encountered were evaluated.
Results:
A total of 7 robotic-assisted liver resections were performed, including 2 robotic-assisted single-port access liver resections with the da Vinci-Si Surgical System (Intuitive Surgical Sunnyvalle, Calif.) USA. The mean age was 44.6 years (range, 21–68 years); there were 5 male and 2 female patients. The mean operative time (± SD) was 61.4 ± 26.7 minutes; the mean operative console time (± SD) was 38.2 ± 23 minutes. No conversions were required. The mean blood loss was 100.7 mL (range, 10–200 mL). The mean hospital stay (± SD) was 2 ± 0.4 days. No postoperative morbidity related to the procedure or death was encountered.
Conclusion:
Our initial experience with robotic liver resection confirms that this technique is both feasible and safe. Robotic-assisted technology appears to improve the precision and ergonomics of single-access surgery while preserving the known benefits of laparoscopic surgery, including cosmesis, minimal morbidity, and faster recovery.
INTRODUCTION
Over the past decade, minimally invasive liver surgery has gained acceptance with proliferation worldwide. This was a slow process, evolving from small peripheral resections to formal hepatic lobectomies. Significant skepticism and often vocal resistance were observed during this evolution. Not until several large series and a consensus statement were published did laparoscopic hepatic surgery achieve a routine place in hepatic surgery.1–4 Several subsequent studies have confirmed the oncologic equivalence of laparoscopic liver resection with open liver resection. In this setting, laparoscopic liver resection has become the standard of care for left lateral sectionectomy. Recent studies have confirmed the benefits of laparoscopic liver resection in patients undergoing repeat hepatectomy or as a bridge to subsequent liver transplantation.3,5 The benefits of laparoscopic liver resection, which include shorter operative and recovery times, less blood loss, and a lower incidence of postoperative adhesions, make this technique highly desirable.2,4–7
Despite the numerous benefits of laparoscopic liver resections, there are several inherent limitations to this technique. These include restricted instrument motion, 2-dimensional imaging, complex ergonomics, and unstable operative exposure.8,9 Robotic-assisted technology appears to offer key solutions to these same fundamental limitations of conventional laparoscopic surgery.10–17 Unfortunately, as was witnessed with initial laparoscopic liver resections, the application of robotic technology in liver surgery has been controversial. To date, few large series of robotic liver resection have been reported. This study examines our group's preliminary experience with robotic-assisted liver resections.
METHODS
A retrospective review of our prospectively collected liver surgery database was conducted to include all robotic-assisted liver resections performed between February and August 2011 at a tertiary care center. Demographics, operative time, intraoperative blood loss, pathology, and postoperative outcome were evaluated. Liver resections were defined according to the International Hepato-Pancreato-Biliary Association's Couinaud classification.18 Resection for benign tumors was considered only for symptomatic lesions or for the presence of uncertainty at preoperative biopsy or radiologic evaluation. Resection of colorectal liver metastases was considered only in the absence of peritoneal carcinomatosis or unresectable extrahepatic disease. In patients with hepatocellular cancers, only those with well-compensated cirrhosis (Child-Pugh class A/B, low grade) with no signs of severe portal hypertension (esophageal varices >F2) were eligible for robotic liver resections.
Surgical Technique
Patient and trocar positioning for robotic-assisted laparoscopic liver resection.
While under general anesthesia, the patient was placed in a supine position, and 4 trocars were inserted. A 12-mm trocar port for the robotic camera was placed above the umbilicus by the Hassan technique. Two 8-mm robotic ports were placed at the left upper quadrant and right upper quadrant, and a 12-mm trocar port was placed at the midclavicular line lateral to the umbilicus for the assistant.
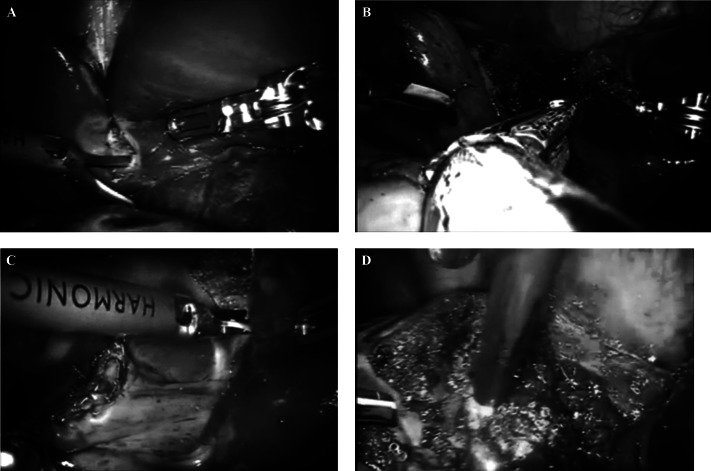
Intraoperative ultrasonography was performed with a 7.5-MHz, 10-mm SSD-1700 linear transducer (BK Medical, Peabody, MA, USA) to examine the remaining liver for undetectable lesions and obtain adequate surgical resection margins. The da Vinci-Si Surgical System (Intuitive Surgical, Sunnyvalle, CA, USA) robot was brought into position over the right shoulder of the patient and docked after placement of the ports. The operator moved to the robot console to control the robotic arms. The assisting surgeon remained at the patient's side to change robotic instruments and performed clipping, stapling, and mobilization through the assistant 12-mm trocar. Figure 1 depicts the position of the trocars. A vascular reticulating endoscopic stapler (Coviden, Norwalk, CT, USA) was used to divide the ligamentum teres hepatis and to control the main branches of the portal veins (Figure 2A). The endoscopic articulating stapler allowed safe control of the major vessels from the hepatic parenchyma (Figure 2B). A Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA) was used to incise the capsule, to divide the parenchyma, and to perform dissection (Figure 2C). A grasper was used to retract the liver. An endoscopic suction device was used to aid in the dissection of the blood vessels. Bile leaks and hemostasis were completed with argon plasma coagulation (Figure 2D).
Figure 1.
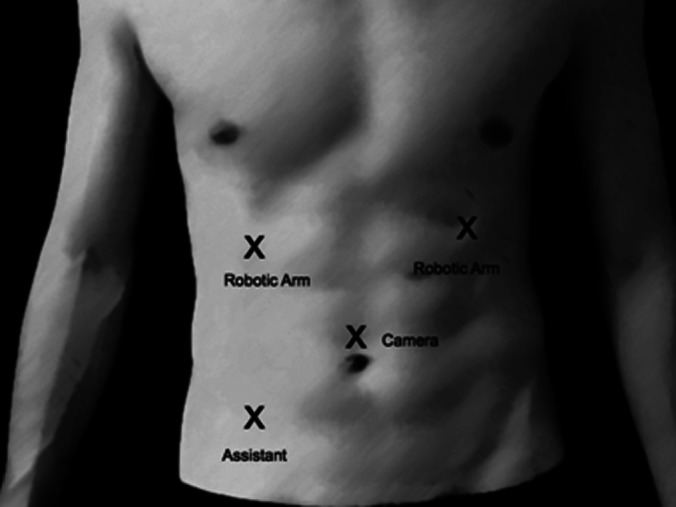
Image of abdomen showing positioning of trocars.
Figure 2.
A. The transected falciform ligament was used to expose the underside of the liver, with dissection of the inferior surface. B. The endoscopic articulating stapler allowed safe control of the major vessels. C. The capsule was incised with the Harmonic scalpel. D. Argon plasma coagulation.
Patient and trocar positioning for robotic-assisted single-port access liver resection.
While under general anesthesia, the patient was placed in a supine position. An incision measuring approximately 3 cm was made above the umbilicus. Dissection was performed down through the fascia to the peritoneum. A single-port device (Applied Medical, Rancho Santa Margarita, CA, USA) was then placed once the abdomen was entered. The single port included 3 small ports that were used for the placement of the robotic arms. The robotic arms were equipped with a Harmonic scalpel, a Prograsper (Intuitive Surgical, Sunnyvale, CA, USA), and a 12-mm camera. In addition, a 12-mm port was placed in the lower left quadrant for the assistant.
The abdomen was then insufflated with 4 L of carbon dioxide. Intraoperative ultrasonography was performed to examine the remaining liver to search for undetectable lesions and obtain adequate surgical resection margins. The da Vinci-Si Surgical System robot was brought into position and docked over the patient's right shoulder after port placements. The operator moved to the console to control the robotic arms. The assisting surgeon remained at the patient's side to change robotic instruments and perform clipping, stapling, and mobilization through the assistant 12-mm trocar. Figure 3A depicts the position of the trocars. Intraoperative ultrasonography was performed to examine the remaining liver to search for undetectable lesions and obtain adequate surgical resection margins. The Harmonic scalpel was used to incise the capsule and to perform dissection. The grasper was used to retract the liver. The main branches of the portal veins were controlled with the application of the endoscopic articulating stapler by use of a white cartridge. The hepatic surfaces were inspected for any evidence of bile leaks, and hemostasis was completed with argon plasma coagulation as needed. Figure 3B shows the surgical incision used for the single-port device.
Figure 3.
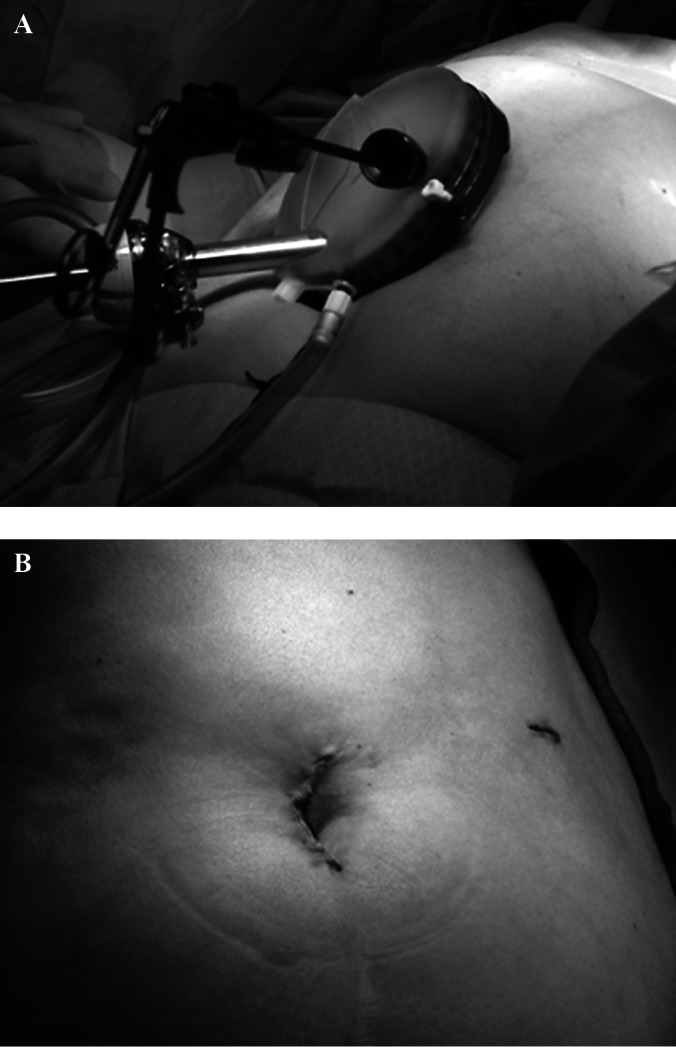
A. Positioning of single-access port. B. Abdomen postoperatively.
RESULTS
Seven of 29 liver resections (24%) were performed with the da Vinci Si-Surgical System during the 6-month study period. Table 1 depicts demographic information, indications for surgery, and characteristics of the lesions. The mean age was 44.6 years (range, 21–68 years), and 71.4% of patients were men. The mean total operative time (± SD) was 61.4 ± 26.7 minutes; the mean operative console time (± SD) was 38.2 ± 23 minutes. The mean blood loss was 100.7 mL (range, 10–200 mL). The mean hospital stay (± SD) was 2 ± 0.4 days (Table 2). No postoperative morbidity related to the procedure or death was encountered. Successful resection was established in all patients without requiring a conversion to the traditional open approach. No patients required a blood transfusion.
Table 1.
Demographic Information, Indications for Surgery, and Characteristics of Lesions for 7 Patients in Cohort
| Case Sequence | Sex | Age, yr | Body Mass Index | Diagnosis | Tumor Size, cm | Resection | Complications | Length of Stay, d |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 45 | 36.5 | Hepatic adenoma | 6 | Left lobe | None | 2 |
| 2 | Male | 58 | 32.1 | Hepatoma | 1.5 | Bisegment 7 and 8 | None | 2 |
| 3 | Male | 21 | 26.4 | Focal nodular hyperplasia | 8 | Left lateral sectionectomy | None | 1 |
| 4 | Male | 28 | 26.4 | Hodgkin lymphoma | 1 | Dx wedge segment 2 | Atelectasis | 1 |
| 5 | Male | 28 | 26.4 | Hodgkin lymphoma | 1 | Dx wedge segment 3 | None | 1 |
| 6 | Female | 64 | 28.1 | Adenoma (procedure performed for suspected metastases) | 1.5 | Single port: left lateral segmentectomy | Delirium and tremors | 5 |
| 7 | Female | 68 | 40.4 | Metastatic adenocarcinoma (colorectal mass) | 1.4 | Single port: left lateral segmentectomy | None | 2 |
Table 2.
Intraoperative Data for Patients in Cohort
| Case Sequence | Estimated Blood Loss, mL | Console Time, min | Docking Time, min | ORa Time, min |
|---|---|---|---|---|
| 1 | 200 | 10 | 60 | 90 |
| 2 | 200 | 6 | 65 | 86 |
| 3 | 200 | 7 | 55 | 79 |
| 4 | 10 | 6 | 10 | 26 |
| 5 | 50 | 6 | 11 | 28 |
| 6 (single-port access) | 15 | 9 | 25 | 51 |
| 7 (single-port access) | 30 | 11 | 42 | 70 |
OR=operating room.
The latter 2 cases were performed by a robotic-assisted single-port access technique. The mean estimated blood loss (± SD) was 22.5 ± 10.6 mL. The mean robot docking time (± SD) was 7 ± 1.7 minutes, the mean operative console time (± SD) was 33.5 ± 12.0 minutes, and the mean total operative time (± SD) was 60.5 ± 13.4 minutes.
DISCUSSION
Over the past decade, laparoscopic liver resection has become an acceptable technique for the management of benign tumors, colorectal metastases, and hepatocellular cancers. Multiple studies have confirmed that laparoscopic liver resection results in a shorter operative time, less blood loss, and a shorter length of hospital stay. Despite considerable controversy, no increases in operative complications or inferior oncologic outcomes were observed. These clinical findings suggest that laparoscopic resection could be expanded to most hepatic resections, including cirrhotic patients and all malignancies.19,20
Unfortunately, conventional laparoscopic liver surgery has several inherent limitations.15 These include challenging exposure, suboptimal visualization, and the complexity of vascular control during major hepatic hemorrhage. Control of major vascular hemorrhage is one of the most important issues in hepatic surgery. These challenges made the use of hand-assisted devices and laparoscopic-assisted open resection (hybrid) attractive options. These devices and techniques afford several benefits, including the ability to use the surgeon's hand to stabilize, mobilize, and control hemorrhage through the use of temporary digital control or direct application of pressure.3,21,22 However, the use of hand-assisted devices or hybrid incisions leads to a greater interruption of the abdominal wall, diluting the potential benefits of minimally invasive surgery.
Robotic-assisted surgery is a new tool that provides a novel way of controlling hemorrhage through stabilized suture repair. Robotic surgery uniquely allows free articulation of the suturing arms, subsequently minimizing the difficulties faced when one is performing conventional laparoscopic liver surgery.10–17 The da Vinci-Si Surgical System provides surgeons with intuitive translation of the instrument handle to the tip movement, eliminating the mirror-image effect. In addition, a remotely controlled camera provides improved visualization with high-quality 3-dimensional images and a stable camera platform with scaling, tremor filtering, and coaxial alignment of the eyes and EndoWrist, with a 360° range of motion, allowing more precise operating techniques (Asheville, NC, USA).5,23–26 Robotic-assisted laparoscopic liver resection is a procedure in evolution. This operative platform potentially increases the diversity of laparoscopic liver resections able to be performed by a surgeon. The ease of robotic suturing opens the surgeon's ability to access the biliary system and repair potential vascular injuries.
However, significant criticism exists over the use of robotic-assisted surgery for liver resection. As was experienced in the early application of laparoscopic liver surgery, significant concerns over safety and efficacy exist. Robotic surgery does in fact separate the surgeon from having direct contact with the patient. This results in significant fear regarding hemorrhage and, in particular, concern about delays in conversion inherent with the use of the robot. Currently, robotic instrumentation has evolved and has become diversified but still lacks a stapling platform or a robotic argon coagulator, necessitating the addition of a qualified surgical assistant. In robotic liver surgery, an experienced assistant surgeon is required to suction, retract, and introduce the vascular stapler. As was seen with the evolution of conventional laparoscopic liver resection, a significant learning curve exists. Robotic liver surgery requires significant experience with the robot both as an assistant and on the console. Competency in robotic liver surgery will require experience in robotic surgery and open hepatic surgery, as well as advanced laparoscopic liver resection. Despite all of these concerns, several small series of robotic-assisted liver resections have been reported with limited conversion rates, reasonable blood loss, and minimal postoperative morbidity, even for major hepatectomy.14,27,28 However, when compared with conventional laparoscopic liver resection, the robotic approach appears to provide similar outcomes.29
An additional potential advantage of robotic-assisted technology is the ability to perform a hepatic resection through the single-port access approach. There are significant data to support the use of single-port laparoscopy because it has gained momentum in multiple disciplines, including laparoscopic cholecystectomy, colectomy, and nephrectomy.30–33 The advantages of single-port laparoscopy have been reported as decreased morbidity, postoperative pain, shorter hospital stay, and faster recovery, in addition to a cosmetic advantage.33 However, to date, there have only been a few case reports using single-port laparoscopic surgery for liver resections because of the complexity of such procedures and the significant and often cumbersome crisscrossing “sword fighting” of instruments during triangulation inside the abdominal cavity. These significant prerequisites restrain the enthusiasm of many surgeons, making single-port laparoscopic liver resections a rather limited field.31
The use of the robotic control through the single-access port appears to limit the occurrence of crisscrossing, improving the ability to use this instrumentation, and allows for a more meaningful use of 3 arms without the frustration or added difficulty with this approach. A recent study from Japan confirmed that robotic single-port liver resection was feasible in a porcine model.34 This study subsequently concluded that single-port laparoscopic liver resection was technically feasible and safe. In our study we elected to use the single-access approach in the last 2 procedures to minimize trauma and port-related complications, such as organ damage, adhesions, bleedings, wound infections, and hernias.33,35
The potential benefits of robotic-assisted single-port access surgery remain to be proven; however, potential therapeutic benefits might include less postoperative pain, a shorter hospital stay, and faster recovery, in addition to the cosmetic advantage. The decrease in abdominal wall trauma could be specifically useful for cirrhotic patients, provided that the incision is made in the supraumbilical location to avoid bleeding from large umbilical veins and to allow a secure closure. The use of the GelPort (Ranchos Margarita, CA, USA) device makes it possible to use large instruments, such as standard laparoscopic ultrasonography probes, LigaSure (Boulder, CO, USA) devices, and staplers. This facilitates the procedure and helps minimize blood loss.
CONCLUSION
Our group has shown that robotic-assisted laparoscopic liver resection is both feasible and safe. We also have reported the first 2 cases of robotic-assisted single-port access liver resection. Our initial experience confirms that robotic liver resection can be practical in select cases. We found no higher incidence of conversion, morbidity, or even death. Robotic liver surgery allows potential advantages not otherwise inherent in conventional laparoscopic liver resection. A robotic approach to single-port access appears technically feasible and safe. Nevertheless, this remains a challenging procedure, requiring both hepatobiliary and laparoscopic experience. Additional experiences are mandatory to assess and examine the safety of this emerging technique.
Contributor Information
Emad Kandil, Division of Endocrine and Oncological Surgery, Tulane Transplant Institute, Department of Surgery, Tulane University School of Medicine, New Orleans, LA, USA..
Salem I. Noureldine, Division of Endocrine and Oncological Surgery, Tulane Transplant Institute, Department of Surgery, Tulane University School of Medicine, New Orleans, LA, USA..
Bob Saggi, Division of Endocrine and Oncological Surgery, Tulane Transplant Institute, Department of Surgery, Tulane University School of Medicine, New Orleans, LA, USA..
Joseph F. Buell, Division of Endocrine and Oncological Surgery, Tulane Transplant Institute, Department of Surgery, Tulane University School of Medicine, New Orleans, LA, USA..
References:
- 1. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection—2,804 patients. Ann Surg. 2009;250(5):831–841 [DOI] [PubMed] [Google Scholar]
- 2. Buell JF, Thomas MT, Rudich S, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–486 [DOI] [PubMed] [Google Scholar]
- 3. Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–830 [DOI] [PubMed] [Google Scholar]
- 4. Cannon RM, Brock GN, Marvin MR, Buell JF. Laparoscopic liver resection: an examination of our first 300 patients. J Am Coll Surg. 2011;213(4):501–507 [DOI] [PubMed] [Google Scholar]
- 5. Panaro F, Piardi T, Cag M, Cinqualbre J, Wolf P, Audet M. Robotic liver resection as a bridge to liver transplantation. JSLS. 2011;15(1):86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17(12):1914–1918 [DOI] [PubMed] [Google Scholar]
- 7. Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc. 2008;22(12):2564–2570 [DOI] [PubMed] [Google Scholar]
- 8. Cadiere GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg. 2001;25(11):1467–1477 [DOI] [PubMed] [Google Scholar]
- 9. Cadière G, Himpens J, Vertruyen M, et al. Evaluation of telesurgical (robotic) NISSEN fundoplication. Surg Endosc. 2001;(9):918–923 [DOI] [PubMed] [Google Scholar]
- 10. Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784 [DOI] [PubMed] [Google Scholar]
- 11. Vibert E, Denet C, Gayet B. Major digestive surgery using a remote-controlled robot: the next revolution. Arch Surg. 2003;138:1002–1006 [DOI] [PubMed] [Google Scholar]
- 12. Choi SB, Park JS, Kim JK, et al. Early experiences of robotic-assisted laparoscopic liver resection. Yonsei Med J. 2008;49:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Lapalorcia LM, Casciola L. Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: a pilot study. J Hepatobiliary Pancreat Surg. 2009;16(4):450–457 [DOI] [PubMed] [Google Scholar]
- 14. Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery. 2011;149:29–39 [DOI] [PubMed] [Google Scholar]
- 15. Idrees K, Bartlett DL. Robotic liver surgery. Surg Clin North Am. 2010;90:761–774 [DOI] [PubMed] [Google Scholar]
- 16. Chan OC, Tang CN, Lai EC, Yang GP, Li MK. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18(4):471–480 [DOI] [PubMed] [Google Scholar]
- 17. Lai EC, Tang CN, Yang GP, Li MK. Multimodality laparoscopic liver resection for hepatic malignancy—from conventional total laparoscopic approach to robot-assisted laparoscopic approach. Int J Surg. 2011;9:324–328 [DOI] [PubMed] [Google Scholar]
- 18. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–355 [DOI] [PubMed] [Google Scholar]
- 19. Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22(10):2208–2213 [DOI] [PubMed] [Google Scholar]
- 20. Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93(1):67–72 [DOI] [PubMed] [Google Scholar]
- 21. Cuschieri A. Laparoscopic hand-assisted hepatic surgery. Semin Laparosc Surg. 2001;8:104–113 [PubMed] [Google Scholar]
- 22. Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392; discussion 92–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidovszky TJ, Smith W, Ghosh J, Ali MR. Robotic cholecystectomy: learning curve, advantages, and limitations. J Surg Res. 2006;136:172–178 [DOI] [PubMed] [Google Scholar]
- 24. D'Annibale A, Morpurgo E, Fiscon V, et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47(12):2162–2168 [DOI] [PubMed] [Google Scholar]
- 25. Camarillo DB, Krummel TM, Salisbury JK., Jr Robotic technology in surgery: past, present, and future. Am J Surg. 2004;188:2S–15S [DOI] [PubMed] [Google Scholar]
- 26. Hashizume M, Tsugawa K. Robotic surgery and cancer: the present state, problems and future vision. Jpn J Clin Oncol. 2004;34(5):227–237 [DOI] [PubMed] [Google Scholar]
- 27. Choi GH, Choi SH, Kim SH, et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc. 2012;26(8):2247–2258 [DOI] [PubMed] [Google Scholar]
- 28. Lai EC, Tang CN, Li MK. Robot-assisted laparoscopic hemi-hepatectomy: technique and surgical outcomes. Int J Surg. 2012;10(1):11–15 [DOI] [PubMed] [Google Scholar]
- 29. Berber E, Akyildiz HY, Aucejo F, Gunasekaran G, Chalikonda S, Fung J. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford). 2010;12(8):583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosok BI, Edwin B. Single-incision laparoscopic liver resection for colorectal metastasis through stoma site at time of reversal of diversion ileostomy: a case report. Minim Invasive Surg. 2011;502176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shetty GS, You YK, Choi HJ, Na GH, Hong TH, Kim DG. Extending the limitations of liver surgery: outcomes of initial human experience in a high-volume center performing single-port laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc. 2011;26(6):1602–1608 [DOI] [PubMed] [Google Scholar]
- 32. Aikawa M, Miyazawa M, Okamoto K, et al. Single-port laparoscopic hepatectomy: technique, safety, and feasibility in a clinical case series. Surg Endosc. 2011;26(6):1696–1701 [DOI] [PubMed] [Google Scholar]
- 33. Gaujoux S, Kingham TP, Jarnagin WR, D'Angelica MI, Allen PJ, Fong Y. Single-incision laparoscopic liver resection. Surg Endosc. 2011;25(5):1489–1494 [DOI] [PubMed] [Google Scholar]
- 34. Sugimoto M, Tanaka K, Matsuoka Y, et al. da Vinci robotic single-incision cholecystectomy and hepatectomy using single-channel GelPort access. J Hepatobiliary Pancreat Sci. 2011;18(4):493–498 [DOI] [PubMed] [Google Scholar]
- 35. Back M, Nimmesgern T, Langwieler TE. Single port access laparoscopy: a review of the most recent development in minimally invasive surgery [in German]. Zentralbl Chir. 2010;135(2):183–187 [DOI] [PubMed] [Google Scholar]