Clinical outcomes of liver transplantation (LT) for hepatocellular carcinoma (HCC) in HIV-coinfected patients were assessed. The study involved 30 HIV-positive patients affected by HCC who underwent LT with 155 HIV-uninfected patients who received the same treatment. LT for HCC is a feasible procedure and the presence of HIV does not particularly affect the post-LT outcome.
Keywords: Liver transplantation, HIV, HCV, Hepatocellular carcinoma
Learning Objectives
Compare clinical outcomes following liver transplant for heptaocellular carcinoma in patients with and without HIV infection.
Identify predictors of mortality following liver transplant for heptaocellular carcinoma in patients with and without HIV infection.
Abstract
Background.
The aim of our work is to assess the clinical outcomes of liver transplantation (LT) for hepatocellular carcinoma (HCC) in HIV-coinfected patients. This is a multicenter study involving three Italian transplant centers in northern Italy: University of Modena, University of Bologna, and University of Udine.
Patients and Methods.
We compared 30 HIV-positive patients affected by HCC who underwent LT with 125 HIV-uninfected patients who received the same treatment from September 2004 to June 2009. At listing, there were no differences between HIV-infected and -uninfected patients regarding HCC features. Patients outside the University of California, San Francisco criteria (UCSF) were considered eligible for LT if a down-staging program permitted a reduction of tumor burden.
Results.
HIV-infected patients were younger, they were more frequently anti-HCV positive, and a higher number of HIV-infected patients presented a coinfection HBV-HCV. Pre-LT treatments (liver resection and or locoregional treatments) were similar between the two groups. Histological characteristics of the tumor were similar in patients with and without HIV infection. No differences were observed in terms of overall survival and HCC recurrence rates.
Conclusion.
LT for HCC is a feasible procedure and the presence of HIV does not particularly affect the post-LT outcome.
Implications for Practice:
This paper provides important hints for the physician involved in the care of HIV-infected patients affected by hepatocellular carcinoma, showing that liver transplantation is a valid option for these patients. The impact of liver transplantation in this setting of patients has never been clearly defined elsewhere. These good results are achieved by the integration of liver surgeons, infectious diseases physicians, gastroenterologists, and oncologists.
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of death among patients with liver cirrhosis and is the fifth most frequent malignant tumor worldwide. Epidemiological data support a foreseeable increase in mortality secondary to HCC in the upcoming years [1]. The most common underlying diseases to this condition are chronic viral hepatitis and alcohol abuse. These conditions are epidemiologically linked with HIV infection, sharing common behavioral risk factors [2]. Several reports have outlined a more aggressive course of HCC in HIV-infected patients. HIV, per se, can boost carcinogenesis with the pivotal role of the HIV-tat protein inducing growth signals and enhancing HCC cell proliferation and antitumor immune response [3–8]. Furthermore, the increased life expectancy of HIV-infected patients, secondary to availability of highly active antiretroviral therapy (HAART) may allow the evolution of underlying liver diseases toward HCC and an increased mortality because of this condition as well as end-stage liver disease (ESLD) [2, 9–11]. Liver transplantation (LT) in patients with HIV and ESLD is a recent indication. Several studies have shown that this procedure is effective, with a survival benefit that is more significant in patients with HBV-HIV coinfection compared with HCV-HIV coinfection [12–14] outlining the need of a focused selection of patients for LT. Liver transplantation in patients with HIV and HCC is still a matter of debate.
Few reports only describe clinical outcome and patient's survival [15–17]. Pre-LT variables that have been associated with clinical end-points include CD4 T-cell count at the time of LT, adherence to HAART, and the MELD score [13]. Moreover, the need of a proper timing for LT is clinically intuitive, considering, in particular, tumor down-staging procedures to reduce tumor burden and recurrence rate.
The aim of this study is to assess clinical outcomes of patients with HIV infection undergoing LT for the treatment of HCC.
Patients and Methods
This is a multicenter comparative study involving three transplant centers in northern Italy. From September 2004 to June 2009, 155 patients with ESLD or chronic hepatitis underwent LT for HCC. Among them, 30 patients were HIV-infected, belonging to the following cohorts: University of Modena and Reggio Emilia, Modena, 12 cases; University of Bologna, 10 cases; and University of Udine, 8 cases. Graft allocation in Italy is based on MELD score; the exception MELD score for HCC candidates is implemented in each center according to HCC stage. Each center manages a single waiting list, with different exception scores.
Inclusion Criteria for HIV-Infected Patients
HIV-infected patients had to fulfill the Italian Protocol for LT in HIV [18]. Inclusion criteria in patients with no previous AIDS-defining events are: CD4 T-cell counts ≥100 cell/μL and HIV viral load (VL) below the limit of detection. Inclusion criteria in patients with previous AIDS-defining events (previous history of CDC “category C” events) is CD4 T-cell counts ≥200 cell/μL. A detectable HIV VL is acceptable in patients intolerant to antiretroviral therapy (ART), if a susceptibility genotypic test is available and a virological suppression post-LT is predicted.
Preoperative Evaluation
A pre-LT multidisciplinary evaluation among transplantation surgeons, gastroenterologists, infectious diseases, and internal medicine physicians sharing clinical, radiological, and laboratory data was performed for all the patients.
HCC was diagnosed with abdominal computed tomography scan, abdominal magnetic resonance, and liver ultrasound, according to the European Association Study liver criteria [19], or with biopsy samples of liver nodules.
Patients with HCC were considered eligible for LT if the University of California, San Francisco (UCSF) criteria were present: single tumor ≤6.5 cm, or up to three tumors with the largest lesion ≤4.5 cm and a total tumor diameter ≤8 cm. Patients outside UCSF criteria were still considered eligible for LT if a proper down-staging program was performed so as to bring these patients within UCSF criteria.
Milan criteria (MC, single tumor ≥2 cm and ≤5 cm, or up to three tumors each ≤3 cm) were used for the purpose of discussion to give better insight into the difference between tumor classification with respect to outcomes and survival [20].
Each patient was evaluated with regard to past medical history and complete blood tests, including serology for viral hepatitis and herpes viruses (HBV, HCV, HDV, HSV1, HSV2, CMV, EBV, VZV, HHV6, and HHV8), Toxoplasma spp., and syphilis. The pre-LT screening also included an upper gastrointestinal endoscopy, a colonoscopy, assessment of pulmonary and cardio-circulatory functions. The severity of the underling liver disease was assessed via the Child-Turcotte-Pugh (CTP) classification and the model for end-stage liver disease (MELD) score. At listing, no priority list was based on HIV serostatus.
HCC Pre-LT Treatment
HCC down-staging was performed with trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), or liver resection.
TACE was used as the first-choice treatment in case of uni- or multifocal tumors less than 3 cm in diameter in CTP A-B patients both before and during listing; in cases of wider tumors, RFA was implemented. If incomplete treatment of the tumor was outlined via imaging or because of a clinically significant increase in the α-fetoprotein serum level (AFP), TACE was repeated.
RFA was used alone for CTP C patients with a single lesion less than 3 cm in diameter. Liver resection was used in CTP A patients whenever a minor hepatectomy was possible. One month after the down-staging procedure, the patient was re-evaluated and considered eligible for LT only if the postprocedural imaging showed a reduction of tumor mass/lesions so as to consider the patient within UCSF criteria. Follow-up imaging was performed with abdominal computed tomography scan and abdominal magnetic resonance at 1, 6, and 12 months after the procedure, and a liver ultrasound at 3 and 9 months. Complete blood tests and AFP were performed monthly. Patients were dropped from the list in cases of extrahepatic extension of HCC, or portal vein tumor thrombus diagnosed by clear radiological criteria or by biopsy samples [20, 21].
Surgical Technique and Postoperative Management
In all three centers, LT was performed with whole organs from deceased donors with the standard piggy-back procedure; hepatic venous outflow was granted with an end-to-side cavo-cavostomy; portal and hepatic artery continuity were reconstructed with end-to-end anastomoses. Finally, the biliary reconstruction was performed with duct-to-duct reconstruction or Roux-en-Y hepatic-jejunostomy in cases of pre-existing biliary anomalies, wide incongruity in caliber of the biliary tract between graft and the recipient, or in cases of retransplantation.
Postoperative immunosuppressive therapy consisted of tacrolimus or cyclosporine with a steroid-sparing schedule. A switch to rapamycin was made in cases of severe adverse events as a result of the use of calcineurin inhibitors, such as nephro- or neuro-toxicity, diabetes onset, in case of de novo tumors, and of a high tumor burden on pathological analysis [22, 23].
In HIV-infected patients, HAART was given up to LT and then discontinued until liver function had stabilized. The choice of the antiretroviral combination was based on the tolerability profile, pre-LT resistance test, and the history of virological suppression, trying to maintain the same HAART as prior to LT.
After transplantation, both HIV-infected and -uninfected patients underwent weekly examinations for the first month, every 2 weeks for the next 3 months, then every 4 weeks until the sixth month, and then monthly until the end of the first postoperative year, or otherwise as required by the clinical conditions. During each examination, blood samples were taken to analyze liver and renal functions, blood cell count, level of immunosuppression, and CMV pp65 antigenemia. For HIV-infected individuals, HIV VL, CD4, and CD8 T-cell counts and HHV6 and HHV8 plasma VL were added.
As imaging follow-up, all patients underwent chest and abdominal computed tomography scans at 1, 6, and 12 months after LT and then every year. Liver and abdominal ultrasound scans were performed every 3 months.
Statistical Analysis
Continuous data are reported as mean ± standard deviation and/or range and were compared by using the two-sided Student's t test. Serum AFP values were converted to square root to normalize the distribution so as to apply Student's t test. Comparisons between groups for categorical variables were performed using the χ2 test with Yates' correction or Fisher's exact test when appropriate. In the analysis of HCC-recurrence-free survival, we considered the evidence of HCC recurrence as an event, whereas the patient who died without HCC recurrence was censored at the time of death. Patient survival and time to HCC recurrence were evaluated using the Kaplan-Meier method and compared with the log-rank test and variables that resulted p < 0.2 were included in a multivariable Cox proportional hazards regression analysis with backward conditional stepwise model and confirmed by forward conditional stepwise model. Included variables were: age, sex, HIV infection, viral etiology, HBV- or HCV-positive serology, pre- and postoperative MC and UCSF criteria, AFP serum level, liver resection pre-LT, RFA, waiting time on list, and microvascular invasion.
Statistical significance was set at p < .05. Statistical analysis was performed with SPSS 15.0.
Results
Demographic and Clinical Characteristics of Patients Before LT
Patients with HIV infection, compared with HIV-uninfected patients, were younger (mean age 47.5 ± 5 vs. 57.6 ± 7.6, p < .001), more frequently anti-HCV positive (80% vs. 57.6%, p = .039), and HBV-HCV coinfected (30% vs. 2.4%, p > .001). Notably, in the anti-HCV group, HCV RNA was negative in six (25%) HIV-infected patients and in seven (9.7%) controls (p = .12).
Nonviral etiology of cirrhosis, including alcohol abuse, NASH, Wilson disease, sclerosing cholangitis, and cryptogenic cirrhosis was present in HIV-uninfected patients only (p = .025).
Waiting time on the list for LT was significantly lower among HIV-infected versus -uninfected patients (8.38 ± 7.61 versus 19.07 ± 14.73 months, p < .001). Nevertheless, no differences can be found in the time from HCC diagnosis to LT in both groups (Table 1). Also the dropout rate between the two group was comparable and it was reported as 26% and 24%, respectively, in HIV-infected and -uninfected patients (p = .99).
Table 1.
General features of HIV-infected and HIV-uninfected patients
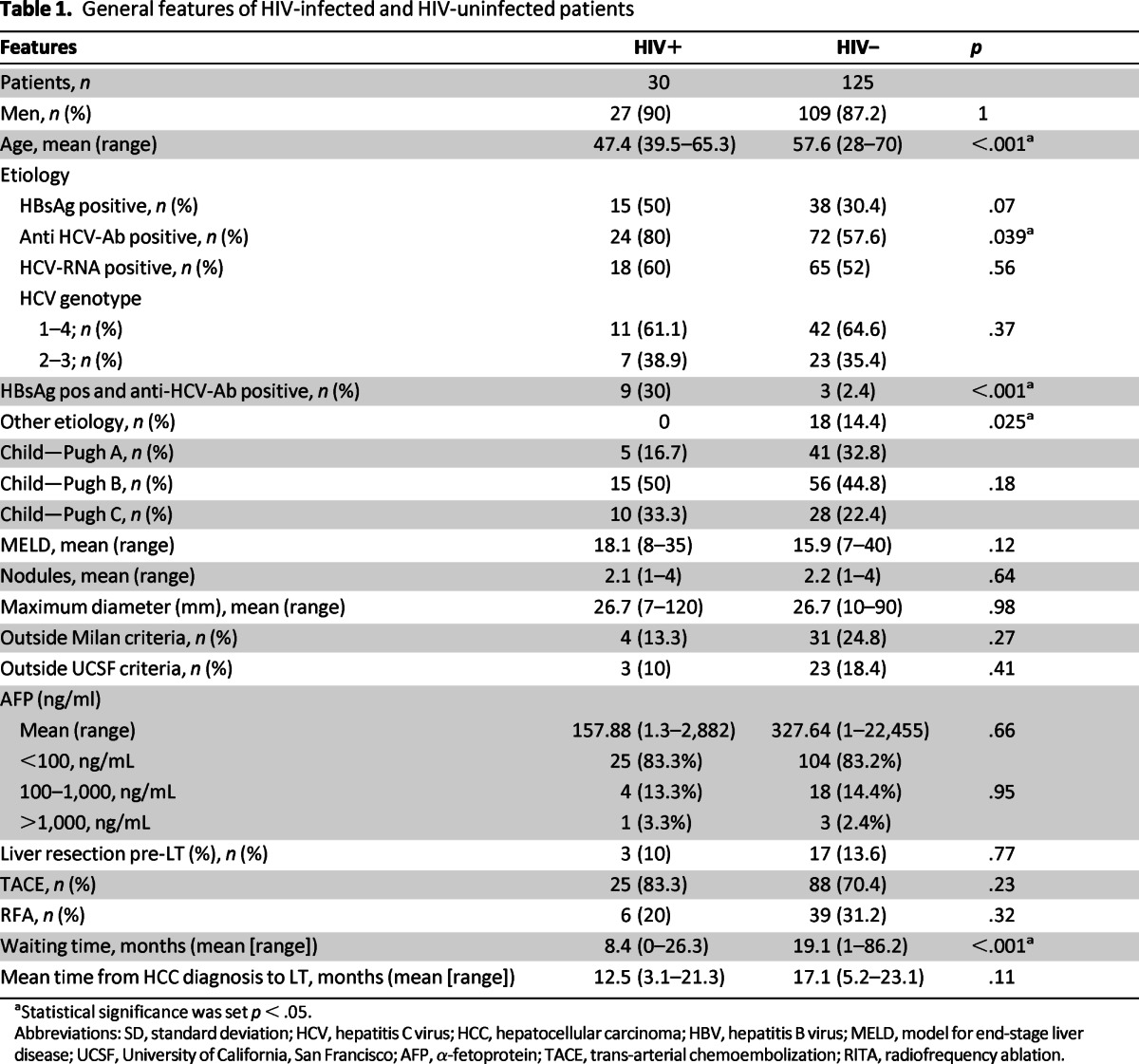
aStatistical significance was set p < .05.
Abbreviations: SD, standard deviation; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; MELD, model for end-stage liver disease; UCSF, University of California, San Francisco; AFP, α-fetoprotein; TACE, trans-arterial chemoembolization; RITA, radiofrequency ablation.
HIV-infected patients at the time of listing had a median CD4 T-cell count of 352.7 cell/μL (range 129–956). Four patients had detectable HIV VL before LT being intolerant to ART.
Analytical descriptions of the patients' clinical characteristics and outcomes are shown in Tables 2 and 3.
Table 2.
General and viro-immunological preoperative features and outcome of HIV-infected patients

Abbreviations: HAART, highly effective antiretroviral therapy; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; APV, fosamprenavir; T20, enfuvirtide; TDF, tenofovir; 3TC, lamivudine; LPV, lopinavir; RTV, ritonavir; ABC, abacavir; NFV, nelfinavir; ATV, atazanavir; FTC, emtricitabin; EFV, efavirenz; RAL, raltegravir; SQV, saquinavir; d4T, stavudine.
Table 3.
HCC features of HIV-infected patients

Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma; UCSF, University of California, San Francisco; TACE, trans-arterial chemoembolization; RFA, radiofrequency.
HCC Treatment Before LT
Considering the whole population, 35 patients were outside MC and 26 patients were outside UCSF criteria. All these patients were within acceptable criteria after down-staging procedures before listing (Table 1). In particular, in the HIV-infected group, four (13.3%) were outside MC and three out of these outside UCSF criteria (10%); in the HIV-uninfected group: 31 (24.8%) were outside MC and 23 (18.4%) outside UCSF criteria.
Pre-LT treatments (liver resection, TACE, and RFA) were similar between the two groups (Table 1). HIV-infected patients underwent a maximum of two TACE and one RFA.
Patient 17 underwent a right hepatectomy because of a large HCC; following this, he developed a postoperative liver failure and underwent an urgent liver transplantation. This patient was outside UCSF criteria (four nodules, the largest 120 mm); he would not have been eligible for LT in election, but in this case LT was performed only as life-saving treatment.
Pathological Characteristics of Tumors
Histological characteristics of the tumor were similar in patients with and without HIV infection (Table 4). At pathological specimen examination, eight (26.7%) HIV-infected patients were outside MC and, among these, six (20%) were outside UCSF criteria. In HIV-uninfected patients, 31 (24.8%) were outside MC and, of these, 23 (18.4%) were outside UCSF criteria.
Table 4.
Pathological features of HIV+ and HIV− patients

Abbreviations: MC, Milan criteria; UCSF, University of California, San Francisco; HCC, hepatocellular carcinoma.
In this study, the number and size of the nodules were considered regardless of the percentage of necrosis. Six out of eight HIV-infected patients who were outside UCSF criteria presented more than 60% necrosis.
The underlying liver parenchyma was cirrhotic in all cases in both groups (chronic liver disease with mild to severe activity in cirrhotic stage).
In regards to the Edmondson and Steinert grading classification, no differences were seen between the two groups. Gx was used when the nodules were completely necrotic and no grading score was available.
Outcomes
Mean post-LT follow-up time was 32.4 ± 21.8 months (range 1.3–68.8) and 31.9 ± 19.4 months (range 0.43–76), respectively, for HIV-infected and -uninfected patients (p = .91). In the HIV-infected group, no deaths with 30 days post-LT were observed. Two patients experienced primary graft nonfunction and underwent re-LT. One patient experienced portal thrombis in the second postoperative day necessitating surgical redo anastomosis, and one patient underwent relaparotomy for hemoperitoneus secondary hepatic artery rupture. Post-LT, all HIV-infected patients underwent effective ART and none of them developed AIDS-defining events.
Recurrence
In HIV-infected and -uninfected individuals, the HCC recurrence rates were 6.7% (2 out of 30 patients) and 14.4% (18 out of 125 patients), respectively (p = .15). Time to HCC recurrence onset was 27.1 ± 17.7 months (range 14.54–39.6) and 9.9 ± 5.3 months (range 1.55–19.11), respectively (p = .003). One- and 3-year HCC-disease-free survival for patients with and without HIV infection was 100% and 95.2% and 91.4% and 83.6%, respectively (p = .32; Fig. 1).
Figure 1.
HCC recurrence-free survival of HIV-infected and HIV-uninfected patients.
Patient 8, 39.6 months post-LT, presented a 2-cm hepatic nodule in the VI segment. Thus, soon after this diagnosis, he underwent hepatic resection that revealed a G3 HCC without vascular invasion and satellites. Following this, he showed a pulmonary node suspicious for HCC, and he underwent a pulmonary resection that showed a metastatic HCC (8 mm in diameter). After the confirmation of bilobar hepatic and pulmonary metastatic disease, the patient was initially given liposomal doxirubicine, then sorafenib (Nexavar) 200 mg orally twice a day. The patient is still alive 55.5 months post-LT.
Patient 15 showed a bilobar HCC hepatic recurrence at 14.5 months post-LT. He was not eligible for any treatments, and he died 20 months post-LT.
Survival
At 1 year and 3 years post-LT, overall survival was 77% and 65% versus 86.4% and 70%, respectively (p = .32), for HIV-infected and -uninfected patients. Figure 2 shows the Kaplan-Mayer survival rate in HIV-infected and -uninfected patients.
Figure 2.
Overall survival of HIV-infected and HIV-uninfected patients.
Twelve out 30 HIV-infected patients (40%) died (Fig. 2). Causes of death were: HCV recurrence in four patients, sepsis in three patients, and HCC recurrence, cardiac failure, visceral bleeding, de novo anus carcinoma, and adrenal carcinoma in one patient each.
No perioperative deaths were observed in the HIV-infected patients group, and the shortest survival after LT was 1.28 months (patient 1).
To get a more comprehensive comparison of the two groups of patients, a subset survival analysis was performed considering patients whose explants fell within or outside MC. No differences were observed in both cases: in patients within MC, 1 and 3 years overall survival was 82% and 65% versus 89% and 76%, respectively (p = .29), for HIV-infected and -uninfected patients (Fig. 3). Furthermore, for patients outside MC, 1 and 3 years overall survival was 63% and 63% versus 83% and 62%, respectively, for both groups.
Figure 3.

Overall survival of HIV-infected and HIV-uninfected patients within Milan criteria after pathological examination.
Thirty-eight out of 125 HIV-uninfected patients (30.4%) died. Causes of death were HCC recurrence (14 patients), HCV recurrence (12 patients), sepsis (7 patients), cardiovascular and respiratory failure (2 patients), and other causes (3 patients).
Multivariable Cox proportional hazard analysis identified the following variables independently associated with survival: age greater than 60 years at LT (p = .1; hazard ratio: 2.16; 95% confidence interval: 1.2–3.9) and waiting time on the list more than 12 months (p = .03; hazard ratio: 2.5; 95% confidence interval: 1.38–4.5).
Discussion
This is the largest multicenter study performed so far on LT for HCC in HIV-infected patients. This clinical condition is a leading cause of non-AIDS solid cancers and a major cause of death in HIV-infected patients [3].
The key message of this study is that LT is a valid option for HCC treatment in HIV-infected patients.
So far, only one paper has described HCC outcome in patients with HIV after LT [15]. In this study, 21 HIV-infected patients were compared with 65 uninfected individuals with viral cirrhosis who were listed for LT during the same period. The authors observed no differences in survival and HCC recurrence; nevertheless, the study appeared to be underpowered because of the limited number of HIV-infected patients who underwent LT (only 16 patients). A high drop-out rate in the HIV-infected patients group was observed [24]. We were able to confirm the same results with a much wider casuistry. In particular, our results underline that HIV per se was not a predictor of recurrence or mortality; therefore, we suggest that HIV-infected patients must be offered the same LT options for HCC treatment currently provided to HIV-uninfected subjects. From our analysis, only a high waiting time on the list and an age greater than 60 years at LT resulted as predictors of mortality; these suggest the need of proper timing for LT, and there is an advantage for HIV-infected patients that usually are younger at the time of LT compared with HIV-uninfected patients.
No priority was given on the waiting list as regards HIV serostatus; nevertheless, a waiting time resulted that was significantly lower in the group of HIV-infected patients (p < .001). It seems to be secondary to the lower mean waiting time in one of the three transplant centers (Udine: eight cases, mean waiting time in list: 2.1 months).
A high proportion of patients needed a tumor down-staging to fulfill UCSF inclusion criteria for LT. We suggest that aggressive treatment of HCC with liver resection, TACE, and RFA is necessary and is able to qualify patients for LT treatment.
Regardless, the down-staging tumor procedures and pathological specimen examination showed that 26.7% of HIV-infected patients and 43.2% of HIV-uninfected patients were outside MC, and 20% of HIV-infected patients and 36% of HIV-uninfected patients were outside UCSF criteria. This gap between preoperative and histological evaluation can be the result of an underestimation of number and size of HCC nodules on the pre-LT imaging. This unavoidable deviation to inclusion criteria did not appear to impair outcome; therefore, we think, in respect to our previous experience [16, 17, 24, 25], that a push toward wider criteria for LT in patients with HCC can be made and definitively we believe that MC can be substituted with UCSF criteria also in HIV-infected patients.
In the post-LT period, no HIV-infected patients developed AIDS-defining events. It appears very important in our experience to permit an early HAART resumption post-LT. This should prevent the CD4 cell count decline secondary to HIV replication as well as decrease the risk of hepatitis C virus recurrence that is consequent to reduced cellular immune function [26].
Pharmacokinetic interactions between protease inhibitors (PI), as part of HAART, and immunosuppressive drugs are critical elements in the management of HIV-infected patients after LT. More rapid increases in immunosuppressive drug serum levels are observed after initiating ritonavir-boosted PI therapy post-LT than when using unboosted PI [26]. Thus, we prefer this class of PI to lower pharmacological interferences, and in the near future, new drugs, such as entry and integration inhibitors, change the post-LT outcome in HIV-positive patients obtaining an optimal HIV control with low pharmacological interference [27, 28].
The main limitation of this study is that we did not perform an intent-to-treat analysis that could have offered important information both about the drop-out on the waiting list and on the HCC progression in HIV-coinfected patients. Nevertheless, we analyzed only the drop-out rate and we did not find any difference between the HIV-infected and -uninfected patients. Furthermore, in this study, three different transplant centers were involved, with different waiting lists and different allocation systems. Thus, we preferred not to perform an intent-to-treat analysis because of the variability in list management of the three centers that could have been a bias for the study.
We were able to demonstrate that time to HCC recurrence was longer in HIV-infected patients, but mortality was similar in the two groups. It could be hypothesized that HAART could have a role in lowering hepatocarcinogenesis progression and it is suggested, from our data, by the assumption that a higher proportion of HIV-infected patients dies because of HCV recurrence than HCC recurrence [29]. Therefore, we can think that these mortality rates could change in the next few years with the availability of new anti-HCV drugs. Moreover, we think that in patients with favorable genotypes such as 2 and 3 [13, 14] pre-emptive anti-HCV viral treatment with interferon and ribavirin should be considered.
Conclusion
We believe that this experience was possible through a high level of integration between liver surgeons, infectious diseases physicians, gastroenterologists, and oncologists. A multidisciplinary approach is needed in HIV-infected patients undergoing LT. In conclusion, this setting of patients, once bound to palliative care, has now provided a synergy of treatments such as HAART, aggressive HCC down-staging procedures, LT, and ad hoc immunosuppressive therapy, involving m-TOR inhibitors, so as to have a high control both of the tumor and of the cirrhosis.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Disclosures
The authors indicated no financial relationships.
Section editors: Rene Adam: Merck Serono, Roche, Pfizer (H); Kenneth Tanabe: LEK Consulting, Best Doctors (C/A); UpToDate Current Medicine Group, LLC, Springer, Sanofi, Institute for Medical Education and Research (H); AstraZeneca (RF); Andrew Zhu: Onyx, ImClone, Novartis, Bayer (C/A); Bayer (RF)
Reviewer “A”: None
Reviewer “B”: Bayer, Aloka, Intrasense (C/A)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.El-Serag HB. Hepatocellular carcinoma: An epidemiologic view. J Clin Gastroenterol. 2002;35(suppl 2):S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: The D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Berretta M, Garlassi E, Cacopardo B, et al. Hepatocellular carcinoma in HIV-infected patients: Check early, treat hard. The Oncologist. 2011;16:1258–1269. doi: 10.1634/theoncologist.2010-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serraino D, Boschini A, Carrieri P, et al. Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS. 2000;14:553–559. doi: 10.1097/00002030-200003310-00011. [DOI] [PubMed] [Google Scholar]
- 5.Altavilla G, Caputo A, Lanfredi M, et al. Enhancement of chemical hepatocarcinogenesis by the HIV-1 tat gene. Am J Pathol. 2000;157:1081–1089. doi: 10.1016/S0002-9440(10)64622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel J, Hinrichs S, Napolitano L, et al. Liver cancer in transgenic mice carrying the HIV tat gene. Cancer Res. 1991;51:6686–6690. [PubMed] [Google Scholar]
- 7.Corallini A, Altavilla G, Pozzi L, et al. Systemic expression of HIV-1 tat gene in transgenic mice induces endothelial proliferation and tumors of different histotypes. Cancer Res. 1993;53:5569–5575. [PubMed] [Google Scholar]
- 8.Berretta M, Cinelli R, Martellotta F, et al. Clinical presentation and outcome of colorectal cancer in HIV-positive patients: A clinical case-control study. Onkologie. 2009;32:319–324. doi: 10.1159/000215719. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Pequignot F, Delarocque-Astagneau E, et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: Evidence for the role of HIV coinfection and alcohol consumption. J Hepatol. 2008;48:200–207. doi: 10.1016/j.jhep.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Salmon-Ceron D, Rosenthal E, Lewden C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736–745. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal E, Salmon-Ceron D, Lewden C, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalité 2005 survey, ANRS EN19) HIV Med. 2009;10:282–289. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg EA, Stock P the AST Infectious Diseases Community of Practice. Solid organ transplantation in the HIV-infected patient. Am J Transplant. 2009;9(suppl 4):S131–S135. doi: 10.1111/j.1600-6143.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooper C, Kanters S, Klein M, et al. Liver transplant outcomes in HIV-infected patients: A systematic review and meta-analysis with synthetic cohort. AIDS. 2011;25:777–786. doi: 10.1097/QAD.0b013e328344febb. [DOI] [PubMed] [Google Scholar]
- 14.Duclos-Vallee JC, Feray C, Sebagh M, et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407–417. doi: 10.1002/hep.21990. [DOI] [PubMed] [Google Scholar]
- 15.Vibert E, Duclos-Vallée JC, Ghigna MR, et al. Liver transplantation for hepatocellular carcinoma: The impact of human immunodeficiency virus infection. Hepatology. 2011;53:475–482. doi: 10.1002/hep.24062. [DOI] [PubMed] [Google Scholar]
- 16.Di Benedetto F, De Ruvo N, Berretta M, et al. Hepatocellular carcinoma in HIV patients treated by liver transplantation. Eur J Surg Oncol. 2008;34:422–427. doi: 10.1016/j.ejso.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Di Benedetto F, De Ruvo N, Berretta M, et al. Don't deny liver transplantation to HIV patients with hepatocellular carcinoma in the highly active antiretroviral therapy era. J Clin Oncol. 2006;24:e26–e27. doi: 10.1200/JCO.2006.06.1374. [DOI] [PubMed] [Google Scholar]
- 18.Italian protocol for liver transplantation in HIV-infected patients. [Accessed April 23, 2011]. Available at http://www.salute.gov.it/imgs/C_17_pubblicazioni_1104_allegato.pdf.
- 19.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 20.Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: An intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piscaglia F, Gianstefani A, Ravaioli M, et al. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16:658–667. doi: 10.1002/lt.22044. [DOI] [PubMed] [Google Scholar]
- 22.Di Benedetto F, Di Sandro S, De Ruvo N, et al. Kaposi's sarcoma after liver transplantation. J Cancer Res Clin Oncol. 2008 Jun;134(6):653–658. doi: 10.1007/s00432-007-0329-3. Epub 2007 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Benedetto F, Di Sandro S, De Ruvo N, et al. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation. 2010 Mar 27;89(6):733–738. doi: 10.1097/TP.0b013e3181c7dcc0. doi: 10.1097/TP.0b013e3181c7dcc0. [DOI] [PubMed] [Google Scholar]
- 24.Di Benedetto F, Tarantino G, Montalti R, et al. The impact of human immunodeficiency virus infection in liver transplantation for hepatocellular carcinoma. Hepatology. 2011;53:1777–1778. doi: 10.1002/hep.24235. [DOI] [PubMed] [Google Scholar]
- 25.Di Benedetto F, Di Sandro S, De Ruvo N, et al. Human immunodeficiency virus and liver transplantation: Our point of view. Transplant Proc. 2008;40:1965–1971. doi: 10.1016/j.transproceed.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 26.Guaraldi G, Cocchi S, Motta A, et al. Differential dose adjustments of immunosuppressants after resuming boosted versus unboosted HIV-protease inhibitors post liver transplant. Am J Transplant. 2009;9:2429–2434. doi: 10.1111/j.1600-6143.2009.02778.x. [DOI] [PubMed] [Google Scholar]
- 27.Latinovic O, Kuruppu J, Davis C, et al. Pharmacotherapy of HIV-1 infection: Focus on CCR5 antagonist maraviroc. Clin Med Ther. 2009;1:1497–1510. doi: 10.4137/cmt.s2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heredia A, Gilliam B, Latinovic O, et al. Rapamycin reduces CCR5 density levels on CD4 T cells and this effect results in potentiation of Enfuvirtide (T-20) against R5 HIV-1 in vitro. Antimicrob Agents Chemother. 2007;51:2489–2496. doi: 10.1128/AAC.01602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow WA, Jiang C, Guan M. Anti-HIV drugs for cancer therapeutics: Back to the future? Lancet Oncol. 2009;10:61–71. doi: 10.1016/S1470-2045(08)70334-6. [DOI] [PubMed] [Google Scholar]




