Abstract
Skin depigmentation represents a well established treatment for extensive vitiligo and may likewise be suited to prevent tumor recurrences and as a prophylactic treatment of familial melanoma, as common bleaching agents are cytotoxic to melanocytes. Effective melanoma prevention requires a bleaching agent-induced loss of exposed melanocytes supported by an immune response to distant pigment cells. Studies on human explant cultures treated with depigmenting agents, 4-tertiary butyl phenol (4-TBP) or monobenzyl ether of hydroquinone (MBEH) revealed a significant increase in the migration of Langerhans cells towards the dermis only upon MBEH treatment thus suggesting selective elicitation of an immune response. To assess the depigmenting potential of bleaching agents in vivo, 4-TBP and MBEH were topically applied to C57BL/6 wild type as well as k14-SCF transgenic, epidermally pigmented mice. MBEH induced significant skin depigmentation in both strains, not observed upon 4-TBP treatment. Cytokine expression patterns in MBEH treated skin support activation of a Th1 mediated immune response corresponding to an influx of T cells and macrophages. Importantly, despite insensitivity of tumor cells to MBEH induced cytotoxicity, significantly retarded tumor growth was observed in B16 challenged k14-SCF mice pretreated with MBEH, likely due to an abundance of cytotoxic T cells accompanied by an increased expression of Th1 and Th17 cytokines. These data support the use of MBEH as a prophylactic treatment for melanoma.
Keywords: MBEH - Mono benzyl ether of hydroquinone, 4-TBP - 4-Tertiary butyl phenol, TRP-1- tyrosinase related protein-1, TRP-2-tyrosinase related protein-2, MART-1 –melanoma antigen recognized by T cells
INTRODUCTION
Monobenzyl ether of hydroquinone or MBEH is currently in use as an FDA approved agent to induce depigmentation in vitiligo patients [1]. Depigmenting qualities of the compound were first described following attempts to identify a cause for skin depigmentation in an occupational setting, which led to the description of ‘occupational vitiligo’ as a separate category of patients undergoing gradual depigmentation [2; 3]. Several phenolic compounds have been described that affect depigmentation either temporarily (such as hydroquinone) or in a permanent fashion, including MBEH [1]. As treatments for vitiligo are largely ineffective, and patients with advanced depigmentation of their skin sometimes prefer an even skin tone, MBEH is included as the active ingredient of monobenzone cream, used to depigment the remainder of the skin over the course of several weeks to months [4].
The working mechanism of MBEH and other depigmenting agents is less well understood. Permanent depigmentation of the skin observed in most patients does suggest that the drug is capable of selectively eliminating melanocytes from the epidermis. Recent data showing selective cytotoxicity of MBEH towards melanocytes and not keratinocytes do support this viewpoint [5]. Its selective action on melanocytes has been explained by structural homology to tyrosine, the natural substrate for melanogenesis. When combined with the rate limiting enzyme for melanogenesis, tyrosinase, MBEH is converted into a highly reactive quinone suggested to react with surrounding enzymatic compounds within the confines of the melanosomal organelle [6]. As expression of melanosomal enzymes is restricted to cells of the melanocyte lineage, the above sequence of events can explain a selective cytotoxic effect of MBEH on melanocytes. It should be considered that different phenolic compounds can follow markedly different depigmentation strategies. In this respect, whereas MBEH is thought to bind tyrosinase, 4-tertiary butyl phenol appears to interact with TRP-1 instead [7; 8].
Quinones binding to melanosomal compounds occurs in a process described as haptenization whereby the hosting compound undergoes slight structural alterations [7]. Melanosomal enzyme modification is significant as TRP-2, MART-1, gp100 and tyrosinase itself are immunogenic molecules and minor changes in amino acid composition or even haptenization have been shown to alter their recognition by T cells reactive with the native peptide [9]. Under increased affinity for the modified peptide, immune tolerance can be broken and improved reactivity will likely ensue. This process is significant for an understanding of the depigmentation process in vitiligo.
Studying the effects of MBEH exposure for melanocyte viability, it was found that sensitivity to MBEH exposure is not directly correlated with the expression of a single melanosomal compound molecule. Rather, there was an inverse relationship noted for sensitivity to MBEH and expression of melanin within the cell. More intensely pigmented cells are thus increasingly resistant to the compound [5]. This can have implications for the treatment of ethnic skin with MBEH.
Besides an understanding of the direct effects of bleaching phenols on pigmented cells, any consequences for cell viability will indirectly affect immune responses to follow. In this regard, the markedly different effects of MBEH and 4-TBP on melanocytes manifest themselves in the induction of necrosis and apoptosis, respectively, in exposed cells [5]. Both death pathways are thought to have markedly different consequences on dendritic cell activation and it is thus likely that 4-TBP and MBEH exposure have markedly different kinetics of depigmentation in an in vivo setting.
The mechanism of action of bleaching phenols becomes particularly important when considering the specificity for cells of the melanocytic lineage. Besides normal epidermal and ocular melanocytes, cells potentially sensitive to the effects of MBEH and 4-TBP can thus include melanoma cells. Systemic toxicity however limits the application of bleaching phenols in a therapeutic setting. The toxicity of both MBEH and 4-TBP towards fibroblasts, for example, is significant [5; 8]. To nevertheless make use of the unique qualities of bleaching phenols towards the treatment of melanoma, two supportive factors are considered here. First, eliminating melanocytes from the skin will prevent precursor cells from converting to melanoma cells by malignant transformation, thus this process may be effective as a chemical alternative to elective surgery for patients familial melanoma, or to prevent recurrences in patients following surgical removal of a primary tumor. Second, cell death among a portion of melanocytes in the skin may elicit an immune response that carries over to an effective anti-tumor response by the recognition of antigens shared between melanocytes and melanoma cells [10; 11; 12]. Familial melanoma is a condition affecting approximately 10% of melanoma cases, or an estimated 6000 newly diagnosed patients per year in the United States alone [13]. If induction of vitiligo can spare patients from agonizing returning visits to the dermatologist to check for newly arising dysplastic nevi, treatment with bleaching phenols may be a more effective and permanent way to address familial disease.
To study the potential of prophylactic treatment using bleaching phenols for familial melanoma, it is necessary to first identify the in vivo effects of the agents under study in an in vivo setting, in particular to address anticipated involvement of an immune response. Here, the effects of bleaching agents 4-TBP and MBEH were studied initially in normal human skin, in order to quantify Langerhans cell emigration in response to either agent. Organotypic cultures of human skin are ideal for these studies as emigration can be studied without new influx of immune cells [14]. Since Langerhans cells migrate upon antigen specific stimulation, the same approach has been followed to distinguish allergenic compounds from skin irritants [15; 16]. This same factor limits the use of skin cultures to assess T cell influx, and these studies were executed in mouse models instead. To this end, bleaching agents were applied to the skin of wildtype mice as well as a transgenic model designed to express melanocytes in the epidermal compartment of the skin. In the k14-SCF mouse stem cell factor is expressed under the k14 promoter within keratinocytes to support melanocyte maintenance within the skin environment, important to follow topical application of the bleaching agents [17]. Depigmentation was quantified in both models and T cell influx, immigration of macrophages and cytokine expressions were measured in the skin. To understand whether melanoma cells are directly sensitive to bleaching agents when exposed in a topical setting, the cytotoxicity of 4-TBP and MBEH towards melanoma cells and melanocytes was compared in vitro before returning to mice to perform a B16 tumor challenge. In this experiment, mice were pretreated with MBEH and subsequently challenged with tumor cells, thus relying solely on immune effects to contain the tumor. Finally, the cytokine environment in the remaining tumor tissue and infiltration by CD8+ T cells were quantified. Taken together, these data shed important light on the prophylactic potential of bleaching agents for the treatment of familial melanoma.
MATERIALS AND METHODS
Mouse models
Two stains of mice, C57BL/6J from Jackson Labs (Bar Harbor, ME) and k14-SCF transgenic mice [17], were used in the depigmentation experiments. In the k14-SCF mice, mouse stem cell factor is expressed under the k14 promoter which maintains melanocytes within the epidermis, thus rendering k14-SCF mice a close mimic of human skin. Group sizes were 5 per group for C57BL/6J mice and 4 per group for k14-SCF mice unless stated. A l l experiments were approved by Loyola University Medical Center’s Institutional Animal Care and Use Committee.
Preparation of bleaching agents and treatment
4-TBP (Sigma-Aldrich, St Louis, MO) was prepared as a stock solution of 3M in 70% ethanol. MBEH (Sigma) was dissolved in 20% dimethyl sulfoxide (Sigma) and mixed with 70% ethanol for a stock concentration of 3M. The use of a vehicle control refers to the use of 20% dimethyl sulfoxide in 70% ethanol. The chemicals were stored as a stock at −20°C until further use. Before necessary treatments chemicals were mixed with Eucerin calming cream (Beierdorf, Wilton, CT) to a final concentration of 1 or 1.5M. Mice were prepared for depigmention treatment by biweekly ventral hair removal with Nair (Church and Dwight Co., Princeton, NJ). C57BL/6 mice were topically treated with 100μl of 1.5 M 4-TBP or MBEH applied to a 3 cm2 area of Naired skin on alternate days for 6 weeks. The k14-SCF mice were similarly treated with 100μl of 1M concentration of 4-TBP or MBEH for 14 weeks. Mice were anesthetized during hair removal and bleaching phenol treatments using an isofluorane gas chamber (Euthanex Corp , Plamer, PA).
Organotypic culture of treated human skin
Organotypic culture was performed as described previously [14]. In brief, 4mm biopsies were punched from otherwise discarded foreskin tissue obtained after routine circumcision. Explants were cultured in DMEM containing 10% inactivated normal human serum at the air–liquid interphase with epidermis facing up in 12 well transwell plates with 0.4 μM pore size (Corning, Teterboro, NJ). After topical treatment with 5 μl of 250 mM of 4-TBP or MBEH daily for 3 days, explants were embedded in optimal cutting temperature compound (OCT) (Sakura Finetek USA, Torrance, CA), snap frozen and maintained at −80 °C for future sectioning. All studies performed with human tissue were carried out in adherence with the Declaration of Helsinki Principles and were approved by the Institutional Review Board of the institution where circumcisions were performed.
Cell culture
Mouse melanocyte cultures were initiated from untreated k14-SCF mice skin samples. After euthanasia mouse skin samples were homogenized O/N in an enzymatic cocktail (thermolysin 0.05mg/ml, collagenase 1mg/ml, trypsin 0.1mg/ml, DNase 0.01mg/ml) prepared in DMEM (Media-Tech, Manassas, VA). Cells were cultured in Ham’s F-12 medium (Media-Tech, Herndon, VA) with 10% heat-inactivated fetal bovine serum (Gemini Bio-products, West Sacramento,CA), 2mM glutamine (Media Tech), 100IU/ml penicillin, 100μg/ml streptomycin 100μg/ml amphotericin (Media Tech), 0.1mM IBMX (Sigma), 10ng/ml 12-O-tetradecanoyl phorbol-13-acetate (Sigma), and 0.03% bovine pituitary extract (InVitrogen).
B16F10 mouse melanoma cells were maintained in DMEM (Media-Tech) with 10% heat-inactivated fetal bovine serum (Gemini Bio-products), 100IU/ml penicillin, 100μg/ml streptomycin and 100μg/ml amphotericin (Media Tech).
Evaluating depigmentation
Depigmentation was evaluated by flatbed scanning of the ventral side of the mice under anesthesia, and subsequent image analysis using Adobe Photoshop software (Adobe Systems Inc., San Jose, CA) as described previously[18]. Depigmentation experiments were repeated thrice and representative experiment was shown. Briefly, depigmentation was evaluated by quantifying mean luminosity of inverted images for the treated area and comparing the data to untreated mice. Statistical analysis of data was performed by comparing relevant groups by Student’s T-test using Excel software.
Immunohistology
Biopsies of treated mouse skin, treated human skin explants, or mouse tumor tissue were embedded in OCT compound (Sakura Finetek USA, Torrance, CA), snap-frozen on dry ice. Eight μm cryostat sections were fixed in cold acetone and stored at −20 °C until use. Human explant sections were stained with antibody CD207 to Langerin (mouse monoclonal: Immunotech, Marselle, France). Vehicle application was used as a negative control. Recruitment of Langerhans cells was evaluated in 3 human skin biopsies per group.
Mouse skin sections were stained with biotinylated hamster antibody 145-2C11 to CD3ε antibody (Pharmingen, San Diego, CA, or goat T-16 ab to CD68 (Santa Cruz Biotechnology Inc, Santa Cruz, CA). Peroxidase labeled anti-mouse or anti-goat antisera or peroxidase labeled streptavidin (Southern Biotechnologies, Birmingham, Alabama) were used in the second step and color was developed using aminoethyl carbazole (AEC) as a substrate (Sigma) as described earlier [19]. Quantifications were performed in 2 serial sections from tissues of 4 mice/group.
Tissues from 3 remaining tumor/group were engaged in the evaluation of infiltrating CD8+T cells. Tumor sections were stained with biotinylated 53-6.7 rat antibody to CD8a (Biolegend, San Diego, CA). Anti-rat alexa flour 568 (Invitrogen, Carlsbad, CA) was used as a secondary antibody. Images were captured by an Olympus BX41 bright field microscope (Olympus, Center Valley, PA).
Stained cells were counted within the epidermis for CD3+T and Langerin +cells or to a depth of twice or four times of the epidermal thickness in the dermal regions for quantifying Langerin +, CD68+ cells respectively ; tissue length or area was calculated using Adobe Photoshop CS4 software (Adobe Systems Inc).
Cytotoxicity Assay
Cell viability was measured by MTT assays (Bioassay system, Hayward, CA) according to manufacturer’s instructions. In brief 20,000 cells/well were plated in triplicate wells of a 96 well plate to attach overnight. Cells were either treated with vehicle alone or with 250, 500 or 900μM of 4-TBP or MBEH for 24 hours. MTT reagent (tetrazole) was added to the cells and incubated in a 37°C humidified chamber for 4 hours. Tetrazole is converted to formazan in mitochondria of living cells. The formazan crystals formed were solubilized in buffer and the wavelength was read in a reader at 562 nm (BMG labtech inc, Durham, NC). Cell viability was calculated as a percentage of absorbance of the samples relative to vehicle treated control.
Evaluating DC maturation
Bone marrow derived monocytes were prepared by culturing murine bone marrow cells using protocols modified from Boudreau, et al [20] . Briefly, bone marrow cells were flushed aseptically from the femurs and tibia of mice. Monocytes were enriched using the EasySep mouse monocyte enrichment kit (Vancouver, BC) according to manufacturers instructions. To generate DC, the monocytes were grown in RPMI (Mediatech Inc., Manassas, VA) supplemented with 10% FBS and 100 U/ml penicillin, 100 μg/ml streptomycin, 50 ng/ml murine GM-CSF (Peprotech, Rocky Hill, NJ) and 12.5 ng/ml murine IL-4 (Peprotech). Cells were plated at 2×106cells per 5 ml of media in Teflon containers (Savillex, Minnetonka, MN) to prevent adherence. Half of the media was replaced every other day for seven days. On day seven, 125 μM of 4-TBP, 63μM or 125 μM of MBEH, or 1μg/ml lipopolysaccharide (LPS) (Sigma) was added to the cells. After 24 h, cells were gently spun and stained with Hamster anti-mouse CD11c, CD80, CD83, CD86, and MHCII labeled with V450, APC, PE, PE-Cy and FITC fluorochromes respectively (BD Biosciences, Sparks MD). Initial gating was performed on live non-debris singlets, with subsequent gating towards CD11c cells using FACS LSR-II equipment (BD Biosciences).
Real time PCR
Cytokine profiling was performed in treated mouse skin in remaining subcutaneous tumor tissue. Sample sizes were 5 per group for skin samples and 3 for remaining tumor tissue obtained from vehicle and 1.5M MBEH treated animals. RNA was isolated from tissue sections using TRIzol reagent (Invitrogen; Carlsbad, CA) and further purified using an RNeasy mini kit (Qiagen, Valencia, CA). Two μg of RNA was reverse-transcribed with superscript III (Invitrogen) and 10% of resultant cDNA was included in the real time PCR reaction. Real time PCR was performed using Quantitect SYBR green master mix and samples were run in triplicate and amplified at 95°C for 10 minutes, 95°C for 15 seconds, 55°C for 30S, 72°C for 30S for 50 cycles. Standard mouse primer sets and probes for IFNγ, IL-2, IL12b, IL17a, TNFα, IL-10, IL-4, and GAPDH were purchased from Qiagen. Relative fold change in gene expression was calculated using the ΔΔ Ct method after normalizing with vehicle treatment. Experiments were repeated thrice with skin samples and twice with tumor samples. Representative data from a single experiment was shown.
Tumor challenge
The k14-SCF mice were treated with 1.5M of MBEH or vehicle for 3 weeks after removing the pelage by application of Nair (Church and Dwight Co). A 100μl volume of 5×105 B16F10 melanoma cells in PBS (Media-tech) was injected subcutaneously in the right flank of MBEH treated and control mice. Tumor volume was recorded daily from day 10 onwards using calipers. Data were pooled from two independent experiments for a total of 8 vehicle treated and 10 MBEH treated animals. Statistical significance of differences among treatment groups was evaluated using Student’s t-test.
RESULTS
Eliciting immune responses by 4-TBP and MBEH in human skin explants
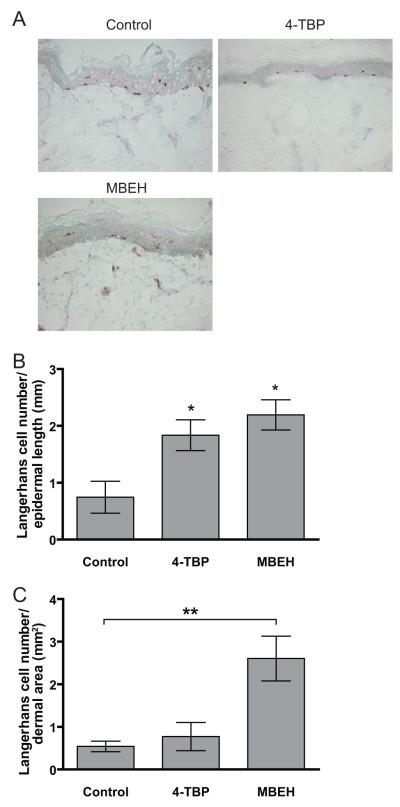
Langerhans cell abundance and migration were monitored as a measure of the elicitation phase of an immune response in human explant cultures treated with 250mM of 4-TBP or MBEH for 3 days (Fig 1). Representative staining for each treatment was shown in Fig 1a. Although the total number of Langerin positive cells in the epidermis remained constant (data not shown), agents 4-TBP and MBEH respectively induced a 2.4 and 2.9 fold increase in migration of Langerhans cells towards the basal layer of the epidermis in comparison to vehicle treatment alone (p<0.05)(Fig 1b). In addition, MBEH and not 4-TBP treatment caused a 2.0 fold increased migration into the dermis over control treatment (p<0.01) (Fig.1b). These data support that the immune activation particularly upon MBEH treatment.
Fig 1. Langerhans cell emigration from 4-TBP and MBEH treated human skin explants.
Explant skin cultures were treated with 250 mM of 4-TBP or MBEH for 3 days and stained for presence of Langerhans cells. Vehicle treatments alone served as controls. (a) Representative image of Langerin staining for each treatment is shown. (b) Quantification of Langerhans cells in the basal layer represented as number of cells/mm epidermal length. (b) Quantification of Langerhans cells in the reticular dermis expressed as number of cells/mm2 . Mean and SE were calculated and statistical significance was evaluated by Students’ t-test (n=3) for epidermal measurements and (n=4) for dermal measurements. ‘*’ indicates p<0.05
Effect of phenolic agents on dendritic cell maturation
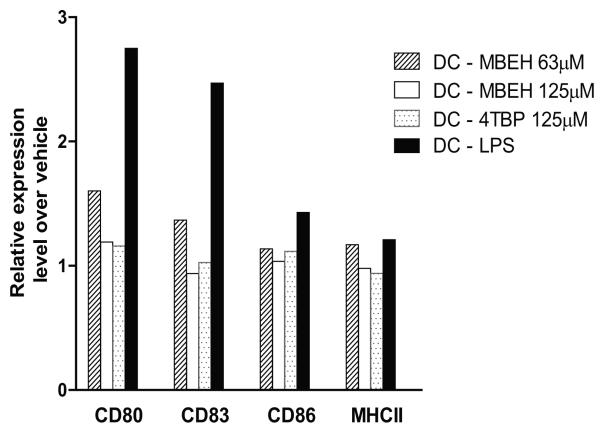
Immature myeloid dendritic cells derived from bone marrow precursors were treated with 63 or 125 μM of MBEH, 125 μM of 4-TBP for 24 hours. Cells stained for activation markers were quantified in live CD11c positive cells (Fig.2). While no activation was observed upon 4-TBP treatment, 1.6, 1.3, 1.1 and 1.1 fold activation for CD80, CD83, CD86 and MHCII respectively was observed upon MBEH treatment. Such limited activation and maturation may contribute to the migratory behavior of DC subsets in response to MBEH, but not 4TBP treatment.
Fig 2. Effect of phenolic agents on dendritic cell maturation.
Immature dendritic cells derived from bone marrow precursors were treated with 125μM of 4-TBP, 63 μm or 125μm of MBEH for 24 hours. Live Cd11c+cells were then stained for dendritic cell activation markers. Vehicle treatment served as a negative control while LPS treatment served as a positive control. Experiments were repeated thrice and data from a representative experiment are shown in Fig 2.
Differential response upon topical application of bleaching agents in mice
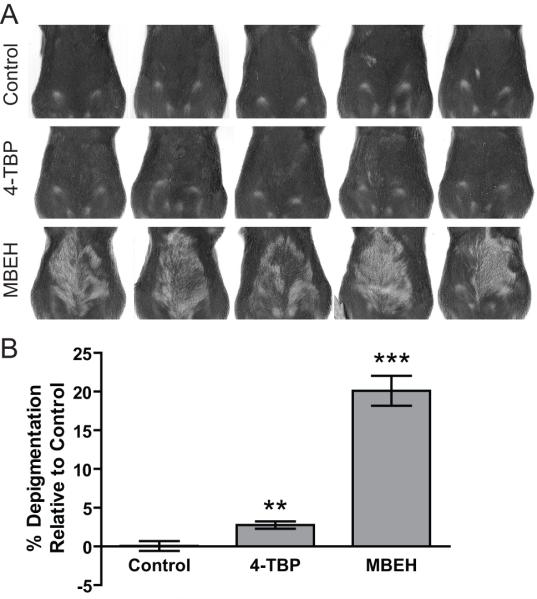
C57BL/6 mice were shaved biweekly to remove the ventral pelage and topically treated with 100ul of 1.5 M MBEH or 4-TBP on alternating days for 6 weeks. Two weeks after cessation of treatment, mice were scanned on a flat bed scanner and changes in pigmentation of the pelage in the treatment area were measured by image analysis. MBEH treatment induced a 20.5% reduction in pigmentation (p<0.0001), while 4-TBP treatment induced 2.8% depigmentation (p<0.01) relative to vehicle treatment (Fig 3b). Scanned mice images were shown in Fig.3a. These data demonstrate that MBEH penetrates the mouse skin at least to the follicular level.
Fig 3. Differential response upon topical application of bleaching agents in mice.
C57BL/6 mice were shaved biweekly to remove ventral hair and topically treated with 100ul of 1.5 M MBEH or 4-TBP on alternating days for 6 weeks. Two weeks after cessation of treatment, mice were scanned on a flat bed scanner and changes in depigmentation quantified. (a) Scanned images of C57BL/6 mice for each treatment immediately following the cessation of the experiment. (b) Change in percent depigmentation with respect to the vehicle treatment. Mean and SE were calculated and statistical significance was evaluated by Student T test (n=5). ‘**’ indicates p<0.01: ‘***’ indicates p<0.0001.
Modeling epidermal pigmentation more akin to human skin
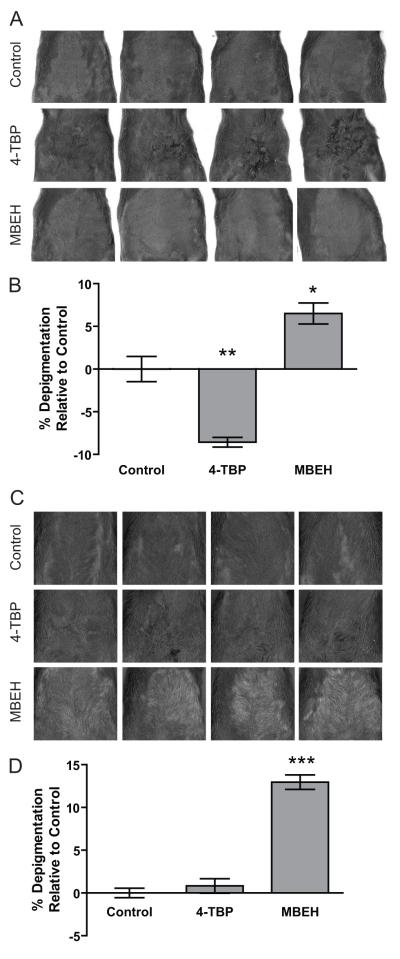
Shaven ventral skin of k14-SCF mice was topically treated with 1M concentration of 4-TBP or MBEH on alternating days for 14 weeks. Changes in the pigmentation levels upon treatment with vehicle, 4-TBP or MBEH were shown in the scanned images of k14-SCF mice skin as well as among the regrown pelage in Fig 4a and 4c. The percent change in pigmentation was quantified relative to vehicle treatment. 4-TBP treatment increased epidermal pigmentation by 8.5% (p<0.01) possibly representing postinflammatory hyperpigmentation of the skin, though no significant change in pigmentation level was observed for regrown hair (Fig 4b). MBEH, however, caused 6.5% depigmentation (p<0.05) in epidermal skin, and 12.9% (p<0.0001) in hair when compared to vehicle treatment (Fig 4d). This dataset demonstrates that MBEH has a depigmenting effect on both skin and hair follicles in mice.
Fig.4. Modeling epidermal pigmentation more akin to human skin.
Shaven ventral skin of k14-SCF mice was topically treated with 1M concentration of 4-TBP or MBEH on alternating days for 14 weeks. Changes in pigmentation were measured in hairless skin as well as among regrown hair after the final treatment. (a) Scanned images of hairless skin of k14-SCF mice upon different treatments. (b) The bar graphs represent change in percent depigmentation of the skin with respect to vehicle treatment. (c) Scanned images of the same k14-SCF mice after regrowth of their hair. (d) Bar graphs represent percent depigmentation levels of regrown hair with respect to vehicle treatment. Mean and SE were calculated and statistical significance was evaluated by Student’s t-test (n=4). ‘*’ indicates p<0.05, ‘**’ indicates p<0.01: ‘***’ indicates p<0.0001.
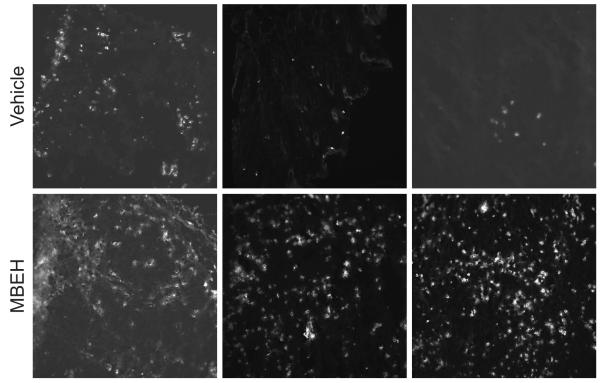
Immune cell influx in response to MBEH versus 4-TBP exposure observed in vivo
T cell and macrophage influx to the skin was quantified by staining for CD3+ and CD68+ cells in epidermal and dermal skin regions respectively treated with 1M concentration of either bleaching agents in k14-SCF mice for 14 weeks. MBEH treatment induced a 2.0 fold increase in T cell infiltration (p<0.05), whereas 4-TBP treatment resulted in an 0.5 fold reduction in the T-cell number when compared with control treated skin (NS) as shown in Fig 5a. A similar trend was observed upon macrophage infiltration to the dermal area of treated k14-SCF skin. MBEH treatment induced a 2.4 fold increase in CD68+ cells (p<0.01), while 4-TBP treatment did not result in a significant increase in macrophage infiltration compared with vehicle treated skin (Fig 5b). This further supports the concept that MBEH activates an immune response while no difference was observed upon 4-TBP treatment.
Fig 5. Immune cell influx in response to MBEH versus 4-TBP exposure observed in vivo.
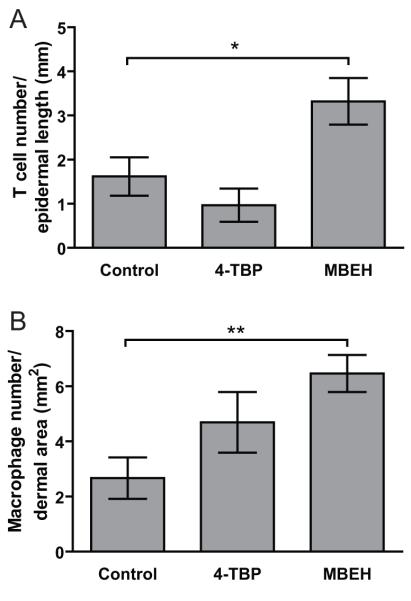
T cell and macrophage influx to the skin was quantified by staining for CD3+ and CD68+ cells in epidermal and dermal skin regions treated with 1M concentration of either bleaching agents in k14-SCF mice for 14 weeks. (a) Influx of CD3+ cells in the epidermal region of the mouse skin was quantified in 2 independent experiments and expressed as number of stained cells/mm epidermal length. (n=6 for 4-TBP and MBEH treatment; n=4 for vehicle treatment) (b) Influx of macrophages in the dermal regions of k14-SCF mice skin samples were quantified and expressed as the number of positive cells/dermal area (mm2). Mean and SE were calculated and statistical significance was evaluated by Student one-tailed T test. ‘*’ indicates p<0.05, ‘**’ indicates p<0.01.
Cytokine profiling in MBEH treated mouse skin
Cytokine environment was studied in frozen C57BL/6 mice skin after treatment with vehicle or 1.5M MEBH for 6 weeks (Fig 6). Increases in the relative transcript levels of TNF-α, IFN-γ and IL-2 were observed at a 2.8, 2.4, and 2.2 fold, respectively over vehicle treatment. Expression levels of IL-10, IL-4, IL-12 and IL-17 were not altered thus supporting a Th1 mediated immune response.
Fig 6. Cytokine profiling in C57BL/6 mice skin treated with MBEH.
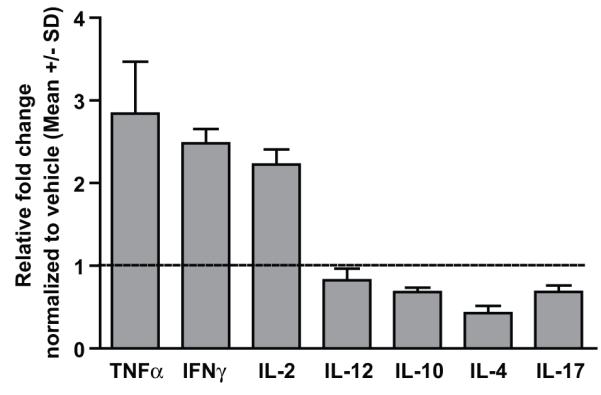
Transcript levels of pro and anti-inflamatory cytokines evaluated by qRT-PCR in mice skin samples treated with vehicle or 1.5 M MBEH for 6 weeks. Relative fold change in the expression levels are shown upon normalization with vehicle treatment. Mean and standard deviation were calculated from 3 replicates. Experiments were repeated thrice and data from a representative experiment are shown.
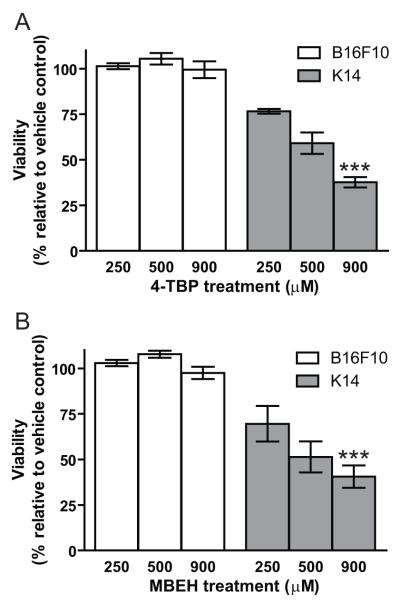
Cytotoxicity of bleaching agents towards B-16 melanoma cells and mouse melanocytes
The viability of cultured B16 melanoma cells and melanocytes (derived from k14-SCF mice) in response to 250, 500 and 900 μM concentration of 4-TBP or MBEH for 24 hours was determined by MTT assays. In Fig.7 data are shown from two independent experiments. Vehicle treatment served as a control for all cell types. The viability of mouse melanocytes was reduced by 23%, 40% and 62% upon 250, 500 and 900 μM 4-TBP treatment, respectively, while no significant change in viability was observed in B16F10 melanoma cells (Fig 7a). Similarly, a 30%, 48% and 59% reduction in mouse melanocytes was observed upon 250, 500 and 900 μM of MBEH treatment respectively. However, no significant difference was observed in the viability of B16F10 melanoma cells even at the highest concentration of MBEH (Fig 7b). This confirms that mouse melanocytes were sensitive to bleaching agents 4-TBP and MBEH as previously shown for human melanocytes, however, melanoma cells are highly resistant to the same chemicals.
Fig 7. Cytotoxicity of bleaching agents towards B16 melanoma cells and mouse melanocytes.
Cultured B16F10 melanoma cells and melanocytes derived from k14-SCF mice treated with 250, 500 or 900 μM concentration of 4-TBP or MBEH for 24 hours. Percent viability was quantified in MTT assays. (a) Viability of B16F10 melanoma cells and k14-SCF melanocytes upon treatment with 4-TBP. (b) Viability of B16F10 melanoma cells and k14-SCF melanocytes upon treatment with MBEH. Data provided from 2 independent experiments with triplicate values for each experiment. Mean and SE were calculated and statistical significance was evaluated by Student’s t-test. ‘***’ indicates p<0.0001.
Tumor challenge of k14-SCF mice with B-16 melanoma cells
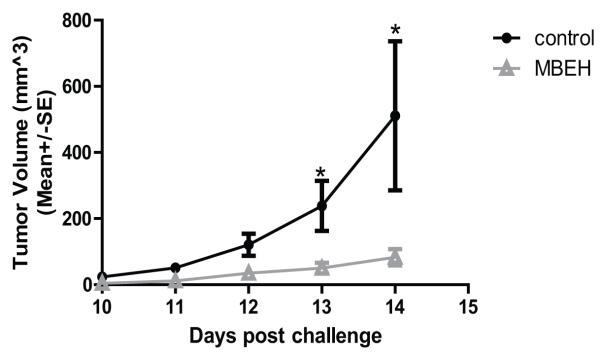
A 1.5M MBEH cream or vehicle alone was applied to the skin of k14-SCF mice for 3 weeks prior to a subcutaneous challenge with 500,000 B16F10 melanoma cells. Treatment was stopped a week before challenging the mice with tumor. The tumor volume was measured daily from day 10 onwards. Data from two independent experiments were combined as Fig.8. A significant difference in tumor growth between the vehicle group and the MBEH treated group was observed at days 11, 12, 13 and 14 post challenge. At day 14 an average tumor volume of 401mm3 was measured for vehicle treated mice had whereas MBEH treated mice displayed an average tumor volume of 91mm3 (p<0.05). Mice were euthanized at day 14.
Fig 8. Tumor challenge of k14-SCF mice with B16 melanoma cells.
Treatment of k14-SCF mice with 1.5M of MBEH or vehicle alone for 3 weeks preceded a tumor challenge with B16F10 melanoma cells subcutaneously in the flank region. The volume of the tumor was measured daily from day-10 onwards. Closed circles represent vehicle treated (control) mice, and open triangles represent the MBEH treated group.Data were pooled from two independent experiments for a total of 8 vehicle treated and 10 MBEH treated animals. Statistical significance of differences among treatment groups was evaluated using Student’s t-test ‘*’ indicates p<0.05
Cytokine profiling in the tumor environment
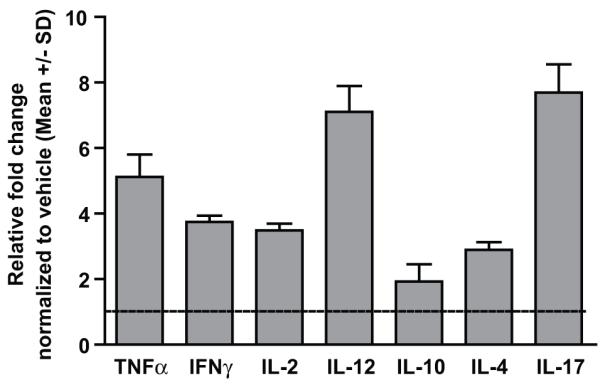
Expression levels of pro and anti-inflammatory cytokines were quantified using qRTPCR in remaining tumor tissue after challenging MBEH pretreated k14-SCF mice with B16 melanoma cells (Fig 9). In parallel with the cytokine environment observed in C57BL/6 mice skin after treatment with MBEH, increased expression levels of TNF α, IFN-γ, IL-2, IL-12 were observed with the relative increase of 5.1, 3.7, 3.4, 7.1 fold over vehicle treatment. Interestingly, in addition to a Th1 environment, substantial levels of IL-17 were also observed with a 7.6 fold increase over vehicle treatment. Expression levels of IL-4 and IL-10 were also observed to be 2.8 and 1.9 fold increased respectively over vehicle treatment, yet overall these data confirm a Th1 mediated immune response correlating with increased anti tumor reactivity.
Fig9. Cytokine profiling in the tumor environment.
Transcript levels of pro and anti-inflamatory cytokines evaluated by qRT-PCR in mice tumor samples. Relative fold change in the expression levels are shown upon normalization with vehicle treatment. Mean and standard deviation were calculated from 3 replicates. Experiments were repeated thrice and data from a representative experiment are shown.
Infiltration of cytotoxic T cells in the tumor tissue
The consequence of a Th1 cytokine environment was further established in the remaining tumor tissue, by quantifying the abundance of cytotoxic CD8+T cells. Representative staining are shown for 3 mice/group (Fig 10). CD8+T cell infiltration was 4.7 fold increased in remaining tumor tissue from 3 animals compared to tumors resected from 3 vehicle treated animals, which was significant at p<0.01 in Student’s t-test. These data strongly suggest that anti-tumor protection provided by MBEH pre-treatment is mediated by cytotoxic T cells.
Fig10. Th1 mediated -tumor protection.
Infiltration by CD8+ T cells were quantified in remaining day 14 tumor tissue of k14-SCF mice pre treated with vehicle or 1.5M MBEH for 3 weeks. Representative microscopic fields are shown in Fig 10.
DISCUSSION
Vitiligo patients with advanced disease can opt for depigmentation therapy, wherein topical application of bleaching agents is used to remove any remaining pigmentation [1]. Here we analyze the mechanism of depigmentation within a tissue context, exploring the exciting concept that such bleaching agents may be used in chemoprevention of malignant melanoma.
The data presented here demonstrate that depigmentation in response to MBEH can be reproduced in an in vivo mouse model. In accordance with our earlier studies, the increased sensitivity of melanocytes to MBEH as compared to 4-TBP in vitro [5] is accompanied by actual greater treatment efficacy in vivo. Contrary to expectation, depigmentation of the pelage occurred more readily than depigmentation of the skin. This may in part be a consequence of the densely populated and heavily melanized epidermis of k14-SCF mice, as we have previously reported that melanin may protect against the cytotoxic effects of MBEH [5].
Such data are of particular importance as the elimination of melanocytes from the skin and hair follicles can theoretically serve as an alternative to ‘elective surgery’ offered for patient with mutations in tumor-associated genes [21; 22]. For example, such elective surgery is offered to patients with mutations in BRCA-1 or BRCA-2 where the odds of developing breast- or ovarian cancer can increase from ~1% to virtually 100% [22]. As preventive surgery is out of the question for familial melanoma patients, the concept of chemoprevention proposed here can offer a much needed alternative for patients with mutations in Ink4A/Arf or CDK4 genes [23; 24].
As we have previously established, sensitivity to MBEH is specific for melanocytes in the epidermis, but other cell types can display similar sensitivity to both MBEH and 4-TBP, including fibroblasts [5]. This precludes the use of bleaching phenols in systemic applications, which is desirable in a therapeutic setting for melanoma. Indeed, several authors have suggested the use of bleaching agents in the treatment of melanoma[25].
It is an intriguing concept that bleaching agents can serve as a prodrug to be selectively converted by enzymes uniquely expressed by melanocytes and melanoma cells to generate toxic quinones. In fact, it has been proposed that resulting quinones may in turn convert molecules in close proximity into increasingly immunogenic moieties according to the ‘haptenization theory’ [6; 7]. In this regard, the accelerated depigmentation in response to MBEH in the order of magnitude observed here further suggests that part of the depigmentation effects observed in vivo may actually be indirect.
We postulate that the necrotic pathway of cell death induced by MBEH as opposed to apoptosis following exposure to 4-TBP may differentially affect processing and presentation of melanocyte antigens and selectively lead to an immune response after MBEH exposure [5; 26; 27]. Indeed, the data presented here strongly support elicitation of an immune response in response to MBEH that is not found after 4-TBP application. First, we observed Langerhans cell emigration from the epidermis of human skin in response to MBEH, whereas after 4-TBP application these cells were merely found in basal position, thus remaining within the epidermis. It is likely that antigens processed by intra-epidermal Langerhans cells will not be presented to T cells in draining lymph nodes, thus preventing an influx of T cells to the skin. Such investigations required moving from ‘ex vivo’ model in human tissue to ‘in vivo’ studies by engaging C57BL/6 or k14-SCF mice with epidermal melanocytes. In these in vivo studies, it was observed that both macrophages and T cells primarily driven by Th1 cytokines infiltrate the skin in response to MBEH, but not 4-TBP. A combination of infiltrating macrophages and T cells is similarly observed in vitiligo and other auto immune disease and this combined effect might be responsible for the removal of target cells under stress [28; 29; 30].
In order to assess whether the inflammatory conditions encountered in MBEH treated skin were accompanied by reactivity to cells of the melanocyte lineage, as well as to test for actual anti-tumor protection of the treatment at hand, mice depigmenting in response to MBEH were then challenged with B16 melanoma cells subcutaneously introduced in depigmented areas. It should be noted that MBEH treatments were ceased well before tumor challenge, rendering the circumstances unlikely for a direct (biochemical) effect on B16 melanoma cells. Such biochemical responses to bleaching agents were further investigated in vitro, and it became apparent that similar to human melanoma cells, B16 cells are highly insensitive to direct exposure to bleaching phenols (unpublished). It is possible, that reduced sensitivity to MBEH towards melanoma cells can be explained by toxin pumps activated in response to malignant transformation that likewise render tumor cells increasingly insensitive to anti proliferative agents [31; 32]
Finally, elevated levels of Th1 Th17 cytokines along with a marked infiltration of CD8+ T cells strongly suggest that significant inhibition of tumor growth upon MBEH pretreatment was primarily due to activation of cytotoxic T.
It has been reported that elimination of melanocytes by MBEH is permanent [1]. Therefore it can be expected that memory responses are established by depigmentation treatment. For melanocytes that are not eliminated by MBEH treatment or due to the immune response that follows, it may be important to drive recall responses in patients where remaining melanocytes malignantly transform at a later time point. This question was addressed by van den Boorn et al in their recent publication where MBEH treatment was combined with adjuvants imiquimod and CpG [12]. Anti-tumor reactivity was observed even 165 days after cessation of the treatment, thus supporting lasting effects of MBEH.
As melanocytes are involved in photo protection, removal of melanocytes can increase the risk of non-melanoma skin cancer occurrence. However, erythema rapidly develops in depigmented skin in response to UV and treated patients avoid sun exposure. Indeed this explains infrequent occurrence of non-melanoma skin cancer in vitiligo patients. [33; 34]
In conclusion, our data support that the greatest potential of bleaching phenols in anti-tumor treatment revolve around prophylactic applications. We have demonstrated that treatment by bleaching phenols can be mimicked in mice closely reflecting human skin pigmentation by the presence of melanocytes in the epidermis. Also, the data clearly demonstrate that the application of MBEH as a single agent should be restricted to topical use, whereas there is little to no efficacy towards melanoma tumor cells. Most excitingly, these data support for the concept that topical application of bleaching phenols can serve as a prophylactic agent to support the prevention of tumor growth in familial melanoma patients and patients concerned about tumor recurrences. Such protection is offered in a 2-tierd approach, by eliminating precursor cells of the melanocyte lineage that can otherwise malignantly transform, and by eliciting an immune response to melanocytes to support removal of melanocytes from sites not exposed to MBEH, as well as to melanoma cells that share expression of melanocyte differentiation antigens. In particular, gp100 and MART-1 and tyrosinase are among the most immunogenic molecules expressed by melanoma cells [6; 35; 36]. Taken together, the data offer pre-clinical support for the establishment of topical depigmentation as adjuvant prophylactic treatment against melanoma.
ACKNOWLEDGEMENTS
Funding from NCI Grant RO3 CA128068 and internal support from Loyola’s Research Funding Committee to CLP and an Arthur J. Schmitt Dissertation fellowship to Vidhya Hariharan are gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bolognia JLLK, Somma S. Depigmentation therapy. Dermatol Ther. 2001;14:29–34. [Google Scholar]
- [2].Oliver EASL, Warren LH. Occupational leukoderma. Arch Dermatol Syphilol. 1940;42:993–998. [Google Scholar]
- [3].Klauder JV, Hardy MK. Occupational Leukoderma. Arch of Dermatol and Syphilol. 1948;57:778–779. [PubMed] [Google Scholar]
- [4].Mosher DB, Parrish JA, Fitzpatrick TB. Mono-benzyl-ether of hydroquinone-retrospective study of treatment of 18 vitiligo patients and a riview of literature. Br J Dermatol. 1977;97:669–679. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- [5].Hariharan V, Klarquist J, Reust MJ, Koshoffer A, McKee MD, Boissy RE, Le Poole IC. Monobenzyl ether of hydroquinone and 4-tertiary butyl phenol activate markedly different physiological responses in melanocytes: relevance to skin depigmentation. J Invest Dermatol. 2010;130:211–220. doi: 10.1038/jid.2009.214. [DOI] [PubMed] [Google Scholar]
- [6].Westerhof W, d’Ischia M. Vitiligo puzzle: the pieces fall in place. Pigm Cell Res. 2007;20:345–359. doi: 10.1111/j.1600-0749.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- [7].Manini P, Napolitano A, Westerhof W, Riley PA, d’Ischia M. A Reactive ortho-Quinone Generated by Tyrosinase-Catalyzed Oxidation of the Skin Depigmenting Agent Monobenzone: Self-Coupling and Thiol-Conjugation Reactions and Possible Implications for Melanocyte Toxicity. Chem Res Toxicol. 2009;22:1398–1405. doi: 10.1021/tx900018q. [DOI] [PubMed] [Google Scholar]
- [8].Manga P, Sheyn D, Yang F, Sarangarajan R, Boissy RE. A role for tyrosinase-related protein 1 in 4-tert-butyl phenol-induced toxicity in melanocytes - Implications for vitiligo. Am J Pathol. 2006;169:1652–1662. doi: 10.2353/ajpath.2006.050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Douat-Casassus C, Marchand-Geneste N, Diez E, Aznar C, Picard P, Geoffre S, Huet A, et al. Covalent modification of a melanoma-derived antigenic peptide with a natural quinone methide. Preliminary chemical, molecular modelling and immunological evaluation studies. Mol Biosyst. 2006;2:240–249. doi: 10.1039/b518044a. [DOI] [PubMed] [Google Scholar]
- [10].Wankowicz-Kalinska A, Le poole C, Van Den Wijngaard R, Storkus WJ, Das PK. Melanocyte-specific immune response in melanoma and vitiligo: Two faces of the same coin? Pigm Cell Res. 2003;16:254–260. doi: 10.1034/j.1600-0749.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- [11].Sanchez-Perez L, Kottke T, Daniels GA, Diaz RM, Thompson J, Pulido J, Melcher A, et al. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- [12].van den Boorn JG, Konijnenberg D, Tjin EPM, Picavet DI, Meeuwenoord NJ, Filippov DV, van der Veen JPW, et al. Effective Melanoma Immunotherapy in Mice by the Skin-Depigmenting Agent Monobenzone and the Adjuvants Imiquimod and CpG. Plos One. 2010;5 doi: 10.1371/journal.pone.0010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hansson J. Familial melanoma. Surg Clin N Am. 2008;88:897–916. doi: 10.1016/j.suc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [14].Lepoole IC, Vanden Wijngaard R, Westerhof W, Dormans J, Vanden Berg FM, Verkruisen RP, Dingemans KP, et al. Organotypic culture of human skin to study melanocyte migration. Pigm Cell Res. 1994;7:33–43. doi: 10.1111/j.1600-0749.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- [15].Pistoor FHM, Rambukkana A, Kroezen M, Lepoittevin JP, Bos JD, Kapsenberg ML, Das PK. Novel predictive assay for contact allergens using human skin explant cultures. Am J Pathol. 1996;149:337–343. [PMC free article] [PubMed] [Google Scholar]
- [16].Rambukkana A, Pistoor FHM, Bos JD, Kapsenberg ML, Das PK. Effects of contact allergens on human langerhans cells in skin organ culture: Migration, modulation of cell surface molecules, and early expression of interleukin-1 beta protein. Labor Invest. 1996;74:422–436. [PubMed] [Google Scholar]
- [17].Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patino JA, Le Poole IC. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Inves Dermatol. 2008;128:2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lepoole IC, Vandenwijngaard R, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions-an immunohistochemical investigation. J Invest Dermatol. 1993;100:816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- [20].Boudreau J, Koshy S, Cummings D, Wan Y. Culture of myeloid dendritic cells from bone marrow precursors. J Vis Exp. 2008 doi: 10.3791/769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Diamond JR, Borges VF, Eckhardt SG, Jimeno A. BRCA in breast cancer:From risk assesment to therapeutic prediction. Drug News Perspect. 2009;22:603–608. doi: 10.1358/dnp.2009.22.10.1440985. [DOI] [PubMed] [Google Scholar]
- [22].Panchal S, Bordeleau L, Poll A, Llacuachaqui M, Shachar O, Ainsworth P, Armel S, et al. Does family history predict the age at onset of new breast cancers in BRCA1 and BRCA2 mutation-positive families? Clin Genet. 2010;77:273–279. doi: 10.1111/j.1399-0004.2009.01328.x. [DOI] [PubMed] [Google Scholar]
- [23].Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, CordonCardo C, Horner JW, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Develop. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sharpless NE, Kannan K, Xu J, Bosenberg MW, Chin LD. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene. 2003;22:5055–5059. doi: 10.1038/sj.onc.1206809. [DOI] [PubMed] [Google Scholar]
- [25].Riley PA, Cooksey CJ, Johnson CI, Land EJ, Latter AM, Ramsden CA. Melanogenesis-targeted anti-melanoma pro-drug development: Effect of side-chain variations on the cytotoxicity of tyrosinase-generated ortho-quinones in a model screening system. E J Cancer. 1997;33:135–143. doi: 10.1016/s0959-8049(96)00340-1. [DOI] [PubMed] [Google Scholar]
- [26].Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- [27].Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- [28].Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. 2008;10:227–243. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- [29].Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, Giachino C, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp. Dermatol. 2008;17:139–140. doi: 10.1111/j.1600-0625.2007.00666_1.x. [DOI] [PubMed] [Google Scholar]
- [30].Le Poole IC, Wankowicz-Kalinska A, van den Wijngaard R, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Invest Dermatol. 2004;9:68–72. doi: 10.1111/j.1087-0024.2004.00825.x. [DOI] [PubMed] [Google Scholar]
- [31].Gillet J-P, Gottesman MM. Mechanisms of Multidrug Resistance in Cancer. 2010. [DOI] [PubMed]
- [32].Baguley BC. Multidrug Resistance in Cancer. 2010. [DOI] [PubMed]
- [33].Schallreuter KU, Tobin DJ, Panske A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed Caucasian patients with vitiligo. Dermatol. 2002;204:194–201. doi: 10.1159/000057881. [DOI] [PubMed] [Google Scholar]
- [34].Nordlund JJ. Nonmelanoma skin cancer in vitiligo patients. J Am Acad Dermatol. 2009;61:1080–1081. doi: 10.1016/j.jaad.2009.06.072. [DOI] [PubMed] [Google Scholar]
- [35].Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, et al. Melanoma-specific CD4(+) T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Expe Medicine. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kawakami Y, Rosenberg SA. Immunobiology of human melanoma antigens MART-1 and gp100 and their use for immuno-gene therapy. Int Rev Immunol. 1997;14:173–192. doi: 10.3109/08830189709116851. [DOI] [PubMed] [Google Scholar]