Disparity exists between patients with lung cancer enrolled in clinical trials and patients treated in the community setting. This study assessed the real-world effectiveness of cytotoxic agents that became available for the treatment of non-small cell lung cancer (NSCLC) in the last 2 decades using the Surveillance, Epidemiology, and End Results–Medicare database. Study findings support the effectiveness of currently approved drugs for the treatment of advanced NSCLC in the real-world oncology practice.
Keywords: SEER–Medicare, Lung cancer, Chemotherapy, Effectiveness, Survival, Real world
Abstract
Objectives.
Disparity exists between patients with lung cancer enrolled in clinical trials and patients treated in the community setting. This study assessed the real-world effectiveness of cytotoxic agents that became available for the treatment of non-small cell lung cancer (NSCLC) in the last 2 decades.
Methods.
We employed the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database for patients diagnosed with stage IIIB/IV NSCLC between 1988 and 2005 to assess the effectiveness of newly approved agents. Effectiveness of specific agents was assessed at time periods immediately following the approval of the agent for NSCLC: baseline, 1988–1994; platinum, 1995–1999; docetaxel, 1999–2003; pemetrexed and bevacizumab, 2004–2005. Significant associations between specific drug treatment and survival improvement were determined using the Kaplan-Meier method, Cox proportional hazard model, and propensity score analyses. Significant differences were established by log-rank test.
Results.
This analysis employed data from 143,548 patients by sex (58% male, 42% female), cancer stage (35% stage IIIB, 65% stage IV), and age (12% 20–64 years, 22% 65–69 years, 45% 70–79 years, 22% 80 years and older). There was temporal improvement in survival for patients treated with newly approved chemotherapy (1-year survival rates: 32.41% in 1988–1994, 32.95% in 1995–1998, 37.40% in 1999–2003, and 39.55% in 2004–2005). Patients treated with a newly approved drug during the relevant treatment era had a significant reduction in the risk of death when compared with patients treated with chemotherapy other than the newly approved agent (hazard ratios [95% confidence interval] were 0.76 [0.71–0.81] for platinum, 0.73 [0.70–0.75] for docetaxel, 0.40 [0.37–0.44] for pemetrexed, and 0.33 [0.27–0.40] for bevacizumab; p < .001). Propensity score adjustment did not significantly alter these results.
Conclusions.
Currently approved drugs for the treatment of advanced NSCLC are associated with improved survival in the U.S. Medicare patient population. Our findings support the effectiveness of these agents in the real-world oncology practice.
Implications for Practice:
The U.S. Food and Drug Administration has approved several new drugs for the treatment of lung cancer in the last 15 years, based mainly on the results of clinical trials conducted in small groups of patients. The current study used the Surveillance, Epidemiology, and End Results (SEER)–Medicare diagnosis and treatment record to assess whether drugs approved through this approach benefit regular patients treated in the community. Our results show that the majority (approximately 70%) of patients with advanced lung cancer do not receive treatment with these approved drugs. However, treatment with an approved drug was associated with a greater likelihood of the patient living longer when compared to patients not treated with any chemotherapy or to those treated with chemotherapy drug other than an approved drug. Our results support the use of clinical trials to evaluate new drugs prior to regulatory approval and also highlight the need for oncologists to consider the use of these drugs for appropriate patients.
Introduction
Lung cancer affects approximately 220,000 new patients annually in the U.S. and causes the most cancer deaths worldwide [1, 2]. Newly approved drugs for the treatment of advanced lung cancer in the last 15 years have led to a greater number of therapeutic options, thereby improving the outlook for non-small cell lung cancer (NSCLC) [3–8]. Regulatory approval for new treatment agents for NSCLC by the U.S. Food and Drug Administration (FDA) is based primarily on the efficacy demonstrated by clinical trials conducted in carefully selected patient populations. However, such clinical trials are more likely to enroll relatively younger patients with limited comorbidities, whereas the majority of lung cancer patients are diagnosed at an advanced age [9–11].
A recent analysis of data submitted to the FDA in support of new drug applications or biologics license applications for new therapeutic agents for NSCLC in the decade extending from 2000 to 2010 greatly underscored the potential negative consequences of the disparity between clinical trial populations and patients treated with the FDA-approved agents in the community setting [12]. A comparison of the 8,795 patients enrolled in these pivotal NSCLC trials to the Surveillance, Epidemiology, and End Results (SEER) registry data for newly diagnosed NSCLC during the same period showed significant disparity in age, sex, and ethnicity of the clinical trial patients relative to the general U.S. patient population expected to be treated with the approved agents [12]. This disparity raised the concern that efficacy from clinical trials may not translate to real-world effectiveness in the general patient population. Indeed, a study conducted in a U.S. academic medical center showed that more than 50% of the patients with lung cancer who were treated at the institution would not qualify for an FDA-approved treatment regimen if treatment selection was based strictly on the eligibility criteria employed for the pivotal trial [13]. We therefore employed the linked SEER-Medicare database to assess the effectiveness of systemic therapy options that became available for advanced NSCLC over the last 2 decades for patients treated outside the controlled clinical trial setting.
Patients and Methods
We employed the linked SEER-Medicare database containing treatment information from the Medicare insurance program and diagnostic information from the population-based cancer registry program SEER [14]. The SEER database is a quality-assured national cancer registry that covers approximately 25% of the whole U.S. population and contains complete data set on treatment information for approximately 93% of all eligible patients [15–18]. This study was approved by the SEER program managers and the institutional review board of Emory University.
Overall survival data were available through December 31, 2007, whereas Medicare claims data were available through 2005. Patients with lung cancer who were treated with chemotherapy were identified by merging the SEER data set of eligible patients with chemotherapy procedure and administration data set from each Medicare claims file (MEDPAR, DME, HHA, HSPS, NCH, and OUTSAF) for every year from 1988 to 2005. We employed the International Statistical Classification of Diseases and Related Health Problems (ICD)-09-CM codes V58.1, V58.11, V66.2, V67.2, 99.25 and Healthcare Common Procedure Coding System codes 96400–96499, 96500–96599, 99555, J8510, J8520, J8521, J8530-J8999, J9000-J9999, C9415, G0355-G0363, S9329-S9331, S9379, Q0083-Q0085, C8953, C8954, and C8955. Patients treated with the drugs of interest were identified using the following drug-specific codes: vinorelbine, J9390; pemetrexed, J9305; docetaxel, J9170; bevacizumab, J9035; paclitaxel, J9265; cisplatin, J9060, J9062, and C9418; carboplatin, J9045; gemcitabine, J9201. Patients were coded as having received chemotherapy (yes/no) through the chemotherapy procedure or administration codes and as having received (yes/no) for each specific drug code using the claim files.
Study Population
Patients were eligible if diagnosed with advanced NSCLC (stage IIIB/IV) between 1988 and 2005 (inclusive), excluding those with additional diagnosis of cancer arising outside the lung. Tumor stage was classified according to the third and sixth editions of the American Joint Commission on Cancer manual for patients diagnosed between 1988 and 2003 and between 2004 and 2005, respectively. Both classifications were merged into a single variable.
Analytic Approach
Age was categorized as 20–64 years, 65–69 years, 70–79 years, and 80+ years. Race was classified as white, black, Asian, Hispanic, Native American, and unknown as reported by the patients. Histology was categorized as squamous cell carcinoma, adenocarcinoma, or others.
Temporal Analysis (Indirect Evidence)
Survival for each patient was calculated from the time of initial diagnosis. We used survival data for the period extending from 1988 to 1994 when the efficacy of systemic therapy for advanced lung cancer had not been established as a baseline. We assessed temporal survival changes across different eras which approximated the period extending from the initial year of FDA approval of an agent until the year of approval of another therapeutic agent for advanced NSCLC: platinum agents (1995–1998), docetaxel (1999–2003), and pemetrexed and bevacizumab (2004–2005). We also assessed temporal changes in survival for patients who did not receive any chemotherapy to establish whether other factors such as improved supportive care and stage migration could account for our findings.
Analysis Limited to Defined Era of Treatment (Direct Evidence)
To assess the survival impact of a specific drug, we first compared the survival of patients treated with any chemotherapy to that of patients not treated with any chemotherapy during the same period. Subsequently, we compared the survival of patients treated with the newly approved drug to the survival of patients treated with other chemotherapy agents during the same period. Significant association of survival benefit with a newly approved drug was assessed at several levels: a temporal survival improvement during the period of interest over the preceding periods, superior survival in patients treated with chemotherapy compared with patients not treated with chemotherapy during a defined period, and superior survival in patients treated with the specific chemotherapy of interest over patients treated with other types of chemotherapy during the defined period. Additional analysis for survival impact of newly approved agents was performed within clinically relevant subgroups defined by tumor stage (III vs. IV) and tumor histologies (for pemetrexed and bevacizumab).
Statistics
Differences in ethnicity, sex, age, stage, defined treatment era, radiation, histology, and Medicare status between patients treated and not treated with chemotherapy were assessed by χ2 test. The Kaplan-Meier method was used to estimate survival functions for overall survival (OS) rates and calculate the 2- and 5-year survival rates. The log-rank test was used to assess the difference in OS rates between different groups. Multivariable Cox proportional hazards models were employed to estimate the adjusted effect on OS rates of chemotherapy, the era of therapy, and specific chemotherapy agents after adjusting for age, sex, ethnicity, stage, histology, and Medicare status. To explore how much of the observed survival improvement over time is directly attributable to specific chemotherapy versus improvement or changes in other factors such as supportive care or stage migration, we calculated the relative ratio of the HR (current era HR divided by the HR for each of the preceding eras). We subsequently performed a p trend analysis to test the statistical significance of the relative ratios.
To better estimate the true association between specific treatment and survival improvement, propensity score analysis was employed to adjust for potential imbalances between treatment groups so as to minimize any “healthy cohort” effect that could have influenced the decision to select specific patients for treatment. Multivariable logistic regressions were used to calculate the propensity score of receiving chemotherapy and the specific chemotherapy agent: platinum, docetaxel, pemetrexed, and bevacizumab during the defined period based on the covariates: age, sex, race, stage, histology (histology excluded for bevacizumab), and Medicare status, respectively. An adjusted Cox proportional hazards model that included propensity score as a covariate was then employed to reassess the effect of each treatment of interest.
The significance levels were set at p ≤ .05 for all tests. The SAS statistical package V9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com) was used for data analyses.
Results
We identified 146,159 patients with stage IIIB/IV NSCLC from the SEER record. In all, 143,548 were eligible, whereas 2,611 were excluded because of other cancer diagnoses. Eligible patients were mostly white (82.4%) and aged 65 years or older (87.7%). Age (91%) and disability (9%) were the most frequent qualifying events for Medicare enrolment. Only 29% of the patients received systemic therapy, with a higher rate in the more recent treatment eras: 20.5% in 1988–1994, 26.7% in 1995–1998, 32.1% in 1999–2003, and 33.8% in 2004–2005 (p < .001).
There was a significant difference in the proportion of treated patients based on Medicare qualifying events, with 29% for patients enrolled based on age, 14% for age with end-stage renal disease (ESRD), 31% for disability, 28% for disability with ESRD, and 24% for ESRD only (p < .001). There was also a lower rate for black and Hispanic patients (27.1%) compared with white (29.6%) and Asian patients (31.4%; p < .001). Chemotherapy administration was lower with increasing age: 29.6% for patients aged 20–64 years, 37.5% for 65–69 years, 31.2% for 70–79 years, and 16.1% for patients ≥80 years (p < .001). There was a higher rate of radiation use in patients treated with chemotherapy (53% vs. 42%; p < .001; Table 1).
Table 1.
Clinical and demographic characteristics of patients, including treatment received and Medicare eligibility status
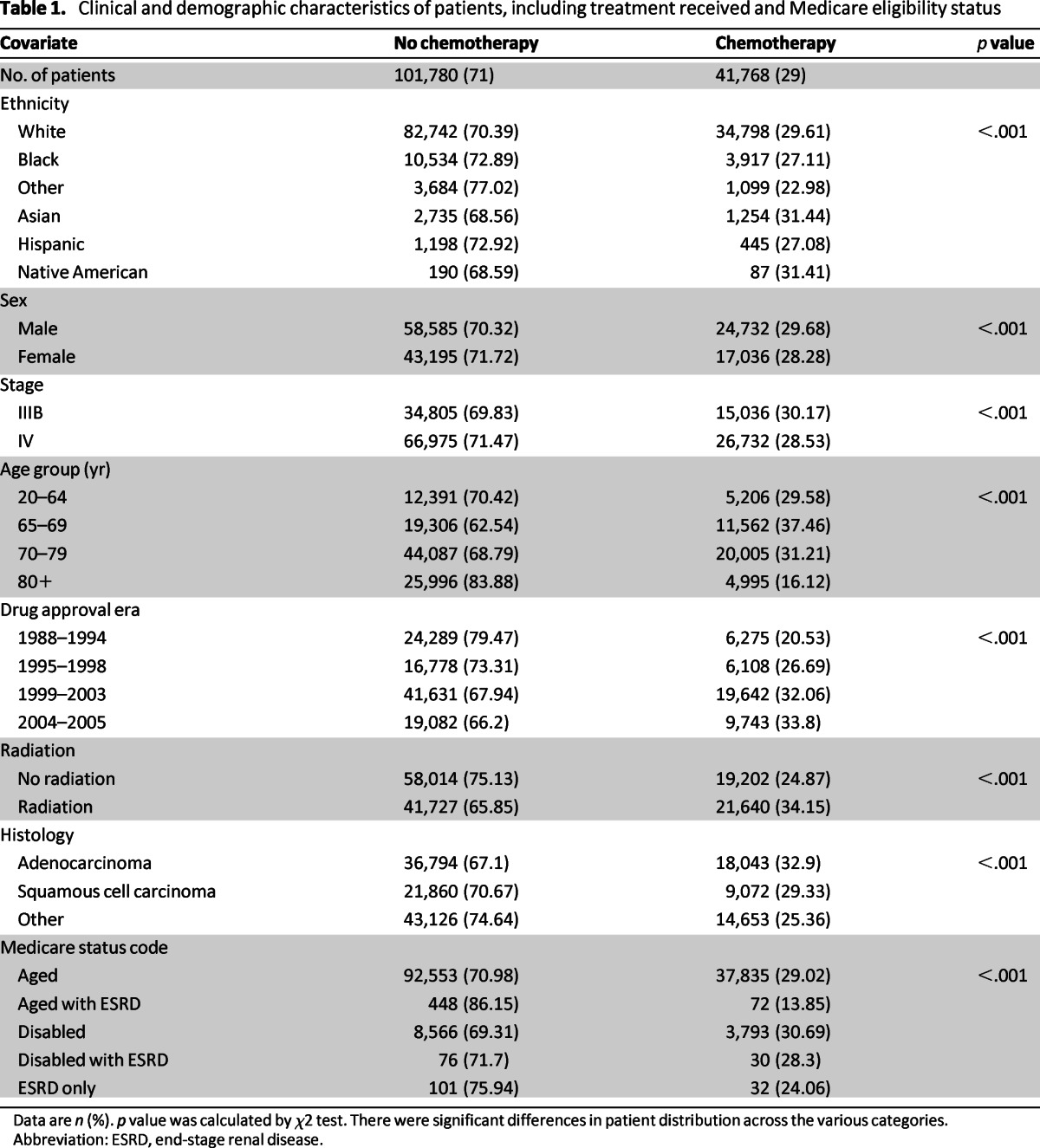
Data are n (%). p value was calculated by χ2 test. There were significant differences in patient distribution across the various categories.
Abbreviation: ESRD, end-stage renal disease.
Survival Analysis: Temporal Analysis
Patients diagnosed during the baseline period had 1-, 2-, and 5-year overall survival rates of 20.28%, 9.22%, and 3.09%, respectively. Higher survival rates were found for patients treated with chemotherapy (overall survival rates: 1-year, 32.41% vs. 16.81%; 2-year, 14.54% vs. 7.69%, and 5-year, 4.26% vs. 2.76%, respectively). The median survival was also higher for patients treated with chemotherapy during this period compared with patients who did not receive chemotherapy (8 vs. 4 months; hazard ratio [HR]: 0.66; 95% confidence interval [CI]: 0.64–0.68; p < .001; Table 2).
Table 2.
Survival analysis for the baseline population and for patients treated with chemotherapy based on the era of drug approval
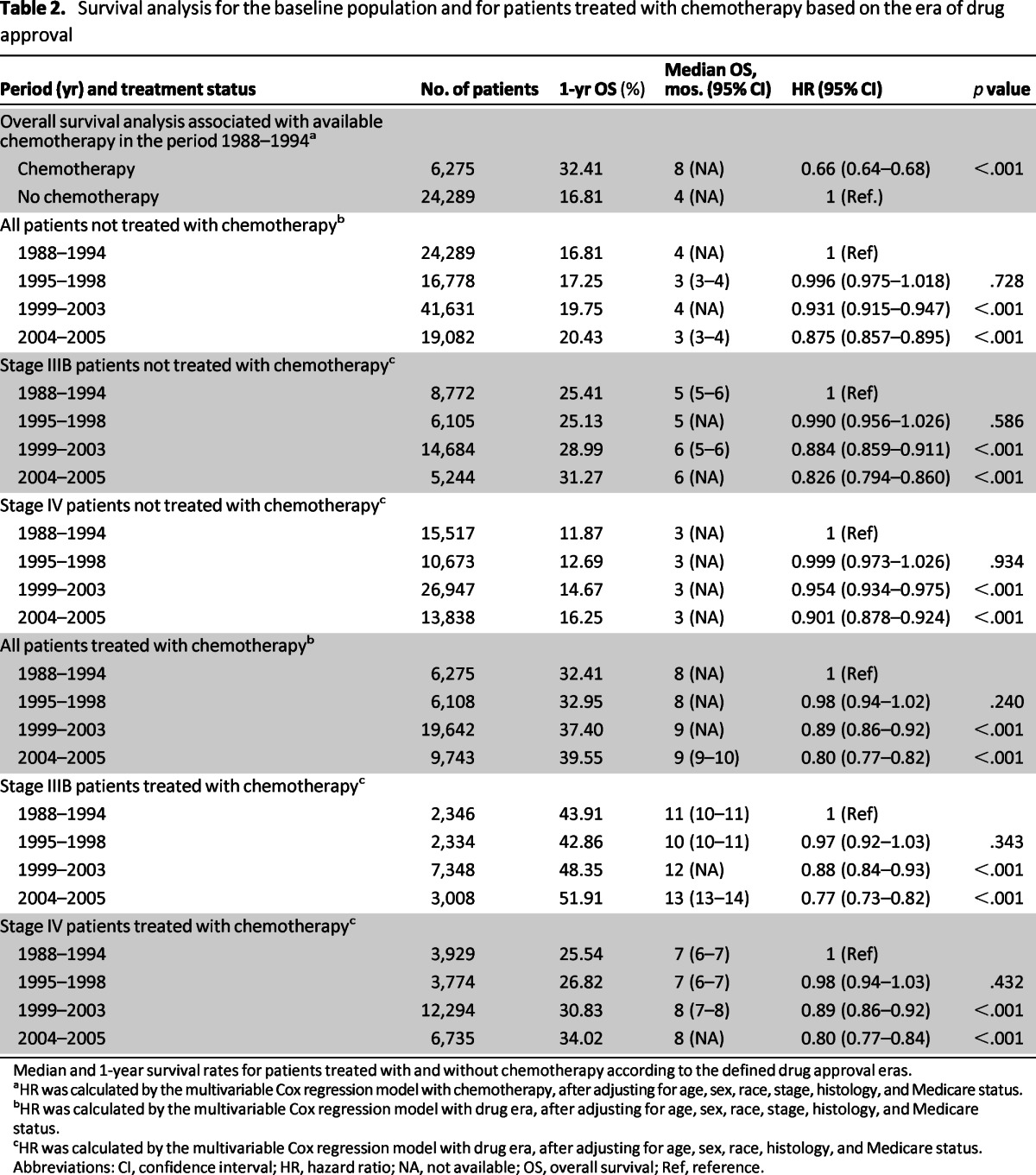
Median and 1-year survival rates for patients treated with and without chemotherapy according to the defined drug approval eras.
aHR was calculated by the multivariable Cox regression model with chemotherapy, after adjusting for age, sex, race, stage, histology, and Medicare status.
bHR was calculated by the multivariable Cox regression model with drug era, after adjusting for age, sex, race, stage, histology, and Medicare status.
cHR was calculated by the multivariable Cox regression model with drug era, after adjusting for age, sex, race, histology, and Medicare status.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; OS, overall survival; Ref, reference.
Increased survival over the baseline period was recorded across all the defined treatment eras, with a higher magnitude of improvement observed in the more recent era as new treatment agents received FDA approval. Although the median survival for all categories of untreated patients remained low (3–6 months) over the entire period covered by this study, there was incremental survival improvement associated with a new drug gaining approval. The 1-year survival rates for untreated patients showed only a modest improvement over time (16.81% in 1988–1994, 17.25% in 1995–1998, 19.75% in 1999–2003, and 20.43% in 2004–2005) compared with the 1-year survival rates in chemotherapy-treated patients, which increased from 32.41% at baseline (1988–1994) to 32.95% in 1995–1998, 37.40% in 1999–2003, and 39.55% in 2004–2005. The improved survival trend was observed for both stage IIIB and IV patients (Table 2). The p trend analysis of the relative ratios of the HR for patients treated with chemotherapy over those not treated with chemotherapy across the defined treatment was significant (p < .001; Table 3).
Table 3.
Relative hazard ratio calculated across different eras of drug approvals
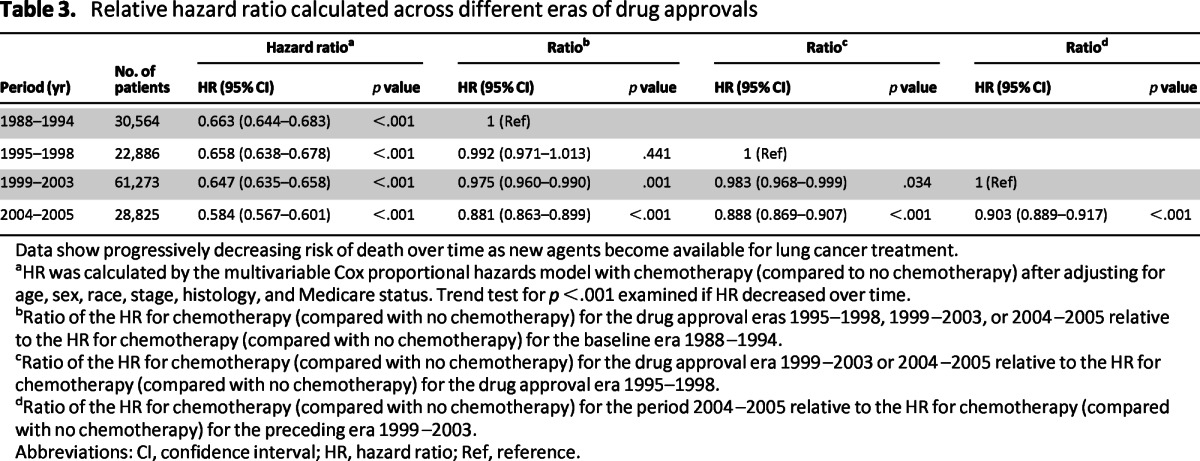
Data show progressively decreasing risk of death over time as new agents become available for lung cancer treatment.
aHR was calculated by the multivariable Cox proportional hazards model with chemotherapy (compared to no chemotherapy) after adjusting for age, sex, race, stage, histology, and Medicare status. Trend test for p <.001 examined if HR decreased over time.
bRatio of the HR for chemotherapy (compared with no chemotherapy) for the drug approval eras 1995–1998, 1999–2003, or 2004–2005 relative to the HR for chemotherapy (compared with no chemotherapy) for the baseline era 1988–1994.
cRatio of the HR for chemotherapy (compared with no chemotherapy) for the drug approval era 1999–2003 or 2004–2005 relative to the HR for chemotherapy (compared with no chemotherapy) for the drug approval era 1995–1998.
dRatio of the HR for chemotherapy (compared with no chemotherapy) for the period 2004–2005 relative to the HR for chemotherapy (compared with no chemotherapy) for the preceding era 1999–2003.
Abbreviations: CI, confidence interval; HR, hazard ratio; Ref, reference.
Survival and Specific Chemotherapy Agents
Platinum Chemotherapy (1995–1998)
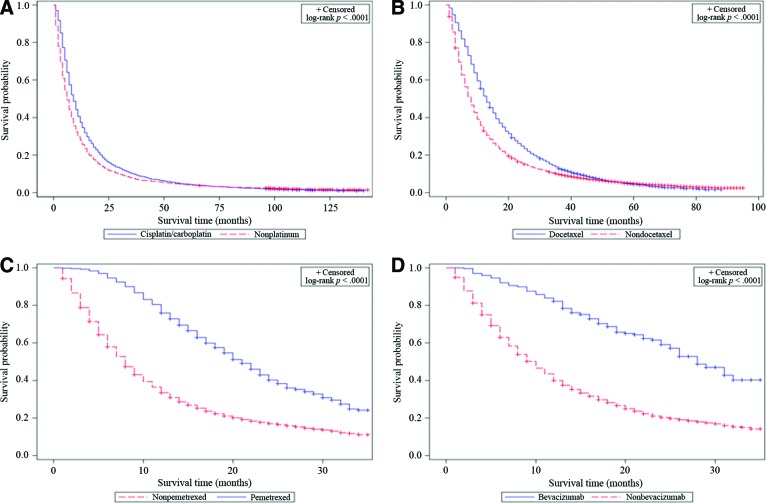
Treatment with cisplatin or carboplatin was associated with higher survival rates than treatment with nonplatinum chemotherapy (1-, 2-, and 5-year OS rates of 37.47% vs. 27.89%, 16.59% vs. 12.43%, and 4.57% vs. 4.4%, respectively; HR: 0.78; 95% CI: 0.74–0.83; p < .001; Fig. 1). The benefit associated with platinum therapy was observed for both stage IIIB and stage IV disease, whereas platinum doublet therapy appeared superior to single-agent platinum therapy (Table 4).
Figure 1.
Kaplan-Meier overall survival curves for patients treated with platinum (A), docetaxel (B), pemetrexed (C), and bevacizumab (D) compared with patients treated with other types of chemotherapy within their respective eras.
Table 4.
Comparisons between patients treated or not treated with a platinum agent and between various platinum combination regimens and single-agent platinum therapy
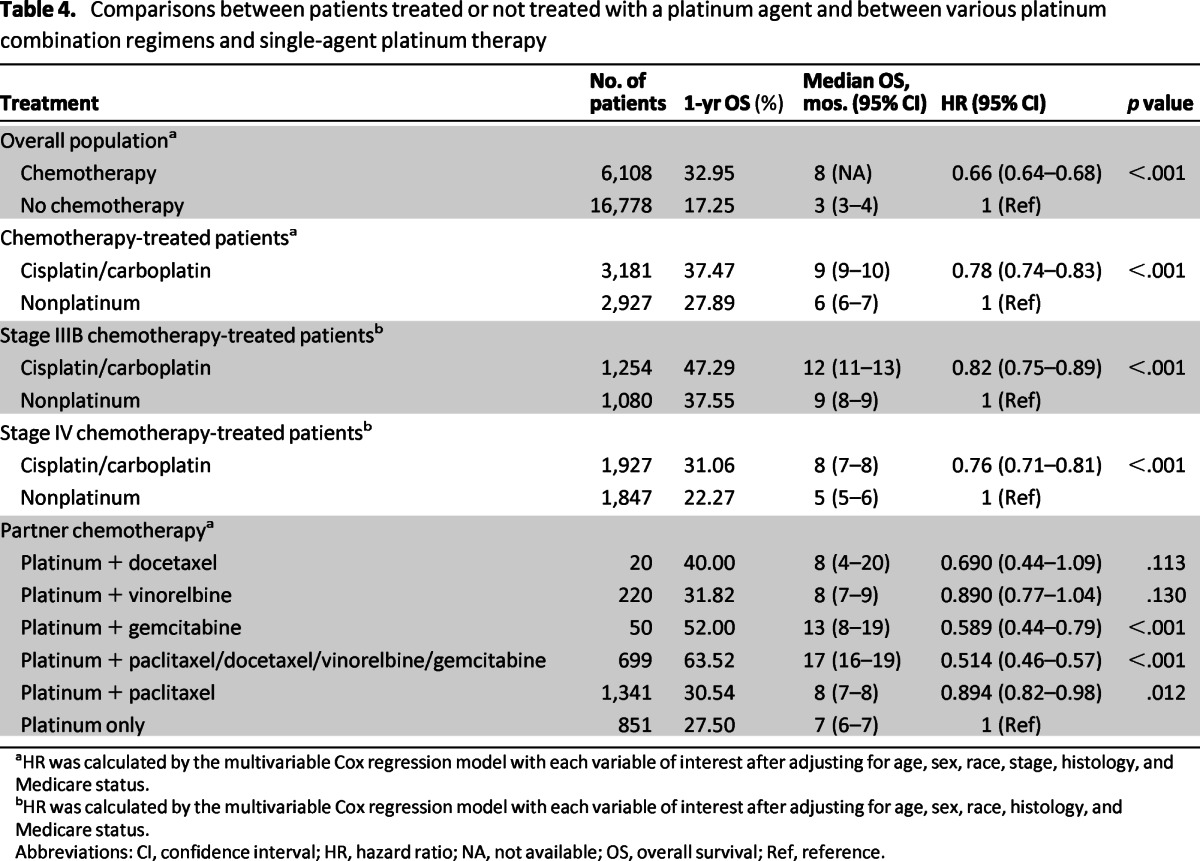
aHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, stage, histology, and Medicare status.
bHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, histology, and Medicare status.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; OS, overall survival; Ref, reference.
Docetaxel (1999–2003)
Although treatment with any chemotherapy was associated with better survival during this period (HR: 0.65; 95% CI: 0.64–0.66; p < .001), patients treated with docetaxel achieved superior survival compared with patients treated with a nondocetaxel chemotherapy (HR: 0.73, 95% CI: 0.70–0.75, p < .001). This was observed for both stage IIIB (HR: 0.80, 95% CI: 0.76–0.85, p < .001) and stage IV patients (HR: 0.69, 95% CI: 0.66–0.72, p < .001; Table 5).
Table 5.
Survival analysis associated with treatment with docetaxel, pemetrexed, and bevacizumab in advanced non-small cell lung cancer
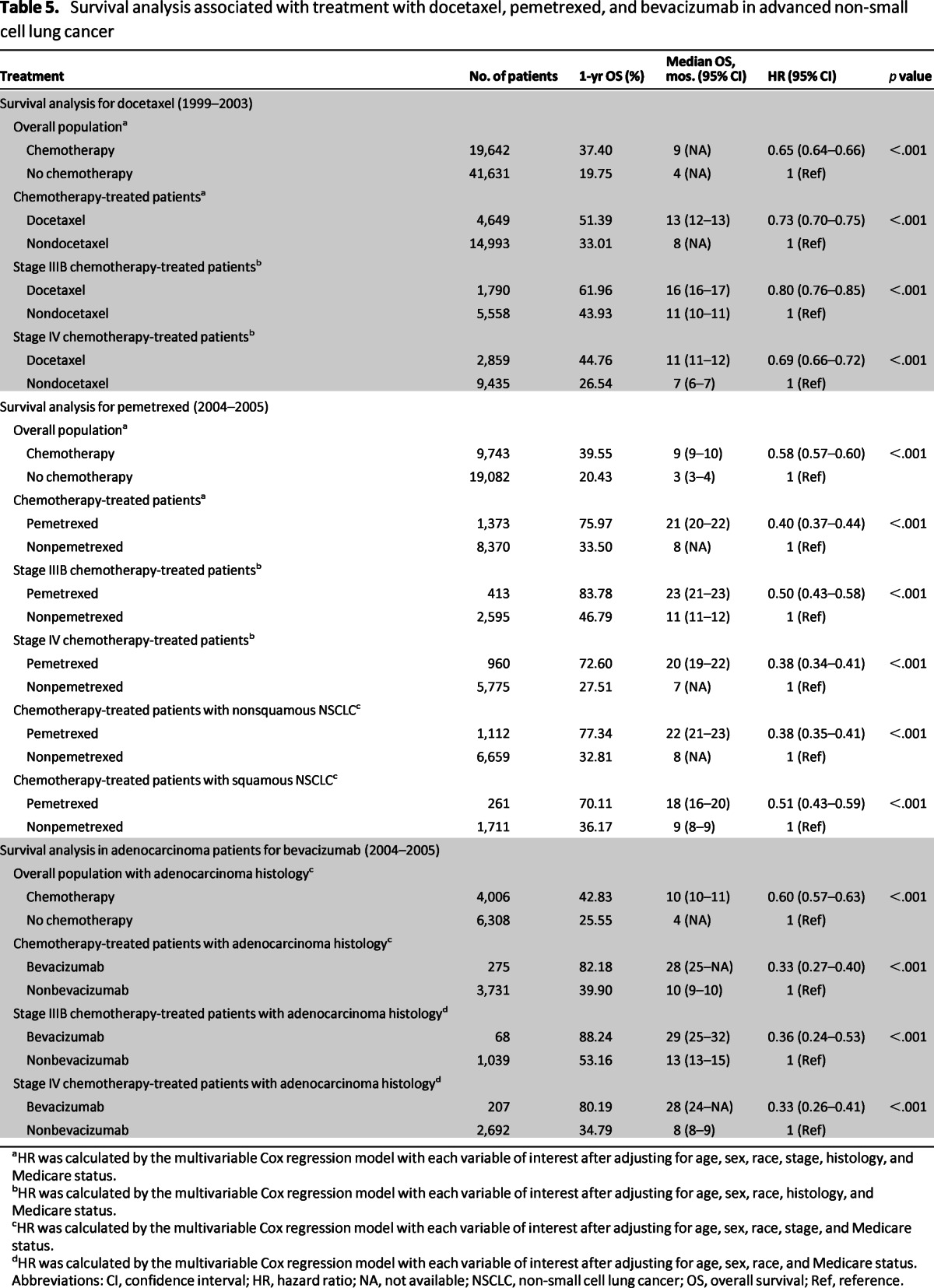
aHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, stage, histology, and Medicare status.
bHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, histology, and Medicare status.
cHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, stage, and Medicare status.
dHR was calculated by the multivariable Cox regression model with each variable of interest after adjusting for age, sex, race, and Medicare status.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; NSCLC, non-small cell lung cancer; OS, overall survival; Ref, reference.
Pemetrexed (2004–2005)
Treatment with chemotherapy during this period was associated with reduced risk of death (HR: 0.58, 95% CI: 0.57–0.60, p < .001). Pemetrexed therapy specifically was associated with superior survival over all other types of chemotherapy (HR: 0.40, 95% CI: 0.37–0.44; p < .001; Table 5, Fig. 1). To avoid an unfair comparison with patients potentially receiving docetaxel after failing pemetrexed as the second-line therapy, repeat comparison was conducted after excluding patients treated with both docetaxel and pemetrexed. This repeat analysis showed similar improvement in outcome for pemetrexed therapy (HR: 0.52, 95% CI: 0.47–0.57; p < .001; Fig. 2).
Figure 2.
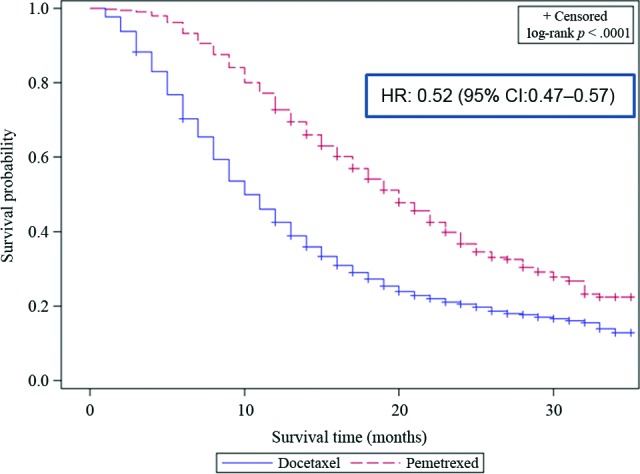
Kaplan-Meier overall survival curves comparing patients treated with pemetrexed versus patients treated with docetaxel after excluding patients treated with both agents.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Bevacizumab (2004–2005)
Approximately 7% of patients with adenocarcinoma NSCLC received bevacizumab (275 of 4,006 patients). Bevacizumab therapy was associated with superior survival compared with patients not treated with bevacizumab, with a 1-year survival rate of 82.18% vs. 39.90% (HR: 0.33, 95% CI: 0.27–0.40, p < .001). This association was observed in both stage IIIB and IV patients (Table 5, Fig. 1).
Propensity Score-Adjusted Analysis
Propensity score-adjusted models confirmed the findings from the multivariable survival analysis. There were significant improvements in overall survival for patients treated with chemotherapy compared with those not treated with chemotherapy in the baseline period of 1988–1994; for all the defined treatment eras over the baseline period among chemotherapy-treated patients; for all chemotherapy-treated patients compared with not-treated patients; and for each specific therapy of interest compared with other types of chemotherapy in each defined treatment era (Table 6).
Table 6.
Survival comparison for matched patient populations using propensity score-adjusted Cox proportional hazard model to control for potential confounding factors that influenced treatment decisions for lung cancer patients
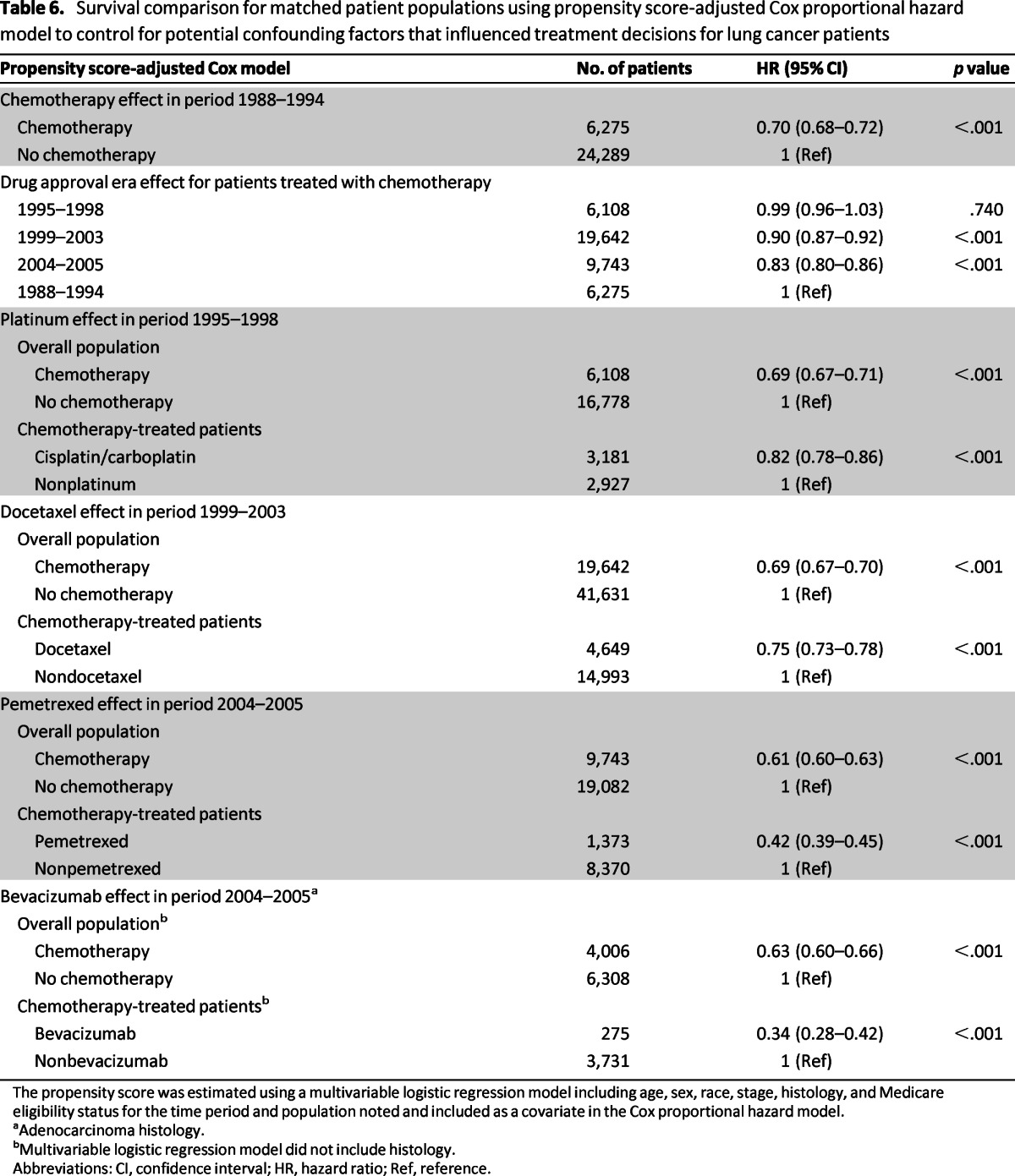
The propensity score was estimated using a multivariable logistic regression model including age, sex, race, stage, histology, and Medicare eligibility status for the time period and population noted and included as a covariate in the Cox proportional hazard model.
aAdenocarcinoma histology.
bMultivariable logistic regression model did not include histology.
Abbreviations: CI, confidence interval; HR, hazard ratio; Ref, reference.
Discussion
We employed a quality-assured registry database to evaluate the effectiveness of FDA-approved chemotherapy agents for NSCLC in the community setting. This approach enabled us to assess whether treatment efficacy established under a controlled clinical trial setting was also effective in patients treated in the real world. Approximately 88% of our analytic patient population was aged 65 years or older, which is comparable to the general population of patients with lung cancer [11]. We observed that only a third of this elderly Medicare patient population received systemic chemotherapy—a finding that reflects the well-described reluctance of oncologists to offer anticancer therapy to elderly patients out of concern for toxicity [19, 20].
The period from 1988 to 1994 provided a good reference period for our temporal analysis because the benefit of chemotherapy for advanced NSCLC was not clearly established at this time [21, 22]. Nonetheless, the 34% reduction in the risk of death for patients treated with any chemotherapy during this baseline period reproduced the initial evidence that came from meta-analysis of relatively small studies showing a potential benefit of systemic chemotherapy for advanced NSCLC [23, 24]. The incremental improvement in overall survival across the time periods that corresponded to the introduction of newer agents provided indirect evidence for the effectiveness of agents that became available during the specific time period. Survival improvement demonstrated for patients treated with an approved agent over patients not treated with the approved agent during the specified time period was considered direct evidence supporting the effectiveness of the newer agents. This approach enabled us to exclude improvements in supportive care options or newer diagnostic techniques and stage migration (the so-called Will Rogers phenomenon) as the likely explanation for the survival benefit associated with the use of newer agents [25]. Specifically, we observed a superior survival in patients treated with platinum chemotherapy. Although we did not replicate previous findings of a superior efficacy of cisplatin over carboplatin, [26, 27], we were able to demonstrate the efficacy of various platinum doublet chemotherapy [27–29].
Effectiveness of docetaxel was demonstrated by the 2-year survival rate of 25%, which compared favorably with the 21% rate reported in the pivotal clinical trial [7]. The high survival rate associated with pemetrexed therapy in this elderly Medicare population is consistent with the findings in a subset analysis of the pivotal pemetrexed study in which patients older than 70 years had a longer median OS (9.5 vs. 7.8 months) and PFS (4.6 vs. 3.0 months) [3, 30]. The superior efficacy of pemetrexed for nonsquamous NSCLC in the clinical trial setting was also observed in our data set [31]. Intriguingly, pemetrexed showed a more modest benefit in squamous NSCLC patients, possibly due to pathologic misclassification, which is a well-recognized challenge in lung cancer diagnosis [32]. However, one could speculate that this finding may suggest a real but limited benefit of pemetrexed in squamous NSCLC.
Bevacizumab is a targeted agent approved for the treatment of nonsquamous NSCLC [6]. Several meta-analyses of studies conducted in different parts of the world also demonstrated the survival benefit of bevacizumab when combined with chemotherapy [33, 34]. Our results suggested that the observed efficacy in clinical trials translated into real-world effectiveness of this agent. This is, however, discordant with the findings by Zhu et al., who failed to demonstrate a significant survival benefit for bevacizumab in Medicare patients, despite finding a numerical increase in median OS time to 9.7 months from 8.9 and 8.0 months in the two comparator arms [35]. The discrepancy between their findings and ours may in part reflect differences in methodology and the nonoverlapping patient populations despite employing the same SEER–Medicare database. They compared survival outcome in patients treated with bevacizumab, paclitaxel, and carboplatin between 2006 and 2007 with survival for patients treated with paclitaxel and carboplatin alone between 2002–2005 and 2006–2007. By contrast, we assessed the benefit of bevacizumab irrespective of the partner chemotherapy in patients diagnosed between 2004 and 2005. More importantly, the two cohorts of control patients in the study by Zhu et al. had almost twice as many stage IIIB patients as the bevacizumab-treated patients (29.1% vs. 30.6% vs. 17.6%; p < .001). Consistent with historical data, we observed better survival outcome for stage IIIB over stage IV patients whether treated with bevacizumab (1-year OS rate: 88.2% vs. 80.2%) or without bevacizumab (1-year OS rate: 53.2% vs. 34.8%). Because disease stage is one of the strongest prognostic factors in lung cancer, a statistical modeling algorithm with propensity scoring may not have been sufficient to compensate for the stage disparity between the analytic cohorts. Although it is conceivable that this stage imbalance contributed to their failure to observe a survival benefit in association with bevacizumab therapy, both results should be considered hypothesis-generating given the retrospective design and limited sample size in comparison with the prospective study population.
Pertinent limitations of our study are worthy of careful consideration, including the retrospective design, which limited our ability to fully control for potential confounders and biases in the treatment decision for specific chemotherapy agent. Also, possible imbalance between comparator groups in clinically relevant factors such as comorbid illnesses, performance status, specific genetic aberrations, and concurrent smoking could have significantly affected treatment outcome. Furthermore, we could not specifically establish when a patient progressed on a specific chemotherapy and when a patient was started on a new agent, thereby limiting our ability to establish whether newly approved agents were used according to the indicated approval guidelines. Moreover, the use of orally administered agents such as erlotinib and experimental treatment options that were not captured in the Medicare record could not be accounted for. However, we expect the large sample size to balance out these differences across the comparator groups. Reassuringly, repeat analysis with propensity score adjustment for some of these factors did not alter the results, thereby suggesting that any potential confounders were evenly distributed across treatment groups and consequently had minimal impact on the observed differences.
In conclusion, our study demonstrated a significant association between improved survival and treatment with approved cytotoxic and biologic agents that have become standard-of-care options for advanced NSCLC, thus providing evidence in support of the effectiveness of these agents in the community setting. Despite the limitations of observational studies, this approach still remains valuable for bridging the knowledge gap between controlled clinical trials, which are the gold standard for establishing new treatment paradigm, and the real-life effectiveness of such therapies. Because randomized controlled clinical trials are unable to answer every important clinical question, observational studies such as this may bridge the gap and provide answers relevant to routine patient care and testable hypotheses for future prospective trials. We hope that the real-world effectiveness indicated by our study will encourage appropriate and greater use of these approved agents and ultimately result in overall improvement in patient outcome.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) of the National Institute of Health (Grants 1K23CA164015 to T.K.O., P01CA116676 to F.R.K., and P01CA116676-5S1 to F.R.K.) and the Georgia Cancer Coalition (T.K.O., S.S.R., and F.R.K.).
This study used the linked the Surveillance, Epidemiology, and End Results (SEER)–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI's SEER Program under Contract N01-PC-35136 awarded to the Northern California Cancer Center, Contract N01-PC-35139 awarded to the University of Southern California, and Contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
We thank Anthea Hammond, Ph.D., for editorial assistance and proofreading the manuscript prior to submission.
Taofeek K. Owonikoko and Camille Ragin contributed equally to this work.
Presented in part at the American Society of Clinical Oncology 2010 Annual Meeting, Chicago, IL.
Author Contributions
Conception/Design: Taofeek K. Owonikoko, Camille Ragin, Suresh S. Ramalingam, Fadlo R. Khuri
Collection and/or assembly of data: Taofeek K. Owonikoko, Camille Ragin, Zhengjia Chen
Data analysis and interpretation: Taofeek K. Owonikoko, Camille Ragin, Zhengjia Chen, Sungjin Kim, Johann C. Brandes, Nabil F. Saba, Rebecca Pentz, Suresh S. Ramalingam, Fadlo R. Khuri
Manuscript writing: Taofeek K. Owonikoko, Camille Ragin, Zhengjia Chen, Sungjin Kim, Johann C. Brandes, Nabil F. Saba, Rebecca Pentz, Suresh S. Ramalingam, Fadlo R. Khuri
Final approval of manuscript: Taofeek K. Owonikoko, Camille Ragin, Zhengjia Chen, Sungjin Kim, Johann C. Brandes, Nabil F. Saba, Rebecca Pentz, Suresh S. Ramalingam, Fadlo R. Khuri
Disclosures
Taofeek K. Owonikoko: Novartis Oncology, Celgene Corporation, Medarex Pharmaceuticals (RF); Suresh S. Ramalingam: Agennix, Boehringer Ingelheim, Genentech, Lilly, Teva (C/A).
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA: Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA: Cancer J Clin. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 10.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 11.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: An analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 12.Shakun M, Farrell AT, Pazdur R. Poor representation of women, older age group and minorities in us registration trials for non-small cell lung cancer: FDA review. J Thorac Oncol. 2011;6:S474. [Google Scholar]
- 13.Somer RA, Sherman E, Langer CJ. Restrictive eligibility limits access to newer therapies in non-small-cell lung cancer: The implications of Eastern Cooperative Oncology Group 4599. Clin Lung Cancer. 2008;9:102–105. doi: 10.3816/CLC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results Program. SEER*Stat database: Incidence—SEER 9 Regs research data (1973–2008) [Accessed December 1, 2009]. Available at http://www.seer.cancer.gov.
- 15.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV55–IV61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the surveillance, epidemiology, and end results registry population: Factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50:939–945. doi: 10.1016/s0895-4356(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 18.Monane M, Bohn RL, Gurwitz JH, et al. Noncompliance with congestive heart failure therapy in the elderly. Arch Intern Med. 1994;154:433–437. [PubMed] [Google Scholar]
- 19.Davidoff AJ, Tang M, Seal B, et al. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 20.Hillner BE, McDonald MK, Desch CE, et al. A comparison of patterns of care of nonsmall cell lung carcinoma patients in a younger and Medigap commercially insured cohort. Cancer. 1998;83:1930–1937. doi: 10.1002/(sici)1097-0142(19981101)83:9<1930::aid-cncr8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Comella P, Frasci G, Panza N, et al. Randomized trial comparing cisplatin, gemcitabine, and vinorelbine with either cisplatin and gemcitabine or cisplatin and vinorelbine in advanced non-small-cell lung cancer: Interim analysis of a phase III trial of the Southern Italy Cooperative Oncology Group. J Clin Oncol. 2000;18:1451–1457. doi: 10.1200/JCO.2000.18.7.1451. [DOI] [PubMed] [Google Scholar]
- 22.Le Chevalier T, Brisgand D, Douillard JY, et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: Results of a European multicenter trial including 612 patients. J Clin Oncol. 1994;12:360–367. doi: 10.1200/JCO.1994.12.2.360. [DOI] [PubMed] [Google Scholar]
- 23.Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: How much benefit is enough? J Clin Oncol. 1993;11:1866–1872. doi: 10.1200/JCO.1993.11.10.1866. [DOI] [PubMed] [Google Scholar]
- 24.Marino P, Pampallona S, Preatoni A, et al. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest. 1994;106:861–865. doi: 10.1378/chest.106.3.861. [DOI] [PubMed] [Google Scholar]
- 25.Chee KG, Nguyen DV, Brown M, et al. Positron emission tomography and improved survival in patients with lung cancer: The Will Rogers phenomenon revisited. Arch Intern Med. 2008;168:1541–1549. doi: 10.1001/archinte.168.14.1541. [DOI] [PubMed] [Google Scholar]
- 26.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Liang X, Zhou X, et al. A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2007;57:348–358. doi: 10.1016/j.lungcan.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 29.Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: A meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Weiss GJ, Langer C, Rosell R, et al. Elderly patients benefit from second-line cytotoxic chemotherapy: A subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:4405–4411. doi: 10.1200/JCO.2006.06.7835. [DOI] [PubMed] [Google Scholar]
- 31.Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:64–70. doi: 10.1097/JTO.0b013e3181f7c6d4. [DOI] [PubMed] [Google Scholar]
- 32.Grilley-Olson JE, Hayes DN, Moore DT, et al. Validation of interobserver agreement in lung cancer assessment hematoxylin-eosin diagnostic reproducibility for non-small cell lung cancer: The 2004 World Health Organization classification and therapeutically relevant subsets. Arch Pathol Lab Med. 2013;137:32–40. doi: 10.5858/arpa.2012-0033-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 34.Botrel TE, Clark O, Clark L, et al. Efficacy of bevacizumab (Bev) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): Systematic review and meta-analysis. Lung Cancer. 2011;74:89–97. doi: 10.1016/j.lungcan.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Sharma DB, Gray SW, et al. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307:1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]