Abstract
Purpose of review
Recent studies have identified a new family of ammonia-specific transporters, Rh glycoproteins, which enable NH3-specific transport. The purpose of this review is to summarize recent evidence regarding the role of Rh glycoproteins in renal ammonia transport.
Recent findings
The Rh glycoproteins, RhAG/Rhag, RhBG/Rhbg and RhCG/Rhcg, transport ammonia in the form of molecular NH3, although there is some evidence suggesting the possibility of NH4+ transport. RhAG/Rhag is expressed only in erythrocytes, and not in the kidney. Rhbg and Rhcg are expressed in distal nephron sites, from the distal convoluted tubule through the inner medullary collecting duct, with basolateral Rhbg expression and both apical and basolateral Rhcg expression. Whether Rhbg contributes to renal ammonia transport remains controversial. Rhcg expression parallels ammonia excretion in multiple experimental models and genetic deletion studies, both global and collecting duct-specific, demonstrate a critical role for Rhcg in both basal and acidosis-stimulated renal ammonia excretion. X-ray crystallography has defined critical structural elements in Rh glycoprotein-mediated ammonia transport. Finally, Rh glycoproteins may also function as CO2 transporters.
Summary
No longer can NH3 transport be considered to occur only through diffusive NH3 movement. Transporter-mediated NH3 movement is fundamental to ammonia metabolism.
Keywords: acid–base, ammonia, CO2, collecting duct, Rh glycoprotein
Introduction
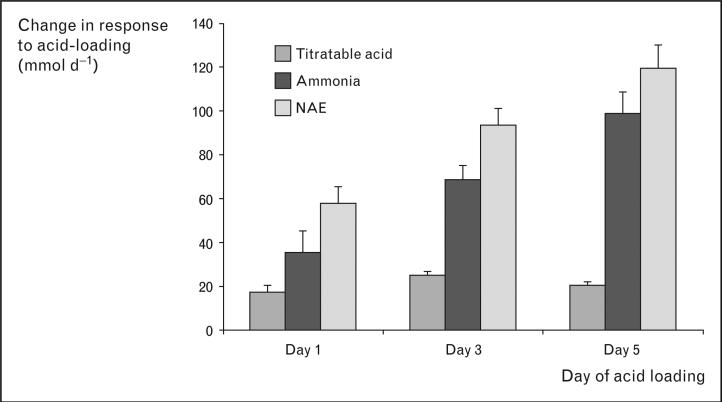
The kidneys have two major functions in acid–base homeostasis: reabsorbing filtered bicarbonate and generating new bicarbonate. In the normal adult, daily filtered bicarbonate averages approximately 4200 mmol/day and must be essentially completely reabsorbed. New bicarbonate generation involves titratable acid and ammonia excretion. Ammonia excretion is the predominant component of net acid excretion under basal conditions and is the primary component of the response to acid–base disturbances (Fig. 1). In this review we use the term ‘ammonia’ to refer to the combination of NH3 and NH4+. When referring to a specific molecular form, we will specifically identify either ‘NH3’ or ‘NH4+’.
Figure 1. Relative roles of titratable acid and ammonia excretion in the response to metabolic acidosis in humans.
Changes from basal for titratable acid, ammonia and net acid excretion are shown. Data calculated from [1].
Important new insights into the molecular mechanisms of ammonia transport have brought about fundamental changes in our understanding of renal ammonia excretion. In particular, Rh glycoproteins transport NH3 and likely mediate a critical role in renal ammonia metabolism. We will review the basics of renal ammonia metabolism and then discuss important new evidence regarding the role of Rh glycoproteins in renal ammonia metabolism.
Ammonia
Ammonia exists in two molecular forms, NH3 and NH4+. Renal ammonia transport has been thought classically to involve passive NH3 diffusion and NH4+ ‘trapping’. However, both NH3 and NH4+ have limited permeability across plasma membranes and are transported by specific mechanisms.
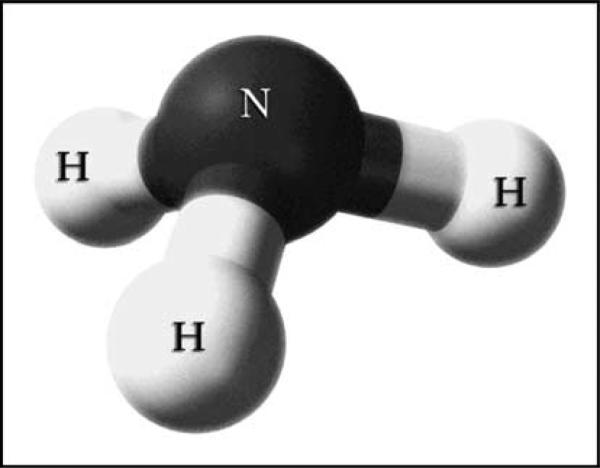
NH3, although uncharged as a whole, has an asymmetric arrangement of positively charged hydrogen surrounding its central nitrogen (Fig. 2). This makes NH3 a polar molecule, with a molecular dipole moment of 1.47 D, and limits its plasma membrane permeability [2]. In this respect NH3 is similar to H2O and urea, two other small, uncharged, yet polar molecules that utilize specific integral membrane proteins for efficient transmembrane transport. NH4+ has negligible permeability across lipid bilayers in the absence of specific transport proteins. However, many proteins transport NH4+, including NHE-3, NKCC-2, K+ channels, and Na+-K+-ATPase, and their role in renal ammonia metabolism was recently reviewed [3].
Figure 2. Charge distribution of molecular NH3.
Ball and stick model of NH3 shows asymmetric distribution of hydrogen nuclei around the central nitrogen.
Renal ammonia metabolism
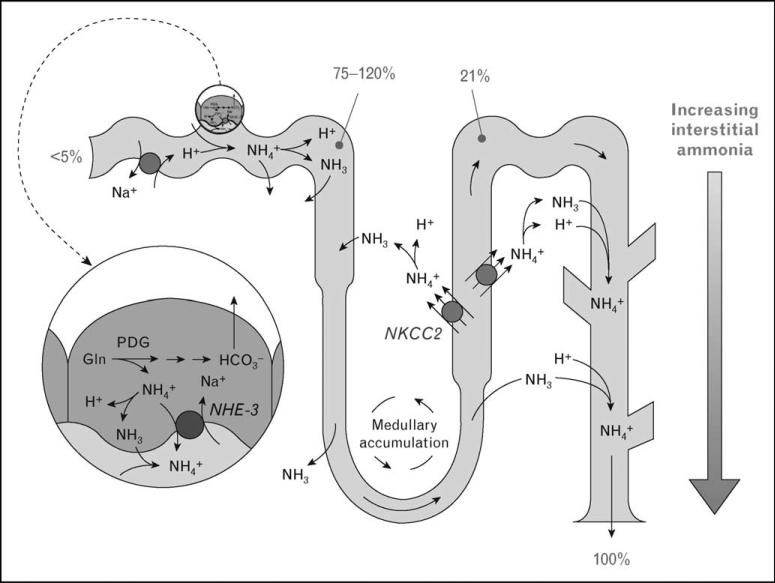
Renal ammonia metabolism involves coordinated ammoniagenesis and renal epithelial ammonia transport. Figure 3 summarizes key elements of renal ammonia metabolism. Unlike other renal solutes, essentially no urinary ammonia derives from glomerular filtration. Instead, ammonia is produced in the kidney, primarily by proximal tubule glutamine metabolism, which yields equimolar NH4+ and HCO3–. Ammonia then undergoes highly regulated transport by renal epithelial cells. NH4+ produced in the proximal tubule is preferentially secreted into the luminal fluid, partially reabsorbed in the late proximal tubule, and undergoes reabsorptionand recycling in the loop of Henle. Ammonia transport in Henle's loop has two major results. First, it generates an axial gradient in renal interstitial ammonia concentration. Second, it reduces ammonia delivery to the distal tubule to only approximately 20% of final urinary ammonia content.
Figure 3. Summary of ammonia metabolism in the kidney.
The proximal tubule produces ammonia, as NH4+, from glutamine. NH4+ is secreted preferentially into the luminal fluid, primarily by NHE-3, and is reabsorbed in the TAL, primarily by NKCC2. Ammonia delivery to the distal nephron accounts for approximately 20% of urinary ammonia; approximately 80% is secreted in the collecting duct through parallel NH3 and H+ transport. Numbers indicate proportion of total urinary ammonia present at the indicated sites under basal states.
Collecting duct ammonia secretion accounts for the remaining approximately 80% of urinary ammonia. Collecting duct ammonia secretion involves parallel NH3 and H+ transport, with little to no NH4+ permeability [4]. Until recently, collecting duct NH3 secretion was thought to involve only passive NH3 diffusion. However, studies only a few years ago showed that the primary mechanism of apical and basolateral ammonia transport by cultured collecting duct cells was transporter-mediated NH3 movement [5,6], questioning the previous paradigm of collecting duct NH3 secretion.
Rh glycoproteins
In 2000, the ‘Rh-associated glycoprotein’ (RhAG/Rhag; by convention, Rh A glycoprotein is abbreviated as RhAG in human tissues and Rhag in non-humans; a similar terminology is used for RhBG/Rhbg and RhCG/Rhcg), and a previously unknown Rh glycoprotein, now known as Rh C glycoprotein (RhCG/Rhcg; the term Rh glycoprotein – kidney (RhGK), was used in this initial report. RhGK is now known to be Rh C glycoprotein), which was shown to transport ammonia when expressed in yeast deficient in endogenous ammonia transporters [7]. Since 2000, knowledge regarding the role of Rh glycoproteins in ammonia transport has exploded. A third Rh glycoprotein, Rh B glycoprotein (RhBG/Rhbg), was identified. Rh glycoproteins were found in essentially all tissues that transport ammonia. Heterologous expression studies, with few exceptions, showed that they transport ammonia and have no other naturally occurring substrates. Multiple experimental models examined their involvement in renal ammonia metabolism, and gene knock-out studies showed that they are critical for normal renal ammonia metabolism. X-ray crystallographic studies identified key features of their tertiary structure, including the likely mechanism of specific transport of gaseous ammonia, NH3.
RhAG/Rhag
Rhag is a component of the erythrocyte Rh complex that is central to transfusion medicine. Multiple studies examining heterologous expression, gene knock-out, and human erythrocytes with genetic lack of RhAG show that RhAG/Rhag transports ammonia as NH3. Because RhAG/Rhag is expressed only in erythrocytes and erythroid-precursor cells and not in the kidney [8], it appears unlikely to contribute directly to renal ammonia metabolism.
RhBG/Rhbg
RhBG/Rhbg was initially identified in 2001 as a homologous EST to Rhag [9]. Although initial studies found Rhbg only in kidney, liver and skin, subsequent studies showed extremely wide tissue distribution, including stomach, intestinal tract and lung [10,11]. However, for reasons discussed below, substantial controversy surrounds its role in renal ammonia metabolism.
Heterologous expression studies uniformly show that RhBG/Rhbg is an ammonia-specific transporter with an affinity for ammonia of approximately 2–4 mM; the only other molecule it is known to transport is a methyl derivative of ammonia, methylammonia. However, different studies reached different conclusions regarding whether Rhbg transports NH3 or NH4+. Most studies show electroneutral NH3 transport [12–14], although one identified only electrogenic NH4+ transport [15]. The explanation underlying the different results is not known at present.
In the mouse and rat kidney, Rhbg is expressed in the basolateral plasma membrane in distal sites, from the distal convoluted tubule (DCT) to the inner medullary collecting duct (IMCD), with greater expression in intercalated cells than in principal cells [16,17]. The only exceptions are the IMCD, in which only intercalated cells express Rhbg, and the cortical collecting duct (CCD) B-type intercalated cell, which does not express significant Rhbg [17]. Thus, it is ideally located to mediate an important role in collecting duct ammonia secretion. Whether RhBG is present in the human kidney is unclear. High levels of RhBG mRNA are present in the human kidney [9], but a recent study using a variety of antibodies did not detect RhBG protein expression [18].
Different studies have drawn different conclusions regarding Rhbg's role in renal ammonia metabolism. Evidence suggesting that Rhbg contributes to renal ammonia transport includes the following: metabolic acidosis increases Rhbg mRNA in mouse kidney [19]; in acid-loaded mice, Rhbg protein expression is increased by collecting duct-specific Rhcg deletion, suggesting Rhbg may improve residual collecting duct ammonia secretion [20••]; and pendrin null mice have decreased Rhbg expression, which may help prevent metabolic alkalosis [21]. Although metabolic acidosis did not change Rhbg expression in the rat kidney [22], Rhbg has a carboxy-terminus phosphorylation site that regulates both transport activity and membrane insertion [23] and could enable metabolic acidosis to regulate ammonia transport without changes in steady-state protein expression. Evidence against a role for Rhbg in renal ammonia excretion is that Rhbg-null mice have normal basal acid–base parameters, normal responses to NH4Cl-induced metabolic acidosis, and normal basolateral NH3 and NH4+ transport in microperfused CCD segments [24]. Additional studies are needed to clarify Rhbg's role in ammonia metabolism.
RhCG/Rhcg
RhCG/Rhcg is widely expressed in ammonia-transporting organs, including kidneys, CNS, testes, lung, liver, stomach and intestinal tract [10,11]. In the kidney, Rhcg is expressed from the DCT through the IMCD, and intercalated cell Rhcg expression exceeds principal cell expression [17,25]. Similar to Rhbg, Rhcg is not expressed significantly in type B intercalated cells or in nonintercalated cells in the IMCD.
Also like Rhbg, multiple studies show that RhCG/Rhcg transports NH3 [12,14,26,27]. One study identified both NH3 and NH4+ transport [26]. The explanation for these differing observations is unclear.
Rhcg may contribute to both apical and basolateral ammonia transport. In the human, rat and mouse kidney, Rhcg exhibits both apical and basolateral immunoreactivity [18,22,28–30]. Rhcg is present in the apical plasma membrane and subapical cytoplasmic vesicles in collecting duct cells, and subcellular redistribution enhances apical plasma membrane Rhcg during metabolic acidosis [30]. Rhcg is also expressed in the basolateral plasma membrane, except in the non-A, non-B cell [28]; in the rat outer medullary collecting duct (OMCD) in the inner stripe, basolateral expression is approximately 20% of total cellular expression [30]. In the mouse, the abundance of basolateral Rhcg varies among strains [28]; these variations correlate with differences in urinary ammonia excretion in response to metabolic acidosis (unpublished observations). Although initial studies did not detect basolateral Rhcg in either rat or mouse kidney, subsequent studies using improved immunohistochemistry techniques and the same antibodies as in the initial reports did demonstrate basolateral expression [28].
Rhcg's expression parallels ammonia excretion in several conditions of altered ammonia metabolism. Chronic metabolic acidosis increases Rhcg protein expression in the OMCD and IMCD through post-transcriptional regulation [22]. In reduced renal mass, in which single nephron ammonia secretion increases, Rhcg expression increases [31]. Cyclosporine A decreases Rhcg expression and impairsammoniaexcretion;thesechangeslikelycontribute to cyclosporine A-induced metabolic acidosis [32]. Inmetabolicacidosisandreducedrenalmass,apicalandbasolateral Rhcg increases in intercalated cells and principal cells, suggesting that both cell types contribute to ammonia secretion and that Rhcg transports NH3 across both the apical and basolateral plasma membranes. Changes in Rhcg plasma membrane expression appear to contribute to the renal response in a wide variety of conditions.
Most recently, genetic deletion studies confirmed Rhcg's role in renal ammonia excretion. Rhcg deletion, whether global or only from the collecting duct, decreased basal ammonia excretion and impaired urinary ammonia excretion in response to metabolic acidosis [20••,33]. Moreover, Rhcg deletion decreases transepithelial ammonia permeability in perfused collecting duct segments and decreases apical membrane NH3 permeability [33].
Rhcg may have important roles in other critical biological functions. Rhcg is expressed in testes and epididymis and Rhcg deletion decreases male fertility [33]. In the colon, Rhcg may contribute to portal ammonia transport [34].
Mechanism of NH3 transport by Rh glycoproteins
X-ray crystallography has provided important information on the mechanisms whereby Rh glycoproteins enable NH3-specific transport. Initial studies examined Amt proteins, bacterial orthologs of Rh glycoproteins [35]. Although the crystal structure of mammalian Rh glycoproteins has not yet been determined, the Rh glycoprotein of Nitrosomonas europaea, NeRh50, has been characterized by crystallographic studies. Amt proteins and NeRh50 have similar structures; differences are noted below. Amt proteins and NeRh50 have 11 transmembrane-spanning segments; mammalian Rh glycoproteins likely have 12 membrane-spanning segments due to an extended amino terminus. The protein exists as a homotrimer; each of the monomers has a hydrophobic central pore through which ammonia transport occurs. In the middle of this central pore is a twin-His site that enables NH3, but NH4+, H2O, Na+ or K+, transport. Specific phenylalanines appear to gate this central pore and specific residues link a carboxy-terminal helix to phenylalanine blockage [36••,37]. Rh glycoproteins appear to lack an extracellular vestibule present in Amt orthologs; this absence likely accounts for the decreased ammonia affinity of Rh glyco-proteins compared with Amt proteins [36••,37].
Nonglycosylated Rh proteins
Two nonglycosylated Rh proteins, RhCE and RhD, exist. They appear to be ancestral duplications of the Rh30 gene. They appear to be present only in erythrocytes and studies to date show they do not transport ammonia [12].
Do Rh glycoproteins transport CO2?
Several lines of evidence indicate that Rh glycoproteins can also transport CO2. In the green algae Chlamydomonas reinhardtii, inhibiting expression of the Rh glycoprotein Rh1 blunts normal responses to extracellular CO2, but does not alter ammonia transport, suggesting a primary role in CO2 transport [38]. Analysis of evolutionary conservation and diversification supports a role in CO2 transport [39]. More recently, RhAG was shown to transport CO2 [40••,41] and examination of the X-ray crystallographic structure of N. europea Rh glycoprotein identified a CO2 binding site at a cytosolic site adjacent to the central pore [37]. Whether Rhbg and Rhcg transport CO2 has not yet been reported, but their tissue distribution [10] and the observation that genetic deletion of Rhbg or Rhcg does not inhibit urine acidification [20••,24,33], which depends on CO2 transport across plasma membranes of intercalated cells, suggests CO2 transport is not a critical function of Rhbg or Rhcg.
Conclusion
The discovery of Rh glycoproteins has profoundly changed our understanding of renal ammonia metabolism.
Collecting duct NH3 transport does not occur primarily through lipid-phase diffusion, but rather is protein-mediated. Rh glycoproteins are ammonia-specific transporters that are expressed in the kidney. Rhcg is an NH3-specific transporter expressed in distal nephron sites critical for ammonia excretion. Its expression in both the apical and basolateral plasma membranes is regulated in parallel with ammonia excretion and gene knock-out studies confirm its fundamental role in renal ammonia secretion. Rhbg's role in renal ammonia metabolism remains controversial. Figure 4 shows our current model of collecting duct ammonia secretion. Whether Rhbg and Rhcg also have important roles as CO2 transporters is not known at present.
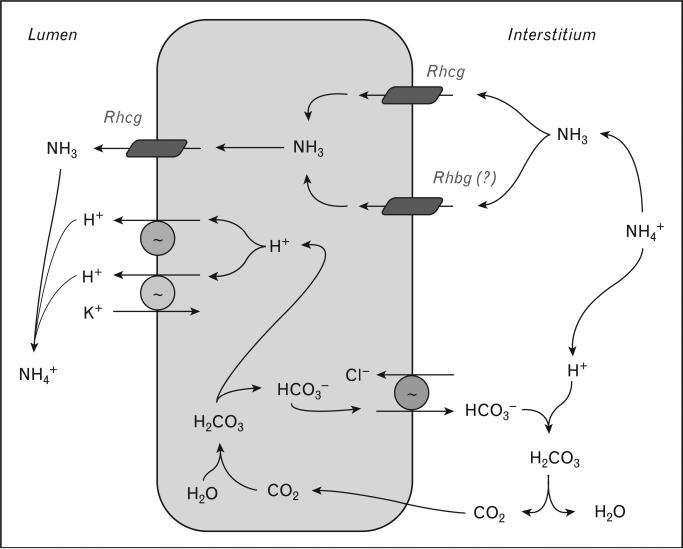
Figure 4. Model of collecting duct ammonia secretion.
Interstitial NH4+ is in equilibrium with NH3 and H+. NH3 is transported across the basolateral membrane by Rhcg, and possibly by Rhbg. In the IMCD, Na+-K+-ATPase transports NH4+ (not shown). Cytosolic NH3 is secreted across the apical plasma membrane by Rhcg. Minor diffusive NH3 components are not shown. Intracellular H+ and HCO3– are formed from hydration of CO2; H+ is secreted by H+-ATPase and H+-K+-ATPase, and then combines with luminal NH3 to form NH4+. Basolateral Cl–/HCO3– exchange transports HCO3– across the basolateral membrane, when it combines with H+ released from NH4+, forming CO2 and H2O. CO2 can recycle into the cell, supplying CO2 used for cytosolic H+ generation.
Acknowledgement
The preparation of this review was funded in part through funds from the NIH, grant R01-DK45788.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 509).
- 1.Sartorius OW, Roemmelt JC, Pitts RF, et al. The renal regulation of acid-base balance in man. IV. The nature of the renal compensations in ammonium chloride acidosis. J Clin Invest. 1949;28:423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature. 1994;368:332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- 3.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;69:317–340. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol. 1991;1:1193–1203. doi: 10.1681/ASN.V1111193. [DOI] [PubMed] [Google Scholar]
- 5.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol. 2005;289:F347–F358. doi: 10.1152/ajprenal.00253.2004. [DOI] [PubMed] [Google Scholar]
- 6.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol. 2004;287:F628–F638. doi: 10.1152/ajprenal.00363.2003. [DOI] [PubMed] [Google Scholar]
- 7.Marini AM, Matassi G, Raynal V, et al. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 8.Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hypertens. 2004;13:533–540. doi: 10.1097/00041552-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Peng J, Mo R, et al. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 10.Han KH, Mekala K, Babida V, et al. Expression of the gas transporting proteins, Rh B glycoprotein and Rh C glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol. 2009;297:L153–L163. doi: 10.1152/ajplung.90524.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner ID. Expression of the nonerythroid Rh glycoproteins in mammalian tissues. Transfus Clin Biol. 2006;13:159–163. doi: 10.1016/j.tracli.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mak DO, Dang B, Weiner ID, et al. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol. 2006;290:F297–F305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, et al. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J. 2005;391:33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, et al. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol. 2004;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- 16.Quentin F, Eladari D, Cheval L, et al. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol. 2003;14:545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- 17.Verlander JW, Miller RT, Frank AE, et al. Localization of the ammonium transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 18.Brown ACN, Hallouane D, Mawby WJ, et al. RhCG is the major putative ammonia transporter expressed in human kidney and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol. 2009;296:F1279–F1290. doi: 10.1152/ajprenal.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheval L, Morla L, Elalouf JM, Doucet A. Kidney collecting duct acid-base ‘regulon’. Physiol Genomics. 2006;27:271–281. doi: 10.1152/physiolgenomics.00069.2006. [DOI] [PubMed] [Google Scholar]
- 20••.Lee HW, Verlander JW, Bishop JM, et al. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol. 2009;296:F1364–F1375. doi: 10.1152/ajprenal.90667.2008. [Collecting duct-specific Rhcg deletion is sufficient to completely inhibit Rhcg's role in both basal and acidosis-stimulated ammonia metabolism, demonstrating the specific role of renal Rhcg for renal ammonia transport.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, Verlander JW, Matthews SW, et al. Intercalated cell H+/OH-transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol. 2005;289:F1262–F1272. doi: 10.1152/ajprenal.00206.2005. [DOI] [PubMed] [Google Scholar]
- 22.Seshadri RM, Klein JD, Kozlowski S, et al. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290:F397–F408. doi: 10.1152/ajprenal.00162.2005. [DOI] [PubMed] [Google Scholar]
- 23.Sohet F, Colin Y, Genetet S, et al. Phosphorylation and ankyrin-G binding of the C-terminal domain regulate targeting and function of the ammonium transporter RhBG. J Biol Chem. 2008;283:26557–26567. doi: 10.1074/jbc.M803120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambrey R, Goossens D, Bourgeois S, et al. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol. 2005;289:F1281–F1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
- 25.Eladari D, Cheval L, Quentin F, et al. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol. 2002;13:1999–2008. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- 26.Bakouh N, Benjelloun F, Hulin P, et al. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- 27.Mayer M, Schaaf G, Mouro I, et al. Different transport mechanisms in plant and human AMT/Rh-type ammonium transporters. J Gen Physiol. 2006;127:133–144. doi: 10.1085/jgp.200509369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HY, Verlander JW, Bishop JM, et al. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol. 2009;296:F545–F555. doi: 10.1152/ajprenal.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han KH, Croker BP, Clapp WL, et al. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006;17:2670–2679. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshadri RM, Klein JD, Smith T, et al. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290:F1443–F1452. doi: 10.1152/ajprenal.00459.2005. [DOI] [PubMed] [Google Scholar]
- 31.Kim HY, Baylis C, Verlander JW, et al. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol. 2007;293:F1238–F1247. doi: 10.1152/ajprenal.00151.2007. [DOI] [PubMed] [Google Scholar]
- 32.Lim SW, Ahn KO, Kim WY, et al. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Experimental Nephrol. 2008;110:e49–e58. doi: 10.1159/000153245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biver S, Belge H, Bourgeois S, et al. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature. 2008;456:339–343. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- 34.Worrell RT, Merk L, Matthews JB. Ammonium transport in the colonic crypt cell line, T84: role for Rhesus glycoproteins and NKCC1. Am J Physiol Gastrointest. 2008;294:G429–G440. doi: 10.1152/ajpgi.00251.2006. [DOI] [PubMed] [Google Scholar]
- 35.Khademi S, Stroud RM. The Amt/MEP/Rh family: structure of AmtB and the mechanism of ammonia gas conduction. Physiology. 2006;21:419–429. doi: 10.1152/physiol.00051.2005. [DOI] [PubMed] [Google Scholar]
- 36••.Lupo D, Li XD, Durand A, et al. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci U S A. 2007;104:19303–19308. doi: 10.1073/pnas.0706563104. [Along with reference 37 are first studies of structure of a Rh glycoprotein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Jayachandran S, Nguyen HH, Chan MK. Structure of the Nitrosomonas europaea Rh protein. Proc Natl Acad Sci U S A. 2007;104:19279–19284. doi: 10.1073/pnas.0709710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci U S A. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci U S A. 2005;102:15512–15517. doi: 10.1073/pnas.0507886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci U S A. 2009;106:5406–5411. doi: 10.1073/pnas.0813231106. [RhAG can transport CO2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2007;22:64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]