Despite advances in therapy options, few guidelines or reviews address the optimal timing or methodology for the radiographic detection of metastatic disease in patients with advanced prostate cancer. This review discusses the current status of predicting the presence of metastatic disease, with a particular emphasis on the detection of the M0 to M1 transition, and reviews current data on newer imaging technologies that are changing the way metastases are detected.
Keywords: Imaging, Lymph nodes, Magnetic resonance imaging, Neoplasm metastasis, Prostatic neoplasms, Radionuclide imaging
Abstract
The therapeutic landscape for the treatment of advanced prostate cancer is rapidly evolving, especially for those patients with metastatic castration-resistant prostate cancer (CPRC). Despite advances in therapy options, the diagnostic landscape has remained relatively static, with few guidelines or reviews addressing the optimal timing or methodology for the radiographic detection of metastatic disease. Given recent reports indicating a substantial proportion of patients with CRPC thought to be nonmetastatic (M0) are in fact metastatic (M1), there is now a clear opportunity and need for improvement in detection practices. Herein, we discuss the current status of predicting the presence of metastatic disease, with a particular emphasis on the detection of the M0 to M1 transition. In addition, we review current data on newer imaging technologies that are changing the way metastases are detected. Whether earlier detection of metastatic disease will ultimately improve patient outcomes is unknown, but given that the therapeutic options for those with metastatic and nonmetastatic CPRC vary, there are considerable implications of how and when metastases are detected.
Implications for Practice:
Many new agents are approved for metastatic castrate resistant prostate cancer (CRPC) yet no guidelines exist on imaging which is essential to confirm the presence of metastatic disease. Herein we review and discuss current data and techniques for the detection of metastatic disease. Today there is a greater depth in understanding of factors contributing to the risk of metastatic disease as well as a greater appreciation of the strengths and weaknesses of various radiographic techniques. Understanding these issues has direct clinical relevance.
Introduction
The vast majority of patients diagnosed with prostate cancer in the U.S. have clinically localized disease at diagnosis [1]. Although radical prostatectomy (RP) or radiation therapy is given with curative intent, 20%–40% of patients will eventually experience biochemical recurrence; most patients will then receive androgen-deprivation therapy (ADT) [2]. Biochemical recurrence following ADT, despite castrate levels of testosterone, represents development of castration-resistant prostate cancer (CRPC), which, in many cases, is followed by M1 metastatic disease; bone is the most common site of detectable metastasis, followed by the lymph nodes [3–6].
The decision to image for metastatic screening is made to gather information that will optimize patient management. Historically, the standard modality for such imaging of bones has been 99mTc-polyphosphonate bone scintigraphy (BS). However, the sensitivity and specificity of BS are poor in comparison with newer imaging modalities such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI) [7]. Similarly, the sensitivity and specificity of newer imaging modalities, such as ultrasmall superparamagnetic iron oxide (USPIO) MRI and diffusion-weighted USPIO MRI, provide vast improvements on standard techniques for investigating lymph node involvement (e.g., pelvic lymph node dissection, morphologic MRI, and computed tomography [CT]) [8, 9]—although, frustratingly, these techniques are not yet widely available in clinical practice. As technology develops, the question naturally arises as to whether techniques other than BS and CT should be used to detect smaller metastases (bone or soft tissues); if so, would this information produce any clinical benefit? Likewise, following verification in wider patient populations, it is important to determine how newer methods of lymph node assessment fit into the diagnosis/assessment paradigm to maximize their clinical value.
This question, as well as the need to reassess the optimal methodology and timing of metastasis detection by imaging techniques, are brought into sharper focus by the rapidly changing treatment landscape, particularly in metastatic CRPC. On the basis of improved overall survival, the U.S. Food and Drug Administration (FDA) has recently approved cabazitaxel, abiraterone, and enzalutamide in the post-docetaxel setting, and radium-223 is expected to be approved in the near future. However, it is the asymptomatic or minimally symptomatic metastatic CPRC patient population that is of particular relevance to this review. Sipuleucel-T is already approved in this setting. Abiraterone has been recently approved in this setting on the basis of the phase III Cougar 302 study [10]. In addition, phase III trials including asymptomatic and minimally symptomatic patients are ongoing with orteronel (NCT01193244) [11], enzalutamide (NCT01212991) [12], ipilimumab (NCT01057810) [13], and Prostvac (Bavarian Nordic, Mountain View, CA) (NCT01322490) [14]. As more therapies become available for the treatment of asymptomatic and minimally symptomatic disease, it is reasonable to ask whether the earlier detection of smaller volume metastases would provide better outcomes for these therapeutic options. Answers to this question will await further trials.
Irrespective of the details of imaging and therapeutic developments, there are currently few guidelines or reviews to aid clinicians in the decision to attempt imaging techniques for the first evidence of metastasis [15–17]. Thus, we feel that it is timely to review the literature on the prevalence, risk, and prediction of metastasis in prostate cancer, discuss current and future imaging practice, and highlight potential unmet needs around the imaging workup diagnosis of M1 disease.
This review is based on key literature searches of the PubMed database and initial discussions at a clinical advisory board. Search terms included the following and words derived from the following: prostate cancer; biochemical recurrence; bone metastasis; bone metastatic; metastatic castrate resistant; hormone refractory; prostate-specific antigen; PSA doubling time; biomarker; imaging; bone scan, bone scintigraphy; radionuclide bone scintigraphy; PET scan; F-18 fluorodeoxyglucose positron emission tomography; computed tomography; CT; MRI; radiographic imaging; prostatectomy, radiation therapy; radiotherapy; salvage radiotherapy; nomogram; predictive.
Prevalence and Risk of Metastatic Disease
The decision to image for metastases should be based on the likelihood of detection. It is therefore important to assess and understand the natural history of the disease. In the following section, we discuss a number of retrospective and prospective analyses that have been made in postdefinitive therapy, ADT-naïve patients, and post-ADT patients to assess time to progression and predictive factors.
ADT-Naïve Patients
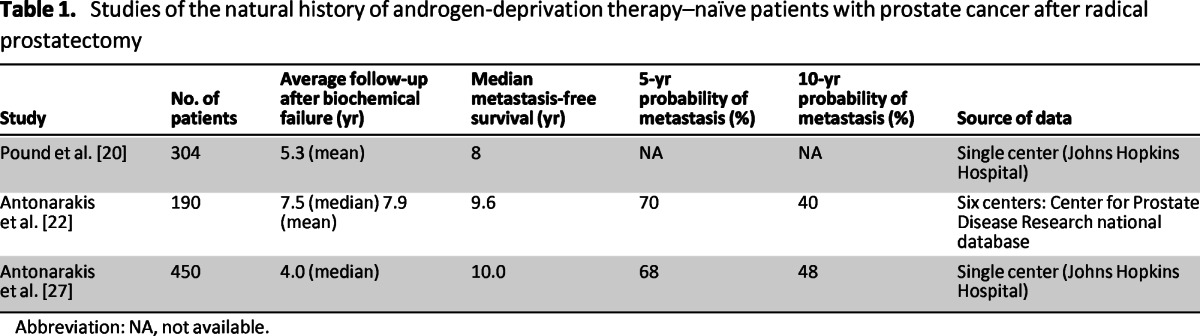
ADT-naïve, post-RP patients with biochemical recurrence can remain free from metastatic disease (detectable by BS or x-ray CT) for an extended period [18–21]. Several large retrospective studies of the natural disease history have found median metastasis-free survival after prostate-specific antigen (PSA) recurrence in this population, in the absence of adjuvant therapy, salvage therapy, or ADT, to be in the order of a median of 8–10 years (Table 1) [20–22]. We note that these studies rarely represent present-day clinical populations in which there is a high rate of ADT use prior to the onset of metastatic disease [23, 24].
Table 1.
Studies of the natural history of androgen-deprivation therapy–naïve patients with prostate cancer after radical prostatectomy
Abbreviation: NA, not available.
Numerous studies have evaluated disease factors such as PSA, PSA velocity, PSA doubling time (PSADT), Gleason score, and time to biochemical recurrence following definitive therapy, which might predict a patient's risk of progression to M1 disease in the absence of treatment. PSA at time of positive scan appears to be highly variable and, as a sole parameter, provides little indication of when to perform BS (Fig. 1) [25, 26]. Loeb et al. reported on 193 post-RP, ADT-naïve patients with newly diagnosed bone metastases; 25.9% had PSA <10 ng/mL, 50.8% had PSA 10–100 ng/mL, and 23.3% had PSA >100 ng/mL [25]. In a small, retrospective study of 128 patients, Okotie et al. found a significant interaction between PSADT and PSA at time of CT or BS. In patients with PSADT <6 months and PSA >10 ng/mL, probability of a positive scan was 46% compared to 11% for PSA <10 ng/mL [19]. The same authors failed to find the Gleason score or time to PSA recurrence to be predictive of a positive scan. Analysis of PSA at time of bone metastasis in the Johns Hopkins Hospital database (n = 126) found an inverse correlation between PSADT and PSA at metastasis, with median PSA values of 40.4, 35.4, 26.4, and 19.3 ng/mL for PSADT of ≤3, 3–9, 9–15, and ≥15 months, respectively [27]. These results may, however, represent a bias toward the inevitable higher PSA that occurs if scans are performed at regular intervals in patients with varying PSADTs.
Figure 1.
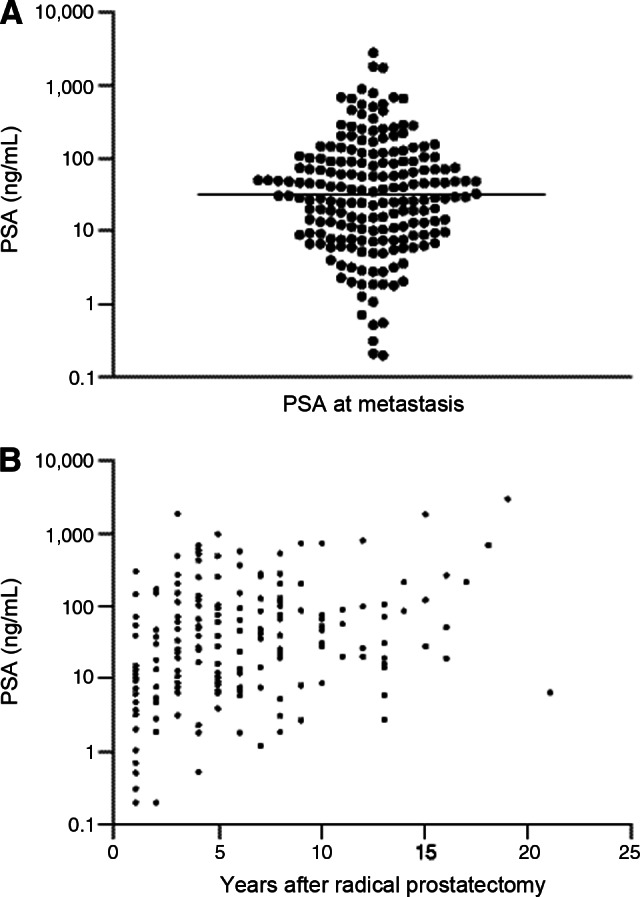
Distribution of prostate-specific antigen at metastasis to bone on log scale (A) and by years from radical prostatectomy to metastasis (B). Adapted from [25] with permission from Elsevier and the American Urological Association.
Abbreviation: PSA, prostate-specific antigen.
Multivariate analysis of both the multicenter Center for Prostate Disease Research cohort and the single-center Johns Hopkins Hospital database (Table 1) found PSADT to be an independent predictor of metastasis-free survival [21, 22]. The importance of PSADT is particularly stressed in the multivariate analysis of the Johns Hopkins Hospital database [21]. Patients with PSADT of >15 months were assigned a relative risk of metastases of 1.0. For patients with PSADT of <3 months, the hazard ratio was 33.3; for PSADT of 3.0–8.9 months, the hazard ratio was 8.0. Analysis of the same database also identified the Gleason score to be independently predictive (hazard ratio for Gleason score 8–10: 4.5; 95% confidence interval [CI]: 1.7–11.9; p = .002) [21].
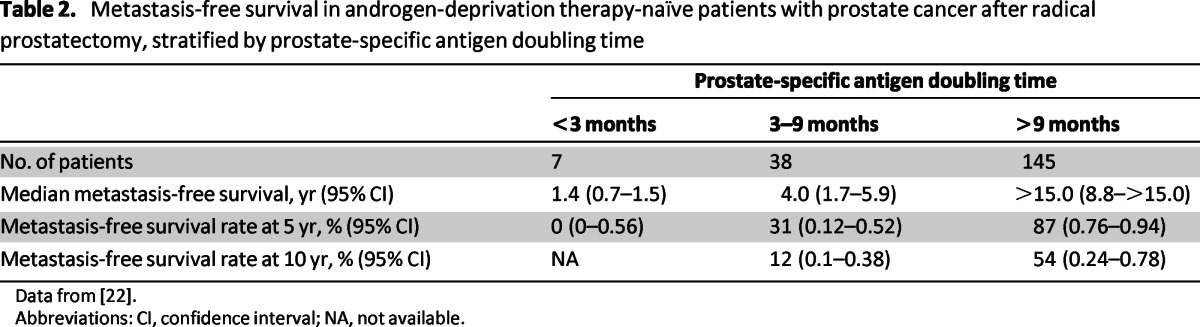
Gleason score, time to PSA relapse, and PSADT were all found to be predictive of metastasis-free survival in a univariate analysis in the Center for Prostate Disease Research study, but PSADT was identified as the strongest determinant of metastasis-free survival in PSA-recurrent disease [22]. The authors of both studies provided roughly similar estimates of the timing of metastasis, stratified according to PSADT (Table 2) [21, 22]. In a recent retrospective multivariate analysis of patients receiving investigational therapy, baseline PSADT, baseline PSA slope, on-study change in PSADT, and on-study change in PSA slope were found to be independently predictive of metastasis-free survival. For patients with or without any decrease in PSA slope 6 months after treatment, median time to metastasis was 63.5 months (95% CI: 34.6–not reached) and 28.9 months (95% CI: 13.5–68.0), respectively [28].
Table 2.
Metastasis-free survival in androgen-deprivation therapy-naïve patients with prostate cancer after radical prostatectomy, stratified by prostate-specific antigen doubling time
Data from [22].
Abbreviations: CI, confidence interval; NA, not available.
Thus, although the highly variable time to metastasis in the post-RP, ADT-naïve population presents a challenge in identifying the best time to image for metastasis, there are general predictive factors, particularly rapid PSADT, that indicate time to metastatic disease. However, all of the above studies were retrospective and subject to a number of limitations. Interpretation of retrospective data is difficult because the frequency of scans is often not reported. In addition, there is the possibility of a lack of standardization of biochemical and/or pathological assessment. The authors of the Johns Hopkins Hospital database cite possible bias in their studies because of nonrandom exclusion of patients receiving adjuvant or salvage therapy, thereby possibly eliminating those with worse prognosis. Furthermore, the radiographic studies are bone-centric and the incidence of developing lymph node and visceral metastases remains largely unstudied.
Post-ADT Patients
The need for more accurate assessment of the probability of metastasis has been emphasized by recently published data, indicating that the frequency of metastases in patients considered to have nonmetastatic CRPC is much higher than previously thought. Analysis of screening failures in the global phase III ENTHUSE study found that 32% of 2,577 men screened for a trial enrolling patients with M0 CRPC did, in fact, have metastatic disease (detectable by BS or CT/MRI), although further interpretation of these data is limited because details of PSA levels, PSADT, and location of metastases were not provided [29].
Prospective data on the natural history of CRPC are provided by the placebo arms of three randomized, double-blind, phase III trials in patients with nonmetastatic CRPC (Table 3), the most recent of which was the denosumab study (Amgen 147) in which the cohort consisted only of patients considered to be at high risk of metastasis (PSA ≥8.0 ng/mL, PSADT ≤10.0 months, or both). In the placebo arms of these trials, ∼26% of patients developed bone metastases (detectable by BS) within 1 year of randomization, whereas the proportion with bone metastases within 2 years ranged from 33% to 46% (Table 3) [30–32]. Median time to bone metastasis was 25–30 months (Table 3) [30–33].
Table 3.
Comparison of the placebo arms of three prospective trials in patients with nonmetastatic castration-resistant prostate cancer
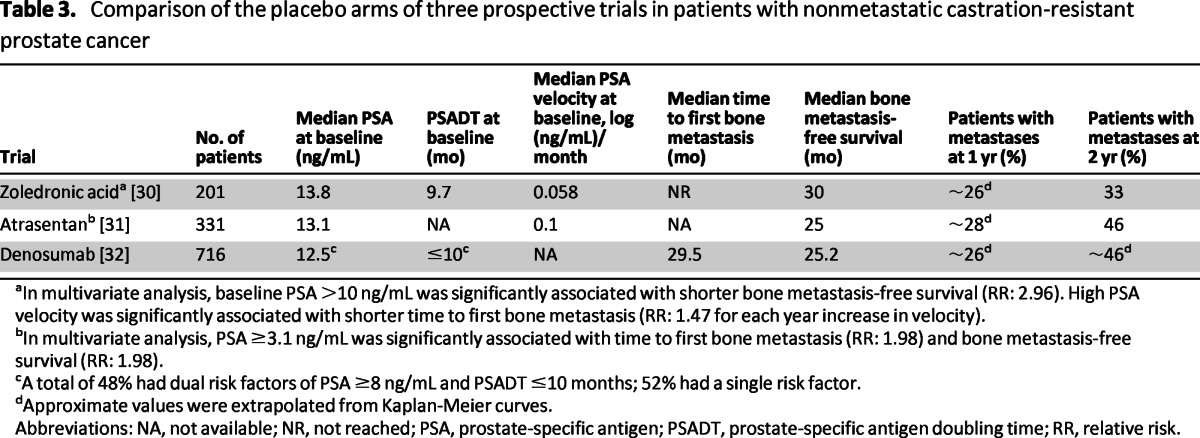
aIn multivariate analysis, baseline PSA >10 ng/mL was significantly associated with shorter bone metastasis-free survival (RR: 2.96). High PSA velocity was significantly associated with shorter time to first bone metastasis (RR: 1.47 for each year increase in velocity).
bIn multivariate analysis, PSA ≥3.1 ng/mL was significantly associated with time to first bone metastasis (RR: 1.98) and bone metastasis-free survival (RR: 1.98).
cA total of 48% had dual risk factors of PSA ≥8 ng/mL and PSADT ≤10 months; 52% had a single risk factor.
dApproximate values were extrapolated from Kaplan-Meier curves.
Abbreviations: NA, not available; NR, not reached; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RR, relative risk.
As in the ADT-naïve setting, PSA and PSADT are predictors of positive BS in post-ADT patients [30, 31]. Multivariate analysis of the zoledronic acid trial found baseline PSA levels >10 ng/mL and high PSA velocity (increase in relative risk of 4.34 for each 0.01 increase in PSA velocity) to be significantly associated with shorter time to first bone metastasis and shorter bone metastasis-free survival. In the atrasentan study, baseline PSA levels of ≥13.1 ng/mL were significantly associated with shorter bone metastasis-free survival, shorter overall survival, and shorter time to first bone metastasis (Table 3).
Data from the zoledronic acid, atrasentan, and denosumab trials—although being the best currently available for broadly stratifying patients with CRPC according to risk—are nonetheless subject to limitations that make them imperfect for the accurate assessment of the probability of metastasis as a function of time. Each of the protocols relied on BS for first-line detection and none specified serial screening for soft-tissue metastasis by cross-sectional imaging.
Predicting Metastatic Disease
Most clinicians use the disease parameters discussed above, such as PSA and PSADT, to assess the risk of metastatic disease in individual patients and to inform decisions on when to start imaging. Nevertheless, tools that can improve predictive accuracy of a patient's risk are potentially attractive to improve clinical decision-making.
Of the predictive tools that are available, nomograms are considered the most accurate by many investigators [34–36] and a number exist for post-RP patients. Slovin et al. used a prospective study of 148 patients with rising PSA and PSADT <12 months after RP or radiation therapy to develop a nomogram for predicting the probability of 1- and 2-year progression-free survival [37], whereas Dotan et al. used retrospective data to develop a tool to predict the risk of current BS positivity [38]. Inman et al. developed an algorithm that predicts the risk of metastases from any time point after RP, although the frequency of scanning in the cohort analyzed to develop the tool was not defined [39].
Gotto et al. recently presented an abstract on the first nomogram for predicting current BS positivity in ADT-treated patients, based on retrospective analysis of 1,650 patients from the Memorial Sloan-Kettering Cancer Center database who were followed from the start of ADT treatment to first positive scan or last hospital visit (scanning frequency not specified) [40]. Based on clinical relevance and data availability, 13 variables were included in the nomogram model: number of previous negative BS, PSA, PSADT, prostatectomy, radiotherapy, brachytherapy, chemotherapy, immunotherapy, estrogen therapy, cryotherapy, clinical trial enrollment, and most recent primary and secondary Gleason grades. Concordance index for the nomogram was 0.76. This important nomogram indicates that a variety of factors—some pre-ADT and others post-ADT—affect time to radiographic metastasis. Additional validation of this nomogram is required before applying it to the general patient population.
Evidence from the ADT-naïve prostate cancer setting [41] and other therapeutic areas [42] indicates that nomograms tend to outperform the predictions of medical experts, providing a strong rationale for a greater role for nomograms in this disease setting should the validity of these initial attempts be verified. Nomograms will need to change to accommodate the use of newer imaging techniques as they become more commonly used.
Current and Future Imaging Methods: Bone Metastases
Planar 99mTc-methylene diphosphonate BS has long been the standard modality for first-line detection of metastases in prostate cancer [7], but issues around its sensitivity and specificity, combined with advances in other imaging technologies, have led to uncertainties regarding its sensitivity in the diagnosis of M1 disease in the current clinical environment. 99mTc-methylene diphosphonate binds to areas of calcium deposition and acts as a nonspecific marker of osteoblastic activity that localizes at sites of active bone mineralization [43]. Thus, although it accumulates at sites of tumor growth, it also identifies a number of nonmalignant bone conditions, such as recent fractures, areas of inflammation, mechanical stress, and degenerative joint disease [44–46]. Low specificity often leads to ambiguity in the interpretation of scans that may or may not be subsequently resolved by conventional x-ray imaging. Although sensitivities in the range of 62%–89% have been reported compared to detection by MRI [47], BS cannot detect sites of recent metastatic cell seeding because only areas of relatively advanced tumor growth with active osteoblastic remodeling are detectable. Indeed, we know very little regarding when the transition from M0 to M1 actually occurs using BS imaging in “typical” patients. More recent trials have included only patients perceived to be at higher risk of metastatic disease. In the following sections, we focus on the relevant literature that has been reported for SPECT, PET, and MRI; for a more in-depth discussion, please see the review by Tombal and Lecouvet [7].
SPECT
The accuracy of standard anterior and posterior planar BS in detecting lumbar metastases can be improved by additional SPECT to generate a three-dimensional image [48–50], with minimal increases in radiation dose. Several studies have demonstrated that SPECT outperforms planar BS alone with respect to sensitivity, specificity, positive predictive value, and negative predictive value [50, 51].
PET
There is some controversy surrounding the suitability of the most common PET isotope, [18F]-fluorodeoxyglucose, as a metabolic marker of prostate cancer metastases, due to its low sensitivity in some reports [52]. However, there are a number of alternative [18F]- or [11C]-labeled markers that may be more appropriate for detection of prostate cancer bone metastases, with clear evidence suggesting improvements in sensitivity and specificity for PET using these markers over conventional BS [50]. [18F]-fluoride PET is approved by the FDA for bone imaging and [11C]-choline PET was also approved very recently [53].
In small prospective studies, McCarthy et al. reported good sensitivity and specificity using [18F]-fluoromethylcholine PET in patients with CRPC [54]. Even-Sapir et al. found that [18F]-fluoride PET-CT was highly sensitive and specific for the detection of bone metastases in patients with high-risk prostate cancer. Furthermore, [18F]-fluoride PET-CT fusion imaging was more specific than [18F]-fluoride PET alone, and more sensitive and specific than planar and SPECT BS [50]. A small retrospective study found [11C]-choline PET-CT to have similar sensitivity for detection of bone and lymph node metastases (96.6%), and slightly lower specificity (76.5%) to that reported for [18F]-fluoride PET-CT in the Even-Sapir et al. study [55]. Other markers that require future investigation of their suitability for imaging of prostate cancer metastases [56] include [18F]-dihydroxyphenylalanine, [18F]-fluorocholine [57–59], [18F]- or [11C]-acetate [57, 60], [11C]-methionine [56], [18F]-fluorodihydrotestosterone [61–63], and [89Zr]-desferrioxamine B-J591, an antibody targeted at PSA [64].
MRI of the Axial Skeleton
MRI has an enhanced sensitivity for the early detection of neoplastic infiltration of the bone marrow in “osteophilic” cancers and hematologic malignancies. MRI studies of the marrow combine conventional anatomic (generally T1-weighted images), which are sensitive to the alteration of the balance between fat/nonfat cells by the cancer cells within the marrow, and diffusion-weighted images, which are sensitive to the particular organization of tumoral tissues (increase in cell density, cellular organelles, etc.).
Currently, access to and availability of MRI are too low for widespread adoption as a first-line detection modality. However, the specificity, sensitivity, and early detection advantages, compared to BS and BS/targeted x-rays, suggest that MRI may, in the future, have a place in standard practice for patients at high risk of metastasis.
In terms of anatomic targets, MRI either covers the whole skeleton (whole-body MRI [WB-MRI]), enabling a comprehensive but time-consuming screening (30–45 minutes), or it can focus on the axial skeleton (i.e., the spine and pelvis), shortening the time of acquisition (<30 minutes) and bringing sufficient diagnostic effectiveness because most metastases affect these red marrow-containing skeletal areas [65]. The whole body approach, however, has the advantage of a combination of bone metastasis screening (M staging) and lymph node screening (N screening), relying on diffusion-weighted imaging to detect bone and node metastases, and anatomic T1 images to confirm and measure them [65].
MRI of the axial skeleton (MRIas) is highly sensitive for the detection of metastases in prostate cancer; it also offers the ability to measure tumor responses. MRIas detects metastases considerably earlier than is possible with BS. In a prospective investigation of 66 high-risk prostate cancer patients, Lecouvet et al. compared MRIas with a standard sequential workup: BS followed by targeted x-rays in patients with equivocal findings, with MRI obtained on request in patients with inconclusive or discrepant BS/targeted x-rays [66]. MRIas identified metastases in 7 of 23 patients identified as negative by BS, and demonstrated sensitivity and specificity of 100% and 88%, respectively [66]. Although there was some concern regarding the classification of equivocal BS scans in this study [67], such classification was used when the findings could not be confidently categorized as positive or negative and was applied uniformly across all imaging tests; therefore, it is unlikely to have introduced bias.
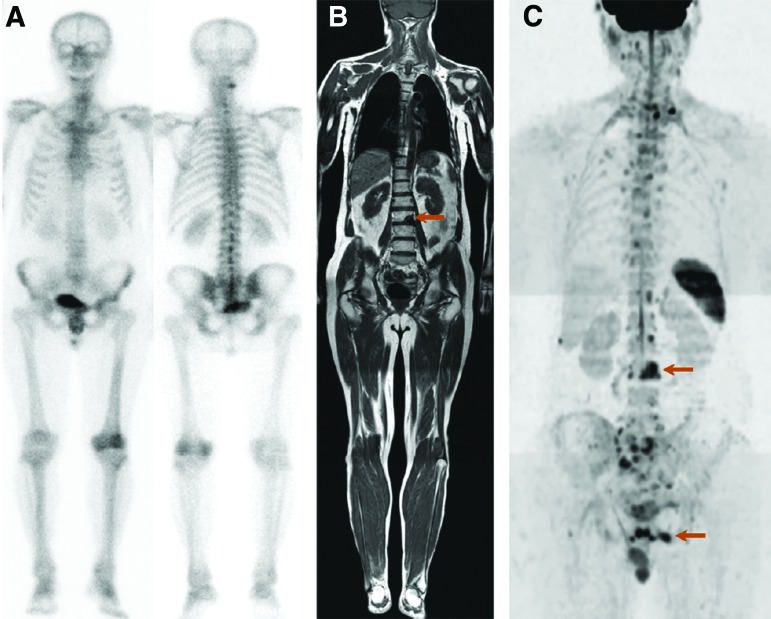
WB-MRI has also been investigated in several small prospective studies and, thus far, has been found to be equivalent to MRIas for the detection of bone metastases [68, 69]. These results were confirmed recently in a prospective trial comparing WB-MRI with diffusion-weighted imaging against 99mTc-BS and CT in the detection of metastases in 100 patients with high-risk prostate cancer [65]. In that trial, the sensitivity of BS/targeted x-rays and WB-MRI for detecting bone metastases was 86% and 98%–100%, respectively (p < .04), and specificity was 98% and 98%–100%, respectively, providing confirmation that WB-MRI outperforms BS/targeted x-rays in detecting bone metastases (Fig. 2).
Figure 2.
Positive identification of bone metastases with whole-body magnetic resonance imaging versus bone scintigraphy. (A): Bone scintigraphy (anterior-posterior and posterior-anterior views) shows no significant lesion. Coronal T1 (B) and diffusion-weighted (C) magnetic resonance images of the body confirm bone metastases within L3 and the left iliac bone. Reprinted from [65] with permission from Elsevier.
Currently, access to and availability of MRI are too low for widespread adoption as a first-line detection modality. However, the specificity, sensitivity, and early detection advantages, compared to BS and BS/targeted x-rays, suggest that MRI may, in the future, have a place in standard practice for patients at high risk of metastasis. The financial cost and patient benefits of this endeavor have yet to be evaluated.
Current and Future Imaging Methods: Lymph Node Metastases
The current criterion standard for evaluation of lymph node involvement in prostate cancer is pelvic lymph node dissection [70, 71], an invasive procedure associated with morbidity, which may not be able to sample all potential lymph node areas [72]. CT and MRI are also routinely used as staging tools in clinical practice, with size as the major determining factor in identification of potentially metastatic lymph nodes [73]. However, evidence indicates that these techniques are not precise enough for the evaluation of regional tumor spread [74–76]. A more recent meta-analysis of the diagnostic accuracy of CT and MRI concluded that both techniques were equally ineffective in detecting lymph node metastases [76]; the pooled sensitivity for CT was 0.42 (95% CI: 0.26–0.56) and for MRI was 0.39 (95% CI: 0.22–0.56) [76].
PET
[11C]-choline PET-CT can also be used to investigate nodal involvement [77], with a higher sensitivity than MRI- or PET-only scanning, including detection of subcentimeter disease [78]. However, due to its inability to accurately assess intraprostatic tumor and small lymph nodal involvement, this technique is not presently recommended for initial staging [79]. However, it is now FDA approved for recurrent disease at the Mayo Clinic.
MRI Combined With Other Techniques
Combining MRI with other techniques can improve the specificity and accuracy of the method. MRI has been combined with USPIO: the nanoparticles are readily taken up by lymph nodes in humans [80] and internalized by macrophages, resulting in changes in magnetic properties that are detectable by MRI [81]. Evidence suggests that USPIO MRI improves the sensitivity of conventional MRI (90.5% vs. 35.4%; p < .001) [8]. However, the authors reported that sensitivity of this approach was substantially lower in nodes <5 mm.
Another approach that has been investigated is the combination of diffusion-weighted MRI and USPIO. Diffusion-weighted MRI USPIO was compared to conventional USPIO MRI in a series of 21 consecutive patients with bladder and/or prostate cancer who underwent 3-T MRI before and after administration of lymphotropic USPIO using conventional and diffusion-weighted MRI sequences. Diffusion-weighted MRI USPIO analysis was significantly quicker than USPIO MRI (13 vs. 80 minutes, respectively; p < .0001) while providing comparable diagnostic accuracy (90% per patient or per pelvic side) [9]. Further studies are required to verify this technique in larger cohorts and confirm its benefits. Most importantly, the future availability of USPIO has been seriously questioned. Hence, most research is now focused on diffusion-weighted MRI, which already performs as well or better than cross-sectional imaging, with the potential for its specificity to be improved by the implementation of apparent diffusion coefficient measurements to discriminate benign from malignant nodes [65].
Current Guidelines and Future Needs
Currently, available guidelines focus on treatment options for metastatic disease rather than detection. The National Comprehensive Cancer Network workup for patients with post-RP biochemical recurrence (defined as failure of PSA to fall to undetectable levels or PSA detectable and rising on two or more subsequent determinations) recommends a clinical history-dependent choice of BS, CT/MRI, PSADT, or prostate biopsy; the workup is less specific for detection in patients with biochemical recurrence following medical or surgical castration [16]. The American Urological Association indicates BS for post-RP, pre-ADT patients with biochemical recurrence, but it acknowledges the variability of PSA at time of positive BS (vide supra), and consequently it does not recommend BS in patients with a postlocal therapy rise in PSA [17]. The European Association of Urology cites rapidly increasing PSA level, high PSA velocity, and short PSADT as indicative of distant metastases after PSA recurrence. It does not recommend BS, CT, or MRI for the routine follow-up of asymptomatic patients with biochemical recurrence after primary therapy, but it does suggest BS for patients with elevated PSA levels for whom the findings will affect the treatment decision [15].
Current and ongoing developments in both imaging technology and therapeutics for metastatic prostate cancer raise the question of whether imaging screening earlier than is currently standard practice might lead to better patient outcomes. There is no evidence to date to support this hypothesis, but given that several treatments that can or may improve survival or progression outcomes are approved or in development for asymptomatic or minimally symptomatic metastatic CRPC, it seems worthy of further assessment.
Newer imaging techniques should be validated and incorporated into future trials assessing the M0 to M1 transition. Information provided by different techniques may have different prognostic values, and any future trials of therapeutic agents using earlier detection will have to be carefully planned due to the risk of lead-time bias that might falsely demonstrate longer overall survival or progression-free survival in patients treated with an investigational therapy earlier in the course of disease.
Current and ongoing developments in both imaging technology and therapeutics for metastatic prostate cancer raise the question of whether imaging screening earlier than is currently standard practice might lead to better patient outcomes. There is no evidence to date to support this hypothesis, but given that several treatments that can or may improve survival or progression outcomes are approved or in development for asymptomatic or minimally symptomatic metastatic CRPC, it seems worthy of further assessment. Irrespective of the developing therapeutic landscape, current practices for first detection of metastases might be considered suboptimal, as evidenced by data suggesting that a high proportion of patients with metastatic CRPC thought to have M0 disease do, in fact, have M1 disease detectable by BS or CT/MRI [29], in addition to studies indicating that negative BS is not necessarily indicative of lack of metastasis [66]. The consequences of not identifying metastatic disease in patients believed to be in the M0 stage are unknown. However, at this time, it clearly narrows the range of therapeutic options immediately available because multiple therapies are indicated for metastatic CRPC but not for nonmetastatic CPRC.
Acknowledgments
This work was supported by Dendreon Corporation. Writing and editorial assistance was provided by Sam Yarwood, Ph.D., of Complete HealthVizion (contracted by Dendreon Corporation).
Author Contributions
Conception/Design: Oliver Sartor, Mario A. Eisenberger, Michael W. Kattan, Bertrand Tombal, Frédéric Lecouvet
Manuscript writing: Oliver Sartor, Mario A. Eisenberger, Michael W. Kattan, Bertrand Tombal, Frédéric Lecouvet
Final approval of manuscript: Oliver Sartor, Mario A. Eisenberger, Michael W. Kattan, Bertrand Tombal, Frédéric Lecouvet
Disclosures
Oliver Sartor: Dendreon, Johnson & Johnson, Takeda, Sanofi (C/A, RF); Johnson & Johnson (OI); Michael W. Kattan: Dendreon, Merck, Pfizer, Caris Life Sciences (C/A); Frederic Lecouvet: Johnson & Johnson, Amgen (H). The other authors indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Moul JW. Biochemical recurrence after definitive prostate cancer therapy. Part I: defining and localizing biochemical recurrence of prostate cancer. Curr Opin Urol. 2005;15:181–186. doi: 10.1097/01.mou.0000165552.79416.11. [DOI] [PubMed] [Google Scholar]
- 3.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88(Suppl):2989–2994. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Oudard S, Banu E, Beuzeboc P, et al. Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23:3343–3351. doi: 10.1200/JCO.2005.12.187. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Tombal B, Lecouvet F. Modern detection of prostate cancer's bone metastasis: Is the bone scan era over? Adv Urol. 2012;2012:893193. doi: 10.1155/2012/893193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 9.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761–769. doi: 10.1016/j.eururo.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CJ, Smith MR, de Bono JS, et al. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2012;30(Suppl):LBA4518. [Google Scholar]
- 11.Saad F, Akaza H, Eisenberger MA, et al. A phase III, randomized study of the investigational agent TAK-700 plus prednisone for patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2011;29:TPS184. [Google Scholar]
- 12.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slovin SF, Beer TM, Higano CS, et al. Initial phase II experience of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2009;27(Suppl):5138. [Google Scholar]
- 14.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidenreich A, Bolla M, Joniau S, et al. European Association of Urology. Guidelines on Prostate Cancer. [Accessed September 27, 2012]. Available at http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf.
- 16.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer v3.2012. [Accessed September 27, 2012]. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 17.American Urological Association. Prostate-specific antigen best practice statement: 2009 update. [Accessed September 27, 2012]. Available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf.
- 18.Kane CJ, Amling CL, Johnstone PA, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 19.Okotie OT, Aronson WJ, Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171:2260–2264. doi: 10.1097/01.ju.0000127734.01845.99. [DOI] [PubMed] [Google Scholar]
- 20.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 21.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012;109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: Analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108:378–385. doi: 10.1111/j.1464-410X.2010.09878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciscano AE, Hardee ME, Sanfilippo N. Management of high-risk localized prostate cancer. Adv Urol. 2012;2012:641–689. doi: 10.1155/2012/641689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2011;29:647–653. doi: 10.1016/j.urolonc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeb S, Makarov DV, Schaeffer EM, et al. Prostate specific antigen at the initial diagnosis of metastasis to bone in patients after radical prostatectomy. J Urol. 2010;184:157–161. doi: 10.1016/j.juro.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Makarov DV, Humphreys EB, Mangold LA, et al. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161. doi: 10.1016/j.juro.2007.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis ES, Keizman D, Carducci MA, et al. The effect of PSA doubling time (PSADT) and Gleason score on the PSA at the time of initial metastasis in men with biochemical recurrence after prostatectomy. J Clin Oncol. 2011;29(Suppl):16. [Google Scholar]
- 28.Antonarakis ES, Zahurak ML, Lin J, et al. Changes in PSA kinetics predict metastasis-free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: Combined analysis of 4 phase II trials. Cancer. 2012;118:1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu EY, Miller K, Nelson J, et al. Detection of previously unidentified metastatic disease as a leading cause of screening failure in a phase III trial of zibotentan versus placebo in patients with nonmetastatic, castration resistant prostate cancer. J Urol. 2012;188:103–109. doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–2085. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami S, Numao N, Okubo Y, et al. Development, validation, and head-to-head comparison of logistic regression-based nomograms and artificial neural network models predicting prostate cancer on initial extended biopsy. Eur Urol. 2008;54:601–611. doi: 10.1016/j.eururo.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Shariat SF, Karakiewicz PI, Roehrborn CG, et al. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113:3075–3099. doi: 10.1002/cncr.23908. [DOI] [PubMed] [Google Scholar]
- 36.Steuber T, Graefen M, Haese A, et al. Validation of a nomogram for prediction of side specific extracapsular extension at radical prostatectomy. J Urol. 2006;175:939–944. doi: 10.1016/S0022-5347(05)00342-3. [DOI] [PubMed] [Google Scholar]
- 37.Slovin SF, Wilton AS, Heller G, et al. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 38.Dotan ZA, Bianco FJ, Jr., Rabbani F, et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol. 2005;23:1962–1968. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inman BA, Frank I, Boorjian SA, et al. Dynamic prediction of metastases after radical prostatectomy for prostate cancer. BJU Int. 2011;108:1762–1768. doi: 10.1111/j.1464-410X.2011.10208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotto G, Changhong Y, Bernstein M, et al. Development of a nomogram model using a very large sample of patients (pts) to predict the risk of 99mTc-bone scan (BS) positivity in pts receiving androgen deprivation therapy (ADT) for prostate cancer (PCa) J Clin Oncol. 2012;30(Suppl):4552. [Google Scholar]
- 41.Kattan MW, Yu C, Stephenson AJ, et al. Comparing the ability of clinicians and a nomogram model at predicting future 99mTc-bone scan (BS) positivity in patients (pts) with rising prostate-specific antigen (PSA) following radical prostatectomy (RP) for prostate cancer (PCa) J Clin Oncol. 2012;30(Suppl):4652. [Google Scholar]
- 42.Ross PL, Gerigk C, Gonen M, et al. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol. 2002;20:82–88. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 43.Einhorn TA, Vigorita VJ, Aaron A. Localization of technetium-99m methylene diphosphonate in bone using microautoradiography. J Orthop Res. 1986;4:180–187. doi: 10.1002/jor.1100040206. [DOI] [PubMed] [Google Scholar]
- 44.Eustace S, Tello R, DeCarvalho V, et al. A comparison of whole-body turboSTIR MR imaging and planar 99mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol. 1997;169:1655–1661. doi: 10.2214/ajr.169.6.9393186. [DOI] [PubMed] [Google Scholar]
- 45.Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 46.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64. [PubMed] [Google Scholar]
- 47.Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: Comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:229–236. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 48.Schirrmeister H, Glatting G, Hetzel J, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800–1804. [PubMed] [Google Scholar]
- 49.Han LJ, Au-Yong TK, Tong WCM, et al. Comparison of bone single-photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med. 1998;25:635–638. doi: 10.1007/s002590050266. [DOI] [PubMed] [Google Scholar]
- 50.Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 51.Nozaki T, Yasuda K, Akashi T, et al. Usefulness of single photon emission computed tomography imaging in the detection of lumbar vertebral metastases from prostate cancer. Int J Urol. 2008;15:516–519. doi: 10.1111/j.1442-2042.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- 52.Ghanem N, Uhl M, Brink I, et al. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur J Radiol. 2005;55:41–55. doi: 10.1016/j.ejrad.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration. FDA approves production of imaging agent that helps detect prostate cancer. [Accessed September 27, 2012]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm319201.htm.
- 54.McCarthy M, Siew T, Campbell A, et al. 18F-fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: Prospective comparison with standard imaging. Eur J Nucl Med Mol Imaging. 2011;38:14–22. doi: 10.1007/s00259-010-1579-x. [DOI] [PubMed] [Google Scholar]
- 55.Eschmann SM, Pfannenberg AC, Rieger A, et al. Comparison of 11C-choline-PET/CT and whole body-MRI for staging of prostate cancer. Nuklearmedizin. 2007;46:161–168. doi: 10.1160/nukmed-0075. [DOI] [PubMed] [Google Scholar]
- 56.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–292. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Kotzerke J, Volkmer BG, Glatting G, et al. Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25–30. [PubMed] [Google Scholar]
- 58.Beheshti M, Vali R, Waldenberger P, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol. 2009;11:446–454. doi: 10.1007/s11307-009-0217-0. [DOI] [PubMed] [Google Scholar]
- 59.Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: A comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766–1774. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]
- 60.Yu EY, Muzi M, Hackenbracht JA, et al. C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: Relative findings and response to therapy. Clin Nucl Med. 2011;36:192–198. doi: 10.1097/RLU.0b013e318208f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanzonico PB, Finn R, Pentlow KS, et al. PET-based radiation dosimetry in man of 18F-fluorodihydrotestosterone: A new radiotracer for imaging prostate cancer. J Nucl Med. 2004;45:1966–1971. [PubMed] [Google Scholar]
- 62.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16b-18F-fluoro-5a-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 63.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 64.Holland JP, Divilov V, Bander NH, et al. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lecouvet FE, El Mouedden J, Collette L, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62:68–75. doi: 10.1016/j.eururo.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Lecouvet FE, Geukens D, Stainier A, et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: Diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007;25:3281–3287. doi: 10.1200/JCO.2006.09.2940. [DOI] [PubMed] [Google Scholar]
- 67.Venkitaraman R, Sohaib A, Cook G. MRI or bone scan or both for staging of prostate cancer? J Clin Oncol. 2007;25:5837–5838. doi: 10.1200/JCO.2007.14.3875. [DOI] [PubMed] [Google Scholar]
- 68.Venkitaraman R, Cook GJ, Dearnaley DP, et al. Whole-body magnetic resonance imaging in the detection of skeletal metastases in patients with prostate cancer. J Med Imaging Radiat Oncol. 2009;53:241–247. doi: 10.1111/j.1754-9485.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 69.Lecouvet FE, Simon M, Tombal B, et al. Whole-body MRI (WB-MRI) versus axial skeleton MRI (AS-MRI) to detect and measure bone metastases in prostate cancer (PCa) Eur Radiol. 2010;20:2973–2982. doi: 10.1007/s00330-010-1879-3. [DOI] [PubMed] [Google Scholar]
- 70.Allaf ME, Partin AW, Carter HB. The importance of pelvic lymph node dissection in men with clinically localized prostate cancer. Rev Urol. 2006;8:112–119. [PMC free article] [PubMed] [Google Scholar]
- 71.Stone NN, Stock RG. Laparoscopic pelvic lymph node dissection in the staging of prostate cancer. Mt Sinai J Med. 1999;66:26–30. [PubMed] [Google Scholar]
- 72.Parkin J, Keeley FX, Jr., Timoney AG. Laparoscopic lymph node sampling in locally advanced prostate cancer. BJU Int. 2002;89:14–17. doi: 10.1046/j.1464-410x.2002.02566.x. [DOI] [PubMed] [Google Scholar]
- 73.McSherry SA, Levy F, Schiebler ML, et al. Preoperative prediction of pathological tumor volume and stage in clinically localized prostate cancer: Comparison of digital rectal examination, transrectal ultrasonography and magnetic resonance imaging. J Urol. 1991;146:85–89. doi: 10.1016/s0022-5347(17)37720-0. [DOI] [PubMed] [Google Scholar]
- 74.Engeler CE, Wasserman NF, Zhang G. Preoperative assessment of prostatic carcinoma by computerized tomography. Weaknesses and new perspectives. Urology. 1992;40:346–350. doi: 10.1016/0090-4295(92)90386-b. [DOI] [PubMed] [Google Scholar]
- 75.Perrotti M, Kaufman RP, Jr., Jennings TA, et al. Endo-rectal coil magnetic resonance imaging in clinically localized prostate cancer: is it accurate? J Urol. 1996;156:106–109. [PubMed] [Google Scholar]
- 76.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 77.de Jong IJ, Pruim J, Elsinga PH, et al. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J Nucl Med. 2003;44:331–335. [PubMed] [Google Scholar]
- 78.Contractor K, Challapalli A, Barwick T, et al. Use of [11C]choline PET-CT as a noninvasive method for detecting pelvic lymph node status from prostate cancer and relationship with choline kinase expression. Clin Cancer Res. 2011;17:7673–7683. doi: 10.1158/1078-0432.CCR-11-2048. [DOI] [PubMed] [Google Scholar]
- 79.Picchio M, Giovannini E, Messa C. The role of PET/computed tomography scan in the management of prostate cancer. Curr Opin Urol. 2011;21:230–236. doi: 10.1097/MOU.0b013e328344e556. [DOI] [PubMed] [Google Scholar]
- 80.Harisinghani MG, Saini S, Weissleder R, et al. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: Radiographic–pathologic correlation. AJR Am J Roentgenol. 1999;172:1347–1351. doi: 10.2214/ajr.172.5.10227514. [DOI] [PubMed] [Google Scholar]
- 81.Wunderbaldinger P, Josephson L, Bremer C, et al. Detection of lymph node metastases by contrast-enhanced MRI in an experimental model. Magn Reson Med. 2002;47:292–297. doi: 10.1002/mrm.10068. [DOI] [PubMed] [Google Scholar]