Abstract
Dietary supplementation of selenium and green tea holds promise in cancer prevention. In this study, we evaluated the efficacies of selenium and green tea administered individually and in combination against colorectal cancer in an azoxymethane (AOM)-induced rat colonic carcinogenesis model and determined the underlying mechanisms of the protection. Four-week old Sprague-Dawley male rats were fed with diets containing 0.5% green tea extract, 1ppm selenium as selenium-enriched milk protein, or combination of 1ppm selenium and 0.5% green tea extract. Animals received 2 AOM (15 mg/kg) treatments to induce colonic oncogenesis. Rats were killed 8 or 30 wk later after the last AOM to examine the effect of dietary intervention on aberrant crypt foci (ACF) formation or tumor development. On sacrifice, colons were examined for ACF and tumors, the mRNA levels of SFRP5 and Cyclin D1, and the proteins levels of ß-catenin, COX-2, Ki-67, DNMT1 and acetyl histone H3. The combination of selenium and green tea resulted in a significant additive inhibition of large ACF formation, this effect was greater than either selenium or green tea alone, P<0.01; the combination also had a significant additive inhibition effect on all tumor endpoints, the effect of the combination diet on tumor incidence, multiplicity and size was greater than selenium or green tea alone, P<0.01. Rats fed the combination diet showed marked reduction of DNMT1 expression and induction of histone H3 acetylation, which were accompanied by restoration of SFRP5 mRNA in normal-appearing colonic crypts. The combination diet also significantly reduced ß-catenin nuclear translocation, Cyclin D1 expression and cell proliferation. These data show, for the first time, that combination of selenium and green tea is more effective in suppressing colorectal oncogenesis than either agent alone. The preventive effect is associated with regulation of genetic and epigenetic biomarkers implicated in colonic carcinogenesis.
Introduction
Colorectal Cancer (CRC) is one of the leading causes of cancer death worldwide; different to other cancers, the importance of environmental exposure (especially diet) in the aetiology of CRC is highlighted by the fact that <50% of the causation can be attributed to familial factors, whereas dietary factors contribute up to 70% [1]. Specific dietary strategies might prove valuable in protecting against cancer [2]. In this regard, associations between diet and lifestyle have been established for CRC prevention, and it has been estimated that 30–50% of CRC could be potentially preventable by consuming a healthy diet [3].
In recent years, naturally occurring agents present in what we eat or drink have drawn a great deal of attention with their potential ability for cancer prevention and/or treatment because of their various health benefits and wide safety margin [2], [4]. Selenium (Se), an essential trace micronutrient, and green tea, the most common beverage consumed worldwide, have been identified as chemopreventive agents for various cancers. Epidemiological, clinical and preclinical studies suggest an inverse relationship between Se intake, green tea consumption and the risk of certain cancers [5]–[7]. Studies with animal models also have shown that Se and green tea have a wide range of preventive activity against CRC [8]–[10]. Despite promising results in preclinical settings, current clinical trial data related to Se and green tea supplementation are not convincing enough to allow a general recommendation for using Se or green tea as an effective agent for chemoprevention of cancer in humans [11]–[13]. The limitation of any single dietary agent for effective prevention might be due to lower potency of dietary agents, whereas natural compounds have shown greater activity when they are present in a mixture [14]. It might be possible to achieve additive or synergistic preventive effects by combining dietary agents that exert complementary mechanisms in their anti-carcinogenic actions [2], [15], [16]. Considerable data from animal and human studies indicate that combinations of dietary agents are more effective than a single agent [17], [18]. For instance, green tea has been shown to synergistically or additively increase the efficacy of other drugs or dietary agents in vitro and in vivo [19]–[22].
Despite the increasing interest on the chemopreventive role of Se or green tea, it is unknown whether the combination of Se and green tea has a beneficial chemopreventive effect on CRC. Although preclinical and clinical reports of combining Se and green tea are lacking, combination approaches with Se or green tea have been studied in colon cancer and other cancer models [23]. For instance, a combination of Se and genistein has been shown to inhibit breast cancer in a rat model [24]; a combination of Se and vitamin E has provided greater protection against esophageal carcinogenesis in rats [22]. The combination of green tea and curcumin or the combination of green tea and sulindac has resulted in synergistic chemopreventive effect in an AOM CRC model [25], [26].
Se and green tea are particularly interesting as a combination not only because they can be easily co-administered in the diet, but also because they have potentially complementary mechanisms of action. Apoptotic removal and DNA repair enzyme O6-alkylguanine DNA alkyltransferase (MGMT)-mediated DNA repair are two important innate cellular responses to environmental carcinogen-induced oncogenic DNA lesions. Green tea has been shown to up-regulate MGMT activity in rat colon in our earlier study (unpublished data) by an epigenetic mechanism that would be expected to repair the type of adduct induced by azoxymethane (AOM) [27]. Dietary Se has been shown by our team to activate apoptotic deletion of AOM-affected cells [28]. We hypothesise that the risk of developing CRC will be reduced by combining agents that target different aspects of innate cellular responses to oncogenic DNA lesions. This is justified by the fact that CRC has a long initial latency period, involves multiple steps and pathways, and a combinational approach may simultaneously regulate multiple molecular and cellular targets involved in the process of CRC [29].
Our increased understanding of CRC at the epigenetic/genetic levels also opens up opportunities to interrupt and reverse the initiation and progression of CRC and provides many targets for dietary intervention [30]. The AOM-induced CRC model has been extensively used in both mechanistic and chemepreventive studies [31] because it recapitulates many of the clinical, pathological, and molecular features of human CRC, such as preneoplastic lesions, aberrant crypt foci (ACF), mutations in K-ras oncogene, and deregulation of signalling pathways in WNT/ß-catenin and inflammation [31], [32]. Although the roles of WNT-antagonists (such as secreted frizzled related proteins (SFRPs)), DNA methyltransferases (DNMT) and histone deacetylation in human colonic carcinogenesis are well documented, the expression of DNMT1, SFRR5 and acetylation of histone H3 in this model are largely unknown; which play crucial roles in the development and progression of human colon cancers [33]–[35]. The present study was designed to evaluate the chemopreventive effect of combining dietary agents of Se and green tea against colonic carcinogenesis, using ACF and colon tumors as endpoints. The effects of this combination on genetic/epigenetic biomarkers were also examined.
Methods
Reagents
AOM and green tea extract were purchased from Sigma-Aldrich Pty. Ltd. Green tea extract (P1204) contains 65% catechin including 34.5% Epigallocatechin Gallate (EGCG). Milk protein (0.34ppm Se) and Se-enriched milk protein (5ppm Se) were provided by Tatura Milk Industries (VIC, Australia) [28]. Se-enriched milk protein contains 83% selenomethionine, 5% selenocysteine and 4% unknown components [36].
Animals
A total of 160 male Sprague-Dawley rats were obtained from the Animal Resource Centre, Adelaide University, Australia. The study was approved by the Animal Welfare Committee at Flinders University (# 651/07). Two experiments were performed, 60 rats for an ACF experiment and 100 for a long-term tumor experiment.
For each experiment, rats were divided randomly into 4 equal experimental groups (with comparable initial body weights), housed in plastic cages (four per cage) and maintained in a temperature and humidity-controlled animal facility with a 12 h light/dark cycle at 22±2°C temperature and 80±10% humidity. Rats were given free access to water, weighed weekly and were monitored closely for clinical signs of ill health throughout the study. Rats appearing sick were euthanized immediately.
Diets
The experimental diets were based on a modified AIN-76A diet and contained 19% sunflower oil by weight so as to “humanize” the fat contribution to energy intake to ∼35% [28]. Milk protein was used as the protein source for control and green tea diets; Se-enriched milk protein was used as protein as well as a supplemental Se source for the high Se diet and the combination diet; control diet contained neither Se nor green tea. Choice of 0.5% green tea was based on our earlier study that this intake significantly increased MGMT expression (mRNA and activity) in rat colons (unpublished data). This dose contains 172.5 mg EGCG/100 g diet and provide 25.9 mg EGCG/rat/day, which when calculated on a per body weight basis would provide the equivalent intake in an adult human of 3–4 cups of green tea per day. This feeding regimen was well tolerated by animals [37]. 1ppm Se was chosen because our previous studies showed that Se-enriched milk protein at 1ppm significantly protected against colon cancers in mice [28]. The diets were prepared fresh at 4-weekly intervals, pelleted and stored at −20°C until used. Details of diets are provided in Table 1.
Table 1. Composition of experimental diets (g/100 g diet).
| Ingredient | Control | Green tea | Se | Se + green tea |
| Milk protein# | 20 | 20 | ||
| Se-enriched milk protein #,† | 20 | 20 | ||
| Sucrose | 20 | 20 | 20 | 20 |
| Corn starch | 31.15 | 30.65 | 31.15 | 30.65 |
| Fiber (alpha cell) | 5 | 5 | 5 | 5 |
| Sunflower oil | 19 | 19 | 19 | 19 |
| Choline | 0.2 | 0.2 | 0.2 | 0.2 |
| Mineral mix‡ | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 | 1 | 1 |
| Methionine | 0.15 | 0.15 | 0.15 | 0.15 |
| Green tea | 0 | 0.5 | 0 | 0.5 |
The experimental diets consisted of a modified AIN-76A diet achieved by adding 19% sunflower oil and 20% protein.
Milk protein was used as source of protein for control diet and green tea diet
Se-enriched milk protein was used as source of protein and Se for Se diet and the combination diet.
No Se was included in mineral mix in the diets.
Experiment 1 (ACF study)
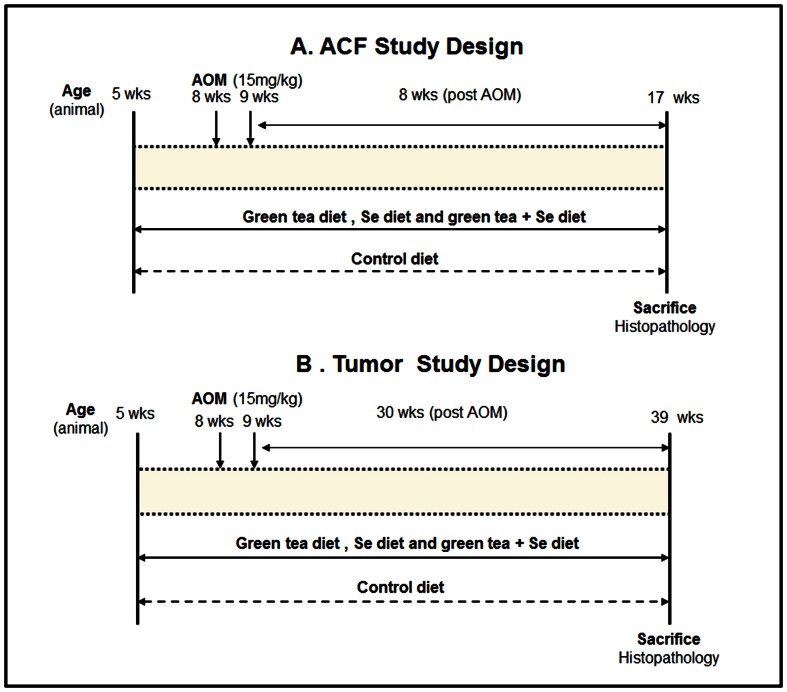
Beginning at 5 wk of age, rats (15/group) were fed to each of four diets. After 2 wk on diets, rats received 2 AOM injections (15 mg/Kg body weight) one wk apart. Rats remained on the same diet throughout the study until killed by CO2 asphyxiation 8 wk after the last AOM injection (Figure 1A). Colons were opened flat overnight on hibond C paper for ACF analysis. After analysis, a distal segment of normal-appearing colon (2 cm) was cut and stained with Ki-67, COX-2 and β-catenin antibodies (12/group).
Figure 1. Experimental design for evaluation of Se, green tea and the combination diet for chemopreventive effects against colonic carcinogenesis in an AOM-induced rat CRC model.
ACF study (A): groups of rats (n = 15) were fed control, Se, green tea or diet containing Se and green at age of 5 wk. 2 wk later, rats were given 15 (mg/Kg/body weight) AOM, once a week for 2 wk. 8 wk after last AOM treatment, rats were euthanized and colons were evaluated for ACF; normal-appearing crypts were also examined for β-catenin, COX-2 and Ki-67 expression. Tumor study (B) : groups of rats (n = 25) were fed control, Se, green tea or diet containing Se and green at age of 5 wk. Rats were given two weekly AOM treatments similar to ACF study. 30 wk after AOM treatment, rats were euthanized and colons were historically evaluated for tumor outcomes; normal-appearing crypts were also examined for β-catenin, DNMT1, Ac-H3 as well as SFRP5 and Cyclin D1 expression.
Experiment 2 (tumor study)
5 wk old rats (25/group) were fed to each of four diets, similar to ACF study. Rats received 2 weekly AOM injections (15 mg/kg) but were killed 30 wk after the last AOM (Figure 1B). Colons were examined for tumor endpoints and further processed for histopathologic evaluation. A distal segment of normal-appearing colon (2 cm) free of neoplasms was dissected for immunohistochemistry of β-catenin, DNMT1 and acetylated histone H3 (Ac-H3) (12/group) or frozen in liquid nitrogen for western blot analysis (6/group) or placed in RNAlater (Ambion) for quantitative RT-PCR analysis (6/group).
Quantification of ACF
Colons were stained with 0.1% methylene blue solution and evaluated at ×40 magnification using a dissecting microscope in a blind scoring procedure. Total number of ACF in the entire colon was scored from the distal to the proximal end of the colon. ACF were distinguished from the surrounding normal crypts by their increased size, elevated appearance and the slit like shape of the luminal opening. Crypt multiplicity was defined as the number of crypts in each focus, and categorized as small (1–3 crypts/focus) and large ACF (4 or more crypts/focus).
Histopathology of tumors
The number, location and size of each tumor were scored by an independent observer unaware of the dietary treatment. The tumors were categorized as adenomas and adenocarcinomas as described previously by us [28]. Endpoints were colon tumor incidence (i.e. proportion of rats with tumors, with adenomas or adenocarcinomas), tumor multiplicity (number of tumors/rat) and tumour size (tumor size/rat). Tumor size was calculated by the formula: log10 [∑(π(diameter1 + diameter 2))2/2] [38].
Immunohistochemical analysis
The primary antibodies against Ki-67 (MIB-5, #M7248) were purchased from Dako, Australia; COX-2 (M-19-R, #SC-1747-R), β-catenin (E5, #SC-7963) and DNMT1 (H-300 #SC-2701) from Santa Cruz Biotechnology, Australia and Ac-H3 (Lys9/Lys14, #9677) from Cell signalling, USA. The detailed procedures for immunohistochemical analysis were reported previously [28]. In brief, antigen retrieval was carried out by heating sections in 0.1 M citrate buffer in pressure cooker plastic tub for 1 hour. Sections were incubated with Ki-67 antibody (1∶1000), COX-2 antibody (1∶500), β-catenin antibody (1∶1,000), DNMT1 antibody (1∶2,000), and Ac-H3 antibody (1∶5,000) overnight after incubation in 3% H2O2 for 20 mins. Detection for Ki-67 and COX-2 was biotinylated secondary rabbit-anti-mouse antibody (1∶200) (Dako) for 30 mins and avidin/biotinylated peroxidase complex (Signet Laboratories) for 20 mins. Detection for DNMT1 and β-catenin was by a HRP polymer link. Slides were visualized by incubating with 3′-diaminobenzamine substrate and counterstained with hematoxylin. A positive staining was identified by a reddish brown precipitate in the nucleus for Ki-67, DNMT1 and Ac-H3, in the cytoplasm for COX-2 and in membrane/nucleus for β-catenin. 20 intact perpendicular well-oriented normal-appearing crypts (extending from the muscular mucosa to the colonic lumen) were examined for each colon sample. The index of colonic crypt cells expressing Ki-67, COX-2, DNMT1 and Ac-H3 was calculated as the number of positive cells per crypt column divided by the total number of cells and multiplied by 100. Membrane and nuclear stained β-catenin cells were counted separately. The abnormal expression of β-catenin, DNMT1 and Ac-H3 was also examined in tumor tissues.
Western Blot analysis
Aliquots of normal-appearing colon tissues from six rats per group were pooled and homogenized in ice-cold lysis buffer (50 mM Tris, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 2 mM PMSF and protease inhibitors) and centrifuged (14,000×g for 25 min at 4°C). The concentration of protein in supernatants was determined using the Bio-Rad protein assay. Equal amounts of proteins (30 µg) were separated on 4–20% SDS-PAGE gels, proteins were transferred to a nitrocellulose membrane using semi-dry transfer and the membrane was blocked with 5% skim milk, probed with β-catenin, DNMT1 and Ac-H3 antibodies and stained with secondary antibody (a horseradish peroxidase-labelled anti-rabbit IgG or goat anti-mouse IgG). Western blot was repeated three times for each sample. Immunoreactive proteins were detected using the enhanced chemiluminescence light detecting kit. Each membrane was re-probed with anti-β-actin antibody (Sigma) or anti-histone H3 antibody (#4499, Cell signalling). Band intensities for β-catenin, DNMT1were quantified by Image J and normalized with β-actin, whereas band intensity of Ac-H3 was normalized with H3 histone. Results are expressed as ratio to β-actin or H3 histone.
Quantitative RT-PCR
Total RNA was extracted from rat colons using a QIAGEN RNeasy Mini Kit (Qiagen, Germany). The concentration and purity of the total RNA was estimated using a NanoDrop® ND-1000 UV-Vis spectrophotometer by measuring the absorbance at 260 and 280 nm. Complementary DNA (cDNA) was synthesized from 0.3 µg total RNA for each sample using a QIAGEN QuantiTect Reverse Transcription Kit. The SFRP5 and Cyclin D1 genes were co-amplified with GAPDH gene, which served as a housekeeping gene. Primers for SFRP5 gene were purchased from Qiagne, Rn_Sfrp5_1_SG QuantiTect Primer Assays (NM_001107591, XM_001055342, XM_219887, XM_347305- Cat # QT01624056). Primers for Cyclin D1gene (NM_171992) were 5′-ATGAGAACAAGCAGATCATCC-3′ (Forward Primer) and 5′-TAGCAGGAGAGGAAGTTGTTG-3′ (Reverse Primer). Primers for the GAPDH gene (NM_017008) were 5′-AACATCATCCCTGCATCCAC-3′ (Forward Primer) and 5′-TTGAAGTCRCAGGAGACAAC-3′ (Reverse Primer). Real-time PCR was conducted with QuantiTect SYBR Green PCR Kit on a Rotor Gene 3000 Cycler (Corbett, Australia). The cycling conditions of 40 cycles were 94°C/15 s, 55°C/30 s and 72°C/30 s. Each sample was run in triplet repeat and normalized by GAPDH. For each PCR run, a non-template reaction was included as negative controls. All PCR data were analysed with Q-Gene software as we described previously [36], [39].
Statistical analyses
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, Illinois) and Stata version 11.1 (StataCorp, Texas). Results are expressed as means ± standard error of the mean for normally distributed data. Between-group comparisons for weight, Ki-67, COX-2, β-catenin, DNMT1, Ac-H3, SFRP5 and Cyclin D1 were performed using one-way ANOVA with correction for multiple comparisons by Tukey's post hoc test, a P-value of <0.05 was considered statistically between the groups. Between-group comparisons of ACF counts and tumor measures were assessed according to the 2×2 factorial designs with green tea and Se as the two main factors. Binary logistic and negative binomial regression models were employed to test for the main effects of green tea and Se and for possible interaction effects between green tea and Se. Post-hoc comparisons for each of the 3 intervention diets with the control diet as the comparison group, as well as comparison for the combination diet with the Se alone or green tea alone were also performed. A P-value of <0.05 was considered statistically significant for each main effect and interaction.
Results
General observation
Diets were well received and consumed by four rat groups. All rats regardless of dietary intervention group showed normal weight gain during the two experiments (data not shown); there was no significant difference in food consumption between the dietary groups. Examination of small intestine, liver and kidney did not reveal any abnormality in either study, indicating dietary supplementation with 1ppm Se or 0.5% green tea, or the combination of Se and green tea did not cause any overt toxicity. Six sick rats were killed before the termination of tumor study; they were distributed across the three treatment groups and tumor endpoints from these rats were not able to be included.
The combination diet effectively inhibits ACF formation in AOM model
AOM-induced ACF were observed predominantly in the distal and middle colon. The effects of Se, green tea and the combination diet on ACF formation are shown in Table 2. Although the total number of ACF/rat from our study was somewhat lower compared to other previously published studies [40], rats fed green tea alone had no effect on total ACF and small ACF/rat, although it significantly reduced large ACF/rat, P<0.05, compared with those fed the control diet. In contrast, all categories of ACF crypt multiplicity (total, small and large ACF/rat) were significantly reduced by Se alone (P<0.05) and the combination diet (P<0.01), in comparison to the control diet. Importantly, the combination diet showed maximal inhibition on large ACF (i.e. ACF containing four or more crypt foci, the most relevant for predicting subsequent invasive tumor lesions) compared with either Se or green tea alone: with approximately 70% greater inhibition versus green tea diet and 66% inhibition greater versus Se diet (mean number = 8.4±1.3, 7.5±1.6 and 2.5±0.5 for green tea, Se and the combination diet respectively, P<0.05 for the combination diet versus each of green tea and Se).
Table 2. Effect of Se alone, green tea alone, and their combination on the formation of AOM-induced ACF in rats.
| Experimental diets | Green tea or Se in diet | Total ACF / per rat# (mean ± SEM) | Small ACF / per rat† (mean ± SEM) | Large ACF / per rat‡ (mean ± SEM) |
| Control diet | 0 | 50.1±4.5a | 37.3±2.6a | 13.6±2.0a |
| Green tea diet | 0.5% | 42.4±7.2a | 34.0±6.2a | 8.4±1.3b |
| Se diet | 1 ppm | 30.7±4.3b | 23.3±3.4b | 7.5±1.6b |
| Se + green tea diet | 1 ppm + 0.5% | 27.3±5.6b | 24.9±5.3b | 2.5±0.5c |
AOM, azoxymethane; Se, selenium; ACF, aberrant crypt foci; SEM, standard errors of mean.
ACF study was undertaken by feeding rats with 4 experimental diets (15 rats per group), containing either control diet, green tea diet, Se diet and the combination diet. Animals received two weekly AOM (15 mg/kg) injections to induce ACF and were killed 8 wk later.
Total number of ACF was calculated as the sum of the small and large ACF.
Small ACF (Crypt multiplicity) was classified by the number of crypts per focus (1–3).
Large ACF (Crypt multiplicity) was classified by the number of crypts per focus (≥4).
Values with different superscripts (a, b, c) in each column were statistically different (P<0.05).
The combination diet effectively inhibits tumor formation in AOM model
The significant decreases in the number of ACF are in keeping with the lower tumor burdens observed in the rats receiving the combination diet (Table 3). There was a significant interaction between Se and green tea, P<0.05, with the combination diet showing additive inhibitory effect on all tumor endpoints relative to a single dietary agent as green tea or Se, P<0.01. Tumor incidence, multiplicity and tumor size were reduced by 73.9, 80.4%, 73.4% versus green tea diet, and by 65.2%, 72.7%, 63% versus Se diet, respectively. Green tea alone did not significantly affect any tumor endpoint, while Se alone was less effective than the combination diet. Analysis of the effect of dietary intervention on cancer formation was specifically performed after histopathological examination of tumors. The incidence of adenoma in the control, green tea, Se and the combination diets were 26.1%, 20.8%, 16.7% and 4.3%, respectively. There was a significant reduction in the rats fed the combination diet, it was reduced by 79.3% versus green tea diet, and by 74.2% versus Se diet, P<0.01, respectively. The incidence of adenocarcinomas was reduced in a similar pattern; 17.4% in rats fed a control diet compared to 4.3% in those fed the combination diet, while in green tea alone the incidence was 12.5% and for Se alone the incidence was 8.3%. Notably, the combination diet reduced the incidence of adenocarcinomas by 65.6% versus green tea diet, and by 48.2% versus Se diet, but numbers were small and the results did not reach significance.
Table 3. Effect of Se and green tea alone, and their combination on tumor incidence, multiplicity and size in AOM-treated rats.
| Tumor incidence | Tumor multiplicity | Tumor size | |||||||||
| Experimental diet | No. rats | Rats with tumors (%) | % of inhibition | Rats with adenoma.(%) | % of inhibition | Rats with Adenocarci-noma#.(%) | % of inhibition | Tumors / rat (Mean ± SEM) | % of inhibition | Tumor / rat (Mean ± SEM) | % of inhibition |
| Control diet | 23 | 10/23(43.5)a | 6/23(26.1%)a | 4/23(17.4%) | 0.61±0.18a | 0.80±0.20a | |||||
| Green tea diet | 24 | 8/24(33.3)a | 23.4% | 5/24(20.8%)a | 20.3% | 3/24 (12.5%) | 28.2% | 0.46±0.15a | 24.6% | 0.64±0.19a | 20% |
| Se diet | 24 | 6/24 (25%)b | 42.5% | 4/24(16.7%)a | 36% | 2/24 (8.3%) | 52.3% | 0.33±0.11b | 45.9% | 0.46±0.17b | 42.5% |
| Se + green tea diet | 23 | 2/23 (8.7%)c | 80% | 1/23 (4.3%)b | 83.5% | 1/23 (4.3%) | 75.3% | 0.09±0.06c | 85.2% | 0.17±0.12c | 78.7% |
AOM, azoxymethane; Se, selenium; SEM, standard errors of mean.
Tumor study was undertaken by feeding animals with 4 different diets (25 rats per group), containing either control diet, green tea diet, Se diet and the combination diet. Animals received two weekly AOM (15 mg/kg) injections to induce tumor formation and killed 30 wk later.
No statistical analysis undertaken for adenocarcinomas due to the small numbers.
Values with different superscripts (a, b, c) in each column were statistically different (P<0.05).
The combination diet prevents AOM-induced ß-catenin nuclear translocation, COX-2 expression and cell proliferation
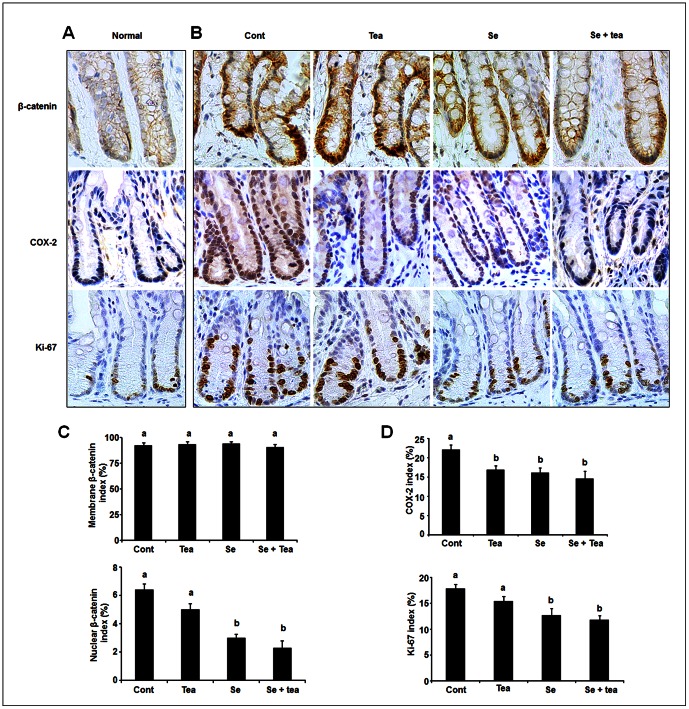
Effects of Se, green tea and the combination diet on ß-catenin, COX-2 and Ki-67 expression were examined in histologically normal-appearing crypts from the ACF study. We first compared the staining pattern of ß-catenin between AOM-untreated normal crypts and AOM-treated crypts. ß-catenin staining in normal crypts of untreated rats showed a low level of ß-catenin staining restricted to cell membrane, with limited nuclear staining (Figure 2A), whereas crypts from AOM-treated rats showed increased staining of ß-catenin in the nucleus (Figure 2B). Comparison of ß-catenin membrane and nuclear staining cells for the 4 dietary groups is shown in Figure 2B and Figure 2C. Se alone, green tea alone and the combination did not affect the percentage of cells showing positive ß-catenin membrane staining. But Se alone (P<0.05) and the combination diet (P<0.01) significantly reduced the percentage of cells showing positive ß-catenin nuclear staining, compared with the control, with no significant difference between Se alone and the combination diet, whereas green tea alone did not reduce ß-catenin nuclear staining. The percentage of cells showing Ki-67 nuclear staining was reduced in a similar pattern by Se alone (P<0.05) and the combination diet (P<0.01), green tea alone did not affect cell proliferation (Figure 2B and 2D), with a low level of Ki-67 staining in AOM-untreated normal crypts (Figure 2A). We also compared the staining pattern of COX-2 between AOM-untreated normal crypts and AOM-treated normal-appearing crypts. AOM untreated rats showed a low level of cytoplasmic staining for COX-2 in the colon (Figure 2A), whereas AOM-treated rats showed increased staining of COX-2 (Figure 2B). We found that the all three diets significantly inhibited COX-2 cytoplasmic staining (P<0.05), compared with control, there was no significant difference between the experiment diets (Figure 2B and 2D).
Figure 2. Effects of dietary Se, green tea and the combination of Se and green tea on β-catenin, COX-2 and Ki-67 expression (ACF study).
A representative section of immunohistochemical staining of β-catenin, Ki-67and COX-2 in AOM-untreated normal crypts (A), AOM-treated normal-appearing crypts from control, green tea, Se and the combination diet (B); labelling index for β-catenin (membrane and nuclear positive cells, calculated as the number of positive cells per crypt column divided by the total number of cells and multiplied by 100 (n = 12) (C); labelling index for COX-2 and Ki-67 (n = 12) (D). β-catenin and Ki-67 nuclear staining was significantly decreased by the combination diet and Se alone, but not green tea. COX-2 cytoplasmic staining was significantly decreased by green tea, Se and the combination diet. Statistical significance of dietary intervention between the groups was analysed by ANOVA, values with different superscripts in each column were statistically different (P<0.05), Bars: mean ± SEM.
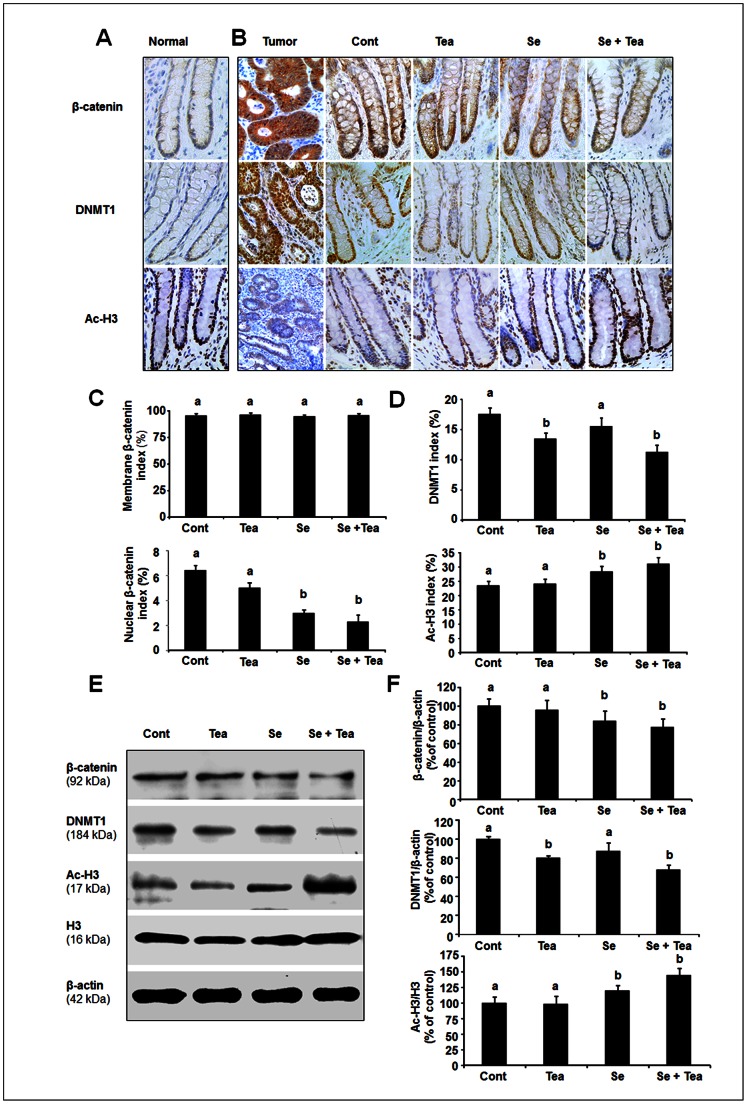
We next compared the staining pattern for β-catenin between AOM-treated normal-appearing crypts and AOM-induced tumors. Increased ß-catenin staining, in particular stronger nuclear staining was significantly higher in colon tumors than in normal-appearing crypts consistent with translocation of ß-catenin (Figure 3B). None of the diets significantly affected membrane staining for β-catenin in normal-appearing crypts from the tumor study, but Se diet alone (P<0.05) and the combination diet (P<0.01) showed consistent inhibitory effects on β-catenin nuclear accumulation (Figure 3C). The effect of dietary intervention on β-catenin expression was further supported by western blot analysis, showing total β-catenin was significant lower in normal-appearing crypts of rats fed the combination diet or Se alone (Figure 3E and 3F).
Figure 3. Effects of dietary Se, green tea and the combination of Se and green tea on β-catenin, DNMT1 and Ac-H3 expression (tumor study).
A representative section of immunohistochemical staining β-catenin, DNMT1 and Ac-H3 in AOM-untreated normal crypts (A), AOM-treated normal-appearing crypts from the control, green tea, Se and the combination diet (B) and tumors; labelling index for β-catenin (membrane and nuclear positive cells) (n = 12) (C) and labelling index for DNMT1 and Ac-H3 (n = 12) (D). Western blot analysis of β-catenin, DNMT1 and Ac-H3 for colon samples (n = 6) (E), expression of β-catenin and DNMT1 was normalized to that of β-actin and expression of Ac-H3 was normalized to that of H3, data was presented as precent of control (F). Increased strong β-catenin nuclear staining and DNMT1 overexpression were displayed in AOM-induced tumors, whereas weaker Ac-H3 expression was noted in colon tumors. β-catenin nuclear staining was significantly decreased by the combination diet and Se alone, but not green tea; DNMT1 was significantly decreased by the combination diet and green tea alone, but not Se; Ac-H3 nuclear staining was significantly increased by the combination diet and Se alone, but not green tea. Statistical significance of dietary intervention between the groups was analysed by ANOVA, values with different superscripts in each column were statistically different (P<0.05), Bars: mean ± SEM.
The combination diet inhibits DNMT1 expression and increased histone H3 acetylation
DNA methyltransferases DNMT and histone modifications play important roles in DNA methylation, with overexpression of DNMT1 and deacetylation of histone H3 or H4 reported in colon cancer [33]. With this in mind we examined the staining pattern of DNMT1 and Ac-H3 in AOM-induced colon tumors, compared with AOM-untreated normal crypts as well as AOM-treated normal-appearing crypts. Strong nuclear staining of DNMT1 and less intense nuclear staining of Ac-H3 were noted in AOM-induced tumors (Figure 3B); relative to normal crypt (Figure 3A) and normal-appearing crypts (Figure 3B), where DNMT1 and AC-H3 positive cells were predominantly located in proliferative compartment (Figure 3B).
To determine whether DNMT1 and Ac-H3 in normal-appearing crypts were regulated by Se alone, green tea alone and the combination diets, we measured the percentage of DNMT1 and Ac-H3 positive cells for the 4 dietary groups (Figure 3D). Significant inhibition of DNMT1 was observed in rats fed the combination diet and green tea alone, P<0.05 versus control, whereas Se alone had no significant effect on DNMT1 expression. When comparing Ac-H3 between the dietary groups, strongest induction of Ac-H3 was noted in rats fed the combination diet, the Se alone also significantly increased Ac-H3, P<0.05, compared with control diet, but green tea alone had no effect (Figure 3D). The effect of dietary intervention on DNMT1 and Ac-H3 expression was also supported by western blot analysis, showing DNMT was significant lower in normal-appearing crypts of rats fed the combination diet or green tea alone, whereas Ac-H3 was significant higher in normal-appearing crypts of rats fed the combination diet or Se alone (Figure 3E and 3F). Our data also showed that changes of Ac-H3 levels were not reflected in total H3 protein because the H3 histone levels were not changed by dietary treatment.
The combination diet restores SFRP5 expression and inhibited CyclinD1 expression
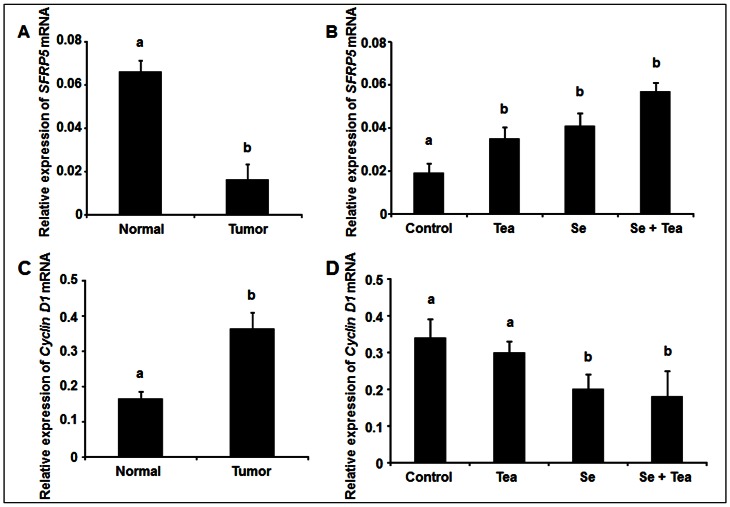
The WNT/β-catenin signalling pathway is regulated by several WNT antagonists, including SFRPs. SFRP1, 2, 5 genes are frequently silenced by promoter hypermethylation in human CRC [41], [42], a high percentage of DNA methylation in SFRP5 gene promoter has been detected by us in AOM-induced colon tumors (unpublished data). It is therefore important to compare the expression level of SFRP5 and the oncogenic WNT/β-catenin downstream gene Cyclin D1 between AOM-untreated normal colons and AOM-induced tumors. We found that levels of SFRP5 mRNA were significantly lower in AOM-induced tumors, compared with AOM-untreated normal colons (Figure 4A), P<0.01, whereas the levels of Cyclin D1 mRNA were markedly higher in AOM-induced tumors, relative to AOM-untreated normal colons (Figure 4C), P<0.05. These data suggest the activation of the WNT/β-catenin signaling pathway in response to AOM treatment. We next examined if dietary intervention regulates the gene expression of SFRP5 and Cyclin D1 in AOM-treated normal appearing-colons. Significant increased expression of SFRP5 mRNA was observed in rats fed Se alone, green tea alone and the combination diet, compared to those fed control diet (Figure 4B), P<0.05. Notably, the level of SFRP5 mRNA in response to the combination diet returned to the level similar to that of AOM-untreated normal colons. With regards to Cyclin D1 expression, Se alone and the combination diet significantly inhibited Cyclin D1 mRNA expression (Figure 4D), versus control, P<0.05, but green tea had no effect on Cyclin D1expression.
Figure 4. Effects of dietary Se, green tea and the combination of Se and green tea on SFRP5 and Cyclin D1 mRNA expression (tumor study).
Quantitative RT-PCR analysis of expression of SFRP5 (A) and Cyclin D1 (C) mRNA in AOM-untreated normal colons and AOM-induced tumors (n = 6); expression of SFRP5 (B) and Cyclin D1 (D) mRNA from the control, green tea, Se and the combination diet (n = 6) in AOM-treated normal-appearing crypts. Significant repression of SFRP5 and marked increase Cyclin D1 mRNA in AOM-induced colon tumors were noted compared with normal colons. SFRP5 was significantly increased by the combination diet, Se alone and green tea alone. Cyclin D1 was also significantly inhibited by the combination diet and Se alone, but not green tea. Statistical significance of dietary intervention between the groups was analysed by ANOVA, values with different superscripts in each column were statistically different (P<0.05), Bars: mean ± SEM.
Discussion
Apoptotic removal and MGMT-mediated DNA repair are two important innate cellular responses to environmental carcinogen-induced oncogenic DNA lesions. Our earlier studies in the AOM model provided evidence that Se-enriched milk protein at 1ppm prevents colon cancer by activating the acute apoptotic response [28]; and 0.5% green tea enhances MGMT expression (unpublished data) in rat colon. We have now extended these findings by showing that the combination of Se and green tea provides a significant protective advantage against the development of early preneoplastic lesions-ACF and colon tumors in the AOM model. The magnitude of inhibition of ACF (in particular large ACF) was consistent with the ability of the combination diet in inhibition of colon tumors (adenomas and adenocarcinomas), supporting the role of ACF as precursors to CRC. Se alone also significantly inhibited most of tumor endpoints, but to a degree less than that found with the combination diet, whereas green tea alone had no significant effect on colonic oncogenesis. Our results are of considerable applied significance because the combination of dietary and/or chemopreventive agents with different modes of actions is emerging as an attractive strategy for chemoprevention [2], [43].
The relative low potency of dietary agents compared with pharmacological compounds makes combination of two distinct dietary agents particularly attractive for achieving greater efficacy for cancer prevention [20]. The challenge is to identify an effective combination, with dietary agents working through similar or different mechanisms to produce an additive or synergistic chemopreventive effect. Se and green tea work through complementary mechanisms targeting different innate cellular responses to oncogenic DNA lesions and so might be valuable. While this is the first study to examine the beneficial effect of a combination of Se and green tea in an animal model of CRC, the concept of combination of Se and green tea is not new. Se-enriched tea powder had a higher antimutagenic effect than Se or tea alone in mice bone marrow, suggesting a possible synergistic interaction between Se and tea ingredients [44]. Supplementation of Se-enriched tea extracts also yielded better inhibitory effects than regular tea on transplanted hepatocellular carcinoma in mice [45]. Thus additional preclinical animal studies of combination of Se and green tea are important before translating this combination regimen to human dietary intervention trials.
Given the challenge for people to drink large amounts of green tea due to its bitterness, a “pill”-based approach to providing green tea is likely to be necessary. Green tea leaves could be ground into micronized powders and added into food to increase the absorption of nutrients in green tea. Oral administration in food appears to be a better route of administration than drinking as this delivered a two-fold increase in EGCG to the small intestine [46]. The choice of Se-enriched milk protein over inorganic Se compounds was simply due to this source being readily incorporable into a range of food products. While the dose of 1ppm Se-enriched milk protein used in the present study was several fold greater than that used for human intervention studies (200 µg Se/day) [5], our previous studies indicated that 1ppm Se was at the lowest range of Se forms to be effective in the AOM model, Se-enriched milk protein at 0.5ppm also showed a trend towards protection [28]. The cancer-protective effects of other Se forms in animals, however, occurred at a level of intake about ten-fold greater than those found to be effective in human [10]. Therefore, it is plausible that supplementation of Se-enriched milk protein at a relatively low doses together with green tea may also be of benefit for CRC prevention.
We further examined the molecular mechanisms that may underlie the combination effects of Se and green tea. Nuclear ß-catenin translocation is the hallmark of activation of the WNT signalling pathway [47] and overexpression of inflammatory markers of COX-2 and increased cell proliferation have been shown in the AOM CRC model from early focal lesions through to adenocarcinomas [48]. Previous studies have suggested that the effects of AOM and dietary agents on biomarkers (such as Cyclin D1and iNOS) are reflected by parallel changes in the ACF and histologically normal-appearing crypts, suggesting the involvement of similar mechanisms between ACF and normal-appearing crypts [49], [50]. In this regard, we examined the effect of the combination diet on ß-catenin, COX-2 and cell proliferation in normal-appearing crypts. In agreement with previous findings [51], increased ß-catenin nuclear translocation was accompanied by increased COX-2 and cell proliferation, which were significantly inhibited by the combination diet in both ACF and tumor studies. Moreover, the chemopreventive effect of Se, green tea and their combination were closely related to the degree of inhibition of ß-catenin and cell proliferation, supporting the view that the WNT signalling pathway is critical for the regulation of colonic crypt renewal and homeostasis [52] and β-catenin is an important biomarker for CRC chemoprevention. Given that the ß-catenin mediated signalling pathway has been identified as a key regulator of epithelial proliferative response [53], the chemopreventive effect of the combination diet might be mediated through a cascade of events in which the primary inhibitory effect may be on ß-catenin, followed by cell proliferation.
Targeting DNA methylation and histone modifications with dietary compounds has emerged as a promising chemopreventive strategy in human clinical trials [54]. Essential micronutrients such as folate, vitamin B-12, Se as well as the dietary tea and sulforaphane are among a growing list of agents that affect epigenetic events as novel mechanisms of chemoprevention [33], [55]. Since Se and green tea have been reported to have DNMT and histone deacetylase (HDAC) inhibition activity in in vitro [56], this prompted us to test the hypothesis that Se and green tea might affect DNMT1 and histone acetylation and thereby inhibit colonic tumorigenesis. The findings of overexpression of DNMT1 and reduced Ac-H3 in AOM-induced tumors suggest the clinical relevance of this model for CRC research. More importantly, we found that although Se alone did not significantly inhibit DNMT1 expression, the expression of DNMT1 was significantly inhibited by the combination diet, green tea alone also markedly inhibited DNMT1. When comparing the effects of dietary intervention on increased expression of Ac-H3, significant effects were achieved only with the combination diet or with Se alone. It has been reported that naturally occurring organoselenium compounds can act as novel HDAC inhibitors because they share structural features with butyrate (a short-chain fatty acid) reported to competitively inhibit HDAC activity [56]. The important finding from the present study is that Se-enriched milk protein might act as an HDAC inhibitor; it has yet to be determined whether a particular chemical form of Se in dairy protein may be responsible for its effect on histone acetylation. Nevertheless, induction of histone acetylation appears to be a potential mechanism for Se in cancer prevention. While green tea may also act as a DNMT inhibitor in AOM model, its inhibitory effect on oncogenesis is not apparent.
The findings that Se and green tea may act as an HDAC or DNMT inhibitor are very interesting. It extends our current understanding of how combination of Se and green tea may prevent CRC by enhancing innate cellular responses to oncogenic lesions, and provides a premise for targeting DNMT1 and histone acetylation for CRC prevention. It is reasonable to propose that the inhibition of DNMT and HDAC in response to combination of green tea and Se may lead to up-regulation of tumor suppression gene or down-regulate proto-oncogenes. This would advance our understanding by postulating that green tea and Se, on top of their already understood actions, bring the epigenetic pathway into play. WNT antagonist gene SFRP5 is a member of SFRPs family and is frequently down-regulated in human CRC [34], [35]. We examined the effect of the combination diet on the expression of SFRP5, and a WNT downstream target gene Cyclin D1 in AOM-treated normal-appearing colons because hypermethylation of promoter SFRP5 (unpublished data), epigenetic silencing of SFRP5 gene and activation of the WNT pathway (accumulation of β-catenin nuclear translocation and increased Cyclin D1 gene expression) were observed by us in AOM model. We found that the combination diet resulted in restoration of SFRP5 gene expression to the levels similar to that of normal colons, which were accompanied by significant changes in the expression of a number of WNT-related gene/proteins in a pattern consistent with inhibition of WNT activation, such as inhibited β-catenin nuclear accumulation, reduced Cylin D1 expression and cell proliferation. These results suggest that epigenetic mechanisms may be involved in activation of the WNT signalling pathway for colon cancer development in AOM model and potential dietary manipulation can be developed using WNT pathway genes/proteins as useful biomarkers for CRC prevention. It has been shown that genistein attenuates the WNT signaling by up-regulating SFRP2 in a human colon cancer cells through changes in demethylation of CpG islands of SFRP2 promoter region [57], and the anthocyanins derived from black raspberries demethylates tumor suppressor genes of SFRP2, 5 and WIF1 through inhibition of DNMT1 and DNMT3B [58]. In a human study, up-regulation of three WNT-antagonist genes by blackberries was associated with decreased DNMT expression [42].
In conclusion, this study showed that a combination of dietary Se and green tea more effectively prevents ACF and colon tumor formation than either agent alone. The preventive effect is associated with regulating genetic and epigenetic biomarkers implicated in colonic carcinogenesis, as evidenced by restoring SFRP5 gene expression, increasing histone H3 acetylation and reducing DNMT1 expression, inhibiting ß-catenin nuclear accumulation, reducing Cyclin D1 expression and cell proliferation in normal-appearing crypts. This combination is thus promising for use in the chemoprevention of CRC but before this can be recommended with confidence, more preclinical studies and clinical trials are needed to validate this novel combination regimen.
Acknowledgments
The authors thank Roshini Somashekar, Joanne Wilkins and Jean Winter for their assistance in immunohistochemistry analysis. The authors thank Tatura Milk Industries Limited for providing milk protein and Se-enriched milk protein.
Funding Statement
Financial support was provided by National Health and Medical Research Council grant and Cancer Council of South Australia (project number 1007501 and 525925). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Half E, Arber N (2009) Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother 10: 211–219. [DOI] [PubMed] [Google Scholar]
- 2. Amin AR, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J Clin Oncol 27: 2712–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiseman M (2008) The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 67: 253–256. [DOI] [PubMed] [Google Scholar]
- 4. Davis CD (2007) Nutritional interactions: credentialing of molecular targets for cancer prevention. Exp Biol Med (Maywood) 232: 176–183. [PubMed] [Google Scholar]
- 5. Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, et al. (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276: 1957–1963. [PubMed] [Google Scholar]
- 6. Yang CS (1997) Inhibition of carcinogenesis by tea. Nature 389: 134–135. [DOI] [PubMed] [Google Scholar]
- 7. Thakur VS, Ruhul Amin AR, Paul RK, Gupta K, Hastak K, et al. (2010) p53-Dependent p21-mediated growth arrest pre-empts and protects HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer Lett 296: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung FL, Schwartz J, Herzog CR, Yang YM (2003) Tea and cancer prevention: studies in animals and humans. J Nutr 133: 3268S–3274S. [DOI] [PubMed] [Google Scholar]
- 9. Xiao H, Hao X, Simi B, Ju J, Jiang H, et al. (2008) Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis 29: 113–119. [DOI] [PubMed] [Google Scholar]
- 10. McIntosh GH, Royle PJ, Lesno S, Scherer BL (2006) Selenised casein protects against AOM-induced colon tumors in Sprague Dawley rats. Nutr Cancer 54: 209–215. [DOI] [PubMed] [Google Scholar]
- 11.Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, et al.. (2011) Selenium for preventing cancer. Cochrane Database Syst Rev: CD005195. [DOI] [PMC free article] [PubMed]
- 12. Novotny L, Rauko P, Kombian SB, Edafiogho IO (2010) Selenium as a chemoprotective anti-cancer agent: reality or wishful thinking? Neoplasma 57: 383–391. [DOI] [PubMed] [Google Scholar]
- 13. Inoue M, Sasazuki S, Wakai K, Suzuki T, Matsuo K, et al. (2009) Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut 58: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 14. Tulp M, Bruhn JG, Bohlin L (2006) Food for thought. Drug Discov Today 11: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 15. Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, et al. (2000) Combinatorial chemoprevention of intestinal neoplasia. Nat Med 6: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 16. Sporn MB, Hong WK (2008) Clinical prevention of recurrence of colorectal adenomas by the combination of difluoromethylornithine and sulindac: an important milestone. Cancer Prev Res (Phila) 1: 9–11. [DOI] [PubMed] [Google Scholar]
- 17. Group CW (1999) Prevention of cancer in the next millennium: Report of the Chemoprevention Working Group to the American Association for Cancer Research. Cancer Res 59: 4743–4758. [PubMed] [Google Scholar]
- 18. Gerner EW, Meyskens FL Jr (2009) Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res 15: 758–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, et al. (2007) Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res 13: 1611–1619. [DOI] [PubMed] [Google Scholar]
- 20. Amin AR, Wang D, Zhang H, Peng S, Shin HJ, et al. (2010) Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem 285: 34557–34565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou JR, Li L, Pan W (2007) Dietary soy and tea combinations for prevention of breast and prostate cancers by targeting metabolic syndrome elements in mice. Am J Clin Nutr 86: s882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang H, Fang J, Jia X, Han C, Chen X, et al. (2011) Chemopreventive effects of early-stage and late-stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis 32: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Telang N, Katdare M (2007) Combinatorial prevention of carcinogenic risk in a model for familial colon cancer. Oncol Rep 17: 909–914. [PubMed] [Google Scholar]
- 24.Hamdy SM, Latif AK, Drees EA, Soliman SM (2011) Prevention of rat breast cancer by genistin and selenium. Toxicol Ind Health. [DOI] [PubMed]
- 25. Ohishi T, Kishimoto Y, Miura N, Shiota G, Kohri T, et al. (2002) Synergistic effects of (-)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett 177: 49–56. [DOI] [PubMed] [Google Scholar]
- 26. Xu G, Ren G, Xu X, Yuan H, Wang Z, et al. (2010) Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem Toxicol 48: 390–395. [DOI] [PubMed] [Google Scholar]
- 27. Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, et al. (2003) Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 3: 7563–7570. [PubMed] [Google Scholar]
- 28. Hu Y, McIntosh GH, Le Leu RK, Woodman R, Young GP (2008) Suppression of colorectal oncogenesis by selenium-enriched milk proteins: apoptosis and K-ras mutations. Cancer Res 68: 4936–4944. [DOI] [PubMed] [Google Scholar]
- 29. McCarty MF (2004) Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther 3: 349–380. [DOI] [PubMed] [Google Scholar]
- 30. Grady WM, Carethers JM (2008) Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 135: 1079–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy BS (2004) Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen 44: 26–35. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K (2000) Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis 21: 1319–1327. [PubMed] [Google Scholar]
- 33. Burdge GC, Lillycrop KA (2010) Bridging the gap between epigenetics research and nutritional public health interventions. Genome Med 2: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, et al. (2004) Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36: 417–422. [DOI] [PubMed] [Google Scholar]
- 35. Ying Y, Tao Q (2009) Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics 4: 307–312. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, McIntosh GH, Le Leu RK, Upton JM, Woodman RJ, et al.. (2011) The influence of selenium-enriched milk proteins and selenium yeast on plasma selenium levels and rectal selenoprotein gene expression in human subjects. Br J Nutr: 1–11. [DOI] [PubMed]
- 37. Volate SR, Muga SJ, Issa AY, Nitcheva D, Smith T, et al. (2009) Epigenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol Carcinog 48: 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu Y, Le Leu RK, Young GP (2005) Absence of acute apoptotic response to genotoxic carcinogens in p53-deficient mice is associated with increased susceptibility to azoxymethane-induced colon tumours. Int J Cancer 115: 561–567. [DOI] [PubMed] [Google Scholar]
- 39. Simon P (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19: 1439–1440. [DOI] [PubMed] [Google Scholar]
- 40. Rao CV, Steele VE, Swamy MV, Patlolla JM, Guruswamy S, et al. (2009) Inhibition of azoxymethane-induced colorectal cancer by CP-31398, a TP53 modulator, alone or in combination with low doses of celecoxib in male F344 rats. Cancer Res 69: 8175–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Najdi R, Holcombe RF, Waterman ML (2011) Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang LS, Arnold M, Huang YW, Sardo C, Seguin C, et al. (2011) Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin Cancer Res 17: 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reddy BS (2007) Strategies for colon cancer prevention: combination of chemopreventive agents. Subcell Biochem 42: 213–225. [DOI] [PubMed] [Google Scholar]
- 44. Xu J, Zhou J, Sheng J, Fang Y, Li F, et al. (2008) Inhibition of cyclophoshamide-induced mutagenicity by microsized powder of selenium-enriched green tea in mice. J Agric Food Chem 56: 3869–3875. [DOI] [PubMed] [Google Scholar]
- 45. Xu J, Yang F, An X, Hu Q (2007) Anticarcinogenic activity of selenium-enriched green tea extracts in vivo. J Agric Food Chem 55: 5349–5353. [DOI] [PubMed] [Google Scholar]
- 46. Hao X, Sun Y, Yang CS, Bose M, Lambert JD, et al. (2007) Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer 59: 62–69. [DOI] [PubMed] [Google Scholar]
- 47. Clevers H (2004) Wnt breakers in colon cancer. Cancer Cell 5: 5–6. [DOI] [PubMed] [Google Scholar]
- 48. Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851. [PubMed] [Google Scholar]
- 49. Suh N, Paul S, Hao X, Simi B, Xiao H, et al. (2007) Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin Cancer Res 13: 350–355. [DOI] [PubMed] [Google Scholar]
- 50. Wali RK, Stoiber D, Nguyen L, Hart J, Sitrin MD, et al. (2002) Ursodeoxycholic acid inhibits the initiation and postinitiation phases of azoxymethane-induced colonic tumor development. Cancer Epidemiol Biomarkers Prev 11: 1316–1321. [PubMed] [Google Scholar]
- 51. Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K (2000) Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis 21: 1117–1120. [PubMed] [Google Scholar]
- 52. de Sousa EM, Vermeulen L, Richel D, Medema JP (2011) Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res 17: 647–653. [DOI] [PubMed] [Google Scholar]
- 53. Eisinger AL, Nadauld LD, Shelton DN, Peterson PW, Phelps RA, et al. (2006) The adenomatous polyposis coli tumor suppressor gene regulates expression of cyclooxygenase-2 by a mechanism that involves retinoic acid. J Biol Chem 281: 20474–20482. [DOI] [PubMed] [Google Scholar]
- 54. Dashwood RH, Ho E (2007) Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol 17: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arasaradnam RP, Commane DM, Bradburn D, Mathers JC (2008) A review of dietary factors and its influence on DNA methylation in colorectal carcinogenesis. Epigenetics 3: 193–198. [DOI] [PubMed] [Google Scholar]
- 56. Lee JI, Nian H, Cooper AJ, Sinha R, Dai J, et al. (2009) Alpha-keto acid metabolites of naturally occurring organoselenium compounds as inhibitors of histone deacetylase in human prostate cancer cells. Cancer Prev Res (Phila) 2: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Chen H (2011) Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med (Maywood) 236: 714–722. [DOI] [PubMed] [Google Scholar]
- 58. Wang LS, Kuo CT, Cho SJ, Seguin C, Siddiqui J, et al. (2013) Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr Cancer 65: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]