This study examined first-line treatment patterns and clinical outcomes in patients with HER2-positive, hormone receptor (HR)-positive metastatic breast cancer in a real-world setting. With or without chemotherapy, dual targeting of HRs and HER2 receptors was found to be associated with significantly prolonged progression-free survival and overall survival times.
Keywords: HER2, Hormone receptor, Treatment, Breast cancer, Metastatic, Trastuzumab
Abstract
Background.
Limited data are available describing the natural history of patients with HER2-positive and hormone receptor (HR)-positive metastatic breast cancer (MBC). We examined first-line treatment patterns and clinical outcomes in patients with HER2-positive, HR-positive MBC in a real-world setting.
Methods.
registHER is a prospective, observational cohort of 1,023 patients with HER2-positive MBC diagnosed within 6 months of enrollment and followed until death, disenrollment, or June 2009 (median follow-up time: 27 months). Demographics, first-line treatment patterns, and clinical outcomes were examined for 530 HER2-positive, HR-positive patients. Progression-free survival (PFS) and overall survival (OS) times were examined. Multivariate analyses adjusted for baseline demographic and prognostic factors.
Results.
HER2-positive, HR-positive patients receiving first-line trastuzumab plus hormonal therapy had significantly longer PFS times than patients who received hormonal therapy only (13.8 vs. 4.8 months; adjusted hazard ratio [HR]: 0.37, 95% confidence interval [CI]: 0.22–0.60); a nonsignificant reduction in OS time was observed (adjusted HR: 0.55, 95% CI: 0.27–1.14). Compared with patients who received first-line trastuzumab plus chemotherapy, patients who received first-line trastuzumab plus chemotherapy and hormonal therapy had longer median PFS times (20.4 months vs. 9.5 months; adjusted HR: 0.53, 95% CI: 0.42–0.68); a statistically significant reduction in risk of death was observed (adjusted HR: 0.50, 95% CI: 0.36–0.70). Sequential use of chemotherapy and hormonal therapy was associated with improved OS times when compared with concurrent use (adjusted PFS HR: 0.81, 95% CI: 0.54–1.21; adjusted OS HR: 0.48, 95% CI: 0.26–0.89).
Conclusions.
These real-world data in patients with HER2-positive/HR-positive MBC provide evidence that, with or without chemotherapy, dual targeting of HRs and HER2 receptors is associated with significantly prolonged PFS and OS times.
Implications for Practice:
This manuscript provides information from a prospective cohort registry study assessing the outcomes of patients with metastatic HER2-positive breast cancer on the basis of hormone receptor status in real-world community and academic practice environments. The results show that survival and progression-free survival are more favorable for the hormone receptor-positive subset. Additionally, hormonal therapy given either concurrently or sequentially with chemotherapy is associated with improved outcome compared with no hormonal therapy. This may aid in treatment planning, in discussions with patients, and in considering hormonal therapy as part of the treatment regimen for patients with HER2-positive and hormone receptor-positive metastatic breast cancer. However, several limitations such as hidden biases are inherent in uncontrolled studies such as this one.
Introduction
Approximately 25% of breast cancer tumors overexpress the HER2 receptor [1]. Trastuzumab, a humanized, monoclonal antibody directed against the extracellular domain of HER2, has demonstrated clinical activity in HER2-positive breast cancer and has become the standard of care for patients with HER2-positive breast cancer [2, 3]. Of HER2-positive breast cancer tumors, approximately 50% are also hormone receptor (HR)-positive; that is, they express estrogen receptor (ER) and/or progesterone receptor (PR) [4]. It has been demonstrated that HR status does not affect the clinical benefit of trastuzumab, whether it is given as a single agent or combined with chemotherapy [4].
HR-positive breast cancer is treated with hormonal therapy (HT) designed to interfere with HR signaling. It has been shown that crosstalk between growth factor and ER-dependent signaling pathways may affect growth regulation in HER2-positive breast cancers [5–8]. On the basis of randomized trials performed in selected populations, it appears that blockade of both pathways is more effective than blocking either pathway alone [9, 10]. Randomized phase III trials in postmenopausal women have shown improvements in PFS times with the addition of trastuzumab or lapatinib to aromatase inhibitor therapy without chemotherapy as initial therapy for HER2-positive and HR-positive advanced breast cancer [11, 12]. In the second-line setting, combining HT with growth factor receptor pathway blockade, using the mammalian target of rapamycin inhibitor everolimus imparted a large benefit in PFS times [8]. A comparison of outcomes of HER2-positive and HR-positive patients with metastatic breast cancer (MBC) from clinical trials relative to those in real-life clinical practice has not been performed.
It is common practice for HR-positive patients to be treated with induction chemotherapy without overlapping HT based on preclinical models showing antagonism of chemotherapy and tamoxifen and a worse outcome in the adjuvant setting with concurrent compared with sequential chemotherapy and tamoxifen [13–17]. However, hormonal/cytotoxic therapy interactions have not been tested in large-scale clinical trials with aromatase inhibitors. Patients typically proceed to hormonal maintenance therapy following a response or stabilization with chemotherapy. However, the assessment of the impact of HT in these patients can be biased because these patients inherently represent those that respond to chemotherapy; “nonresponders” often do not receive endocrine treatment. Thus, the most accurate assessment of the impact of HT is when given as first-line therapy concurrently with cytotoxic and/or biological therapy.
registHER is a large, multicenter, prospective observational study of 1,023 patients with newly diagnosed HER2-positive MBC. registHER includes 530 patients with HR-positive tumors, affording a unique opportunity to examine the natural history of HER2-positive, HR-positive MBC, as well as first-line treatment patterns and clinical outcomes in a real-world setting.
Methods
Study Design
registHER is a multicenter, prospective observational U.S.-based cohort study of patients from community and academic settings with newly diagnosed HER2-positive MBC (either locoregional or distant). The objectives of the registHER study were to describe the natural history of disease and treatment patterns for patients with HER2-positive MBC and to explore associations between demographic and clinical factors, specific therapies, cardiac toxicities, and patient outcomes. The study was approved by all local institutional review boards, and all enrolled patients provided informed consent.
Patient recruitment was conducted from December 2003 to February 2006. Eligible patients included women and men with HER2-positive breast cancer and a first diagnosis of MBC within 6 months of enrollment. HER2 status was determined according to institutional guidelines. Patients were eligible regardless of treatment before enrollment or during study follow-up; treatment with trastuzumab was not a requirement for study participation. To minimize patient selection bias, investigators were encouraged to recruit all eligible patients at their practice.
Data Collection
Patients received care according to their physicians' standard practice without study-specified evaluations. Patient information (including demographics, tumor characteristics based on individual institutional criteria, initial metastatic sites and progressions, systemic treatment received, and response to treatment) were recorded at enrollment and updated every 3 months thereafter. Participating physicians or designated staff entered patient data into electronic case report forms using a web-based electronic data capture system. Formal, prespecified scheduled assessments for tumor response were not required. Tumor progression was reported by physicians according to their standard practice and was categorized as based on imaging or physical findings.
First-Line Treatment Groups After MBC Diagnosis
To minimize potential selection bias and exclude patients who switched to HT after nonresponse to chemotherapy-based regimens, primary analyses of treatment patterns in HR-positive patients (defined as expression of ER and/or PR) focused on first-line treatments. First-line treatment included all treatments given before first progression, including HT only (including tamoxifen, aromatase inhibitors, fulvestrant, gonadotropin-releasing hormone analogue or other HT), trastuzumab and HT, trastuzumab and chemotherapy, and trastuzumab and chemotherapy and HT. Among patients who were treated with trastuzumab, chemotherapy, and HT, two subgroups of patients were created based on whether HT overlapped with chemotherapy. Patients were assigned to the concurrent use subgroup if HT and chemotherapy overlapped for at least 30 consecutive days (including patients whose HT started significantly after chemotherapy with overlap for 30 days). Otherwise, they were assigned to the sequential use subgroup.
Statistical Methods
Analyses incorporated all follow-up data on registHER patients as of June 15, 2009 (database lock). Enrollment of 83 patients whose MBC diagnosis was more than 6 months (up to 9 months) prior to enrollment was permitted; these patients are included in all analyses. Demographic and clinical characteristics were summarized by HR status and by first-line treatment after MBC diagnosis. Progression-free survival (PFS) time was defined as the time from the date of MBC diagnosis until first disease progression (PD) reported after initiation of treatment for MBC or death. Overall survival (OS) time was defined as the time from the date of MBC diagnosis to the date of death from all causes or last follow-up. Patients without PD or death were censored at the last follow-up date as of June 15, 2009. PFS and OS times were estimated using the Kaplan-Meier product-limit method [18]. The median and the 95% confidence intervals and p values from log rank tests are reported for time-to-event data.
Univariate and multivariate Cox proportional hazard models were used to report hazard ratios (HRs) and 95% CIs. Multivariate Cox proportional hazard models included clinically significant predictors of treatment group assignment and prognostic for survival, including age at diagnosis, race/ethnicity, Eastern Cooperative Oncology Group (ECOG) performance status, initial cancer stage at breast cancer diagnosis, and number and location of metastatic sites.
Distant disease-free interval (DDFI) was defined as the time between the end of nonhormonal adjuvant treatment and metastatic diagnosis; it was computed only for patients diagnosed in stages I–III or with more than 14 days between initial and metastatic diagnosis.
Results
Patient and Tumor Characteristics by HR Status
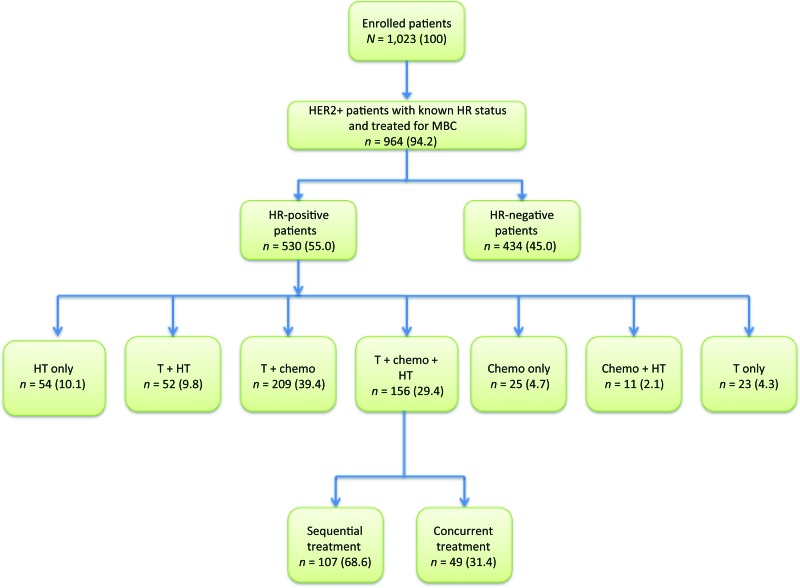
From December 2003 to February 2006, 1,023 patients with HER2-positive MBC were enrolled at 240 sites in the U.S. Only 33 patients (3.1%) did not enroll due to patient refusal (n = 27), investigator decision (n = 3), or other reasons (n = 3). Of 1,023 patients, 964 (94.2%) had HER2-positive tumors, known HR status, and had been treated for MBC, thus making them eligible for this analysis (Fig. 1). Of 964 patients with known HR status, 55.0% (n = 530) had HR-positive tumors. Of those, 62.3% expressed both ER and PR, 32.3% expressed ER only, and 4.7% expressed PR only. As of June 15, 2009, median follow-up time for HR-positive patients in registHER from time of metastatic diagnosis was 28.7 months.
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram for registHER study population and analysis cohort for first-line treatment.
Abbreviations: Chemo, chemotherapy; HR, hormone receptor (estrogen receptor and/or progesterone receptor); HT, hormonal therapy; MBC, metastatic breast cancer; T, trastuzumab.
Patients with HR-positive tumors and HR-negative tumors were similar with respect to age at metastatic diagnosis and race/ethnicity. Median age was 53 years in both groups, and more than three-fourths of patients were white (Table 1). Of patients with HR-positive disease diagnosed with early stage disease receiving adjuvant therapy, about one-fourth (26.0%) received chemotherapy only, nearly 40% received HT with chemotherapy, and 5.6% received adjuvant trastuzumab. In HR-negative patients receiving adjuvant therapy, nearly two-thirds (63.9%) received chemotherapy only and 5.6% received HT with chemotherapy. Approximately 17% of patients in both groups received no prior adjuvant therapy. The majority of patients in both HR-positive and HR-negative groups had MBC diagnosed more than 12 months after initial diagnosis of early stage disease and had two or more metastatic sites. Patients with HR-positive tumors were less likely to have nonvisceral metastasis at MBC diagnosis compared with patients with HR-negative tumors (20.4% vs. 8.5%, respectively) and about half as likely to have central nervous system (CNS) metastasis (4.2% vs. 10.8%, respectively). Patients with HR-positive tumors had a longer DDFI (26.1 vs. 13.1 months). Because of missing data, DDFI should be interpreted with caution.
Table 1.
Demographic and baseline characteristics by hormone receptor status for the primary analysis population (n = 964)

Data are n (%) unless otherwise noted.
aTime from the end of nonhormonal adjuvant treatment to metastatic diagnosis. Computed only for patients diagnosed in stages I-III or more than 14 days between initial and metastatic diagnosis.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hormone receptor, MBC, metastatic breast cancer.
Patient and Tumor Characteristics in HR-Positive Population by First-Line Treatment After MBC Diagnosis
A total of 273 patients with HR-positive MBC received HT as first-line treatment, including 220 patients who received an aromatase inhibitor and 36 patients who received tamoxifen. When examining patients with HR-positive tumors by first-line treatment after MBC diagnosis, 54 (10.1%) were treated with HT only; 52 (9.8%) were treated with trastuzumab and HT; 209 (39.4%) were treated with trastuzumab and chemotherapy; 156 (29.4%) were treated with trastuzumab, chemotherapy, and HT; 25 (4.7%) were treated with chemotherapy only; 11 (2.1%) were treated with chemotherapy and HT; and 23 (4.3%) were treated with trastuzumab only (Fig. 1). Of patients treated with trastuzumab, chemotherapy, and HT, 107 were treated sequentially and 49 were treated concurrently.
Patients with HR-positive tumors treated with trastuzumab only were older at metastatic diagnosis (median: 62.0 years, range: 33–82 years), whereas patients treated with chemotherapy only were younger (median: 49.0 years; range: 32–80 years; Table 2). Patients treated with HT only and patients treated with trastuzumab, chemotherapy, and HT as first-line treatment after MBC were most likely to have received HT with chemotherapy in the adjuvant setting compared with the other treatment groups (50.0% and 45.1%, respectively). In all, about one-fourth of patients treated with trastuzumab and HT in the first-line setting had been treated with HT only (25.6%) or HT with chemotherapy (25.6%) in the adjuvant setting. Patients treated in the first-line setting with chemotherapy only had the longest median DDFI (42.5 months). The majority of patients across treatment groups were diagnosed with stage I–III MBC more than 12 months after initial diagnosis. Patients treated with trastuzumab and HT were most likely to have one metastatic site at diagnosis (61.5%); patients treated with chemotherapy only were most likely to have two or more metastatic sites at diagnosis (60.0%). Visceral sites were the most common type of metastatic site at diagnosis across all treatment groups, followed by nonvisceral for most treatment groups.
Table 2.
Baseline characteristics of hormone receptor-positive patients by first-line treatment after diagnosis of metastatic breast cancer (n = 530)

Data are n (%) unless otherwise noted.
a151 patients who were stage IV at initial diagnosis were not included; percentages are based on nonmissing data.
bTime from the end of nonhormonal adjuvant treatment to metastatic diagnosis. Computed only for patients diagnosed in stages I-III or more than 14 days between initial and metastatic diagnosis.
Abbreviation: MBC, metastatic breast cancer.
Outcomes for HER2-Positive/HR-Positive Patients With MBC Treated in the First-Line Setting
For all HR-positive patients in registHER, the median PFS from time of MBC diagnosis was 11.7 months (95% CI: 10.2–12.6 months) compared with 8.8 months (95% CI: 7.8–10.3 months) for the HR-negative patients (Table 3). Overall survival (OS) from the time of MBC diagnosis was also longer: 41.5 months for HR-positive patients (95% CI: 37.7–44.6) and 28.6 months for the HR-negative patients (95% CI: 26.3–32.0).
Table 3.
Survival from metastatic diagnosis and sites of progression for hormone receptor-positive patients by first-line treatment

Data are n (%) unless otherwise noted. In all, 26 HR-positive patients (4 who received hormonal therapy only, 11 who received trastuzumab plus chemotherapy, and 3 who received trastuzumab plus chemotherapy plus hormonal therapy) and 28 HR-negative patients had PD recorded on or before initiation of treatment for metastatic breast cancer. For these patients, progression-free survival was calculated from time of metastatic diagnosis to the first PD recorded after initiation of metastatic treatment. If hormone therapy and chemotherapy overlapped for at least 30 consecutive days, patients were assigned to the concurrent use subgroup; otherwise, they were assigned to the sequential use subgroup.
aDeath occurred before any disease progression.
Abbreviations: HR, hormone receptor; NR, not reached; PD, progressive disease.
Fewer patients in the HR-positive group experienced a CNS event as their first progression compared with patients in the HR-negative group (16.4% vs. 25.3%). Conversely, patients in the HR-positive group were more likely to have nonvisceral sites as the site of first progression than were patients in the HR-negative group (25.7% vs. 12.8%; Table 3).
Trastuzumab Plus HT Versus HT Only
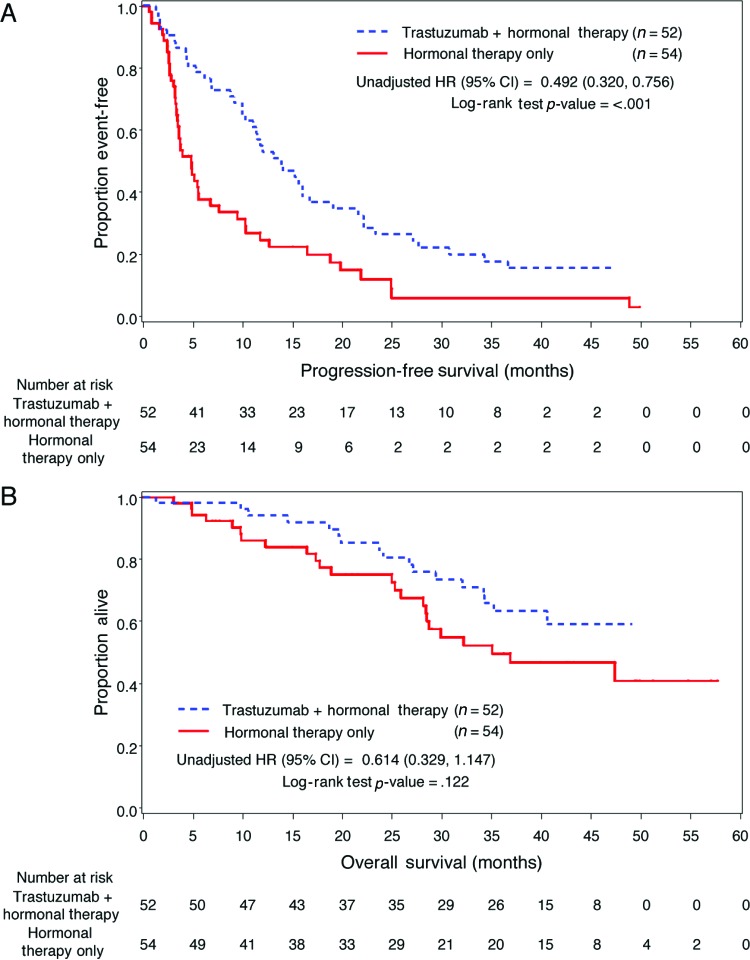
Patients receiving trastuzumab and HT (n = 52) had longer PFS compared with patients who received HT only (n = 54; 13.8 vs. 4.8 months; unadjusted HR: 0.49, 95% CI: 0.32–0.76, p < .001; Table 3, Fig. 2A). Median OS for the trastuzumab plus HT group was not reached, whereas the median OS for the HT only group was 35.1 months (unadjusted HR: 0.61, 95% CI: 0.33–1.15, p = .122; Fig. 2B, Table 3). In multivariate analyses for PFS, the adjusted risk of disease progression in patients treated with trastuzumab plus HT was significantly lower than for patients treated with HT only (adjusted HR: 0.37; 95% CI: 0.22–0.60, p < .001). The adjusted HR for OS was reduced for patients treated with trastuzumab plus HT but was not statistically significant (adjusted HR: 0.55; 95% CI: 0.27–1.14, p = .109).
Figure 2.
Kaplan-Meier estimates. (A): Kaplan-Meier estimated progression-free survival in hormone receptor (HR)-positive patients for first-line treatment with trastuzumab plus hormonal therapy versus hormonal therapy only. (B): Kaplan-Meier estimated overall survival in HR-positive patients for first-line treatment with trastuzumab plus hormonal therapy versus hormonal therapy only.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Trastuzumab Plus Chemotherapy Plus HT Versus Trastuzumab Plus Chemotherapy, and Sequential Versus Concurrent Therapy
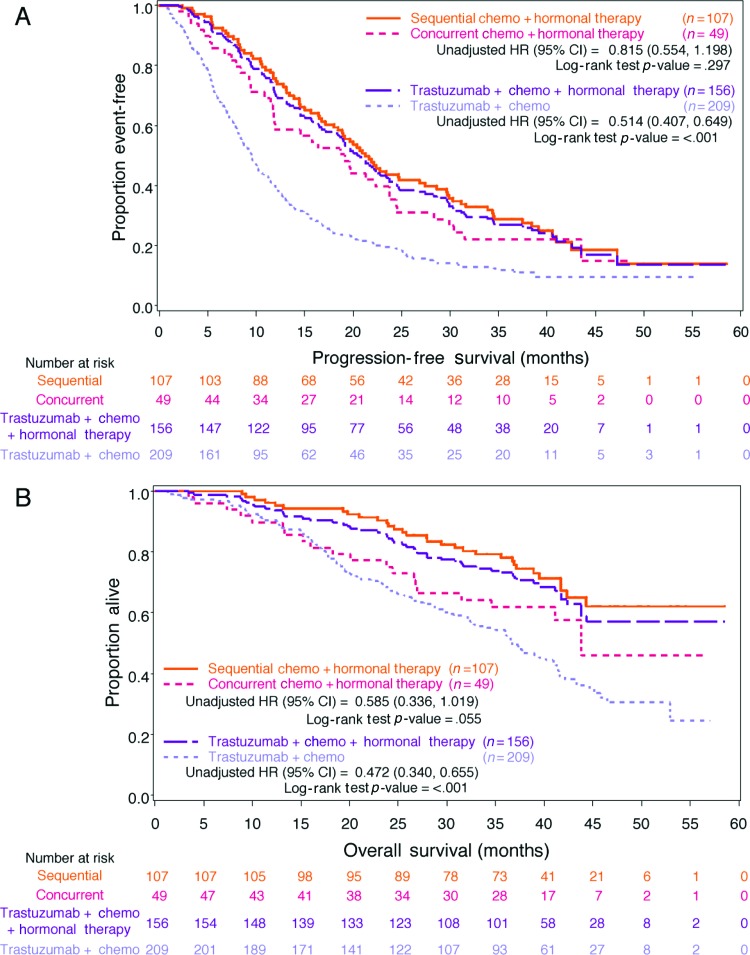
Compared with patients who received only trastuzumab plus chemotherapy (n = 209), patients who received trastuzumab plus chemotherapy plus HT (n = 156) had longer median PFS times (20.4 vs. 9.5 months; unadjusted HR: 0.51, 95% CI: 0.41–0.65; Table 3, Fig. 3A). Median OS time was not reached for the trastuzumab plus chemotherapy plus HT group, whereas the median OS time for the trastuzumab plus chemotherapy group was 36.7 months (unadjusted HR: 0.47, 95% CI: 0.34–0.66; Table 3, Fig. 3B). After adjustment for clinically significant baseline and tumor characteristics, patients in the trastuzumab plus chemotherapy plus HT group had a statistically significant lower risk for disease progression compared with patients treated with trastuzumab plus chemotherapy (adjusted HR: 0.53, 95% CI: 0.42–0.68, p < .001). The risk of death was also statistically significantly lower for patients treated with trastuzumab plus chemotherapy plus HT (adjusted HR: 0.50, 95% CI: 0.36–0.70, p < .001).
Figure 3.
Kaplan-Meier estimates. (A): Kaplan-Meier estimated progression-free survival in hormone receptor (HR)-positive patients for first-line treatment with trastuzumab plus chemotherapy plus hormonal therapy versus trastuzumab plus chemotherapy, and sequential versus concurrent therapy. (B): Kaplan-Meier estimated overall survival in HR-positive patients for first-line treatment with trastuzumab plus chemotherapy plus hormonal therapy versus trastuzumab plus chemotherapy, and sequential versus concurrent therapy.
Abbreviations: Chemo, chemotherapy; CI, confidence interval; HR, hazard ratio.
To account for the bias in patients who received sequential HT to be enriched for those with responsive disease, we analyzed concurrent and sequential first-line HT use separately. In registHER, of the 156 patients who received trastuzumab plus chemotherapy plus HT, 49 patients received chemotherapy and HT concurrently (i.e., chemotherapy and HT overlapped for at least 30 consecutive days) and 107 received these treatments sequentially. Fewer patients in the sequential group experienced a CNS event as their first progression compared with patients in the concurrent group (18.5% vs. 25.0%; Table 3). However, patients in the sequential treatment group were more likely to have nonvisceral progression (32.1% vs. 19.4%) as their first progression. Conversely, patients in the concurrent group were more likely to have visceral disease as their first progression compared with patients in the sequential treatment group (41.7% vs. 35.8%). The duration of trastuzumab treatment for patients in the two subgroups was similar (data not shown). The only difference between the two subgroups was the duration of HT and chemotherapy received: patients in the concurrent subgroup received both chemotherapy and HT for longer. The median duration of HT in the concurrent versus sequential subgroup was 15.4 months (range: 0.9–47.8 months) versus 13.6 months (range: 0.9–54.3 months), respectively. Median duration of chemotherapy in concurrent versus sequential subgroups was 5.1 months (range: 0.4–47.8 months) versus 4.4 months (range: 0.6–13.4 months), respectively.
Kaplan-Meier estimated curves for the concurrent and sequential patient subgroups are shown in Figure 3A and 3B. For the patients who received chemotherapy and HT sequentially, PFS was longer than it was for those who received chemotherapy and HT concurrently (21.3 vs. 19.1 months; Table 3). Patients who received sequential chemotherapy and hormone treatment compared with those who received concurrent treatment demonstrated a nonsignificant lower risk of disease progression (unadjusted HR: 0.82, 95% CI: 0.55–1.20, p = .297; Fig. 3A). The median OS for sequential therapy was not reached; for concurrent therapy, it was 43.8 months. The hazard of death for OS was reduced for patients who received sequential therapy, with borderline statistical significance (unadjusted HR: 0.59, 95% CI: 0.34–1.02, p = .055; Fig. 3B). In multivariate analyses for PFS, the adjusted risk of disease progression in patients treated sequentially was reduced, but it was not statistically significant when compared with patients treated concurrently (adjusted HR: 0.81, 95% CI: 0.54–1.21, p = .303). A statistically significant reduction in risk of death was observed (adjusted HR: 0.48; 95% CI: 0.26–0.89, p = .019).
A sensitivity analysis examining survival in patients with sequential versus concurrent treatment, including patients treated with chemotherapy and HT (n = 11), was conducted. Results for this analysis did not differ from those for the primary analyses and are not included in this report.
Discussion
The registHER data provide real-world evidence that, in HER2-positive/HR-positive patients with MBC, the targeting of both hormone and HER2 receptors is associated with a benefit compared with HER2-based therapy alone. HR-positive patients in registHER had longer median PFS times from time of MBC diagnosis than did HR-negative patients (11.7 vs. 8.8 months) and HER2-positive/HR-positive patients receiving trastuzumab plus HT had significantly longer PFS times than patients who received HT only (13.8 vs. 4.8 months). For patients who received trastuzumab plus chemotherapy, patients who also received HT also had longer median PFS times (20.4 vs. 9.5 months). However, the cohort of patients who received trastuzumab plus chemotherapy plus HT had received more trastuzumab therapy than those in the trastuzumab plus chemotherapy cohort. In addition, the trastuzumab plus chemotherapy plus HT cohort had a longer DDFI between the end of nonhormonal adjuvant treatment and metastatic diagnosis than did patients in the trastuzumab plus chemotherapy cohort (24.9 vs. 35.1 months), indicating that as a group they may have had a better prognosis or more responsiveness to HT.
Multivariate analysis conducted in the registHER study population to adjust for prognostic factors including age, ECOG status, and sites of metastatic disease that could influence treatment choice suggest that, for patients with HER2-positive/HR-positive tumors receiving treatment with trastuzumab and chemotherapy, adding HT may provide an independent beneficial effect (HR: 0.53, p < .001). To account for the inherent bias in patients receiving sequential HT to have been selected due to response or stability to initial therapy, we separated those patients treated with concurrent as opposed to sequential therapy and we found that both HT groups had significantly longer PFS and OS times compared with patients not treated with HT.
Our retrospective analysis shows that there is lack of uniformity in how HT is used for treating HER2-positive breast cancer, with a tendency to use chemotherapy for patients with heavier burden and visceral disease. Such biases in treatment based on clinical factors complicated the analysis of retrospective or prospective registry studies. However, in our analysis, adjustments for these factors showed consistent improved PFS times—and in some cases, OS times—with the addition of HT to either trastuzumab alone or with chemotherapy. In the absence of chemotherapy, randomized trials have shown a benefit from the addition of trastuzumab or lapatinib to aromatase inhibitor HT [11, 12]. However, prospective randomized trials have not formally tested the addition of HT to trastuzumab and optimal therapy for this group is not well defined [19].
Preclinical studies have suggested that endocrine therapy, which prevents cells from proliferating, and chemotherapy, which requires that cells be actively dividing, may be antagonistic [13–16]. Nevertheless, there has not been firm consensus about whether concurrent or sequential treatment with chemotherapy and HT is better [20]. Albain et al. showed that adjuvant chemotherapy with cyclophosphamide, doxorubicin, and fluorouracil (CAF) plus tamoxifen given sequentially is more effective adjuvant therapy than tamoxifen alone for postmenopausal patients with endocrine-responsive, node-positive breast cancer [21]. However, concurrent compared with sequential therapy has not been tested with aromatase inhibitors, which have a different mechanism of action compared with tamoxifen. In a retrospective analysis of 111 patients with advanced HER2- and hormone receptor-positive disease, maintenance endocrine therapy added to trastuzumab upon the completion of chemotherapy was associated with a significant PFS benefit [22]. In an uncontrolled clinical trial that tested a combination of trastuzumab, lapatinib, and letrozole for patients with HER2 and HR-positive cancers, rates of complete pathological response (pCR) of 21% and pCR plus near-pCR of 54% were noted—similar to that of the HR-negative cohort treated without letrozole [23]. Still, data from randomized trials are lacking to demonstrate and quantify the independent role of first-line HT, either concurrently or sequentially with chemotherapy in HER2- and HR-positive advanced breast cancer.
registHER is a real-world prospective, observational study. As such, there may be several limitations that exist with these analyses compared with prospective, randomized controlled trials. The registHER patients may not be fully representative of the population at large as a cohort in a population-based study, although practices were encouraged to enroll all eligible patients. Given the timeframe of this study, only 5.6% of patients (21 of 377) in the HR-positive cohort received the current standard of adjuvant trastuzumab. Therefore, the clinical outcomes from registHER may be different than those that arise from more current cohorts; this issue is being addressed by the ongoing systHERs registry (NCT01615068). Some trends in this study are noted to be nonsignificant and may be subject to immortal time bias, particularly in the sequential group. Additionally, possible “confounding by indication” may be inherently present, due to the nonrandomized, observational nature of the registHER study.
Although data collection for PFS analysis may not be controlled as it would be in a randomized trial with scheduled tumor assessments, it may more closely represent standard real-world practice where treatment is changed almost exclusively due to progression or unacceptable side effects; OS should not be biased in this manner. Residual confounding may also occur as a result of factors unaccounted for or insufficiently captured in this study. Because patients may have had a diagnosis of MBC up to 9 months prior to enrollment and patients with longer survival may be more likely to enroll, OS estimates from time of MBC diagnosis in registHER may be slightly higher than expected in a general population of patients with MBC. There also is the possibility of a selection bias for OS, as there is no accepted method of adjusting OS for all postprogression treatments. Finally, limited information was collected for cause of death (options included only “cancer” and “other”) and was missing for 32 of 538 deaths (>5% of deaths) in the registHER cohort, which precluded the calculation of breast cancer-specific mortality rates in this study.
Conclusions
In this large prospective registry that used standard treatment approaches from the last decade for HER2-positive MBC, HR positivity was associated with a lower likelihood of having received adjuvant chemotherapy. At the time of metastasis, HR-positive patients had a lower degree of disease burden and visceral involvement compared with HR-negative cases. In patients with HER2- and HR-positive disease, the use of either concurrent or sequential HT was associated with improved PFS and OS times when added to trastuzumab either with or without chemotherapy; however, after adjusting for other relevant variables, the OS superiority was only significant in patients receiving chemotherapy. The use of HT—either concurrently or sequentially—appears to improve outcomes when added to trastuzumab-based therapy for HER2- and HR-positive MBC, although further study is needed to understand the causal relationship and degree of this benefit, whether it pertains equally to tamoxifen and aromatase inhibitors, and the impact of concurrent compared with sequential therapy.
Acknowledgments
This work was supported by Genentech, Inc., South San Francisco, California, USA. Copyediting and editorial assistance for this manuscript was provided by Tmirah Haselkorn, Ph.D. (EpiMetrix, Inc.) and was financially supported by Genentech. We thank Iulia Cristina Tudor and Ken Dana for their statistical programming expertise.
Author Contributions
Conception/Design: Debu Tripathy, Peter A. Kaufman, Adam M. Brufsky, Musa Mayer, Marianne Ulcickas Yood, Bongin Yoo, Cheng Quah, Denise Yardley, Hope S. Rugo
Provision of study material or patients: Peter A. Kaufman, Adam M. Brufsky, Marianne Ulcickas Yood, Denise Yardley, Hope S. Rugo
Collection and/or assembly of data: Peter A. Kaufman, Adam M. Brufsky, Marianne Ulcickas Yood, Denise Yardley, Hope S. Rugo
Data analysis and interpretation: Debu Tripathy, Peter A. Kaufman, Adam M. Brufsky, Marianne Ulcickas Yood, Bongin Yoo, Cheng Quah, Denise Yardley, Hope S. Rugo
Manuscript writing: Debu Tripathy, Peter A. Kaufman, Adam M. Brufsky, Musa Mayer, Marianne Ulcickas Yood, Bongin Yoo, Cheng Quah, Denise Yardley, Hope S. Rugo
Final approval of manuscript: Debu Tripathy, Peter A. Kaufman, Adam M. Brufsky, Musa Mayer, Marianne Ulcickas Yood, Bongin Yoo, Cheng Quah, Denise Yardley, Hope S. Rugo
Disclosures
Debu Tripathy: Genentech (C/A, H); Peter A. Kaufman: Genentech (C/A, RF); Adam M. Brufsky: Genentech (C/A, H, SAB); Musa Mayer: Genentech (C/A); Marianne Ulcickas Yood: Genentech (C/A, H, SAB); Bongin Yoo: Genentech (E, OI); Cheng Quah: Genentech (E, OI); Denise Yardley: Genentech (C/A); Hope S. Rugo: Genentech, GlaxoSmithKline, Novartis, Pfizer, Merck (RF).
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Brufsky A, Lembersky B, Schiffman K, et al. Hormone receptor status does not affect the clinical benefit of trastuzumab therapy for patients with metastatic breast cancer. Clin Breast Cancer. 2005;6:247–252. doi: 10.3816/CBC.2005.n.027. [DOI] [PubMed] [Google Scholar]
- 5.Todorovic-Rakovic N, Neskovic-Konstantinovic Z, Nikolic-Vukosavljevic D. Cross-talk between ER and HER2 in breast carcinoma. Arch Oncol. 2006;14:146–150. [Google Scholar]
- 6.Sabnis G, Brodie A. Understanding resistance to endocrine agents: Molecular mechanisms and potential for intervention. Clin Breast Cancer. 2010;10:E6–E15. doi: 10.3816/CBC.2010.n.014. [DOI] [PubMed] [Google Scholar]
- 7.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 10.Buzdar AU. Role of biologic therapy and chemotherapy in hormone receptor- and HER2-positive breast cancer. Ann Oncol. 2009;20:993–999. doi: 10.1093/annonc/mdn739. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 12.Johnston S, Pippen J, Jr., Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg GJ, Froese EK. Antagonism of the cytocidal activity and uptake of melphalan by tamoxifen in human breast cancer cells in vitro. Biochem Pharmacol. 1985;34:763–770. doi: 10.1016/0006-2952(85)90755-5. [DOI] [PubMed] [Google Scholar]
- 14.Osborne CK, Kitten L, Arteaga CL. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J Clin Oncol. 1989;7:710–717. doi: 10.1200/JCO.1989.7.6.710. [DOI] [PubMed] [Google Scholar]
- 15.Hug V, Hortobagyi GN, Drewinko B, et al. Tamoxifen-citrate counteracts the antitumor effects of cytotoxic drugs in vitro. J Clin Oncol. 1985;3:1672–1677. doi: 10.1200/JCO.1985.3.12.1672. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland RL, Hall RE, Taylor IW. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983;43:3998–4006. [PubMed] [Google Scholar]
- 17.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 19.Glück S, Arteaga CL, Osborne CK. Optimizing chemotherapy-free survival for the ER/HER2-positive metastatic breast cancer patient. Clin Cancer Res. 2011;17:5559–5561. doi: 10.1158/1078-0432.CCR-10-2051. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KI. Combining endocrine agents with chemotherapy: Which patients and what sequence? Cancer. 2008;112(3 suppl):718–722. doi: 10.1002/cncr.23189. [DOI] [PubMed] [Google Scholar]
- 21.Albain K, Barlow W, Ravdin P, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montemurro F, Rossi V -CR, Cossu Rocca M, et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer. 2012;118:17–26. doi: 10.1002/cncr.26162. [DOI] [PubMed] [Google Scholar]
- 23.Rimawi MF, Mayer IA, Forero A, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013 Apr 8; doi: 10.1200/JCO.2012.44.8027. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]