Abstract
BACKGROUND
The inpatient and outpatient burden of human metapneumovirus (HMPV) infection among young children has not been well established.
METHODS
We conducted prospective, population-based surveillance for acute respiratory illness or fever among inpatient and outpatient children less than 5 years of age in three U.S. counties from 2003 through 2009. Clinical and demographic data were obtained from parents and medical records, HMPV was detected by means of a reverse-transcriptase polymerase-chain-reaction assay, and population-based rates of hospitalization and estimated rates of outpatient visits associated with HMPV infection were determined.
RESULTS
HMPV was detected in 200 of 3490 hospitalized children (6%), 222 of 3257 children in outpatient clinics (7%), 224 of 3001 children in the emergency department (7%), and 10 of 770 asymptomatic controls (1%). Overall annual rates of hospitalization associated with HMPV infection were 1 per 1000 children less than 5 years of age, 3 per 1000 infants less than 6 months of age, and 2 per 1000 children 6 to 11 months of age. Children hospitalized with HMPV infection, as compared with those hospitalized without HMPV infection, were older and more likely to receive a diagnosis of pneumonia or asthma, to require supplemental oxygen, and to have a longer stay in the intensive care unit. The estimated annual burden of outpatient visits associated with HMPV infection was 55 clinic visits and 13 emergency department visits per 1000 children. The majority of HMPV-positive inpatient and outpatient children had no underlying medical conditions, although premature birth and asthma were more frequent among hospitalized children with HMPV infection than among those without HMPV infection.
CONCLUSIONS
HMPV infection is associated with a substantial burden of hospitalizations and outpatient visits among children throughout the first 5 years of life, especially during the first year. Most children with HMPV infection were previously healthy. (Funded by the Centers for Disease Control and Prevention and the National Institutes of Health.)
Human metapneumovirus (HMPV), A paramyxovirus discovered in 2001, is associated with acute respiratory illness among infants and children worldwide.1–11 In addition, HMPV causes acute respiratory illness and results in hospitalization among older adults and persons with underlying chronic conditions, including asthma, cancer, and chronic obstructive pulmonary disease.12–19 However, the seasonality of HMPV disease and its overall burden in hospitalizations and outpatient visits among young children remain poorly defined. Previously published studies have been limited by their retrospective nature,1–9 the use of data collected from convenience samples over relatively short periods, and the absence of asymptomatic controls.
The Centers for Disease Control and Prevention (CDC) New Vaccine Surveillance Network (NVSN) conducted prospective surveillance for HMPV infection among children less than 5 years of age who had acute respiratory illness or fever during the period from 2000 through 2009 in three U.S. counties.20–23 Earlier NVSN reports of HMPV infection included only inpatients enrolled at two sites over a 3-year period,24,25 whereas the current study extends the investigation to rates of hospitalization, emergency department (ED) visits, and outpatient visits for HMPV infection at three study sites over a period encompassing six seasons (November through May, from 2003 through 2009).
METHODS
STUDY DESIGN
The design and methods of NVSN surveillance have been reported previously.20–23,26 Surveillance was conducted in the counties surrounding Cincinnati, Nashville, and Rochester, New York. The surveillance periods included in this study were November through May, from 2003 through 2009. Healthy controls who were less than 5 years old and residing in the study counties were recruited during the same months from 2003 through 2005 at well-child visits in 8 to 10 primary care practices (the number of practices differed in the 2 years). Children less than 5 years old who had acute respiratory illness or fever were enrolled within 48 hours after hospital admission from Sunday through Thursday; outpatient surveillance was conducted 1 or 2 days per week concurrently. Patients in the ED were enrolled from 1 through 4 days per week. During the study period, surveillance hospitals cared for more than 95% of children in the county.
Children were excluded if they had symptoms for more than 14 days, had chemotherapy-associated neutropenia, had been hospitalized within the previous 4 days, or were newborns who had never been discharged. Written informed consent was obtained from parents or guardians, and the institutional review boards at each site and the CDC approved the study.
DATA COLLECTION AND LABORATORY TESTING
Demographic characteristics and medical and social histories were obtained with the use of standardized questionnaires.21,26 Laboratory and clinical information was obtained from medical records. High-risk conditions included premature birth (<36 weeks of gestation); chronic pulmonary, cardiac, renal, or immunodeficiency disease; cancer; and sickle cell anemia.20,25 Nasal and throat swabs were tested by means of a real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay for HMPV and other viruses.20,22,23,27–29 Children with a positive RT-PCR test for HMPV were considered to have confirmed HMPV infection. Viral load was compared between HMPV-positive ill children and healthy controls with the use of a t-test.
STATISTICAL ANALYSIS
Prospectively collected demographic and clinical data were compared with the use of a Pearson chi-square test or Wilcoxon rank-sum test, as appropriate for categorical or continuous variables. Age-group categories were based on previous studies.20–23,25,26 Logistic regression was used for comparisons between inpatient and outpatient settings among HMPV-positive or HMPV-negative children, with adjustment for age as a continuous variable for each potential risk factor.
The number of hospitalizations per 1000 children was calculated with the use of the seasonal weighted number of hospitalizations attributable to HMPV infection, with adjustment for the number of surveillance days and the proportion of patients enrolled, divided by the county population according to annual U.S. Census intercensal population estimates (www.cdc.gov/nchs/nvss/bridgedrace.htm). The 95% confidence intervals were calculated with the use of 1000 bootstrap samples.21 To estimate population-based rates of outpatient visits, we used National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) data for November through May of each year from 2003 through 2008, encompassing five seasons. We estimated rates of acute respiratory illness or fever attributable to HMPV infection by multiplying the proportion of patients who were HMPV-positive for each age group, clinical setting, and study year by the estimated age-specific rate of acute respiratory illness or fever (from the NAMCS and NHAMCS data). The 95% confidence intervals were calculated with the use of the delta method.20,22 Asymptomatic outpatient controls were not included in the rate calculations. All analyses were performed with the use of R software, version 2.12.2 (www.r-project.org).
RESULTS
STUDY POPULATION
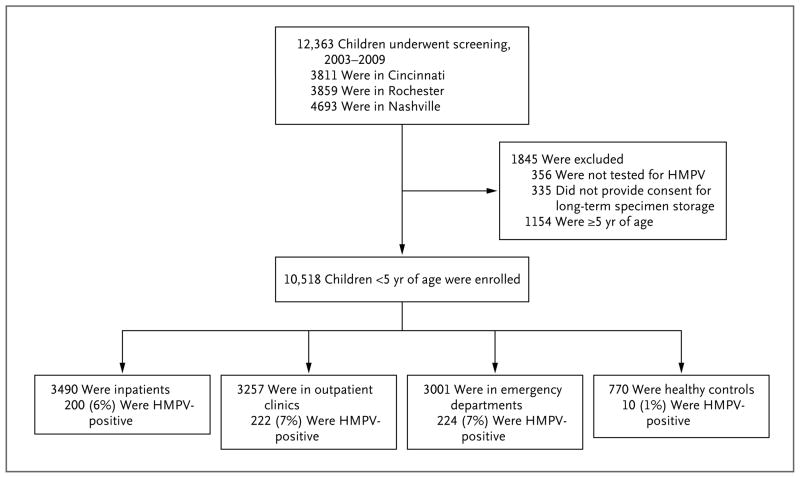
Of 12,363 children enrolled in the overall surveillance program, 10,518 (85%) were included in this study, and 646 ill children and 10 controls had confirmed HMPV infection (Fig. 1). Children whose specimens were unavailable for HMPV testing (356 children), whose parents or guardians did not provide consent for long-term specimen storage (335), or who were more than 5 years old (1154) were excluded.
Figure 1. Study Enrollment.
Of 12,363 children enrolled, 10,518 (85%) were included in the study. A total of 646 children who were inpatients or outpatients had confirmed human metapneumovirus (HMPV) infection.
INPATIENT SURVEILLANCE
HMPV was detected in 200 of 3490 hospitalized children (6%) (Table 1). HMPV-positive inpatients were significantly older than HMPV-negative in-patients (median age, 13 months vs. 6 months; P<0.001), but the groups did not differ significantly with respect to sex, race or ethnic group, or insurance type. However, among hospitalized children, those who were HMPV-positive were more likely than those who were HMPV-negative to have high-risk coexisting conditions (40% vs. 30%, P = 0.002).
Table 1.
Demographic and Clinical Characteristics of Children with and Those without Human Metapneumovirus (HMPV) Infection.*
| Characteristic | Inpatients | Outpatients | ||||
|---|---|---|---|---|---|---|
| HMPV-Positive (N = 200) | HMPV-Negative (N = 3290) | P Value | HMPV-Positive (N = 446) | HMPV-Negative (N = 5812) | P Value | |
| Age — mo | ||||||
|
| ||||||
| Median | 13 | 6 | <0.001 | 17 | 16 | 0.61 |
|
| ||||||
| Interquartile range | 5–26 | 1–19 | 8–32 | 8–32 | ||
|
| ||||||
| Age group — no. (%) | <0.001 | 0.59 | ||||
|
| ||||||
| <6 mo | 56 (28) | 1614 (49) | 71 (16) | 1036 (18) | ||
|
| ||||||
| 6–11 mo | 39 (20) | 443 (13) | 96 (22) | 1148 (20) | ||
|
| ||||||
| 12–23 mo | 49 (24) | 605 (18) | 114 (26) | 1524 (26) | ||
|
| ||||||
| 24–35 mo | 27 (14) | 291 (9) | 77 (17) | 892 (15) | ||
|
| ||||||
| 36–59 mo | 29 (14) | 337 (10) | 88 (20) | 1212 (21) | ||
|
| ||||||
| Sex — no. (%) | 0.16 | 0.35 | ||||
|
| ||||||
| Female | 95 (48) | 1398 (42) | 198 (44) | 2713 (47) | ||
|
| ||||||
| Male | 105 (52) | 1892 (58) | 248 (56) | 3099 (53) | ||
|
| ||||||
| Race or ethnic group — no. (%)† | 0.67 | 0.25 | ||||
|
| ||||||
| White | 95 (48) | 1407 (43) | 124 (28) | 1754 (30) | ||
|
| ||||||
| Black | 62 (31) | 1090 (33) | 220 (49) | 2704 (47) | ||
|
| ||||||
| Hispanic | 26 (13) | 515 (16) | 69 (15) | 901 (16) | ||
|
| ||||||
| Other or unknown | 17 (8) | 278 (8) | 33 (7) | 453 (8) | ||
|
| ||||||
| Type of health insurance — no. (%) | 0.37 | 0.01 | ||||
|
| ||||||
| Public | 120 (60) | 2011 (61) | 312 (70) | 4310 (74) | ||
|
| ||||||
| Private | 72 (36) | 1098 (33) | 100 (22) | 1240 (21) | ||
|
| ||||||
| None | 6 (3) | 166 (5) | 30 (7) | 242 (4) | ||
|
| ||||||
| Unknown | 2 (1) | 15 (<1) | 4 (1) | 20 (<1) | ||
|
| ||||||
| High-risk condition — no. (%)‡ | 81 (40) | 990 (30) | 0.002 | 97 (22) | 1293 (22) | 0.81 |
|
| ||||||
| Premature birth | 48 (24) | 513 (16) | 0.006 | 39 (9) | 585 (10) | 0.34 |
|
| ||||||
| Asthma | 61 (30) | 677 (21) | <0.001 | 83 (19) | 1084 (19) | 0.98 |
|
| ||||||
| Chronic lung disease | 20 (10) | 182 (6) | 0.009 | 1 (<1) | 70 (1) | 0.06 |
|
| ||||||
| Supplemental oxygen — no. (%) | 106 (53) | 1178 (36) | <0.001 | — | — | |
|
| ||||||
| Diagnosis at discharge — no. (%)§ | <0.001 | <0.001 | ||||
|
| ||||||
| Pneumonia | 99 (50) | 731 (22) | 39 (9) | 259 (4) | ||
|
| ||||||
| Bronchiolitis | 44 (22) | 987 (30) | 46 (10) | 544 (9) | ||
|
| ||||||
| Asthma | 29 (14) | 419 (13) | 62 (14) | 530 (9) | ||
|
| ||||||
| Viral illness | 11 (6) | 391 (12) | 152 (34) | 2058 (35) | ||
|
| ||||||
| Fever | 8 (4) | 294 (9) | 19 (4) | 314 (5) | ||
Outpatients were defined as children seen in the emergency department or outpatient clinic. P values were calculated by means of the Pearson chi-square test, except for the median age, which was calculated by means of the Wilcoxon rank-sum test.
Race or ethnic group was determined according to the report of the parent or guardian.
Some children had more than one high-risk condition.
Diagnosis at discharge was reported on the basis of up to 10 discharge diagnoses, ordered as pneumonia, bronchiolitis, asthma, viral illness (including nonspecific viral illness, croup, respiratory syncytial virus infection, and upper respiratory infection), fever, influenza, seizure, bacteremia or septicemia, pharyngitis, and sinusitis. Only diagnoses present in at least 4% of HMPV-positive children are shown. For more details, see Table S4 in the Supplementary Appendix.
CLINICAL CHARACTERISTICS OF INPATIENTS
HMPV-positive children were more likely than HMPV-negative children to require supplemental oxygen (53% vs. 36%, P<0.001), had a longer mean stay in the intensive care unit (ICU) (4.5 days [95% confidence interval (CI), 3.2 to 10.5] vs. 2.0 days [95% CI, 1.0 to 4.0], P = 0.01), and were more likely to undergo chest radiography (86% vs. 70%, P<0.001). However, HMPV-positive children and HMPV-negative children had similar rates of ICU admission (6% and 7%, respectively) and intubation (4% and 3%, respectively). HMPV status was not associated with the overall length of stay or the rate of positive bacterial cultures of the blood or urine, although HMPV-positive children were less likely than HMPV-negative children to have a urine culture obtained (20% vs. 37%, P<0.001) and were also less likely to have a cerebrospinal culture obtained (11% vs. 26%, P<0.001).
HMPV-positive children were more likely than HMPV-negative children to receive a diagnosis of pneumonia (Table 1). Bronchiolitis and asthma were the next most common diagnoses among HMPV-positive patients. Among children with HMPV infection, 21 (10%) had other viruses simultaneously detected by means of PCR assay or culture: 13 children (6%) had respiratory syncytial virus (RSV), 6 (3%) had influenza virus, and 2 (1%) had parainfluenza virus (see Table 3 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
HOSPITALIZATION RATES
From 2003 through 2009, the average annual rate of HMPV-associated hospitalization was 1 per 1000 children less than 5 years of age and 3 per 1000 less than 6 months of age (Table 2). Annual rates ranged from a low of 2 per 1000 children less than 6 months of age in the 2004–2005 season to a high of 4 per 1000 children less than 6 months of age in the 2006–2007 season. The average hospitalization rate among children 6 to 11 months old was 2 per 1000, but the rate varied by year (range, 1 to 3).
Table 2.
Rates of Inpatient and Outpatient Treatment for HMPV Infection, According to November–May Season and Age.*
| Season | Age | ||||
|---|---|---|---|---|---|
| <6 mo | 6–11 mo | 12–23 mo | 24–59 mo | 0–59 mo | |
| no. of HMPV infections/1000 children (95% CI) | |||||
|
| |||||
| Hospitalized children | |||||
|
| |||||
| 2003–2004 | 3 (2–5) | 3 (1–4) | 1 (1–2) | 1 (0–1) | 1 (1–2) |
|
| |||||
| 2004–2005 | 2 (1–4) | 2 (1–3) | 1 (0–2) | 0 (0–1) | 1 (1–1) |
|
| |||||
| 2005–2006 | 3 (1–5) | 1 (0–3) | 2 (1–4) | 1 (0–1) | 1 (1–2) |
|
| |||||
| 2006–2007 | 4 (2–7) | 3 (1–6) | 1 (1–2) | 1 (0–1) | 1 (1–2) |
|
| |||||
| 2007–2008 | 3 (1–6) | 1 (0–2) | 1 (0–2) | 0 (0–0) | 1 (0–1) |
|
| |||||
| 2008–2009 | 3 (1–5) | 3 (1–5) | 0 (0–1) | 0 (0–1) | 1 (0–1) |
|
| |||||
| 2003–2009 | 3 (2–4) | 2 (1–3) | 1 (1–2) | 0 (0–1) | 1 (1–1) |
|
| |||||
| Children at outpatient clinics | |||||
|
| |||||
| 2003–2004 | 37 (12–113) | 134 (73–239) | 76 (42–137) | 52 (34–80) | 63 (47–86) |
|
| |||||
| 2004–2005 | 44 (18–107) | 19 (5–76) | 53 (30–94) | 25 (15–40) | 32 (23–45) |
|
| |||||
| 2005–2006 | 59 (26–130) | 141 (78–253) | 69 (33–143) | 27 (13–56) | 53 (37–76) |
|
| |||||
| 2006–2007 | 58 (26–129) | 118 (62–226) | 114 (68–193) | 57 (36–90) | 73 (54–98) |
|
| |||||
| 2007–2008 | 93 (49–178) | 136 (73–252) | 81 (46–143) | 44 (27–72) | 67 (49–90) |
|
| |||||
| 2008–2009 | 52 (13–201) | 76 (19–297) | 86 (29–257) | 47 (23–98) | 57 (34–96) |
|
| |||||
| 2003–2009 | 58 (39–86) | 102 (73–142) | 75 (56–102) | 39 (31–51) | 55 (46–66) |
|
| |||||
| Children in emergency departments | |||||
|
| |||||
| 2003–2004 | 27 (17–44) | 33 (19–58) | 16 (9–29) | 14 (9–20) | 17 (13–23) |
|
| |||||
| 2004–2005 | 5 (1–20) | 18 (8–37) | 5 (2–14) | 5 (2–10) | 6 (4–10) |
|
| |||||
| 2005–2006 | 19 (9–41) | 24 (11–53) | 33 (19–57) | 11 (6–20) | 17 (12–24) |
|
| |||||
| 2006–2007 | 19 (10–35) | 34 (17–67) | 21 (10–41) | 11 (7–18) | 16 (12–22) |
|
| |||||
| 2007–2008 | 5 (1–20) | 34 (17–66) | 16 (9–31) | 5 (3–11) | 10 (7–14) |
|
| |||||
| 2008–2009 | 15 (6–39) | 34 (19–63) | 22 (12–41) | 8 (4–15) | 14 (10–21) |
|
| |||||
| 2003–2009 | 16 (11–22) | 29 (21–39) | 17 (13–23) | 9 (7–12) | 13 (11–16) |
Surveillance of hospitalized children, children at outpatient clinics, and children in emergency departments was conducted from November through May in the years from 2003 through 2009. Rates of infection among outpatients were calculated by multiplying the national rate of outpatient visits for acute respiratory illness, obtained from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, by the proportion of visits for acute respiratory illness that was attributable to HMPV infection in this study. Controls were not included in the rate calculations.
OUTPATIENT SURVEILLANCE
HMPV was detected in 222 of 3257 children (7%) evaluated in outpatient clinics and in 224 of 3001 (7%) evaluated in the ED. Overall, HMPV-positive and HMPV-negative children did not differ significantly with respect to age, sex, or race or ethnic group (Table 1), but HMPV-positive children were slightly less likely to have public insurance coverage and more likely to have no insurance coverage. The prevalence of high-risk conditions was similar in the two groups, but HMPV-positive children evaluated in the ED were slightly more likely than HMPV-negative children to have been born prematurely (13% vs. 11%, P = 0.009).
HMPV was detected in only 10 of 770 asymptomatic control children (1%) (P<0.001 for the comparisons with both hospitalized and outpatient children). Asymptomatic HMPV-positive controls did not differ from symptomatic children with respect to median age but had a significantly lower HMPV viral load than did HMPV-positive symptomatic children (mean genome copy number, 4×105 vs. 1×107; P<0.001).
CLINICAL CHARACTERISTICS OF OUTPATIENTS
One third (34%) of all outpatient children received a diagnosis of viral illness, and 10% received a diagnosis of bronchiolitis (Table 1). HMPV-positive children were more likely than HMPV-negative children to receive a diagnosis of pneumonia or asthma. Among outpatient children with HMPV infection, 27 (6%) had other viruses simultaneously detected by means of PCR or culture: 14 children (3%) had RSV, 11 (2%) had influenza virus, and 2 (<1%) had parainfluenza virus (Table 3 in the Supplementary Appendix).
RATES OF OUTPATIENT CLINIC AND EMERGENCY DEPARTMENT VISITS
The rate of HMPV-associated outpatient clinic visits was 55 per 1000 children less than 5 years of age (95% CI, 46 to 66) (Table 2). Children 6 to 11 months of age had the highest rate, at 102 per 1000 (95% CI, 73 to 142). Rates of clinic visits associated with HMPV infection were 75 per 1000 (95% CI, 56 to 102) among children who were 12 to 23 months old, and 39 per 1000 (95% CI, 31 to 51) among those who were 24 to 59 months old.
For children less than 5 years of age, the rates of ED visits associated with HMPV infection ranged from 6 per 1000 for the 2004–2005 season to 17 per 1000 for the 2003–2004 and 2005–2006 seasons. The rates of ED visits varied according to age, but children 6 to 11 months old had the highest rate, at 29 per 1000 (95% CI, 21 to 39).
SEASONAL AND ANNUAL VARIATION
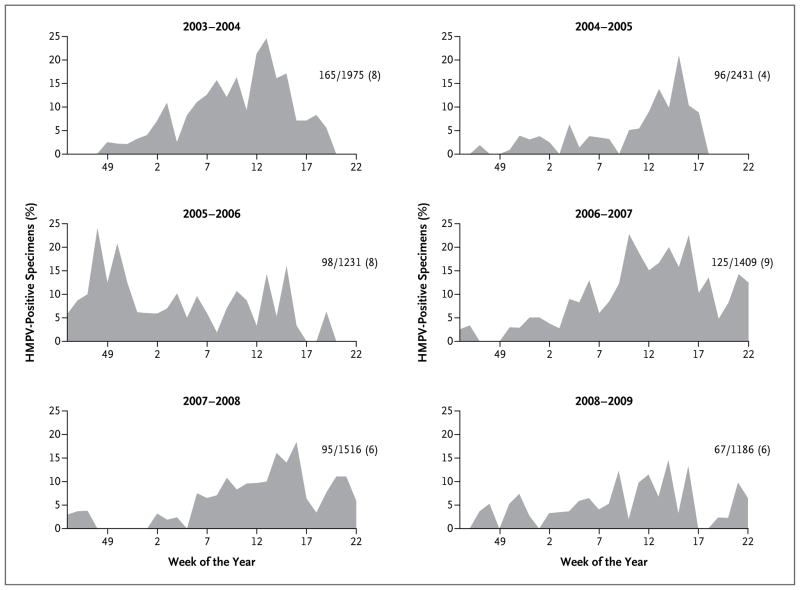
HMPV infections were detected throughout the 7-month surveillance period during all 6 years, but the pattern of detection varied (Fig. 2), with early peaks in some seasons (e.g., 2006–2007) and later ones in others. Overall, the majority (82%) of HMPV infections occurred during the months of January through April, with HMPV infection present in 10% of all specimens from patients with acute respiratory illness during the month of April.
Figure 2. HMPV-Positive Specimens, According to Week of the Year, 2003–2009.
Curves for HMPV-positive specimens are shown for all three sites combined during the six November–May seasons of the study period. The number of HMPV-positive specimens, the total number of specimens, and the percentage of HMPV-positive specimens for that season are shown at the right of each graph.
FACTORS ASSOCIATED WITH HOSPITALIZATION
To determine the effects of host and socioeconomic factors on illness severity, we compared the demographic and clinical characteristics of HMPV-positive inpatients and outpatients. In addition, to determine whether these risk factors were specific to HMPV, as compared with other causes of acute respiratory illness, these characteristics were also compared between HMPV-negative inpatients and outpatients, with adjustment for age (Table 3).
Table 3.
Inpatients and Outpatients with and Those without HMPV Infection, According to Factors Potentially Associated with the Risk of HMPV Infection and Severity of Illness, with Adjustment for Age.
| Variable | Children with HMPV Infection | Children without HMPV Infection | ||||
|---|---|---|---|---|---|---|
| Inpatient (N = 200) | Outpatient (N = 446)* | P Value | Inpatient (N = 3290) | Outpatient (N = 5812)* | P Value | |
| number (percent) | number (percent) | |||||
|
| ||||||
| Age group | 0.008 | <0.001 | ||||
|
| ||||||
| <6 mo | 56 (28) | 71 (16) | 1614 (49) | 1036 (18) | ||
|
| ||||||
| 6–11 mo | 39 (20) | 96 (22) | 443 (13) | 1148 (20) | ||
|
| ||||||
| 12–23 mo | 49 (24) | 114 (26) | 605 (18) | 1524 (26) | ||
|
| ||||||
| 24–35 mo | 27 (14) | 77 (17) | 291 (9) | 892 (15) | ||
|
| ||||||
| 36–59 mo | 29 (14) | 88 (20) | 337 (10) | 1212 (21) | ||
|
| ||||||
| Male sex | 105 (52) | 248 (56) | 0.25 | 1892 (58) | 3099 (53) | <0.001 |
|
| ||||||
| Any high-risk condition | 81 (40) | 97 (22) | <0.001 | 990 (30) | 1293 (22) | <0.001 |
|
| ||||||
| Premature birth | 48 (24) | 39 (9) | <0.001 | 513 (16) | 585 (10) | <0.001 |
|
| ||||||
| Attendance at out-of-home day care | 78 (39) | 207 (46) | 0.65 | 846 (26) | 2473 (43) | <0.001 |
|
| ||||||
| Other child or children in home | 98 (49) | 173 (39) | 0.05 | 1633 (50) | 2492 (43) | <0.001 |
|
| ||||||
| <5 yr old | 76 (38) | 138 (31) | 0.32 | 1329 (40) | 1882 (32) | <0.001 |
|
| ||||||
| 5–17 yr old | 40 (20) | 55 (12) | 0.04 | 578 (18) | 1004 (17) | 0.001 |
|
| ||||||
| Smoking exposure in home | ||||||
|
| ||||||
| Any | 80 (40) | 188 (42) | 0.41 | 1301 (40) | 2357 (41) | 0.03 |
|
| ||||||
| Maternal | 46 (23) | 115 (26) | 0.70 | 746 (23) | 1421 (24) | 0.04 |
|
| ||||||
| History of breast-feeding | 105 (52) | 259 (58) | 0.44 | 1972 (60) | 3134 (54) | <0.001 |
|
| ||||||
| Maternal educational level, high school or less | 125 (62) | 274 (61) | 0.81 | 1896 (58) | 3639 (63) | <0.001 |
Outpatients were defined as children seen in the emergency department or outpatient clinic.
Younger age was associated with hospitalization among both HMPV-positive and HMPV-negative children, particularly for infants less than 6 months of age. Boys and girls had the same rates of hospitalization. The presence of a high-risk condition, most commonly premature birth, was associated with hospitalization for both HMPV-positive and HMPV-negative children. Attendance in out-of-home day care was not associated with hospitalization among HMPV- infected children. There was a trend toward an association between hospitalization for HMPV infection and the presence of other children in the home, but this association was significant only for children between 5 and 17 years old in the home. Status with respect to smoking exposure, breast-feeding status, and maternal level of education were not associated with the rate of hospitalization among HMPV-positive children.
DISCUSSION
Our findings show that among children who were younger than 5 years of age, the annual rate of hospitalization associated with HMPV was 1 per 1000 children, which is the same as the rates of hospitalization associated with influenza virus, at 1 per 1000, and parainfluenza virus types 1, 2, and 3 combined, at 1 per 1000,22,23 but lower than the rate for RSV, at 3 per 1000.20 Thus, the potential inpatient burden of disease associated with HMPV is similar to that associated with other common respiratory viruses (Table 1 in the Supplementary Appendix). Extrapolation of these rates on the basis of U.S. Census data suggests that approximately 20,000 hospitalizations associated with HMPV infection occur annually among children less than 5 years of age.
The rates of outpatient visits associated with detectable HMPV infection were 55 per 1000 clinic visits and 13 per 1000 ED visits, which were lower than the rates of RSV infection among out-patients (80 per 1000 clinic visits and 28 per 1000 visits, according to the NVSN).20 However, the rates of outpatient visits associated with HMPV infection were similar to those for influenza during the 2002–2003 season (50 per 1000 clinic visits and 6 per 1000 ED visits) but were lower than the rates for influenza during the 2003–2004 season (95 per 1000 clinic visits and 27 per 1000 ED visits)22 (Table 2 in the Supplementary Appendix). The average annual rates of HMPV-associated outpatient visits were approximately 20, 50, and 80 times as high as the rates of HMPV-associated hospitalization among children less than 6 months, 6 to 23 months, and 24 to 59 months of age, respectively. Extrapolation of these rates to the U.S. population suggests that 1 million outpatient clinic visits and 263,000 ED visits may be associated with HMPV infection annually among U.S. children less than 5 years of age.
The rates of HMPV-associated clinic and ED visits were highest among children 6 to 11 months of age, in contrast to rates for HMPV-associated hospitalization, which were highest among children less than 6 months old. Furthermore, the rate of HMPV-associated outpatient visits among older children remained similar to the rate among young infants. This pattern is in contrast to that for RSV infection, for which outpatient rates decreased markedly after 1 year of age.20 Thus, HMPV, like influenza virus, may cause clinically significant disease throughout early childhood.22 During most of the study period, there were no commercially available diagnostic tests for HMPV, and the virus is difficult to culture. Thus, few children would have received a diagnosis of HMPV infection in the clinical setting. Rapid diagnostic tools for HMPV detection would identify infection in these children.
In this cohort, 40% of children hospitalized with HMPV infection had underlying high-risk conditions, including premature birth and asthma, whereas only 22% of outpatient children with HMPV infection had a high-risk condition. Other reports have also identified a substantial number of children with high-risk conditions who were hospitalized with HMPV infection.3,4,7,8,30–32 Conversely, nearly two thirds of inpatients and three quarters of outpatients with HMPV infection were otherwise healthy. These data suggest that all children are at risk for HMPV infection requiring medical attention, which provides important information to guide future prevention efforts, including vaccine development.
The clinical features of HMPV infection are similar to those of infections due to other respiratory viruses.9,11,25,30 However, hospitalized children with detectable HMPV infection were more likely than those without HMPV infection to require supplemental oxygen and had longer ICU stays, possibly reflecting underlying conditions that predisposed them to more severe disease. There were no deaths associated with HMPV infection, RSV infection, or influenza in the study cohort.20,25 Nonetheless, the data show that HMPV is capable of causing severe disease, and fatal HMPV infections have been reported.1,7,12,13,16,18,33–41 Furthermore, children with HMPV infection were more likely than those without the infection to undergo chest radiography, possibly owing to the absence of a specific viral diagnosis.
A small minority of children with HMPV had other viruses detected with the use of an RT-PCR assay. HMPV was detected in a few asymptomatic children, although at significantly lower viral loads than those in symptomatic children; higher viral loads have been correlated with more severe illness.42–46 Although parents of control children were asked about concurrent respiratory symptoms, it is possible that some children had an acute respiratory illness before enrollment and were still shedding virus; conversely, these children may have been in the early stage of HMPV infection, and illness that subsequently developed was not recorded. HMPV shedding can last from 1 to 2 weeks after acute illness.11,30,31,47
As with many pathogens, caution is warranted in attributing illness to HMPV when the virus is detected, since detection does not prove causation. However, other studies have shown that HMPV is rarely detected in asymptomatic children.11,27,28 Furthermore, virus challenge with HMPV causes acute respiratory illness in nonhuman primates and small-animal species that is clinically, histologically, and virologically similar to the disease in humans.48–55
Although the peak of HMPV detection varied from year to year, the prevalence of HMPV overlapped with the circulation of other common respiratory viruses. This finding underscores the strength of a multiyear study in delineating the epidemiologic features of HMPV. As with RSV and influenza virus, regular community-based, year-round surveillance is needed to define the onset and duration of HMPV activity. The similarity of clinical symptoms associated with different viruses strongly supports the need for specific molecular diagnostic methods for precise viral identification.
Our study has some limitations. First, our data can only reflect association, and in the absence of a specific intervention, we cannot infer that HMPV was the causal factor in the illnesses we observed. Second, our study population may not be representative of the entire U.S. population or that of other locations, despite the large number of patients enrolled over 6 years at three sites. Third, children were enrolled only from November through May, and some eligible children were excluded owing to missing samples or protocol deviations. Thus, we may have underestimated or overestimated the overall burden of HMPV infection. Fourth, institutional differences in medical practice may have affected hospitalization rates, but we used strict inclusion criteria and prospective data collection. Finally, children with neutropenia who were receiving treatment for cancer were excluded, although HMPV can cause severe and fatal disease in such children.7,12,18,35,56,57 Additional studies in special populations are needed to capture the complete burden of HMPV disease.
In conclusion, HMPV is frequently associated with acute respiratory illness in young children that requires medical attention, and HMPV infection represents a substantial health care burden among both inpatients and outpatients.
Supplementary Material
Acknowledgments
Supported by cooperative agreements with the CDC (U38/CCU417958 and U01/IP000022, to Vanderbilt University; U38/CCU217969 and U01/IP000017, to the University of Rochester; and U38/CCU522352 and U01/IP000147, to Cincinnati Children’s Hospital Medical Center) and by grants from the National Institutes of Health (AI-82417 and AI-085062, to Dr. Williams).
We thank the children and families who participated in this study; all the members of the New Vaccine Surveillance Network, including: Geraldine Lofthus, Kenneth Schnabel, Andrea Marino, Lynne Shelley, Jennifer Carnahan, Linda Anderson, Gladys Lathan, Charlene Freundlich, Christina Albertin, and Rebecca Martinez at the University of Rochester; Jennifer Klemenc, Amy Podsiad, Monika Johnson, Diane Kent, Ann Clay, Erin Keckley, and Jody Peters at Vanderbilt University; Jessica Schloemmer, Linda Jamison, Diana Henderson, David Witte, Pamela Groen, and Joel Mortensen at Cincinnati Children’s Hospital Medical Center; and Ranee Seither, Aaron Curns, Larry Anderson, Carolyn B. Bridges, Jim Alexander, Benjamin Schwartz, Frances Walker, John Copeland, Jennifer Reuer, Minnie Wang, and John Zhang at the CDC.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official views of the CDC.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
This article is dedicated to the memory of Caroline Breese Hall, M.D., a leader in respiratory virus research, a respected mentor, and a valued colleague and friend.
References
- 1.Boivin G, De Serres G, Cote S, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–40. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Døllner H, Risnes K, Radtke A, Nordbø SA. Outbreak of human metapneumovirus infection in Norwegian children. Pediatr Infect Dis J. 2004;23:436–40. doi: 10.1097/01.inf.0000126401.21779.74. [DOI] [PubMed] [Google Scholar]
- 3.Esper F, Martinello RA, Boucher D, et al. A 1-year experience with human meta-pneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freymouth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–4. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Gray GC, Capuano AW, Setterquist SF, et al. Multi-year study of human metapneumovirus infection at a large US Mid-western medical referral center. J Clin Virol. 2006;37:269–76. doi: 10.1016/j.jcv.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–57. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP. Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection. Pediatr Infect Dis J. 2007;26:693–9. doi: 10.1097/INF.0b013e3180621192. [DOI] [PubMed] [Google Scholar]
- 8.McAdam AJ, Hasenbein ME, Feldman HA, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–6. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 9.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–33. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–9. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 13.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 14.Martinello RA, Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53:248–54. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicente D, Montes M, Cilla G, Pérez-Trallero E. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg Infect Dis. 2004;10:1338–9. doi: 10.3201/eid1007.030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JV, Crowe JE, Jr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192:1149–53. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–5. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 22.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–9. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–5. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JV, Edwards KM, Weinberg GA, et al. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–8. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the New Vaccine Surveillance Network. Pediatr Infect Dis J. 2004;23:S188–92. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 27.Ali SA, Gern JE, Hartert TV, et al. Real-world comparison of two molecular methods for detection of respiratory viruses. Virol J. 2011;8:332. doi: 10.1186/1743-422X-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98.e1–104.e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot HK, Shepherd BE, Crowe JE, Jr, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28:682–7. doi: 10.1097/INF.0b013e31819d0d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoogen BG, van Doornum GJ, Fockens JC, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–7. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 31.Ebihara T, Endo R, Kikuta H, et al. Human metapneumovirus infection in Japanese children. J Clin Microbiol. 2004;42:126–32. doi: 10.1128/JCM.42.1.126-132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson EJ, Simões EAF, Buttery JP, et al. Prevalence and characteristics of human metapneumovirus infection among hospitalized children at high risk for severe lower respiratory tract infection. J Pediatr Infect Dis Soc. 2012;1:212–22. doi: 10.1093/jpids/pis069. [DOI] [PubMed] [Google Scholar]
- 33.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44:1152–8. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 34.Cane PA, van den Hoogen BG, Chakrabarti S, Fegan CD, Osterhaus AD. Human metapneumovirus in a haemato-poietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–10. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 35.Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24:1891–901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Liao RS, Appelgate DM, Pelz RK. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility for the elderly in Oregon. J Clin Virol. 2012;53:171–3. doi: 10.1016/j.jcv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Morrow BM, Hatherill M, Smuts HE, Yeats J, Pitcher R, Argent AC. Clinical course of hospitalised children infected with human metapneumovirus and respiratory syncytial virus. J Paediatr Child Health. 2006;42:174–8. doi: 10.1111/j.1440-1754.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 38.Noyola DE, Alpuche-Solís AG, Herrera-Díaz A, Soria-Guerra RE, Sánchez-Alvarado J, López-Revilla R. Human metapneumovirus infections in Mexico: epidemiological and clinical characteristics. J Med Microbiol. 2005;54:969–74. doi: 10.1099/jmm.0.46052-0. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier G, Déry P, Abed Y, Boivin G. Respiratory tract reinfections by the new human metapneumovirus in an immuno-compromised child. Emerg Infect Dis. 2002;8:976–8. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13:324–8. doi: 10.1111/j.1399-3062.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivaprakasam V, Collins TC, Aitken C, Carman WF. Life-threatening human metapneumovirus infections in West of Scotland. J Clin Virol. 2007;39:234–7. doi: 10.1016/j.jcv.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosis S, Esposito S, Osterhaus AD, et al. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42:286–90. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerna G, Sarasini A, Percivalle E, et al. Prospective study of human metapneumovirus infection: diagnosis, typing and virus quantification in nasopharyngeal secretions from pediatric patients. J Clin Virol. 2007;40:236–40. doi: 10.1016/j.jcv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Kunz AN, Englund JA, Kuypers J, Maranich A, Fairchok MP. Detection of multiple respiratory viruses by real-time polymerase chain reaction in infants attending an outpatient clinic. Eur J Clin Microbiol Infect Dis. 2008;27:1245–8. doi: 10.1007/s10096-008-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62:382–8. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48:239–45. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osterhaus A, Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–1. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- 48.Kuiken T, van den Hoogen BG, van Riel DA, et al. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol. 2004;164:1893–900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumino KC, Agapov E, Pierce RA, et al. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis. 2005;192:1052–60. doi: 10.1086/432728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vargas SO, Kozakewich HP, Perez-Atayde AR, McAdam AJ. Pathology of human metapneumovirus infection: insights into the pathogenesis of a newly identified respiratory virus. Pediatr Dev Pathol. 2004;7:478–86. doi: 10.1007/s10024-004-1011-2. [DOI] [PubMed] [Google Scholar]
- 51.Hamelin ME, Yim K, Kuhn KH, et al. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J Virol. 2005;79:8894–903. doi: 10.1128/JVI.79.14.8894-8903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J Virol. 2005;79:10944–51. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyde PR, Chetty SN, Jewell AM, Schoonover SL, Piedra PA. Development of a cotton rat-human metapneumovirus (hMPV) model for identifying and evaluating potential hMPV antivirals and vaccines. Antiviral Res. 2005;66:57–66. doi: 10.1016/j.antiviral.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Biacchesi S, Skiadopoulos MH, Yang L, et al. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J Virol. 2004;78:12877–87. doi: 10.1128/JVI.78.23.12877-12887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skiadopoulos MH, Biacchesi S, Buch-holz UJ, et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–37. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–81. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 57.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–96. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.