Abstract
Despite the importance of nitrous oxide (N2O) in the global radiative balance and atmospheric ozone chemistry, its sources and sinks within the Earth’s system are still poorly understood. In the ocean, N2O is produced by microbiological processes such as nitrification and partial denitrification, which account for about a third of global emissions. Conversely, complete denitrification (the dissimilative reduction of N2O to N2) under suboxic/anoxic conditions is the only known pathway accountable for N2O consumption in the ocean. In this work, it is demonstrated that the biological assimilation of N2O could be a significant pathway capable of directly transforming this gas into particulate organic nitrogen (PON). N2O is shown to be biologically fixed within the subtropical and tropical waters of the eastern South Pacific Ocean, under a wide range of oceanographic conditions and at rates ranging from 2 pmol N L−1 d− to 14.8 nmol N L−1 d−1 (mean ± SE of 0.522±1.06 nmol N L−1 d−1, n = 93). Additional assays revealed that cultured cyanobacterial strains of Trichodesmium (H-9 and IMS 101), and Crocosphaera (W-8501) have the capacity to directly fix N2O under laboratory conditions; suggesting that marine photoautotrophic diazotrophs could be using N2O as a substrate. This metabolic capacity however was absent in Synechococcus (RCC 1029). The findings presented here indicate that assimilative N2O fixation takes place under extreme environmental conditions (i.e., light, nutrient, oxygen) where both autotrophic (including cyanobacteria) and heterotrophic microbes appear to be involved. This process could provide a globally significant sink for atmospheric N2O which in turn affects the oceanic N2O inventory and may also represent a yet unexplored global oceanic source of fixed N.
Introduction
Nitrous oxide (N2O) is an important greenhouse gas that contributes to stratospheric ozone depletion. The transfer of this gas within the Earth System is intimately linked to physical and biological processes occurring in the ocean, atmosphere and soils. In the ocean, N2O saturation levels depend upon oceanographic variables such as temperature and salinity while processes producing N2O are mainly being controlled by organic matter and dissolved O2 [1]. A detailed understanding of its biological production and consumption is necessary to predict the effects of long term changes in N2O on the Earth’s climate [2].
According to current knowledge, nitrification performed by bacteria [3] and archaea [4], via aerobic NH4 + oxidation or nitrifier denitrification (the pathway in which NH4 + is oxidized to NO2 − followed by the reduction of NO2 − to NO, N2O, and N2 [5]) is the main process responsible for most of the N2O production under oxic or even microaerophilic conditions; whereas partial denitrification, via the anaerobic reduction of NO2 − to N2O, can produce this gas under suboxic conditions [3], [6].
Contrary to its production, N2O can only be consumed by photolysis in the stratosphere [7] and by canonical denitrification via dissimilative reduction of N2O to N2, a pathway that only exists under anoxic condition [3], [6], [8]. However, early studies suggest that N2O, and even NO2 −, could act as substrates for the enzymatic nitrogenase complex (NifH) [9], [10]. Indeed, it is also known that given the properties of the NifH, certain diazotrophs are able to reduce not only N2, but also other multi-bonded substrates, such as acetylene, azide, cyanide, methyl isocyanide and even N2O [11], [12]. Regarding N2O, this molecule could bind to metals belonging to the NifH complex through the N-bound nitro form and the N-O bond form [13]. However, there is no direct evidence so far that indicates how or where the N-O bond containing molecule N2O binds to NifH, or that indicates the chemical mechanism of its reduction.
The eastern South Pacific (ESP) region hosts the most extreme range of biogeochemical conditions in the global ocean, from the very eutrophic, nitrate-rich and oxygen-poor waters of the Peruvian and Chilean coastal upwelling (CU ∼71°–76°W) to oxygenated and severely nutrient-limited waters of the central subtropical Pacific gyre (STG ∼110°W) [14]. Dissolved O2 vertical distribution denotes the well-known oxygen minimum zone (OMZ),which has relatively shallow suboxic-anoxic waters (0≥ O2≤11 µmol L−1) in subsurface waters off Peru and northern Chile [15] and also the oxygenated waters towards the subtropical gyre and south of ∼37°S. N2O concentrations in seawater and its concomitant exchange across air-sea interface also reflect the previously mentioned biogeochemical gradients in the ESP. Thus, N2O content in the ESP can be as high as 400% saturation with strong effluxes within coastal upwelling and sub-saturated or slightly supersaturated N2O levels (∼80–105%) with near zero air-sea fluxes within the subtropical gyre (STG) [16], [17]. A number of studies reports sub-saturated N2O values in surface waters throughout the world’s major oceans (Table S1 and Text S1); these low values have been assumed to be analytical artifacts [18] given the absence of any known chemical or biological mechanism able to remove N2O from surface waters. In suboxic subsurface waters, in contrast, such as those found in the OMZ of the ESP (at depths from 50 to 400 m), sub-saturated N2O concentrations (as low as 40–60%) have been solely attributed to canonical denitrification, which is so far the only known process able to use N2O as an electron acceptor instead of dissolved O2. Canonical denitrification along with anaerobic NH4 + oxidation (anammox), both processes particularly active in the ESP [19], [20], are the main sinks for fixed N budgets.
There has been long-standing uncertainty as to whether or not the ocean is losing N faster than it is being incorporated via marine N2 fixation [8], [21]. Important advances in the understanding of regulation, rates and the microorganisms involved in N2 fixation have been made in recent years [22]–[24]. These studies indicate that NifH is present in a diverse range of microbial groups which include the well-studied diazotrophic cyanobacteria, diatom–diazotroph assemblages and gammaproteobacteria [23], [25], [26]. In fact, diazotrophs display high levels of metabolic diversity, much greater than previously thought, among which can be found not only well-known photoautotrophs but also chemo- and heterotrophic diazotrophs [27]. Recent results showing the expression of nitrogenase gene (nifH), suggest that heterotrophic bacteria dominate the diazotrophic community in the oligotrophic waters of the western South Pacific [28] and even in the OMZ of the Arabian Sea [29].
The possible occurrence of biological N2O fixation or rather the well-known process which removes N2O was investigated using an enriched, labeled substrate (15N2O). This process was explored using field samples and cultures of marine cyanobacteria such as Trichodesmium and Crocosphaera. These bacteria, particularly Trichodesmium, are present in most tropical and subtropical gyres, being the dominant diazotrophic species and having global significance in relation to the introduction of new N into the ocean [30], [31].
Results and Discussion
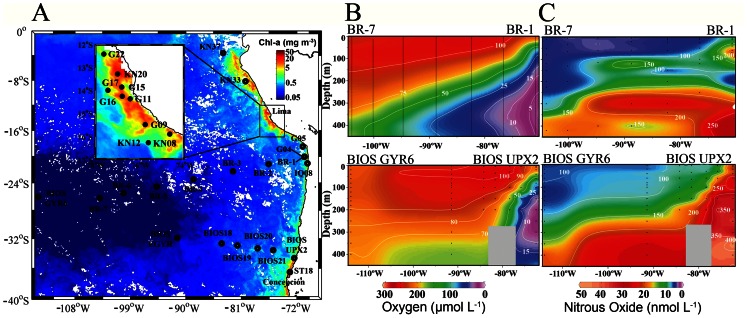
N2O fixation was explored in field experiments carried out during several cruises covering a wide range of geographical locations (13°–36.5°S; 72°–110°W) and depths (from the surface down to 400 m). The study areas have extreme biogeochemical conditions reflected in surface chlorophyll-a (Chl-a; Fig. 1A), dissolved fixed N (mainly NO3 −) and O2. The latter variable varies from the oxygenated waters in the subtropical gyre and south of ∼37°S to suboxic-anoxic waters (0≥ O2≤11 µmol L−1) off Peru and northern Chile. Oxygen deficient waters are clearly observed (Fig. 1B), delimiting an OMZ that has become one of the shallowest and most intense in the world ocean [19]. Oceanographic conditions of the sampled stations are summarized in Table 1.
Figure 1. Study area showing: A) The location of the sampling stations superimposed on a background illustrating mean surface Chlorophyll concentrations for the 2005–2011 period (Color data is available at http://oceancolor.gsfc.nasa.gov/cgi/l3), Chl-a concentration expressed in mg Chl-a m−3 coded according to the color bar); B) Zonal dissolved oxygen transects and its saturation percentage in two transects, from Easter Island to the coast at 20°S (Iquique), and from Easter Island to the coast at 32°S (Valparaiso); and C) Zonal distribution of dissolved N2O and its saturation percentages obtained from the same two transects.
Color scales show dissolved O2 concentrations in (µmol L−1), while the solid lines indicate saturation percentages (%).
Table 1. Location, water depth along with some oceanographic and meteorological variables/parameters obtained from the sampled stations.
| Station | Date mm/dd/yy | Lat. (°S) | Lon. (°W) | Water depth (m) | SST (°C) | Wind (m s−1) | Zm (m) | Surface NO3 − (µmol L−1) | Surface PO4 3− (µmol L−1) | Surface N/P ratio | N2O fix assay | |
| BIOS GYR6 | 11/14/04 | −26.06 | −113.98 | 3078 | 23.2 | 2.87 | 189 | 0.05 | 0.06 | 0.83 | ||
| BIOS EGYR | 11/30/04 | −31.90 | −91.40 | 2996 | 18.3 | 6.47 | 180 | 0.13 | 0.10 | 1.3 | ||
| BIOS 18 | 12/02/04 | −32.66 | −84.20 | 3760 | 17.5 | 6.40 | 174 | 3.64 | 0.36 | 10 | ||
| BIOS 19 | 12/03/04 | −32.94 | −81.63 | 4006 | 17.13 | 2.45 | 128 | 2.74 | 0.36 | 7.6 | ||
| BIOS 20 | 12/04/04 | −33.32 | −78.36 | 3830 | 17.4 | 3.94 | 125 | 0.92 | 0.30 | 3 | ||
| BIOS 21 | 12/05/04 | −33.58 | −75.84 | 4374 | 16.8 | 8.57 | 83 | 0.06 | 0.30 | 0.20 | ||
| BIOS UPX2 | 12/07/04 | −34.65 | −72.47 | 1193 | 12.8 | 8.90 | 40 | 19.48 | 1.30 | 15 | ||
| KN08 | 10/20/05 | −15.91 | −74.65 | 1370 | 15.0 | 5.9 | 28 | 9.6 | 1.7 | 5.7 | * | |
| KN12 | 10/22/05 | −16.28 | −75.61 | 4100 | 15.9 | 4.17 | 27 | 10.3 | 1.4 | 7.4 | * | |
| KN20 | 10/25/05 | −13.3 | −76.99 | 885 | 15.3 | 6.98 | 6 | 7.4 | 1.9 | 3.8 | * | |
| KN33 | 10/30/05 | −8.17 | −80.33 | 328 | 17.4 | 5.76 | 27 | 8.9 | 1.3 | 7 | * | |
| KN37 | 11/02/05 | −3.6 | −83.95 | 3239 | 18.5 | 4.06 | 25 | NA | NA | NA | * | |
| G04 (14.6) | 02/17/07 | −20.06 | −70.75 | 1480 | 21.9 | NA | 17 | 1.4 | 0.64 | 2.19 | * | |
| G05 (14.14) | 02/18/07 | −18.5 | −71.03 | 1203 | 23.9 | 4.47 | 12 | 4.1 | 0.08 | 51.3 | * | |
| G09 (14.21) | 02/19/07 | −15.5 | −75.75 | 3135 | 21.9 | 7.5 | 16 | NA | 0.45 | NA | * | |
| G11 (14.47) | 02/21/07 | −14.38 | −76.42 | 315 | 17.6 | 8.1 | 30 | 10.7 | 0.82 | 13.0 | * | |
| G15 (14.66) | 02/22/07 | −13.87 | −76.8 | 750 | 19.5 | 9.7 | 16 | NA | NA | NA | * | |
| G16 (14.74) | 02/23/07 | −14.27 | 76.78 | 789 | 20.0 | 11.5 | 10 | 6.1 | 0.07 | 87.1 | * | |
| G17 (14.86) | 02/24/07 | −14.01 | −77.42 | 5153 | 20.9 | 6.7 | 19 | 4.9 | 0.48 | 10.21 | * | |
| G22 (14.100) | 02/27/07 | −12.43 | −77.6 | 598 | 21.2 | 4.0 | 14 | 0.6 | 0.18 | 3.33 | * | |
| IQ08 | 09/22/08 | −21.07 | −70.27 | 2200 | 15.6 | NA | 10 | 0.07 | NA | NA | * | |
| ST 18a | 2009 | −36.51 | −73.12 | 92 | 12.6 | 6.8 | 19 | 11.7 | 1.13 | 9.7 | * | |
| BR−1 | 11/20/11 | 20. 05 | −70.47 | 1900 | 19.77 | 8.88 | 14 | 0.33 | 0.94 | 0.35 | * | |
| BR−2 | 11/25/11 | 21.10 | −76.34 | 4695 | 18.0 | NA | 57 | 0.31 | 0.49 | 0.61 | ||
| BR−3 | 11/27/11 | 22.15 | −82.20 | 2572 | 18.0 | NA | 47 | 0,45 | 0.20 | 0.8 | ||
| BR−4 | 12/01/11 | 23.27 | −88.46 | 3941 | 18.7 | NA | 62 | BLD | 0.34 | 0.08 | ||
| BR−5 | 12/04/11 | 24.33 | −94.43 | 3402 | 19.7 | NA | 69 | BLD | 0.27 | >0.01 | ||
| BR−6 | 12/06/11 | 25.33 | −100.08 | 3181 | 20.9 | NA | 52 | BLD | 0.22 | >0.01 | ||
| BR−7 | 12−09/11 | 26.14 | −103.57 | 2691 | 21.9 | 4.11 | 41 | BLD | 0.18 | >0.01 | * | |
They include Sea Surface Temperature (SST), wind speed, mixing layer depth (Zm), surface concentrations of nitrate and phosphate, surface dissolved N/P ratio.
COPAS time series station. Values averaged on austral spring-summer period.
Denotes the stations where assimilative N2O fixation assays were performed. BLD: Below limit of Detection; NA: Not Available.
N2O fixation was observed in 92% of sampled depths, with rates ranging between 2 pmol N L−1 d−1 and 14.8 nmol N L−1 d−1 (mean ± SE of 0.522±1.06 nmol N L−1 d−1, n = 93). These field samples, which came from different trophic (Fig. 1A) and O2 regimes (Fig. 1B), were incubated on board under a light gradient (65% to 4% of surface irradiance) and under dark conditions, and with temperature and dissolved O2 levels maintained close to those found under in situ conditions (Table S2). N2O fixation was blocked in control experiments treated with HgCl2 (total experiments n = 15), confirming the biological nature of this process, hereafter referred to as assimilative N2O fixation.
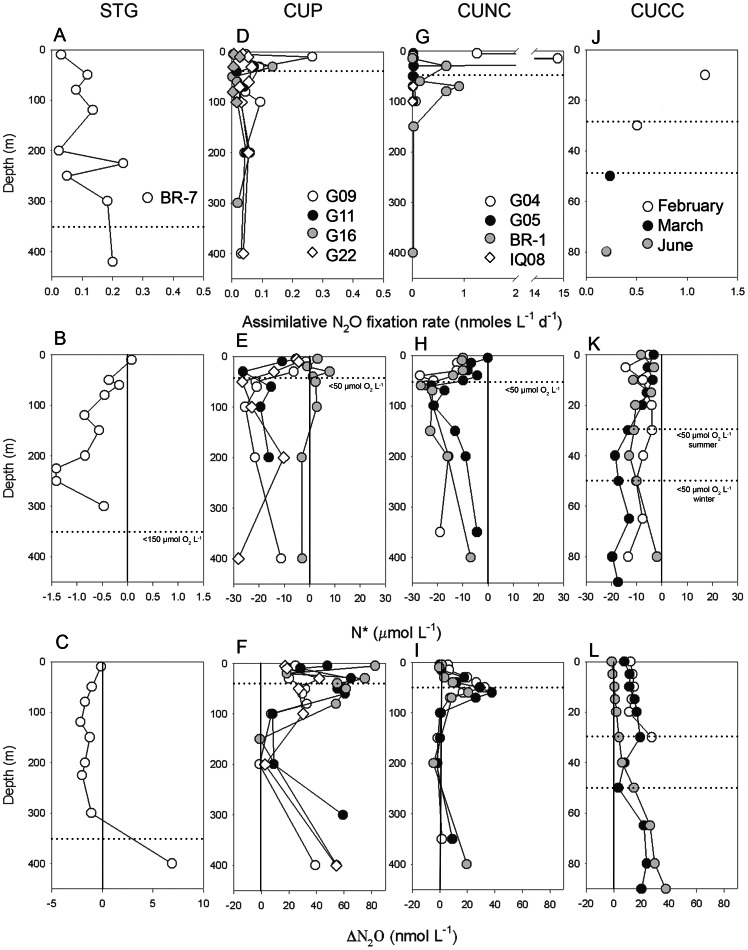
Vertical distributions of N2O fixation rates are shown in Fig. 2A, D, G, J along with the semi-conservative tracer N* [21] (Fig. 2B, E, H, K) and ΔN2O [32] (Fig. 2C, F, I, L). Off Peru (CUP) and northern Chile (CUNC), N2O fixation rates peaked at the surface and/or around the base of the oxycline (Fig. 2D, 2G) and were well correlated with fluorescence and particulate organic carbon and nitrogen (POC/PON) distributions (data not shown). Off central Chile (CUCC, Fig. 2J), N2O fixation rates were higher in the photic-oxic layer decreasing toward deeper waters (90 m depth). At the STG (BR-7 station, Fig. 2A), remarkably, vertical N2O fixation showed a maximum level at the base of the eufothic zone, where chlorophyll fluorescence and POC revealed a maximum level [33]. Additionally, experiments performed in the photic zone during the Big Rapa (BR-1 and BR-7 stations) showed active N2O fixation rates under dark as well as in situ light conditions (see Table S2), but without any significant differences. This supports the idea that microorganisms assimilating N2O are not only photoautotrophs and that part of microbes fixing N2O could be heterotrophs.
Figure 2. Depth profiles of assimilative N2O fixation rates (nmol L−1 d−1) (A, D, G, J) along with N* (B, E, H, K) and apparent N2O production (ΔN2O µmol L−1) (C, F, I, L).
Parameters at selected stations from left to right column are: the STG (St. BR 7); the CUP (Sts. G09, G11, G16 and G22); the CUNC (Sts. G04, G05, IQ08 and BR 1); and the CUCC (St.18 or COPAS sampled in January, February, March, 2009). Note the change in scale in N* and ΔN2O for the STG and in the assimilative N2O fixation rates between the surveyed locations. Vertical line denotes zero for N* and ΔN2O indexes.
Table 2 shows ranges of N2O fixation rates and their statistics, along with basic biogeochemical variables in predefined areas i.e., the STG and coastal upwelling (CU) centers. There was significant variation in N2O fixation rates among the study areas (Kruskal Wallis test, p<0.05), being higher at the CUNC. Further analyses involving a multiple linear regression (p<0.05) showed that 27% of the variation observed in N2O fixation rates was linked to dissolved O2 concentrations, with higher rates observed at lower O2 values; less variance percentage was ascribed to differences in Chl-a and fixed N pools among areas. In this sense, it has been suggested that dissolved O2 controls surface diazotrophic activity which could be enhanced in close proximity to water column denitrification areas [34].
Table 2. Rates of N2O fixation (range and average±SD) along with surface inventories of nitrate (fixed N), nitrous oxide and chlorophyll-a in the sampled area.
| Subtropical South Pacificgyre (STG) | Coastal Upwelling offPeru (CUP) | Coastal Upwelling off northern Chile (CUNC) | Coastal Upwelling off central Chile (CUCC) | |
| Representative for: | 10°–30° S | 10°–19°S | 20°–23°S | 35°–37° S |
| 80°–110°W | 71°–76°W | ∼71°–72°W | 75°–76.5°W | |
| Surface area (km2) | 6.9×106 | 5.3×105 | 1.6×103 | 1.1×104 |
| Assimilative N2O fixation rate(nmol N L−1 d−1) | 0.023–10.64 | 0.002–0.266 | 0.002–14.79 | 0.202–1.172 |
| average±SD | 0.825±1.705 (n = 17) | 0.051±0.048 (n = 63) | 0.485±1.140 (n = 37) | 0.530±0.451 (n = 26) |
| Fixed N inventory(mmol N m−2) | 2.0–36.5 | 33.7–150 | 52.1–120 | 200–818 |
| N2O inventory(µmol N m−2) | 825–1125 | 247–1373 | 500–2061 | 157–2786* |
| Chl-a inventory(mg m−2) | 1.53–20 | >500 | 50–100 | 250–500 |
| Other features | Deep biome | Partial presence of continental shelf | Non presence of continental shelf | Large continental shelf |
| Extremely Fe and N limitation | Moderate Fe and N limitation | Moderate Fe limitation | Non expected Fe limitation Bío-Bío river | |
| Oxygenated water | Permanent OMZ | Permanent OMZ | Seasonal OMZ |
N2O inventories based on data come from the COPAS time series station since 2002 to date.
Assimilative N2O fixation rates in the photic layer samples, under different light intensities, averaged 0.493±2.27 nmol N L−1 d−1 (n = 42). Despite the fact that certain maxima of N2O fixation rates are observed in the photic zone, these values were not significantly different (Mann–Whitney test, p = 0.03) from those measured in the aphotic layer, under the occasional influence of the OMZ, which averaged 0.104±0.190 nmol N L−1d−1 (n = 32).
In addition, all sampled areas in this study share a moderate to severe iron and NO3 − deficiency [35]–[37] which is reflected in the N* index. Indeed, in each predefined area N* profiles started with values close to zero in surface water and decreased with depth, reaching negative N* values as low as −30 (Fig. 2B, E, H and K). Thus, N* indicates a depletion or deficit of N compared to P, and could be a consequence of the lateral and upward transport of denitrified waters from the OMZ belonging to the ESP, causing a (lower than expected) deviation of the Redfield N:P ratio. If PO4 −3 is forced above the Redfield ratio, N fixing organisms may gain a selective advantage, which increases the inventory of NO3 −. Consequently, the impact of N2O fixation may lie in compensating N2O loss caused by canonical denitrification. Particularly, the N deficit relative to P is a common pattern observed at the STG [38] (Fig. 2 B) and may be accentuated by weak vertical mixing and very scarce aeolian dust supply toward this region [14]. There, N* values close to zero or negative zero are usually observed within the gyre explaining the predominance of advected denitrification over N-fixation [21], [38].
On the other hand, within the STG (St. BR-7) noticeable negative values of ΔN2O in oxygenated water were detected (Fig. 2C). However, these could not be possible by heterotrophic denitrification given the oxygenated condition of the entire water column. Therefore, the ΔN2O values suggest that the N2O deficit could be caused by assimilative N2O consumption. In contrast, in the upwelling of Peru, northern Chile ΔN2O values decrease from positive values at surface, peaking sometime at the oxyclines, to close to zero or lightly negative towards the OMZ’s core (Fig. 2F, I); there N2O is consumed by canonical denitrification [39].
It is important to note that N2 fixation rates were detected at the same stations and depths [40] as the detection of N2O fixation rates in our sampled CU areas (in 60% of the samples). In addition, in both CUP and CUNC study areas, dinitrogenase reductase genes (nifH) have been found and phylogenetic analysis revealed a diverse diazotrophic community [40] in which nifH sequences fell within three of the four known clusters for this gene [41], [42]. Importantly however, no sequences associated with cyanobacteria were found during that study [40] which agrees with nifH transcripts retrieved in the western South Pacific gyre that showed active heterotrophic communities, mainly γ-proteobacteria from cluster I [42], able to fix N2 [28].
In order to check whether typical diazotrophic organisms have the same capacity to fix N2O as they have for fixing N2, three of the most studied strains of marine cyanobacteria [43] belonging to Trichodesmium (H-9 and IMS101) and Crocosphaera (W-8501) were cultured under laboratory conditions. It is important to note that they used NifH to transform N2 into PON, but lacked the nosZ genes that encode the catalyzing enzymes of dissimilative N2O reduction to N2 [44], thereby only leaving assimilative N2O fixation as the potential N2O consumption process. By contrast, Synechococcus (RCC 1029) was used as a negative control, given that this species does not possess either nifH or nosZ genes in its genome [45].
Several types of experiments were carried out with these cyanobacterial strains (Table S2). All incubated strains except strain RCC 1029 showed a significant excess of 15N in PON (referred to as atom%) of 2 to 5 fold higher with respect to the natural abundance of 15N in PON (∼0.369 atom%) and its variation seemed to depend on the cell density of each cultured strain (quantified as particulate organic nitrogen “PON”). Time course assays of N2O fixation with these strains at different cell densities (measured throughout PON) are illustrated in Figure 3. When observed, the incorporation of 15N2O increased with incubation time, showing enrichments in the heavier isotope in PON (Fig. 3A, B, C).
Figure 3. Result of typical time course assimilative N2O fixation experiments with a) Trichodesmium (IMS101); b) (Trichodesmium (H-9); c) Crocosphaera (WH-8501); and d) Synechococcus (RCC-1029) strains, showing variation in 15N enrichment (atom%) and in biomass expressed as PON (µg L−1) over incubation time.
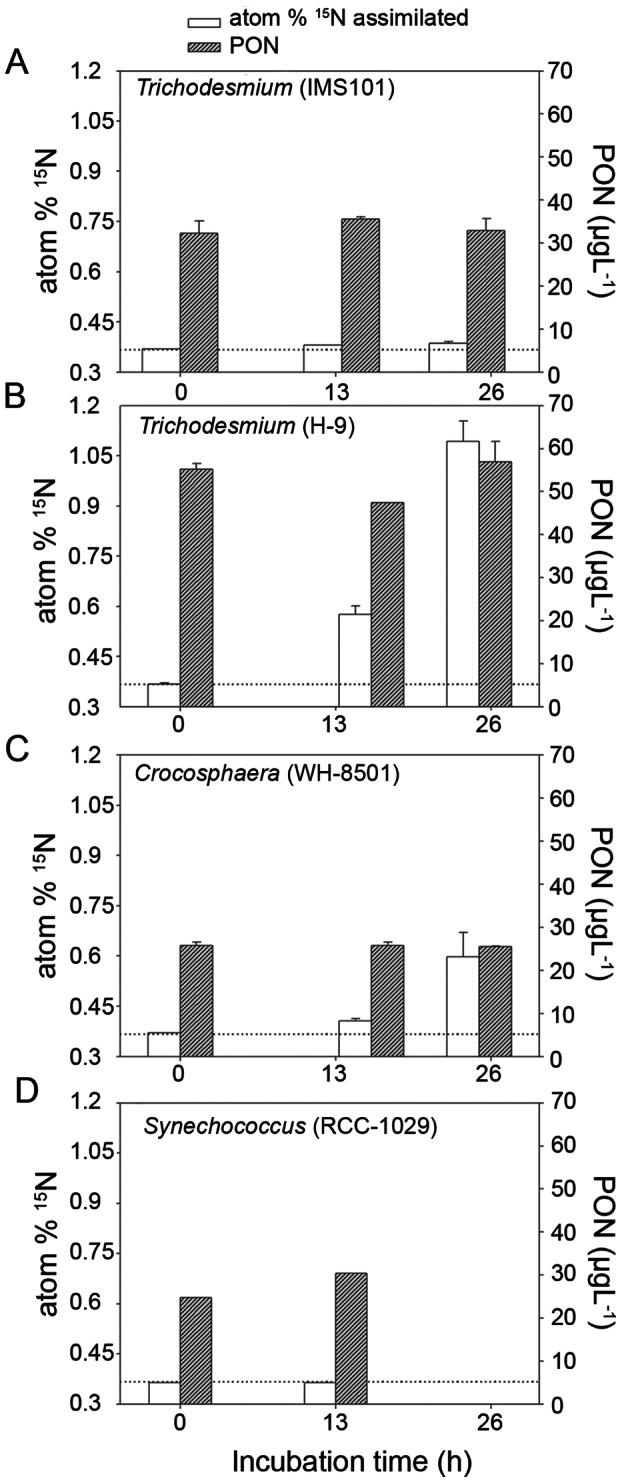
Dotted horizontal line indicates the value of abundance of 15N in PON (∼0.369 atom%).
In addition, different concentrations or doses of dissolved 15N2O were added to the cultures of Trichodesmium (IMS101, Fig. 4A) and Synechococcus sp. (RCC 1029, Fig. 4B) in order to verify the nitrogenase kinetics or the dose/response relationship. Whilst Synechococcus sp. did not show any 15N2O incorporation as the doses increased (confirming its inability to use N2O), Trichodesmium displayed enhanced N2O incorporation rates as concentrations of added N2O increased from 10–400 nmol L−1 final dissolved concentration. This observed trend suggests that nitrogenase has an increasing affinity for N2O as N2O doses increased (Fig. 4A). It is important to remark that a slight enrichment in atom% (and even atom% excess with respect to natural isotopic abundance) was observed at minimal N2O doses (Fig. 4), whose final concentration was close to those environmental levels found in the STG (∼6–10 nmol L−1). This suggests that even at very low N2O concentrations, N2O incorporation may occur. In surface waters associated with coastal upwelling areas, N2O concentrations can reach 100 nmol L−1 or more [46], and if we look at water under the influence of subsurface O2 deficiency, N2O concentrations as high as 400 nmol L−1 have been reported [46]. Assuming that an increase in atmospheric N2O is a likely trend under certain future intensification of eutrophication scenarios [47], and hypoxia and warming [48], this final added concentration of N2O in field experiments could be plausible for some marine environments.
Figure 4. Assimilative N2O fixation experiments showing the response of Trichodesmium (IMS101) and Synechococcus (RC 1029) to different doses of dissolved 15N2O, in increasing order from 10 to 400 nmol L−1 of final concentrations.
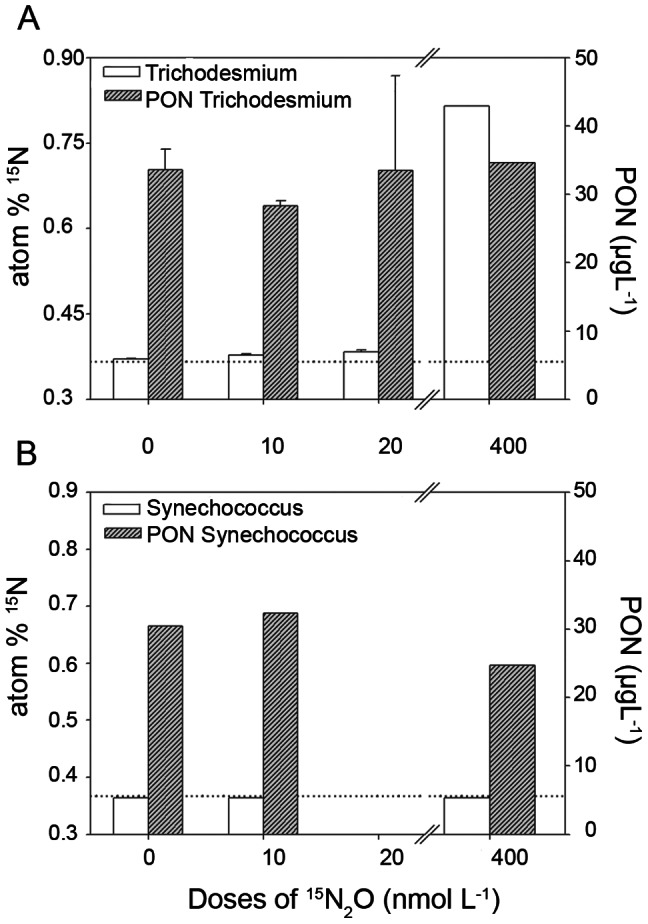
Note change of y-axis scale for experiments.
Given that some of the N2O enrichments used in our field experiments (e.g., the STG Fig. 2A, D, G, J) largely exceeded natural N2O levels in the surface ocean by 100 and 1000 times, rates of N2O fixation obtained for these sampled areas should be considered as a potential. A simple calculation of the N2O turnover time was carried out in surface waters of the STG and CU areas, taking into consideration surface N2O inventories and assimilative N2O fixation rates 100 and 1000 times smaller than those respectively measured, i.e. around 1 to 10 pmol L−1 d−1 (Table 2). Estimates showed that the surface N2O inventory could be removed entirely between 2 and 12 years. Further kinetic studies and half-saturation constant determinations will be necessary in order to assess the ability of diazotrophic organisms to use N2O at its naturally occurring levels in the ocean.
One question that is worth addressing is why N-fixers would fix N2O instead of N2. When answering this question one has to bear in mind two points: 1) that both pathways are associated with non-selective NifH; and 2) that there might be benefits related to energy for using N2O instead of N2. The Gibbs free energy required during N2O assimilation is thermodynamically advantageous compared to that of N2 because the dissociation energy for breaking the N-N bond in the case of N2O is only half that required for the N2 molecule [49], [50]. Thus, if available, N2O may appear to be a more energetically favorable substrate than N2.
It is however possible that this process does not directly occur. Therefore, dissimilative reduction of N2O to N2 followed by the biological fixation of N2 into PON may take place, in which case assimilative N2O fixation does not constitute a process in itself. This possibility was tested by looking at the isotopic composition of dissolved N2 in seawater medium of the cultures (taken thought exetainer) following the addition of 15N2O to the experiments. The lack of detection of any dissolved 15N2 in the exetainers after the addition of 15N2O (during different time incubations) precludes the possibility that N2O fixation takes place via N2 production and further fixation of N2 into PON.
This study provides further arguments in favor of the fact that assimilative N2O fixation should exist in marine waters to reduce uncertainties for the marine N cycle. One such argument is that the current isotopic and isotopomeric composition of accumulated atmospheric N2O cannot yet be precisely constrained by the N and O isotopic (and isotopomeric) compositions of N2O on the ocean surface [51]. A second argument is that it is well-known that there is an imbalance between the sources and sinks of the global N budget [6], [19]. This should become less pronounced if a new N2O utilization pathway is included, in some way affecting the global N2O cycle [52].
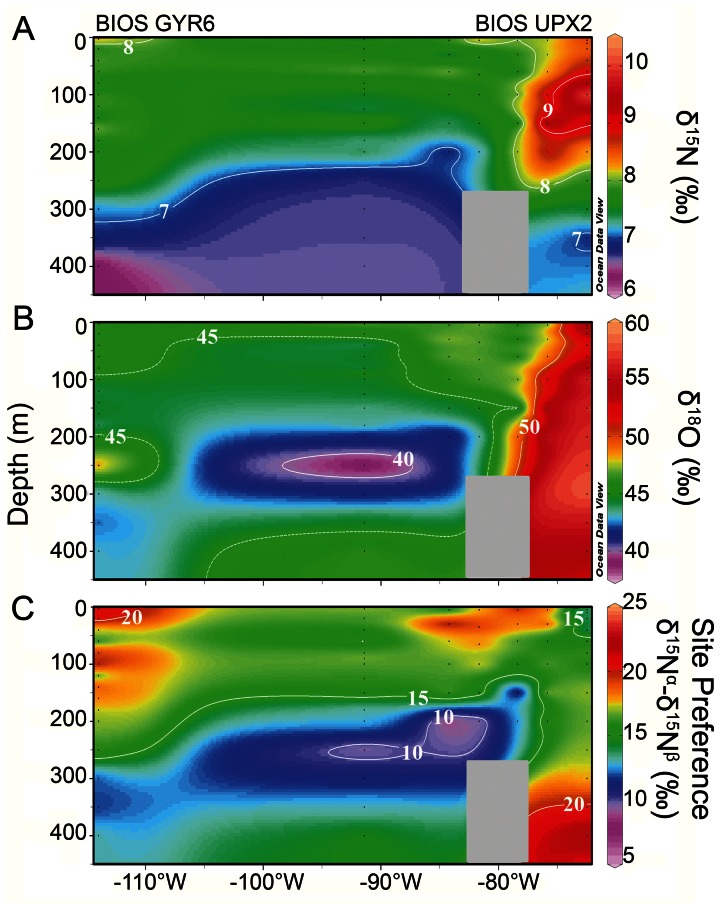
Firstly, the N2O dual isotope signatures (δ 15Nbulk and δ 18O) and their isotopomeric compositions (NαNβO including the site preference SP = δ 15Nα– δ 15Nβ) have been proposed as a more certain indicator of N2O production pathways [51], [53]. The zonal distribution of theses signatures within the ESP is shown in Fig. 5 (Text S2); it reveals important differences in these signatures between the CU and the STG. In the STG, surface values averaged 7.72±0.38, 45.23±1.97 and 16.67‰ (n = 45) for δ 15Nbulk, δ 18O, and SP, respectively (with δ 15N and δ 18O referenced to air-N2, and Vienna Standard Mean Ocean Water VSMOW, respectively). On the other hand, the isotopic values in surface waters of the STG were slightly higher than atmospheric values (δ 15Nbulk = 7.0±0.6‰, δ 18O = 43.7±0.9‰ and SP = 18.70 [51]), and even greater than subsurface water measurements with δ 15Nbulk = 4±1%, δ 18O = 38.5±3% and SP = 4±4% (see Fig. 5). These surface values at the STG coincide with those reported for the subtropical North and South Pacific gyre [54]–[56], and support the idea that the isotopic N2O composition of surface waters cannot simply be the result of mixing between the atmospheric and subsurface marine N2O pools.
Figure 5. Zonal distribution at 32°S of vertical distributions of N2O two-isotope signatures: a) δ 15N bulk; b) δ 18O; and c) the site preference (SP = δ 15Nα – δ 15Nβ).
Color scales indicate isotopic composition in ‰. Data interpolation was done with Ocean Data View.
Therefore, some surface water biological process should operate to maintain the isotopic compositions of δ 15N, δ 18O and SP as observed. In view of the prevailing levels of oxygenation in these waters, nitrification would be expected to be the process of greatest importance. In cultures of ammonia-oxidizing bacteria, regardless of the specific mechanism of N2O production, this gas is produced as a byproduct with much more depleted isotopic compositions for N and O than those observed in the ocean surface. For example, nitrifying bacterial cultures exhibit average signals of δ 15Nbulk from −54.9‰ to −6.6‰, δ 18O = 40‰ and a SP ∼33.5‰ via NH2OH decomposition, but an SP of about −0.8‰ via nitrifier denitrification [57], [58]. With the recent finding that marine Archaea can produce N2O via ammonium oxidation, [4], [59], resulting in a heavier isotopic composition than that observed in ammonia oxidizing bacteria (δ 15Nbulk = 8.7±1.5‰, δ 18O = 34.0. ±0.9‰ and SP = 30.3±1.2), the current constraints on the global isotopic N2O budget are narrowed. However, even when taking into account that N2O production in surface water comes from a mix of Bacteria and Archaea as well as the effect of N2O influx from the atmosphere (Table S3), observed isotopic compositions of N2O in the surface waters of the STG do not match the signal made by N2O produced by nitrifiers in the surface water nor the signals observed in either the atmosphere or the subsurface water.
Here, some type of biological process such as assimilative N2O reduction into PON that enriches the dissolved N2O pool in δ 15Nbulk and δ 18O is able to resolve the observed discrepancies. This process could also explain the estimated negative values of ΔN2O (Fig. 1C) in the STG. However, the negative values of N* and positive values of ΔN2O as observed within the CU do not exclude the possibility that assimilative N2O fixation is taking place but simply indicate that other N2O producing processes (nitrification or partial denitrification), and/or in situ total denitrification along with the advection of denitrified waters are occurring faster than assimilative N2O fixation, camouflaging both expected N* and ΔN2O signals.
Secondly, if N2O fixation is significantly taking place in the world’s oceans, it should have an important effect on the global ocean ΔN2O inventory which is illustrated in Table S3 and Text S3. As part of assimilative N2O fixation appears to be carried by phototrophic diazotrophs, (even if one cannot exclude other micro-organisms), it should be directly linked to diazotroph biomass or abundance. Thus, significant contributions of N2O fixation should be observed in these regions where diazotrophs are of quantitative importance. Indeed, emerging patterns of marine N fixation suggest that the Pacific Ocean, in particular the South Pacific (although poorly studied), has the lowest abundance and diazotroph activity compared with other regions such as the North Atlantic, the Indo-Pacific oceanic region and even the Baltic Sea [22].
Further research on N2O fixation in other oceanic basins is recommended and several insights appear to indicate that assimilative N2O fixation could be higher in regions where diazotrophic microorganisms were more abundant or more active The gathered evidence draws attention to the importance of this pathway for regional and even global N2O removal, preventing part of its potential efflux toward the atmosphere. Thus, N2O represents a form of yet unreported fixed N that could change our vision and understanding of the oceanic N cycle.
Methods
This study covers coastal upwelling centers off Peru (CUP ∼8.2–16°S), northern Chile (CUNC, Arica∼ 19°S and Iquique ∼21°S) and central Chile (CUCC, Concepción ∼36.5°S), as well as the eastern subtropical gyre (STG with two transects from the Chilean coast to Eastern Island, covering 20°–27°S and 73°–110°W). The CUP and CUCN areas were visited four times: in October- November 2005 by KN182-9 cruise (R/V Knorr), in February 2007 by the Galathea-3 cruise (R/V Vædderen), in March 2008 by the IQOX cruise (R/V Purihalar), and in November-December 2010 by the Big Rapa cruise (R/V Melville). Biological samples were obtained during oceanographic expeditions in which work in Chilean territorial waters was authorized by the Chilean Government under control of the SHOA (Servicio Hidrográfico y Oceanográfico de la Armada de Chile; www.shoa.cl). Moreover, a Chilean government observer participated in each of the cruises. The area off central Chile was sampled monthly from September 2008 to September 2009 at the COPAS time series station known as St. 18. In addition, unpublished isotopic and isotopomeric N2O data from the Biosope cruise (October-December 2004; R/V L’Atalante) was also included (data from Leg 2 from Easter Island to Talcahuano, Chile ∼ 73.1° W, 36.7°S). Table 1 summarizes the location, water depth and other oceanographic variables and parameters measured at the sampling stations.
During all cruises, vertical profiles of temperature, salinity, dissolved O2, fluorescence and PAR (Photosynthetically Active Radiation) were obtained using a Conductivity Temperature Depth CTD-O2 probe (Sea-Bird Electronics Inc., USA). The O2 sensors from the upcast CTD-O were calibrated with discrete samples obtained by Winkler titration (see below). In the case of the KN182-9 cruise, O2 sensors were calibrated pre- and post-cruise at Woods Hole Oceanographic Institution (USA). During the Galathea-3 cruise, an ultrasensitive sensor STOX was tested in situ [60]. Water column light irradiance, averaged over the visible spectrum (400–700 nm), was measured using a LI-COR (LI-190) quantum sensor, and fluorescence was measured using a WetStar sensor.
Discrete water samples, for chemical analyses and experiments, from the surface (<2 m), down to 400 m were collected using Niskin bottles (12 L) attached to a rosette sampler. Core parameters, including dissolved O2 and N2O, nutrients (NH4 +, NO3 −, NO2 −, PO4 3−), Chl-a, particulate organic carbon and nitrogen (POC and PON), and their natural C and N isotopic compositions, were determined at all stations. N*, a quasi-conservative tracer defined as a linear combination of NO3 − and PO4 3−, was estimated from nutrient concentrations throughout the water column [21]. Apparent N2O production (ΔN2O) was obtained from the difference between the N2O saturation at equilibrium with the atmosphere and its concentration measured in seawater [61].
In terms of the employed analytical methods, dissolved O2 was analyzed in triplicate by automatic Winkler titration. The samples for N2O analyses were transferred directly into 20-mL glass vials (triplicates), preserved with 50 µL of saturated HgCl2 and sealed with butyl rubber and aluminum cap stoppers. N2O was determined by Helium equilibration in the vial, followed by quantification with a Varian 3380 Gas Chromatograph (GC) using an electron capture detector maintained at 350°C, for more details see [46]. A calibration curve was made with 5 points (He, 0.1 ppm, air, 0.5 ppm, and 1 ppm) and the detector linearly responded to this concentration range. The analytical error for the N2O analysis was less than 3%.
Nutrient samples were collected with a 60 mL plastic syringe and filtered through a glass fiber filter (pore size 0.7 µm) into high-density polypropylene scintillation vials. Samples were stored at –20°C until laboratory analysis, except during the KN182-9 and Galathea-3 cruises when dissolved NO2 − and PO4 −3 concentrations were immediately determined on board [62]. For determination of Chl-a, a fluorometry method was used for filtered seawater through a 45 mm Whatman GF/F filter [63]. Analyses of particulate organic C and N (POC and PON) and their natural 13C and 15N isotopic compositions were carried out after filtering 1 L of seawater through pre-combusted 0.7 µm glass fiber filters (22 mm Whatman GF-F) and stored at –20°C until analysis. Filters were dried at 60°C for 12 h before determining their isotopic composition via continuous-flow isotope ratio mass spectrometry (IRMS; Finnigan Delta Plus). Reproducibility for 13C and 15N was greater than 0.11‰ and 0.02‰, respectively, based on the acetanilide standard used as reference material. Isotope ratios were expressed as per mil deviations from the isotopic composition of Vienna PDB and air, for 13C and 15N, respectively [64]. Significant differences were checked between the enrichment as 15N atom% and its atom% excess of PON in the experiments with respect to the natural background or natural isotopic composition of PON taken at each sampled station and depth.
Additionally, oceanographic/meteorological and biogeochemical variables/parameters shown in Table 1 were measured and estimated. The mixed layer depth (Zm) was obtained from vertical density profiles measured every 1 dbar using the CTD sensor, and the depth of the euphotic zone (irradiation at 1% of its surface value) was estimated from the attenuation coefficient of downwelling irradiance averaged over the visible spectrum (400–700 nm) measured by a LI-COR sensor. In the few instances where light profiling was not possible (night sampling), light profiles were estimated using surface irradiation (assumed 4% surface reflection) and the vertical attenuation coefficient of PAR (K) from the previous day.
In order to detect differences in N2O fixation rates among the sampled areas (i.e., STG, CUP, CUNC and CUCC), non parametric Kruskal Wallis test was carried out using statistical language R [65]. Additionally, a multiple linear regression model was performed to assess the variables that determine the variance of N2O fixation rates, with a prior logarithmic transformation of this dependent variable [65]. Categorical variables associated with the different study areas were also included. A step-wise selection was used to test the significance of each variable in the model. The best model was obtained by determining its heteroscedasticity and p-value (p<0.05). Models were then contrasted using the Akaike Information Criteria (AIC). The comparison among light (65, 30, 4 and 1% of irradiance) and dark treatments for N2O fixation rates was done with a Mann–Whitney test.
N2O Fixation Experiments (Assimilative N2O Reduction into PON)
Experiments for assimilative N2O fixation were performed using an improved stable isotope technique [66] at selected stations listed in Table 1. 15N-labeled N2O gas (99 Atom %; CAMPRO SCIENTIFIC) was offered as a substrate during the experiments to measure N2O fixation rates by incubating samples. Assimilative N2O fixation rates were assayed with both field samples and cultured cyanobacteria strains, both subjected to different experimental treatments (see Table S2).
Field samples were incubated on board, under temperature-controlled conditions, using an in situ range of light intensities, as well as dark conditions. For this purpose, seawater was dispensed from Niskin bottles using a gas-tight Tygon tube, to avoid any oxygenation, into 1.5–2 L double-laminated aluminum-polyethylene or transparent Tedlar® bags. The volume and weight of the filled bags were controlled at the beginning and end of the incubation process, and real volumes were used in rate calculations. As an additional precaution, a permeability test was performed on bags prior to the experiments. The bags were filled with pure helium and monitored for 24 h using gas chromatography. They showed no atmospheric gas (N2O or CH4) intrusions, thus ensuring hermetically sealed conditions. Atmospheric O2 could not be tested in the bags via chromatography (given the sensitivity of the chromatographic method), however tests were performed using atmospheric N2O as a tracer in vacuum emptied bags. As N2O intrusions were not detected inside the bag during the days following the sampling, O2 intrusions during incubations in similar bags were considered unlikely. Each bag had a hose/valve with a septum through which 15N2O tracer and different treatments, i.e., 15N2O, HgCl2 (see Table S2) were injected using gastight syringes. Tracer addition was carried out at a final concentration of 10∼20 µmol L−1 (1 or 2 mL of tracer gas into 1.5–2 L incubation volume). The relative tracer (15N2O) concentration with respect to natural 14N2O background varied between 100 and 1000 fold (depending on seawater N2O levels), taking into consideration solubility and partition coefficients, as well as the ratio between gas and liquid phases of N2O in the bag [61].
Most of the incubations were performed using six deck incubators maintained at sea surface temperature and with light intensities ranging between 65% and 4% of incident light (Lee Filters®). Samples from below the base of the euphotic layer were incubated in the dark in a thermo-regulated bath (Johnson Control®, KN182-9 cruise) or a temperature controlled incubator at temperatures close to in situ. During the Big Rapa cruise, surface duplicate samples were simultaneously incubated under in situ light and dark conditions. The incubations lasted 24 h and they were terminated by gentle filtration onto pre-combusted 0.7 µm glass fiber filters (Whatman GF/F filters) using a vacuum (<100 mm Hg) or a peristaltic pump. Filters were stored at −20°C until laboratory analysis. Off central Chile, samples were incubated at two times (t = 12 and t = 24) with artificial light in a temperature-controlled room.
With samples obtained off central Chile (see above) and with cyanobacterial strains cultivated in the laboratory, assimilative N2O fixation rates (several batches) were assayed as time course experiments. In order to achieve this, samples (triplicates) were amended with 15N2O and incubated for 12 and 24h. Incubations were terminated by filtration as was outlined above. To assay assimilative N2O fixation, 15N2O was offered as a substrate during the experiments with Trichodesmium, Croccosphaera and Synechococcus. These diazotrophic cyanobacteria were isolated by Jon Waterbury at WHOI and correspond to Trichodesmium erythraeum, strain IMS101 (http://img.jgi.doe.gov/cgi-bin/w/); Trichodesmium sp., strain H9-4 (genetically H9 looks like T. tenue); and Crocosphaera watsonii, strain WH-8501 (http://genome.jgi-psf.org/crowa/crowa). Additionally, the Synechococcus sp. Biosope_141 D strain RCC 1029 was obtained from the Rosscof collection (http://www.sb-roscoff.fr/Phyto/RCC/index.php).
The diazotrophic strains were cultivated at 24°C under artificial light according to a daily cycle of 12 h light/12 h dark, in YBCII artificial seawater medium with no nitrogen sources [67], while Synechococcus was cultivated with the same cycle in PCR-S11 medium [68] at 18°C. Several batches of these strains were then transferred to GC bottles (50 or 125 mL), and 50 or 100 µL of 15N2O (depending on of the vials) was injected into each bottle through septa. Different doses were inoculated, with final concentrations going from 10 to 400 nmol L−1 (expected levels in the study area). Cell density was variable depending on the type of cultivated strain and date when experiments were undertaken. PON level (µg L−1) measured during each time incubation was used as a biomass index. No variations of PON (Fig. 3) were recorded during time course experiments, indicating no net growth during incubations.
Prior to each experiment and tracer inoculation, the health and purity of cultures were checked using flow cytometry and microscopy. The IMS101, H-9, WH-8501, RCC-1029 strains were not axenic cultures and, therefore, bacterial cell density was periodically checked in the cultures with a FACSCalibur flow cytometer equipped with an ion–argon laser of 488 nm of 15 mW (Becton Dickinson). The picoplanktonic bacterial abundance was estimated from samples previously stained with SYBR-Green I (10,000 x; Molecular Probes) following Marie et al, 2000 [69]. Cell Quest Pro and Cytow software were used for data acquisition and analysis. In the case of WH-8501, abundances varied from 5×103 to ∼ 4×104 cell mL−1, but the abundance of WH-8501strain was an order of greater magnitude.
Finally, after addition of 15N2O to selected experiments with H-9 and WH-8501 strains, liquid samples were collected in exetainers (LABCO) with particular care to avoid air contamination, and the isotopic composition of N2 was measured in order to check if one-step reaction transforming N2O directly into NH4 + proceeded without intermediary N2. The isotope ratios of N2 were measured in gas mixtures (headspace) using a Thermo Finnigan GasBench+PreCon trace gas concentration system interfaced to a Thermo Scientific Delta V Plus isotope-ratio mass spectrometer (U Davis, USA; http://stableisotopefacility.ucdavis.edu/n2.html).
Supporting Information
Oceanic N2O undersaturation (saturation %) and air-sea flux (µmol m−2 d−1) reported in surface and subsurface/intermediate waters around the global ocean. Depths of hypoxic/suboxic waters are indicted
(DOCX)
Experimental setup used with in natural and cyanobacteria cultured samples to asses assimilative N2O fixation.
(DOCX)
ΔN2O inventories (µmol·m−2) and estimated net N2O production (µmol·m−2·d−1) in surface waters at selected stations along N2O air-sea exchange (µmol·m−2·d−1) and N2O consumption rates by fixation. In some station, denitrification and N2 fixation rates (both based on published data) are available in order to compare with other N2O consuming processes.
(DOCX)
Isotopic and Isotopomeric determination
(DOCX)
N2O mass balance
(DOCX)
Acknowledgments
The authors want to thank the crews of the R.Vs Knorr (USA), Vædderen (Denmark) and Kay Kay (Chile) for their valuable help during the cruises. We are also grateful to O. Ulloa, J. Moffet, B. Thamdrup and D. Repeta for inviting us to participate in the Knorr, Galathea-3 and Big Rapa cruises, respectively. Part of this work was carried out under the auspices of the Danish Expedition Foundation (Dansk Ekspeditionsfond) which contributed to Galathea 3. This work was finalized within the framework of the International Associated Laboratory MORFUN. We thank Dr. R. Escribano and the Kay-Kay staff for running the COPAS time series off Concepción and Salvador Ramirez for bio-informatics support. We especially acknowledge Mauricio Gallegos for his dedicated laboratory and field work, and Laura Bristow, Hermman Bange, Tage Dalsgaard and Francis Chan for critically reading an earlier version of this manuscript. We appreciate the cyanobacterial strains given by the COAS center (Ricardo Letelier) and the support in culturing bacteria offered by Osvaldo Ulloa, and M Lorena Gonzalez. Finally, we thank the staff of Universidad Arturo Pratt and Ruben Moraga for their logistical help in Iquique. It is a contribution to FONDAP (CR2) N° 1511009.
Funding Statement
This work has been supported by the Chilean National Commission for Scientific and Technological Research (CONICYT) through the FONDECYT (grant 1050743 and 3110158) and FONDAP (grant 1501007) programs, as well as by the Betty and Gordon Moore Foundation (MILOCO grant). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bange HW (2008) Gaseous nitrogen compounds (NO, N2O, N2, NH3) in the ocean. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ, editors. Nitrogen in the Marine Environment. Amsterdam: 2nd edition. Elsevier. 51–94.
- 2. Codispoti LA (2010) Interesting Times for Marine N2O. Science 327: 1339–1340 doi:10.1126/science.1184945. Available: http://www.sciencemag.org/content/327/5971/1339.full.pdf. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 3. Codispoti LA, Christensen JP (1985) Nitrification, Denitrification and Nitrous-Oxide Cycling in the Eastern Tropical South-Pacific Ocean. Mar Chem 16: 277–300 doi:10.1016/0304-4203(85)90051-9. [Google Scholar]
- 4. Santoro AE, Casciotti K, Francis CA (2010) Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current. Environ Microbiol 12: 1989–2006 doi:10.1111/j.1462?2920.2010.02205.x. Available: http://onlinelibrary.wiley.com/doi/10.1111/j.1462-2920.2010.02205.x/abstract;jsessionid = 0A8D78C4E783A3AD3B90D6E6C0354C67.d02t04. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 5. Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33: 1723–1732 doi:10.1016/S0038-0717(01)00096-7. Available: http://www. sciencedirect.com/science/article/pii/S0038071701000967. Accessed 2013 April 23. [Google Scholar]
- 6. Elkins JW, Wofsy SC, McElroy MB, Kolb CE, Kaplan WA (1978) Aquatic sources and sinks for nitrous oxide. Nature 275: 602–606 doi:10.1038/275602a0. Available: http://www.nature.com/nature/journal/v275/n5681/pdf/275602a0.pdf. Accessed 2013 April 23. [Google Scholar]
- 7. Cicerone RJ (1989) Analysis of Sources and Sinks of Atmospheric Nitrous-Oxide (N2O). J Geophys Res-Atmos 94: 18265–18271 doi:10.1029/JD094iD15p18265. Available: http://onlinelibrary.wiley.com/doi/10.1029/JD094iD15p18265/abstract. Accessed 2013 April 23. [Google Scholar]
- 8. Codispoti LA, Brandes JA, Christensen JP, Devol AH, Naqvi SWA, et al. (2001) The oceanic fixed nitrogen and nitrous oxide budgets: Moving targets as we enter the anthropocene? Sci Mar 65: 85–105 doi:10.3989/scimar.2001.65s285. Available:http://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/684/700. Accessed 2013 April 23. [Google Scholar]
- 9. Vaughn SA, Burgess BK (1989) Nitrite, a new substrate for nitrogenase. Biochemistry 28: 419–424 doi:10.1021/bi00428a002. doi: 10.1021/bi00428a002. Available: http://dx.doi.org/10.1021/bi00428a002. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 10. Jensen BB, Burris RH (1986) N2O as a substrate and as a competitive inhibitor of nitrogenase. Biochemistry 25: 1083–1088 doi:10.1021/bi00353a021. Available: http://www.ncbi.nlm.nih.gov/pubmed/3516213. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Ortiz JM, Burris RH (1975) Interactions among substrates and inhibitors of nitrogenase. J Bacteriol 123: 537–545. Available: http://jb.asm.org/content/123/2/537.abstract. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed]
- 12. Christiansen J, Seefeldt LC, Dean DR (2000) Competitive substrate and inhibitor interactions at the physiologically relevant active site of nitrogenase. J Biol Chem 275: 36104–36107 doi:10.1074/jbc.M004889200. Available: http://www.jbc.org/content/275/46/36104.full.pdf+html. Accessed 5 January 2013. [DOI] [PubMed] [Google Scholar]
- 13. Burgess BK, Lowe DJ (1996) Mechanism of Molybdenum Nitrogenase. Chem Rev 96: 2983–3012 doi:10.1021/cr950055x. Available: http://www.ncbi.nlm. nih.gov/pubmed/11848849. Accessed 5 January 2013. [DOI] [PubMed] [Google Scholar]
- 14. Claustre H, Sciandra A, Vaulot D (2008) Introduction to the special section bio-optical and biogeochemical conditions in the South East Pacific in late 2004: the BIOSOPE program. Biogeosciences 5: 679–691 doi:. [Google Scholar]
- 15. Thamdrup B, Dalsgaard T, Revsbech NP (2012) Widespread functional anoxia in the oxygen minimum zone of the Eastern South Pacific. Deep-Sea Res Pt I 65: 36–45 doi:10.1016/j.dsr.2012.03.001. Available: http://www. sciencedirect.com/science/article/pii/S0967063712000611. Accessed 2013 April 23. [Google Scholar]
- 16. Charpentier J, Farías L, Pizarro O (2010) Nitrous oxide fluxes in the central and eastern South Pacific. Global Biogeochem Cy 24: GB3011–GB3025 doi:10.1029/2008gb003388. Available: http://dx.doi.org/10.1029/2008GB003388. Accessed 2013 April 23. [Google Scholar]
- 17. Nevison CD, Weiss RF, Erickson DJ (1995) Global Oceanic Emissions of Nitrous-Oxide. J Geophy Res-Oceans 100: 15809–15820 doi:10.1029/95JC00684. Available: http://onlinelibrary.wiley.com/doi/10.1029/95JC00684/abstract. Accessed 2013 April 23. [Google Scholar]
- 18. Cline JD, Wisegarver DP, Kelly-Hansen K (1987) Nitrous oxide and vertical mixing in the equatorial Pacific during the 1982–1983 El Niño. Deep Sea Res Pt I 34: 857–873 doi:10.1016/0198–0149(87)90041–0. Available: http://www.sciencedirect.com/science/article/pii/0198014987900410. Accessed 2013 April 23. [Google Scholar]
- 19. Thamdrup B, Dalsgaard T, Jensen MM, Ulloa O, Farias L, et al. (2006) Anaerobic ammonium oxidation in the oxygen-deficient waters off northern Chile. Limnol Oceanogr 51: 2145–2156 doi:10.4319/lo.2006.51.5.2145. Available: http://www.aslo.org/lo/toc/vol_51/issue_5/2145.html. Accessed 2013 April 23. [Google Scholar]
- 20. Ward BB, Devol AH, Rich JJ, Chang BX, Bulow SE, et al. (2009) Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 461: 78–U77 doi:10.1038/Nature08276. Available: http://www.nature.com/nature/journal/v461/n7260/pdf/nature08276.pdf. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 21. Gruber N, Sarmiento J (1997) Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem Cy 11: 235–266 doi:10.1029/97GB00077.Available: http://onlinelibrary.wiley.com/doi/10.1029/97GB00077/abstract. Accessed 2013 April 23. [Google Scholar]
- 22. Sohm JA, Webb EA, Capone DG (2011) Emerging patterns of marine nitrogen fixation. Nature Rev Microbiol 9: 499–508 doi:10.1038/Nrmicro2594. Available: http://www.nature.com/nrmicro/journal/v9/n7/pdf/nrmicro2594. pdf. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 23. Zehr JP, Kudela RM (2011) Nitrogen Cycle of the Open Ocean: From Genes to Ecosystems. Annual Review of Marine Science 3: 197–225 doi:10.1146/annurev-marine-120709–142819 Available: http://oceandatacenter.ucsc.edu/home/Publications/Zehr,2011_AnnuRev.pdf. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 24. Großkopf T, Mohr W, Baustian T, Schunck H, Gill D, et al. (2012) Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488: 361–364 doi:10.1038/nature11338. Available: http://dx.doi.org/10.1038/nature11338. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 25.Karl D, Michaels A, Bergman B, Capone D, Carpenter E, et al. (2002) Dinitrogen fixation in the world’s oceans. Biogeochemistry 57: 47–98. Available: http://www.princeton.edu/sigman/publications/pdf/KarlBiogeochemistry2002.pdf. Accessed 2013 April 23.
- 26. Zehr JP (2011) Nitrogen fixation by marine cyanobacteria.Trends in Microbiology. 19: 162–173 doi:10.1016/j.tim.2010.12.004. Available: http://www.sciencedirect.com/science/article/pii/S0966842X10002155. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 27. Riemann L, Farnelid H, Steward G (2010) Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat Microb Ecol 61: 235–247 doi:10.3354/ame01431. Available: http://www.int-res.com/abstracts/ame/v61/n3/p235-247/. Accessed 2013 April 23. [Google Scholar]
- 28. Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, et al. (2012) Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. Isme Journal 6: 1238–1249 doi:10.1038/ismej.2011.182. Available: http://www.nature.com/ismej/journal/v6/n6/full/ismej2011182a.html. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jayakumar A, Al-Rshaidat MMD, Ward BB, Mulholland MR (2012) Diversity, distribution, and expression of diazotroph nifH genes in oxygen-deficient waters of the Arabian Sea. FEMS Microbiol Ecol 82: 597–606 doi:10.1111/j.1574–6941.2012.01430.x. Available: http://dx.doi.org/10.1111/j.1574-6941.2012.01430.x. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 30.Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ (2012) Trichodesmium – a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol Rev 1–17.doi: 10.1111/j.1574–6976.2012.00352.x. Available: http://dx.doi.org/10.1111/j.1574-6976.2012.00352.x. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed]
- 31. Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ (1997) Trichodesmium, a Globally Significant Marine Cyanobacterium. Science 276: 1221–1229 doi:10.1126/science.276.5316.1221. Available: http://www.sciencemag.org/content/276/5316/1221.abstract. Accessed 2013 April 23. [Google Scholar]
- 32. Yoshinari T (1976) Nitrous oxide in the sea. Mar Chem 4: 189–202 doi:10.1016/0304–4203(76)90007–4. Available: http://www.sciencedirect.com/science/article/pii/0304420376900074. Accessed 2013 April 23. [Google Scholar]
- 33.Grob C, Ulloa O, Claustre H, Huot Y, Alarcon G, et al. (2007) Contribution of picoplankton to the total particulate organic carbon concentration in the eastern South Pacific. Biogeosciences 4: 837–852. Available: http://www.biogeosciences.net/4/837/2007/bg-4-837-2007.pdf. Accessed 2013 April 23.
- 34. Deutsch C, Sarmiento J, Sigman D, Gruber N, Dunne J (2007) Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445: 163–167 doi:10.1038/nature05392. Available: http://dx.doi.org/10.1038/nature05392. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 35. Bruland KW, Rue EL, Smith GJ, DiTullio GR (2005) Iron, macronutrients and diatom blooms in the Peru upwelling regime: brown and blue waters of Peru. Mar Chem 93: 81–103 doi:10.1016/j.marchem.2004.06.011. Available: http://www.es.ucsc.edu/∧kbruland/Manuscripts/RUE/Brulandetal2005.pdf. Accessed 2013 April 23. [Google Scholar]
- 36.Blain S, Bonnet S, Guieu C (2008) Dissolved iron distribution in the tropical and sub tropical South Eastern Pacific. Biogeosciences 5: 269–280. Available: http://www.biogeosciences.net/5/269/2008/bg-5-269-2008.pdf. Accessed 2013 April 23.
- 37.Bonnet S, Guieu C, Bruyant F, Prasil O, Van Wambeke F, et al. (2008) Nutrient limitation of primary productivity in the Southeast Pacific (BIOSOPE cruise). Biogeosciences 5: 215–225. Available: http://www.biogeosciences.net/5/215/2008/bg-5-215-2008.pdf. Accessed 2013 April 23.
- 38. Deutsch C, Gruber N, Key RM, Sarmiento JL, Ganachaud A (2001) Denitrification and N2 fixation in the Pacific Ocean. Global Biogeochem Cy 15: 483–506 doi:10.1029/2000gb001291. Available: http://dx.doi.org/10.1029/2000GB001291. Accessed 2013 April 23. [Google Scholar]
- 39.Farias L, Castro-Gonzalez M, Cornejo M, Charpentier J, Faundez J, et al. (2009) Denitrification and nitrous oxide cycling within the upper oxycline of the eastern tropical South Pacific oxygen minimum zone. Limnol Oceanogr 54: 132–144. Available: http://www.aslo.org/lo/toc/vol_54/issue_1/0132.pdf. Accessed 2013 April 23.
- 40. Fernandez C, Farias L, Ulloa O (2011) Nitrogen Fixation in Denitrified Marine Waters. Plos One 6. (6): e20539 doi:10.1371/journal.pone.0020539. Available: http://www.plosone.org/article/info:doi/10.1371/journal.pone.0020539. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mehta MP, Butterfield DA, Baross JA (2003) Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca ridge. Appl Environ Microb 69: 960–970 doi:10.1128/Aem.69.2.960–970.2003 Available: http://aem.asm.org/content/69/2/960. full.pdf+html. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5: 539–554 doi:10.1046/j.1462–2920.2003.00451.x. Available: http://onlinelibrary.wiley.com/doi/10.1046/j.1462-2920.2003.00451.x/pdf. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 43.Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, et al.. (2005) Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cy 19: GB2024- GB2041. Artn Gb2024. doi: 10.1029/2004gb002331. Available:http://dornsife.usc.edu/assets/sites/125/docs/Capone_et_al_2005_GBC.pdf. Accessed 2013 April 23.
- 44.Genbank website.Available: http://www.ncbi.nlm.nih.gov/genbank/.Accessed 2013 April 23.
- 45. Palenik B, Brahamsha B, Larimer FW, Land M, Hauser L, et al. (2003) The genome of a motile marine Synechococcus. Nature 424: 1037–1042 doi:10.1038/Nature01943. Available: http://www.nature.com/nature/journal/v424/n6952/full/nature01943.html. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 46. Cornejo M, Farias L, Gallegos M (2007) Seasonal cycle of N2O vertical distribution and air-sea fluxes over the continental shelf waters off central Chile (36° S). Prog Oceanogr 75: 383–395 doi:10.1016/j.pocean.2007.08.018. Available: http://www.sciencedirect .com/science/article/pii/S0079661107001589. Accessed 2013 April 23. [Google Scholar]
- 47. Naqvi SWA, Jayakumar DA, Narvekar PV, Naik H, Sarma VVSS, et al. (2000) Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408: 346–349 doi:10.1038/35042551. Available: http://www.nature.com/nature/journal/v408/n6810/full/408346a0.html. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 48. Gruber N (2011) Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Philos T Roy Soc A 369: 1980–1996 doi:10.1098/ rsta.2011.0003.. Available: http://rsta.royalsocietypublishing.org/content/369/1943/1980.abstract. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 49.Herzberg G (1966) Molecular spectra and molecular structure. In: Nostrand V, editor. Electronic spectra and electronic structure of polyatomic molecule. New York: Reinhold. p. 778.
- 50. Shestakov AF, Shilov AE (2001) On the coupled oxidation-reduction mechanism of molecular nitrogen fixation. Russ Chem B 50: 2054–2059 doi:10.1023/a:1015028713288. Available: http://dx.doi.org/10.1023/A:1015028713288. Accessed 2013 April 23. [Google Scholar]
- 51. Yoshida N, Toyoda S (2000) Constraining the atmospheric N2O budget from intramolecular site preference in N2O isotopomers. Nature 405: 330–334 doi:10.1038/35012558. Available: http://www.nature.com/nature/journal/v405/n6784/full/405330a0.html. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 52. Suntharalingam P, Sarmiento JL (2000) Factors governing the oceanic nitrous oxide distribution: Simulations with an ocean general circulation model. Global Biogeochem Cy 14: 429–454 doi:10.1029/1999GB900032. Available: http://www.gfdl.noaa.gov/bibliography/related_files/pns0001.pdf. Accessed 2013 April 23. [Google Scholar]
- 53. Toyoda S, Yoshida N, Miwa T, Matsui Y, Yamagishi H, et al. (2002) Production mechanism and global budget of N2O inferred from its isotopomers in the western North Pacific. Geophys Res Lett 29: 1037 doi:10.1029/2001gl014311. Available: http://dx.doi.org/10.1029/2001GL014311. Accessed 2013 April 23. [Google Scholar]
- 54. Charpentier J, Farias L, Yoshida N, Boontanon N, Raimbault P (2007) Nitrous oxide distribution and its origin in the central and eastern South Pacific Subtropical Gyre. Biogeosciences 4: 729–741 doi:10.5194/bg-4-729-2007. Available: http://www.biogeosciences.net/4/729/2007/bg-4-729-2007.pdf. Accessed 2013 April 23. [Google Scholar]
- 55. Yamagishi H, Yoshida N, Toyoda S, Popp BN, Westley MB, et al. (2005) Contributions of denitrification and mixing on the distribution of nitrous oxide in the North Pacific. Geophys Res Lett 32: L04603–L04606 doi:10.1029/2004gl021458. Available: http://dx.doi.org/10.1029/2004GL021458. Accessed 2013 April 23. [Google Scholar]
- 56.Popp BN, Westley MB, Toyoda S, Miwa T, Dore JE, et al. (2002) Nitrogen and oxygen isotopomeric constraints on the origins and sea-to-air flux of N2O in the oligotrophic subtropical North Pacific gyre. Global Biogeochem Cy 16. Artn 1064. doi: 10.1029/2001gb001806. Available:http://www.soest.hawaii.edu/oceanography/faculty/sansone/Popp%20et%20al%202002%20GBC.pdf. Accessed 2013 April 23.
- 57.Sutka RL, Ostrom NE, Ostrom PH, Breznak JA, Gandhi H, et al.. (2006) Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. App Environ Microb 72: 638–644. doi 10.1128/Aem.72.1.638–644.2006. Available: http://aem.asm.org/content/72/1/638.short. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed]
- 58. Frame CH, Casciotti KL (2010) Biogeochemical controls and isotopic signatures of nitrous oxide production by a marine ammonia-oxidizing bacterium. Biogeosciences 7: 2695–2709 doi:10.5194/bg-7-2695-2010. Available: http://www.biogeosciences.net/7/2695/2010/bg-7-2695-2010.pdf. Accessed 2013 April 23. [Google Scholar]
- 59. Loscher CR, Kock A, Konneke M, LaRoche J, Bange HW, et al. (2012) Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences 9: 2419–2429 doi:10.5194/bg-9-2419-2012. Available: http://www.biogeosciences.net/9/2419/2012/bg-9-2419-2012.html. Accessed 2013 April 23. [Google Scholar]
- 60. Revsbech NP, Larsen LH, Gundersen J, Dalsgaard T, Ulloa O, et al. (2009) Determination of ultra-low oxygen concentrations in oxygen minimum zones by the STOX sensor. Limnol Oceanogr-Methods 7: 371–381 doi:10.4319/lom.2009.7.371. Available: http://www.aslo.org/lomethods/free/2009/0371. html. Accessed 2013 April 23. [Google Scholar]
- 61.Sarmiento J, Gruber N (2006) Ocean Biogeochemical Dynamics. Princeton University Press Princeton Book. p 503.
- 62.Grasshoff K, Kremling K, Ehrhardt M (1983). Methods of seawater analysis. Weinheim: Verlag Chemie. p 419.
- 63. Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JDH (1965) Fluorometric Determination of Chlorophyll. J Conseil 30: 3–15 doi:10.1093/icesjms/30.1.3. Available: http://icesjms.oxfordjournals.org/content/30/1/3. abstract. Accessed 2013 April 23. [Google Scholar]
- 64. Bohlke JK, Laeter JRd, Bievre PD, Hidaka H, Peiser HS, et al. (2005) Isotopic Compositions of the Elements, 2001. J Phys Chem Ref Data 34: 57–67 doi:10.1063/1.1836764. Available: http://link.aip.org/link/?JPR/34/57/1. Accessed 2013 April 23. [Google Scholar]
- 65.Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN ISBN 3–900051–07–0. Available: http://www.R-project.org. Accessed 2013 April 23.
- 66.Montoya JP, Voss M, Kahler P, Capone DG (1996) A simple, high-precision, high-sensitivity tracer assay for N2 fixation. App Environ Microb 62: 986–993. Available:http://aem.asm.org/content/62/3/986.full.pdf. Accessed 2013 April 23. [DOI] [PMC free article] [PubMed]
- 67. Chen YB, Zehr JP, Mellon M (1996) Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp IMS 101 in defined media: Evidence for a circadian rhythm. J Phycol 32: 916–923 doi:10.1111/j.0022–3646.1996.00916.x. Available: http://onlinelibrary. wiley.com/doi/10.1111/j.0022-3646.1996.00916.x/abstract. Accessed 2013 April 23. [Google Scholar]
- 68. Rippka R, Coursin T, Hess W, Lichtlé C, Scanlan DJ, et al. (2000) Prochlorococcus marinus Chisholm et al. 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a2/b2-containing cyanobacterium (Oxyphotobacteria). Int J Sys Evol Micr 50: 1833–1847 doi:10.1099/00207713-50-5-1833. Available: http://ijs.sgmjournals.org/content/50/5/1833.abstract. Accessed 2013 April 23. [DOI] [PubMed] [Google Scholar]
- 69.Marie D, Simon N, Guillou L, Partensky F, Vaulot D (2000) Flow cytometry analysis of marine picoplankton. In: Diamond RA, DeMaggio S, editors. In living color: protocols in flow cytometry and cell sorting. Springer, Berlin. 421–454.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oceanic N2O undersaturation (saturation %) and air-sea flux (µmol m−2 d−1) reported in surface and subsurface/intermediate waters around the global ocean. Depths of hypoxic/suboxic waters are indicted
(DOCX)
Experimental setup used with in natural and cyanobacteria cultured samples to asses assimilative N2O fixation.
(DOCX)
ΔN2O inventories (µmol·m−2) and estimated net N2O production (µmol·m−2·d−1) in surface waters at selected stations along N2O air-sea exchange (µmol·m−2·d−1) and N2O consumption rates by fixation. In some station, denitrification and N2 fixation rates (both based on published data) are available in order to compare with other N2O consuming processes.
(DOCX)
Isotopic and Isotopomeric determination
(DOCX)
N2O mass balance
(DOCX)