Abstract
The introduction of the hypomethylating agents azacitidine and decitabine has been a major advancement in the treatment of patients with higher-risk myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia who are ineligible for more intensive treatments. This concise drug review summarizes the current state of treatment with azacitidine and decitabine.
Keywords: Azacitidine, Decitabine, Drug profile, DNA methyltransferase inhibitor, Hypomethylating drug
Implications for Practice:
The introduction of the hypomethylating agents azacitidine and decitabine has been a major advancement in the treatment of patients with higher-risk myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia who are ineligible for more intensive treatments, such as allogeneic stem cell transplantation and induction chemotherapy. However, a number of uncertainties remain. Because only about 50% of patients respond to therapy with hypomethylating agents, identification of response-predicting biomarkers is warranted. In addition, the majority of responders relapse within 2 years; thus, identification of mechanisms of resistance is pivotal for further treatment optimization. This concise drug review summarizes the current state of treatment with the hypomethylating agents azacitidine and decitabine.
Introduction
The hypomethylating agents azacitidine and decitabine (5-aza-2′-deoxycytidine) are currently approved for the treatment of several specific forms of myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML), as depicted in Table 1.
Table 1.
Summary of approved indications
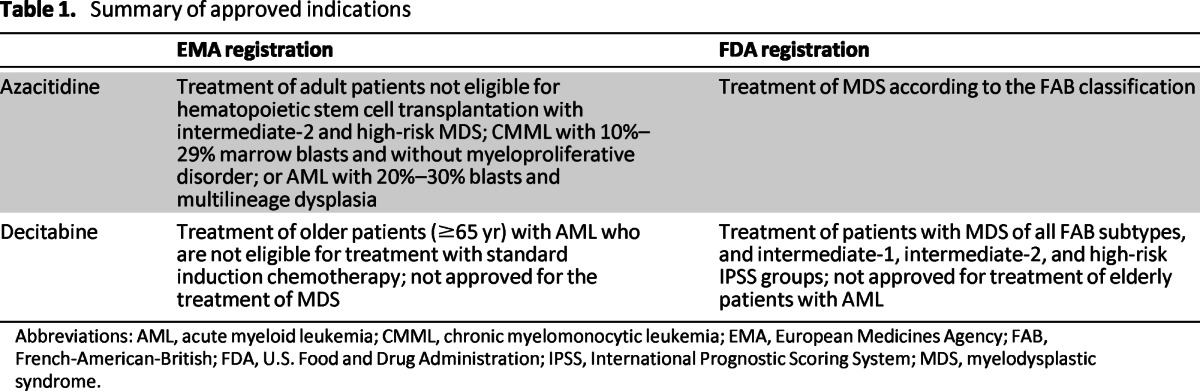
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; EMA, European Medicines Agency; FAB, French-American-British; FDA, U.S. Food and Drug Administration; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome.
The only potentially curative therapy for patients with higher-risk MDS (International Prognostic Scoring System intermediate-2 risk and high risk) is an allogeneic stem cell transplantation (allo-SCT). However, for the majority of patients, allo-SCT is not an option because of advanced age and/or comorbidities. In addition, the results obtained with intensive chemotherapy (when feasible) are often disappointing. For patients with MDS, the probability of complete remission after intensive chemotherapy is generally lower and the remission duration shorter than for patients with primary AML [1]. Therefore, the introduction of the hypomethylating agents has been a major advancement in the treatment of patients with higher-risk MDS who are ineligible for allo-SCT. Table 2 summarizes the key pharmacologic features of these two agents.
Table 2.
Summary table
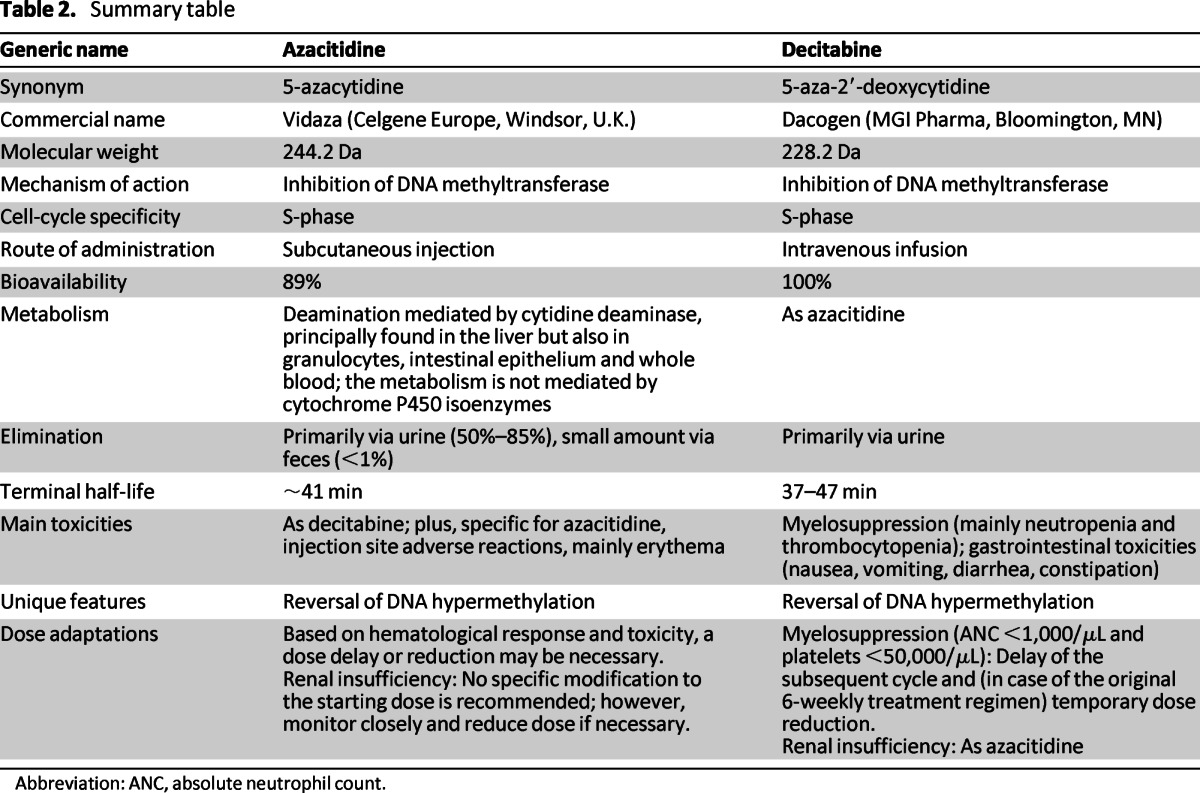
Abbreviation: ANC, absolute neutrophil count.
The pivotal phase III trial investigating azacitidine treatment in patients with higher-risk MDS demonstrated a significant improvement of overall survival compared to supportive care or low-dose cytarabine. However, when compared to intensive chemotherapy, there was no significant difference in overall survival [2]. In the absence of undisputed, comparative data, it remains uncertain whether hypomethylating agents should always be preferred over intensive chemotherapy [3].
In contrast to azacitidine, decitabine showed no beneficial effect on overall survival or time to AML in patients with MDS, even though the response percentages obtained with azacitidine and decitabine were almost the same [2, 4, 5]. The fact that a survival benefit in patients with MDS was found for azacitidine and not for decitabine does not necessarily indicate a discrepant pharmacology. The difference may also be explained by the differences in study design—in particular the inclusion criteria, the number of treatment cycles, and whether or not postprogression treatment was allowed.
Azacitidine and decitabine have also been studied for the treatment of elderly patients with AML. Elderly patients with AML have a poor prognosis with intensive chemotherapy. Because of high therapy-related mortality, less aggressive therapies are often used. A phase III study in elderly patients (≥65 years) with AML who were unfit for intensive chemotherapy compared low-dose decitabine with conventional treatments, including low-dose cytarabine and supportive care. The study demonstrated a significant survival benefit for decitabine compared to conventional treatment (7.7 vs. 5.0 months; hazard ratio [HR]: 0.82, 95% confidence interval [CI]: 0.68–0.99; p = .037). Decitabine improved the complete remission rate (17.8% vs. 7.8%, respectively; p = .001) without hampering treatment safety [6]. Based on these results, in September 2012, decitabine was registered by the European Medicines Agency (EMA) for the treatment of elderly patients (≥65 years) with AML who are not eligible for treatment with standard induction chemotherapy.
An important question is whether therapy with hypomethylating agents in elderly patients with AML may also improve outcome compared with intensive chemotherapy. A retrospective study in 671 elderly patients (≥65 years) with newly diagnosed AML demonstrated that treatment with hypomethylating agents resulted in a lower complete response (CR) rate and overall response rate (ORR) than intensive chemotherapy (CR: 28% vs. 42%; ORR: 29% vs. 47%, respectively). However the 2-year relapse-free survival rates (40% vs. 30%, p = .843) and median survival times (6.5 vs. 6.7 months, p = .413) were similar in the two groups. This suggests that the long-term outcomes for elderly patients with AML who were treated with hypomethylating agents are similar to the long-term outcomes after intensive chemotherapy [7].
Clinical Use
Azacitidine is available as a white lyophilized powder, which has to be reconstituted with Water for Injection immediately prior to administration to obtain a 25 mg/mL suspension. The azacitidine suspension should be injected subcutaneously into the upper arm, thigh, or abdomen. The site of the injection should be rotated. The recommended starting dose for the first treatment cycle of azacitidine (for all patients regardless of baseline hematology laboratory values) is 75 mg/m2 daily for 7 days, followed by a rest period of 21 days. Cycles should be repeated every 4 weeks. In subsequent treatment cycles, the dose should be adjusted based on hematologic response and toxicity. A delay in starting the next cycle or a dose reduction may be necessary [8].
In addition to this 7-day dosing schedule, alternative azacitidine schedules that omit the weekend doses have been evaluated. Although these regimens showed similar benefits regarding transfusion independence and hematologic improvement, effects on time to AML transformation or overall survival were not compared. Furthermore, the study population consisted primarily of patients with lower-risk MDS [9]. Therefore, it remains unknown whether these alternative regimens yield comparable efficacy as the approved 7-day azacitidine schedule.
Decitabine is also available as a white lyophilized powder. It has to be reconstituted with 10 mL sterile Water for Injection in order to obtain a 5 mg/mL solution. Immediately after reconstitution, this solution should be further diluted with 0.9% sodium chloride solution for injection, 5% glucose solution for injection, or lactated Ringer's solution for injection to a final drug concentration of 0.1–1.0 mg/mL [10].
At present, two regimens for decitabine administration are approved by the U.S. Food and Drug Administration: the original 6-weekly regimen of 15 mg/m2 administered by continuous intravenous infusion over 3 hours repeated every 8 hours for 3 days, and the later-approved 4-weekly regimen of 20 mg/m2 by continuous intravenous infusion over 1 hour repeated daily for 5 days [10]. The main advantage of this new regimen is the reduced infusion time, which enables treatment in the outpatient setting [11]. This 4-weekly decitabine regimen is also the dose scheme recently approved by the EMA for treatment of elderly patients (≥65 years) with AML who are not eligible for standard induction chemotherapy [6].
Complete blood counts should be determined at least prior to each cycle to monitor response and toxicity. If myelosuppression is present (absolute neutrophil count <1,000/μL and platelets <50,000/μL), subsequent decitabine cycles should be delayed; for the original 6-weekly treatment regimen, the dose should be temporarily reduced [10].
Duration of Treatment
Azacitidine treatment should be continued for a minimum of six courses before evaluating the effects of treatment. Response to treatment is assessed according to the International Working Group criteria, distinguishing three response categories: complete response, partial response, and hematological improvement [12]. Silverman et al. found that in 91% of the responding patients, initial response was seen within six cycles [13]. Continued azacitidine treatment after initial response further improved the response in 48% of patients. Maximum response was achieved by 92% of responders by cycle 12 [13].
Similarly for decitabine, a broad range was found for the time to initial response. The median time to response was more than 3 months [4, 5]. It is recommended to continue decitabine treatment for a minimum of four cycles (i.e., 6 months), provided that patients are monitored for hematologic and renal toxicities [10]. Treatment with azacitidine or decitabine should be continued as long as the patient shows continued benefit [8, 10]. The median duration of the hematological response was about 13 months in both azacitidine studies [2, 14, 15] and 9–10 months in both decitabine studies [4, 5].
The possibility of retreatment with decitabine at the moment of disease recurrence after initial treatment was investigated. Retreatment with decitabine was found to result in objective responses in 45% of the patients who previously responded to decitabine. However, the quality and duration of the second disease remission were found to be inferior. Therefore, patients who respond to decitabine might possibly derive more clinical benefit from continuation of the initial treatment [16].
Mechanism of Action
Epigenetic changes, such as aberrant DNA methylation, have an important place in the pathogenesis of MDS and AML. The most studied change of DNA methylation is the silencing of tumor suppressor genes by hypermethylation of the CpG islands within the promoter region [17]. In contrast to structural changes such as mutation or deletion causing permanent loss of gene expression, epigenetic changes can be pharmacologically reversed, resulting in gene re-expression and restoration of normal cellular functions [18].
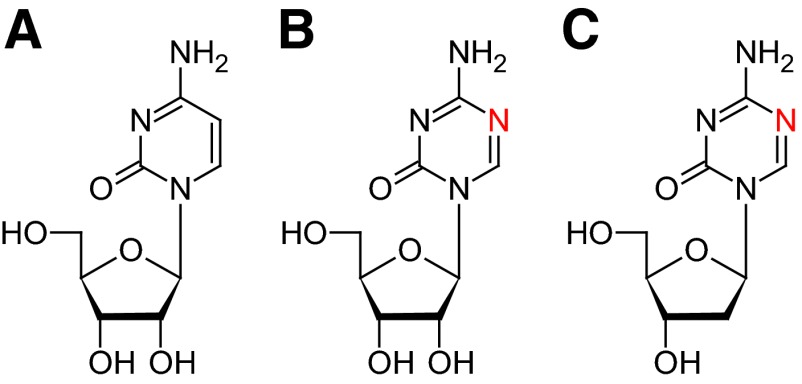
Azacitidine and decitabine (5-aza-2′deoxycytidine) are cytidine analogs in which the carbon atom at position 5 in the pyrimidine ring has been replaced by a nitrogen atom (Fig. 1). Originally, they were intended as cytotoxic drugs. However, it was discovered that a low dose of these drugs could cause DNA demethylation by inactivation of DNA methyltransferase-1 (DNMT-1), the enzyme responsible for methylation of the DNA [19].
Figure 1.
Chemical structures of cytidine (A), azacitidine (B), and decitabine (5-aza-2′-deoxycytidine) (C).
Following cellular uptake, azacitidine and decitabine are converted into their monophosphates, diphosphates, and triphosphates. Decitabine triphosphate is a deoxyribonucleotide that is incorporated only into DNA. Azacitidine is mainly converted to azacitidine triphosphate, which is incorporated into the RNA. A smaller portion of the administered azacitidine, about 10%–20%, is converted to 5-aza-2′-deoxycytidine triphosphate via the enzyme ribonucleotide reductase and is available for incorporation into the DNA. Incorporation into the DNA results in the formation of adducts between the DNA and DNMT-1. At high doses, the DNA is not able to recover and cell death occurs. However, at lower doses the formed adducts are degraded by the proteosome, after which the DNA is restored. DNA synthesis is then resumed in the absence of DNMT-1. As a consequence the aberrant DNA methylation pattern can no longer be reproduced toward the daughter strands [20]. In this way, a low dose of azacitidine or decitabine is able to induce re-expression of previously silenced genes. Reactivation of cell cycle-regulating genes that were initially silenced due to hypermethylation may induce cell differentiation, reduce proliferation, and/or increase apoptosis of the daughter cells [21].
Response Prediction
Several reports have dealt with response prediction, focusing on two different outcomes: response to treatment with hypomethylating agents and longer-term endpoints (mainly overall survival). For patients with higher-risk MDS, several factors were associated with a slightly superior response to hypomethylating therapy: no prior therapy, shorter MDS duration [22], longer MDS duration prior to treatment [5], normal karyotype, a bone marrow blast percentage <15%, and no previous exposure to low-dose cytarabine [23].
For prediction of the overall survival following azacitidine therapy, Itzykson et al. proposed a three-category prognostic classification system based on independent prognostic factors. Predictors for a favorable overall survival were a performance status <2, high-risk cytogenetics, absence of circulating blasts, and no red blood cell transfusion dependency (<4 units per 8 weeks). The prognostic score, based on these four factors, discriminated three groups with median overall survival times of 6, 15, and >26 months, respectively [23]. The obtained prognostic score was validated in several independent patient cohorts receiving azacitidine [24, 25]. Moreover, platelet doubling after the first cycle of azacitidine was found to predict a longer overall survival time for patients with MDS, CMML, or AML [25].
It is nevertheless important to further improve identification of prognostic factors of response to hypomethylating agents. Mutations in the TET2 and DNMT3A genes (both regulating DNA methylation) and the ASXL1 gene (regulating histone modifications) are associated with a favorable response of patients with higher-risk MDS to azacitidine treatment. However, these genetic factors have not yet been clearly associated with differences in survival [26, 27].
Treatment Failure
Only about 50% of patients respond to therapy with hypomethylating agents, and the majority of the responders relapse within 2 years [2]. The outcome after failure of hypomethylating therapy is poor. Retrospective analysis of the outcome of 435 patients with higher-risk MDS who experienced azacitidine treatment failure demonstrated a median overall survival of 5.6 months and a 2-year survival probability of 15% [28]. For 87 patients with MDS or CMML, a median overall survival time of 4.3 months was reported after decitabine failure, with an estimated 1-year survival rate of 28% [29]. The outcome of patients with secondary AML arising from MDS after azacitidine failure was even worse, with a median overall survival time of 3.6 months and a 1-year survival rate of only 8% [30]. There is no standard of care for salvage therapy after failure of hypomethylating therapy. Because of the limited therapeutic options, this is a challenging cohort of patients in a setting that constitutes an important area of research.
One might question whether it could be useful to switch from one hypomethylating agent to the other after treatment failure. Considering the slightly different mechanisms of action of azacitidine and decitabine, as well as the distinct metabolic activation routes concerned, there is not necessarily complete cross-resistance between both hypomethylating agents. Failure of one drug may not exclude activity of the other. A small study investigated the value of decitabine treatment after azacitidine failure [31]. Of the 14 patients with MDS who were analyzed, three patients achieved complete remission and one patient showed hematologic improvement, which corresponded to an overall response rate of 28%. Of the responders, prior azacitidine therapy was stopped because of disease progression (n = 1), lack of response (n = 2), and severe skin toxicity (n = 1) [31], which suggests that changing hypomethylating therapy from azacitidine to decitabine may have some clinical utility. No studies were found investigating a switch in the opposite direction (i.e., the use of azacitidine in decitabine failures).
Combination Therapies
Numerous reports have dealt with the combination of hypomethylating agents and other agents, ranging from growth factors to histone deacetylase inhibitors. A fully comprehensive overview of all combination strategies currently investigated is beyond the scope of this review. However, we would like to highlight the promising combination of a hypomethylating agent with the immunomodulating drug lenalidomide. A phase II study demonstrated that the combination of azacitidine (75 mg/m2 daily for 5 days) and lenalidomide (10 mg/day for 21 days, 28-day cycle) was well tolerated and highly active in patients with higher-risk MDS. Using this combination, 44% of patients achieved CR and the ORR was 72% [32]. These results seem to be better than the response rates obtained with azacitidine monotherapy in the AZA-001 trial (CR: 17%, ORR: 49%) [2], although the discrepant response rates might be attributable to differences between the study populations. Randomized trials comparing this combination regimen, as well as others, with azacitidine monotherapy, are currently ongoing.
Bioanalysis
The bioanalysis of azacitidine and decitabine in human plasma can be exerted by high-performance liquid chromatography coupled with tandem mass spectrometry detection [33, 34].
Pharmacokinetics
Absorption
Azacitidine is rapidly absorbed after subcutaneous administration. Following a standard dose of 75 mg/m2 subcutaneously, maximum plasma concentration (750 ± 403 ng/mL) is reached after 30 minutes. The absolute bioavailability of azacitidine after subcutaneous administration is approximately 89% compared to intravenous infusion [35].
For decitabine, maximum plasma concentration is generally observed at the end of infusion. Following a 3-hour intravenous infusion of 15 mg/m2, the maximum plasma concentration is 64.8–77.0 ng/mL. At this time, steady-state plasma concentration is reached as well [36].
Distribution
Both azacitidine and decitabine are widely distributed over tissues. For azacitidine, the mean volume of distribution after intravenous administration was about 76 L [35]. For decitabine, a volume of distribution of 63–89 L/m2 was found at steady state [36].
Metabolism
Intracellular phosphorylation is a pivotal step for azacitidine and decitabine to become active. Beyond this step, the exact metabolic fate of azacitidine and decitabine is unknown. Both drugs undergo spontaneous hydrolysis in aqueous solution and deamination mediated by cytidine deaminase, which is principally found in the liver but also in granulocytes, intestinal epithelium, and whole blood. In vitro studies indicate that the metabolism of azacitidine and decitabine is not mediated by cytochrome P450 isoenzymes. Clinically significant inhibitory or inductive effects on CYP enzymes are also unlikely [10, 37].
Elimination
Azacitidine is cleared rapidly from plasma. The mean elimination half-life after a standard subcutaneous dose of 75 mg/m2 is about 41 minutes. This is approximately twofold higher than the mean elimination half-life after intravenous administration (22 minutes). The systemic clearance of azacitidine is 147 ± 47 L/hour [35]. Urinary excretion is the primary route of elimination of azacitidine and its metabolites. Following intravenous and subcutaneous administration of 14C-labeled azacitidine, 50%–85% of the administered radioactivity was recovered in urine, whereas less than 1% was recovered in the feces. Unfortunately, parent drug and possible drug metabolites could not be distinguished in these 14C-azacitidine studies. Nevertheless, the longer half-life calculated based on 14C radioactivity (3.4–6.2 hours) suggested the presence of circulating metabolites [38, 39].
The terminal elimination half-life (t1/2) of decitabine is 37–47 minutes. Consequently, steady-state in plasma is reached at the end of the 3-hour infusion. Thereafter, plasma concentrations decline biexponentially and are measurable up to 2 hours postinfusion. The total body clearance of decitabine is 125–132 L/hour per m2. Decitabine plasma pharmacokinetics remained unchanged upon repeated dosing and even from cycle to cycle [36].
Alterations for Special Populations
Because azacitidine, decitabine, and/or their metabolites are primarily excreted by the kidneys, caution is needed in patients with renal impairment. Liver function tests and serum creatinine should be determined prior to initiation of therapy and prior to each treatment cycle. No specific modification to the starting dose is recommended in patients with renal insufficiency prior to starting treatment. However, patients should be closely monitored for toxicity and dose reductions should be implemented if necessary [8]. To our knowledge, there has only been one retrospective study that examined the feasibility of therapy with azacitidine or decitabine in patients with renal insufficiency (creatinine clearance ≤59 mL/min) [40]. Toxicity rates were comparable to those in previous reports in patients with adequate renal function. Most patients were able to tolerate the hypomethylating agents at standard doses. However, dose reductions and treatment interruptions were required for some patients, particularly those with severe renal insufficiency (i.e., creatinine clearance ≤30 mL/min). This group experienced more treatment-related toxicities, mainly myelosuppression and worsening of the renal function [40]. A prospective study may be warranted to better define the pharmacokinetics of hypomethylating agents in patients with renal insufficiency and to determine if dose adjustments at the beginning of treatment would be needed. One trial (NCT00652626) is currently recruiting patients to study the effect of renal impairment on the pharmacokinetics of azacitidine.
Toxicity
Azacitidine and decitabine are generally well tolerated and have a manageable toxicity profile. The most common toxicity is myelosuppression, mainly displaying as neutropenia and thrombocytopenia. However, toxicity can be difficult to assess for patients with severe cytopenias from their disease [2, 14, 41]. Adverse events related to myelosuppression typically occur in the third week of the treatment cycle. Most patients achieve hematologic recovery prior to the next treatment cycle. Otherwise, a delay in starting the next cycle or a dose reduction may be necessary. Hematologic adverse events were most frequently observed during the first two treatment cycles and nadir values for hematologic parameters generally improved during subsequent cycles [4, 41]. Data about an elevated risk for infections or bleeding caused by hypomethylating agents were not very consistent [41]. Patients should be advised to promptly report febrile episodes and to be observant for signs and symptoms of bleeding [8].
The most common nonhematological adverse events were gastrointestinal toxicities such as nausea, vomiting, diarrhea, and constipation, which generally occurred in the first week of the treatment cycle. These events were generally mild and transient. They could be managed with concomitant medications, including antiemetics and antidiarrheals [2, 4].
Azacitidine administration can result in adverse reactions at the site of injection, mainly erythema. Most of these reactions are transient. A minority of patients (<12%) required treatment with corticosteroids and/or antihistamines. The incidence of adverse reactions at the site of injection might be reduced by changing the needle used to load the syringe to a clean needle (i.e., without azacitidine residue) before injection. Additionally, use of warm compresses after injection may alleviate symptoms [41].
Contraindications or Special Precautions
According to the product information, azacitidine is contraindicated in patients with advanced malignant hepatic tumors. There are no adequate data on the use of azacitidine or decitabine in pregnant women. Studies in mice have shown reproductive toxicity. Based on these results and their mechanism of action, azacitidine and decitabine should not be used during pregnancy, especially during the first trimester, unless clearly necessary. Men and women of childbearing potential must use effective contraception during and up to 3 months after treatment. It is not known whether azacitidine, decitabine, or their metabolites are excreted in human milk. Because of potential serious adverse reactions in nursing infants, breastfeeding is contraindicated during treatment. The safety and effectiveness in children younger than 18 years have not been established [8, 10].
Clinical Monitoring
Complete blood counts should be performed at least prior to each cycle to monitor response and toxicity. Possible consequences for the administered dose were previously described. Liver function tests and serum creatinine should be determined prior to initiation of therapy and prior to each treatment cycle. No dose reduction is recommended for patients with hepatic or renal insufficiency prior to starting treatment, but patients should be closely monitored for toxicity [8, 10]. For patients treated with azacitidine, the serum bicarbonate concentrations should be monitored. If unexplained reductions in serum bicarbonate (<20 mmol/L) or elevations of serum creatinine or blood urea nitrogen (at least twofold above baseline and above the upper limit of normal) occur, the dose should be reduced or administration delayed [8]. Decitabine treatment should be postponed if any of the following nonhematologic toxicities are present: serum creatinine ≥177 μmol/L (2 mg/dL); alanine aminotransferase or total bilirubin at least two times the upper limit of normal; or active or uncontrolled infection. Decitabine treatment should not be restarted until the toxicity is resolved [10].
Author Contributions
Conception/Design: Jan Schellens
Collection and/or assembly of data: Ellen Derissen
Data analysis and interpretation: Ellen Derissen
Manuscript writing: Ellen Derissen
Final approval of manuscript: Jos Beijnen, Jan Schellens
Disclosures
The authors reported no financial relationships.
References
- 1.Beran M. Intensive chemotherapy for patients with high-risk myelodysplastic syndrome. Int J Hematol. 2000;72:139–150. [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itzykson R, Fenaux P. Optimizing hypomethylating agents in myelodysplastic syndromes. Curr Opin Hematol. 2012;19:65–70. doi: 10.1097/MOH.0b013e32834ff58a. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 5.Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: Final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120:4840–4845. doi: 10.1182/blood-2012-06-436055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summary of product characteristics: Vidaza 25 mg/mL powder for suspension for injection. [Accessed September 11, 2012]. Available at http://www.ema.europa.eu/docs.
- 9.Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27:1850–1856. doi: 10.1200/JCO.2008.17.1058. [DOI] [PubMed] [Google Scholar]
- 10.Approved labeling dacogen (decitabine) for injection. [Accessed October 2, 2012]. Available at http://www.accessdata.fda.gov.
- 11.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: The alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–3848. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 13.Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. 2011;117:2697–2702. doi: 10.1002/cncr.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 15.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 16.Rüter B, Wijermans PW, Lübbert M. Superiority of prolonged low-dose azanucleoside administration? Results of 5-aza-2′-deoxycytidine retreatment in high-risk myelodysplasia patients. Cancer. 2006;106:1744–1750. doi: 10.1002/cncr.21796. [DOI] [PubMed] [Google Scholar]
- 17.Issa JP. Epigenetic changes in the myelodysplastic syndrome. Hematol Oncol Clin North Am. 2010;24:317–330. doi: 10.1016/j.hoc.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–3946. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 20.McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: Molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15:3927–3937. doi: 10.1158/1078-0432.CCR-08-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian HM, O'Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 23.Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 24.Breccia M, Loglisci G, Cannella L, et al. Application of French prognostic score to patients with International Prognostic Scoring System intermediate-2 or high risk myelodysplastic syndromes treated with 5-azacitidine is able to evaluate overall survival and rate of response. Leuk Lymphoma. 2012;53:982–983. doi: 10.3109/10428194.2011.643408. [DOI] [PubMed] [Google Scholar]
- 25.van der Helm LH, Alhan C, Wijermans PW, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch Azacitidine Compassionate Named Patient Programme. Br J Haematol. 2011;155:599–606. doi: 10.1111/j.1365-2141.2011.08893.x. [DOI] [PubMed] [Google Scholar]
- 26.Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 27.Itzykson R, Fenaux P. Predicting the outcome of patients with higher-risk myelodysplastic syndrome treated with hypomethylating agents. Leuk Lymphoma. 2012;53:760–762. doi: 10.3109/10428194.2011.651618. [DOI] [PubMed] [Google Scholar]
- 28.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prébet T, Gore SD, Thépot S, et al. Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure. Br J Haematol. 2012;157:764–766. doi: 10.1111/j.1365-2141.2012.09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borthakur G, Ahdab SE, Ravandi F, et al. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk Lymphoma. 2008;49:690–695. doi: 10.1080/10428190701882146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–4951. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Rudek MA, He P, et al. Quantification of 5-azacytidine in plasma by electrospray tandem mass spectrometry coupled with high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813:81–88. doi: 10.1016/j.jchromb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Patel K, Guichard SM, Jodrell DI. Simultaneous determination of decitabine and vorinostat (Suberoylanalide hydroxamic acid, SAHA) by liquid chromatography tandem mass spectrometry for clinical studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863:19–25. doi: 10.1016/j.jchromb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Marcucci G, Silverman L, Eller M, et al. Bioavailability of azacitidine subcutaneous versus intravenous in patients with the myelodysplastic syndromes. J Clin Pharmacol. 2005;45:597–602. doi: 10.1177/0091270004271947. [DOI] [PubMed] [Google Scholar]
- 36.Cashen AF, Shah AK, Todt L, et al. Pharmacokinetics of decitabine administered as a 3-h infusion to patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) Cancer Chemother Pharmacol. 2008;61:759–766. doi: 10.1007/s00280-007-0531-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Liu L, Laille E, et al. In vitro assessment of cytochrome P450 inhibition and induction potential of azacitidine. Cancer Chemother Pharmacol. 2010;65:995–1000. doi: 10.1007/s00280-010-1245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troetel WM, Weiss AJ, Stambaugh JE, et al. Absorption, distribution, and excretion of 5-azacytidine (NSC-102816) in man. Cancer Chemother Rep. 1972;56:405–411. [PubMed] [Google Scholar]
- 39.Israili ZH, Vogler WR, Mingioli ES, et al. The disposition and pharmacokinetics in humans of 5-azacytidine administered intravenously as a bolus or by continuous infusion. Cancer Res. 1976;36:1453–1461. [PubMed] [Google Scholar]
- 40.Batty GN, Kantarjian H, Issa JP, et al. Feasibility of therapy with hypomethylating agents in patients with renal insufficiency. Clin Lymphoma Myeloma Leuk. 2010;10:205–210. doi: 10.3816/CLML.2010.n.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santini V, Fenaux P, Mufti GJ, et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol. 2010;85:130–138. doi: 10.1111/j.1600-0609.2010.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]