On May 10, 2012, a conditional marketing authorization valid throughout the European Union was granted for pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphoma. This article summarizes the scientific review of the application leading to approval in the European Union.
Keywords: Pixantrone, Pixuvri, Aggressive non-Hodgkin's lymphoma, EMA, European Medicines Agency
Learning Objectives
Describe the efficacy profile of pixantrone in patients with relapsed or refractory NHL.
Identify the most frequent toxicities associated with pixantrone treatment.
Abstract
On May 10, 2012, the European Commission issued a conditional marketing authorization valid throughout the European Union for pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphoma (NHL). Pixantrone is a cytotoxic aza-anthracenedione that directly alkylates DNA-forming stable DNA adducts and cross-strand breaks. The recommended dose of pixantrone is 50 mg/m2 administered on days 1, 8, and 15 of each 28-day cycle for up to 6 cycles. In the main study submitted for this application, a significant difference in response rate (proportion of complete responses and unconfirmed complete responses) was observed in favor of pixantrone (20.0% vs. 5.7% for pixantrone and physician's best choice, respectively), supported by the results of secondary endpoints of median progression-free and overall survival times (increase of 2.7 and 2.6 months, respectively). The most common side effects with pixantrone were bone marrow suppression (particularly of the neutrophil lineage) nausea, vomiting, and asthenia. This article summarizes the scientific review of the application leading to approval in the European Union. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the European Medicines Agency website (http://www.ema.europa.eu).
Implications for Practice:
On February 16 2012, the European Medicines Agency (EMA) recommended the granting of a conditional marketing authorization for pixantrone, 29 mg, powder for concentrate for solution for infusion for the treatment of patients with multiply relapsed aggressive non-Hodgkin's Lymphoma (NHL) as monotherapy. In the main study submitted for this application, a significant difference in response rate (proportion of complete responses and unconfirmed complete responses) was observed in favor of pixantrone (20.0% vs. 5.7% for pixantrone and physician's best choice, respectively), supported by the results of secondary endpoints of median progression-free and overall survival times (increase of 2.7 and 2.6 months, respectively). The most common side effects are neutropenia, leucopoenia, anaemia, thrombocytopenia, asthenia, pyrexia, cough, decreased ejection fraction, and nausea. Haematological side effects are also the most common associated with grade 3 or 4 toxicity. Detailed recommendations for the use of this product are available on the EMA website.
Introduction
Anthracyclines are one of the most active drug classes in non-Hodgkin's lymphoma (NHL), but the likelihood of cardiotoxicity rises as the cumulative dose increases. The established first-line chemotherapy regimens typically include cyclophosphamide, anthracycline (doxorubicin), vincristine, and corticosteroids (the CHOP regimen). The addition of rituximab has further improved response rates and survival in certain lymphoma entities [1, 2]. However, about 20%–50% of patients either fail to respond to front-line treatment (primary refractory disease) or have relapsing disease. There is no consensus regarding the best regimen for aggressive NHL beyond first relapse in patients not eligible for stem cell transplant or in disease refractory to second-line therapy.
Pixantrone (CTI Life Sciences Limited, Hertfordshire, United Kingdom) is a cytotoxic aza-anthracenedione (Fig. 1). DNA intercalation, nucleic acid compaction, and interference with DNA-topoisomerase II activity resulting in protein-associated DNA strand breaks have been proposed as critical events that lead to drug-induced cell death for mitoxantrone and anthracyclines. Unlike anthracyclines and mitoxantrone, pixantrone is only a weak inhibitor of topoisomerase II. Moreover, unlike anthracyclines and anthracenediones, pixantrone directly alkylates DNA-forming stable DNA adducts and cross-strand breaks. Furthermore, because pixantrone incorporates a nitrogen heteroatom into the ring structure and does not have ketone groups, pixantrone has less potential for generating reactive oxygen species, binding iron, and forming alcohol metabolites that are believed to cause the cardiac toxicity of anthracyclines.
Figure 1.

Structural formula of pixantrone.
The review of this new drug application was conducted by the European Medicines Agency (EMA) Committee of Human Medicinal Products (CHMP). Following the scientific review, a conditional marketing authorization was issued in the European Union (EU) for pixantrone as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphomas. The benefit of pixantrone treatment has not been established in patients when used as fifth-line or greater chemotherapy in patients who are refractory to last therapy.
This article summarizes the scientific review of the application leading to approval of pixantrone in the EU. The detailed scientific assessment report and product information (including the summary of product characteristics [SmPC]) for this product are available on the EMA website (http://www.ema.europa.eu).
Nonclinical Aspects
The mechanistic studies submitted showed that pixantrone binds to DNA in vitro, similarly to mitoxantrone. The interaction of pixantrone with DNA and topoisomerase II was qualitatively similar to that of mitoxantrone, but quantitatively different as shown by the lower amount of DNA single-strand breaks, double-strand breaks, and DNA-protein crosslinks in L1210 leukemia cells in vitro [3]. The in vitro cell-killing effects of pixantrone did not seem to be solely related to stimulation of topoisomerase II-mediated DNA cleavage or to formation of DNA breaks, suggesting that pixantrone may operate by currently undefined mechanisms.
Pixantrone had a broad spectrum of antitumor activity against hematological and solid tumor models. The activity in the hematological tumors was superior to that of standard agents and was present at a wide range of well-tolerated doses. The combination studies demonstrated the potential of pixantrone as a therapeutic agent against a broad range of malignancies.
Some toxicological findings were of relevance to the clinical use of pixantrone. Sudden deaths of rodents during or immediately after i.v. bolus administration of pixantrone were primarily attributable to the injection rate and dose volumes, indicating that pixantrone should be administered as slow infusion. Hematotoxicity and myelotoxicity were shown to be similar to mitoxantrone. The cardiotoxic potential of pixantrone appeared to be lower than that of the reference compounds doxorubicin and mitoxantrone [4].
Pharmacokinetics
Following intravenous administration, plasma concentrations of pixantrone reached the maximal concentration at the end of infusion and then declined polyexponentially. The pharmacokinetics of pixantrone were dose-independent in the 3–105 mg/m2 dose range. No substantial differences were observed when the medicinal product was given as a single agent or in combination studies. Pixantrone exhibited a large volume of distribution of 25.8 L and a weak binding to serum protein (approximately 50%).
Metabolism did not appear to be an important elimination pathway for pixantrone. Acetylated metabolites were pharmacologically inactive and metabolically stable. Pixantrone had a moderate to high total plasma clearance of 72.7 L/hr and a low renal excretion accounting for less than 10% of the administered dose in 0–24 hours. The terminal half-life ranged from 14.5 to 44.8 hr with a mean of 23.3 ± 8.0 hours (n = 14, coefficient of variation = 34%) and a median of 21.2 hours. Plasma clearance was mainly nonrenal. Biliary excretion of unchanged pixantrone appeared to be the major elimination pathway. As a consequence, pixantrone should not be administered to patients with severe liver impairment. Hepatic uptake of pixantrone is possibly mediated by OCT1 active transporters. Biliary excretion by P-glycoprotein and BCRP and agents that inhibit these transporters has the potential to decrease hepatic uptake and excretion efficiency of pixantrone. In addition, pixantrone excretion might be increased with a consequent decrease in systemic exposure in case of coadministration with efflux transport inducers.
Pixantrone had only a weak or no capability to inhibit P-glycoprotein, BCRP, and BSEP transport mechanism in vitro. Pixantrone did inhibit OCT1-mediated metformin transport in vitro, but it is not expected to inhibit OTC1 in vivo at clinically relevant concentrations. Pixantrone was a poor inhibitor of OATP1B1 and OATP1B3 uptake transporters in vitro. A relationship between plasma exposure to pixantrone and neutrophil count has been observed.
Age, sex, and race did not seem to have a significant effect on pharmacokinetics of pixantrone. However, clearance appeared to be dependent on body size measures and dosing based on body surface area has been introduced.
Dose Finding
Three phase I dose-escalation single-agent studies (two in solid tumors and one in NHL/chronic lymphocytic leukemia) explored two different treatment regimens: pixantrone every 3 weeks and pixantrone weekly for 3 consecutive weeks with 1 week rest [5–7]. The dose-limiting toxicity was reversible grade 4 neutropenia of more than 4 days duration. The final schedule selected for phase II development was 85 mg/m2 of pixantrone on days 1, 8, and 15 of a 28-day cycle. A phase II study was conducted in adult patients with relapsed aggressive NHL. The primary efficacy variable was objective response rate (ORR) according to local evaluation based on World Health Organization/Union for International Cancer Control criteria. Thirty-three patients were enrolled and treated with at least one dose of pixantrone. There were nine (27.3%) objective responses. All but one of the confirmed responses were in patients with diffuse large B-cell lymphoma (DLBCL) or other high-grade B-cell lymphoma. There was only one response among the seven patients with mantle cell lymphoma (14.3%) [8].
Clinical Efficacy
The pivotal efficacy study for this application was study PIX 301 (NCT00088530; EudraCT 2004–000480-10) [9]. This study was an international, multicenter, randomized, active controlled, open-label phase III study designed to compare the efficacy and safety of pixantrone against physician's choice of protocol-specified single-agent therapies in patients with relapsed or refractory aggressive NHL who had received at least two prior NHL regimens. Patients in the experimental group received pixantrone 85 mg/m2 by i.v. infusion on days 1, 8, and 15 of each 4-week cycle for up to 6 cycles. In the comparator group, physicians chose one out of six specified single agents—vinorelbine, oxaliplatin, ifosfamide, etoposide, mitoxantrone, gemcitabine—for up to 6 cycles or rituximab using protocol-defined doses and schedules.
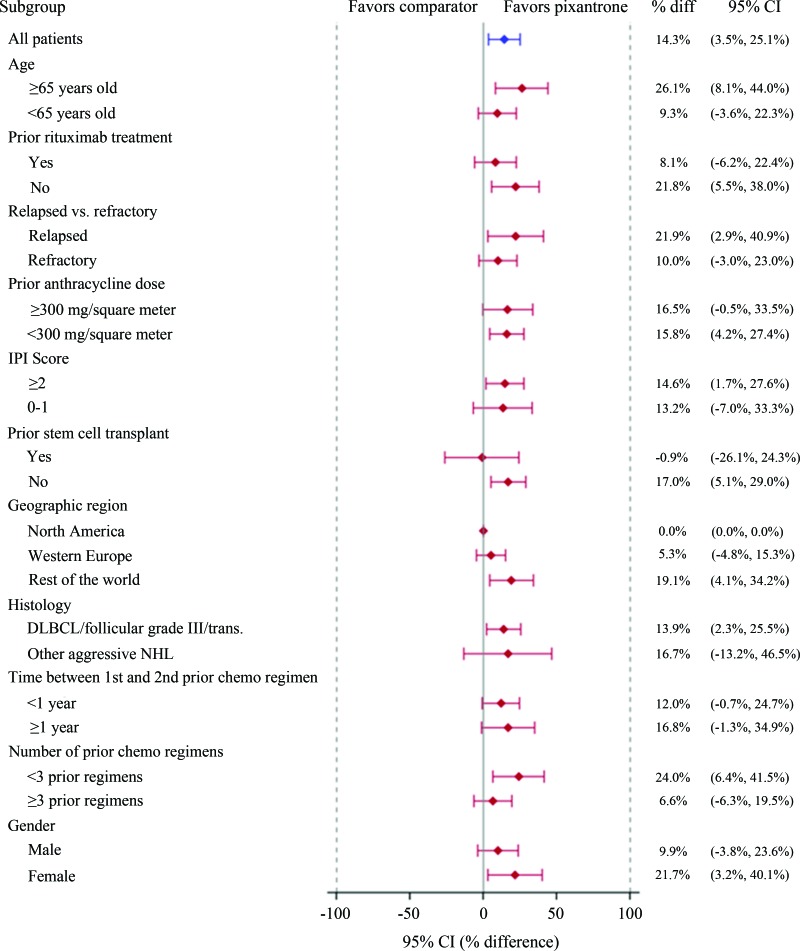
In exploratory subgroup analyses, factors favoring response to pixantrone included age ≥65 years and women. However, the significance of these differences was difficult to interpret. Other factors associated with more favorable response to pixantrone were “rest of world region” (patients recruited outside North America and Western Europe), absence of prior anti-CD20 treatment or stem cell transplant, and less than three prior chemotherapy regimens.
Patients in PIX 301 were required to have been sensitive to prior anthracycline therapy (confirmed or unconfirmed complete response [CR] or partial response [PR]). Types of NHL permitted were (Revised European American Lymphoma Classification/World Health Organization classification): DLBCL, including mediastinal large B-cell lymphoma and primary effusion lymphoma/immunoblastic lymphoma; transformed indolent lymphoma; grade 3 follicular lymphoma; peripheral T-cell lymphoma, including not otherwise specified and diffuse mixed cell lymphoma; and anaplastic large cell lymphoma, including T/null cell or primary systemic type. Patients with mantle cell lymphoma were excluded.
The primary efficacy endpoint was the proportion of CR and unconfirmed complete responses (CRu), as assessed by a blinded independent assessment panel based on the Report of the International Workshop to Standardize Response Criteria. The primary analysis used a database cutoff after the last patient completed the end-of-treatment visit. Overall survival (OS) and progression-free survival (PFS) were secondary endpoints.
A total of 320 patients were originally planned for this study. However, the study closed after randomization of 140 patients due to extremely slow accrual. The applicant company has provided further details of when the decision was made to stop recruitment and confirmed that the sponsor was blinded at this stage. No adjustment for the type I error was considered necessary.
Seventy patients were randomized to each study group. Patient demographics and baseline disease characteristics (Table 1) were generally well balanced between the treatment groups. However, limited data were available for patients previously treated with rituximab (38 patients in the pixantrone arm and 39 patients in the comparator group). Only 20 patients in the pixantrone arm and 16 in the comparator arm completed six cycles of therapy, the major reason being progressive/relapsed disease (28 vs. 39 patients in the pixantrone and comparator group, respectively) and to a lower extent adverse events (15 vs. 9 patients in the pixantrone and comparator arm, respectively).
Table 1.
Baseline patient and disease characteristics
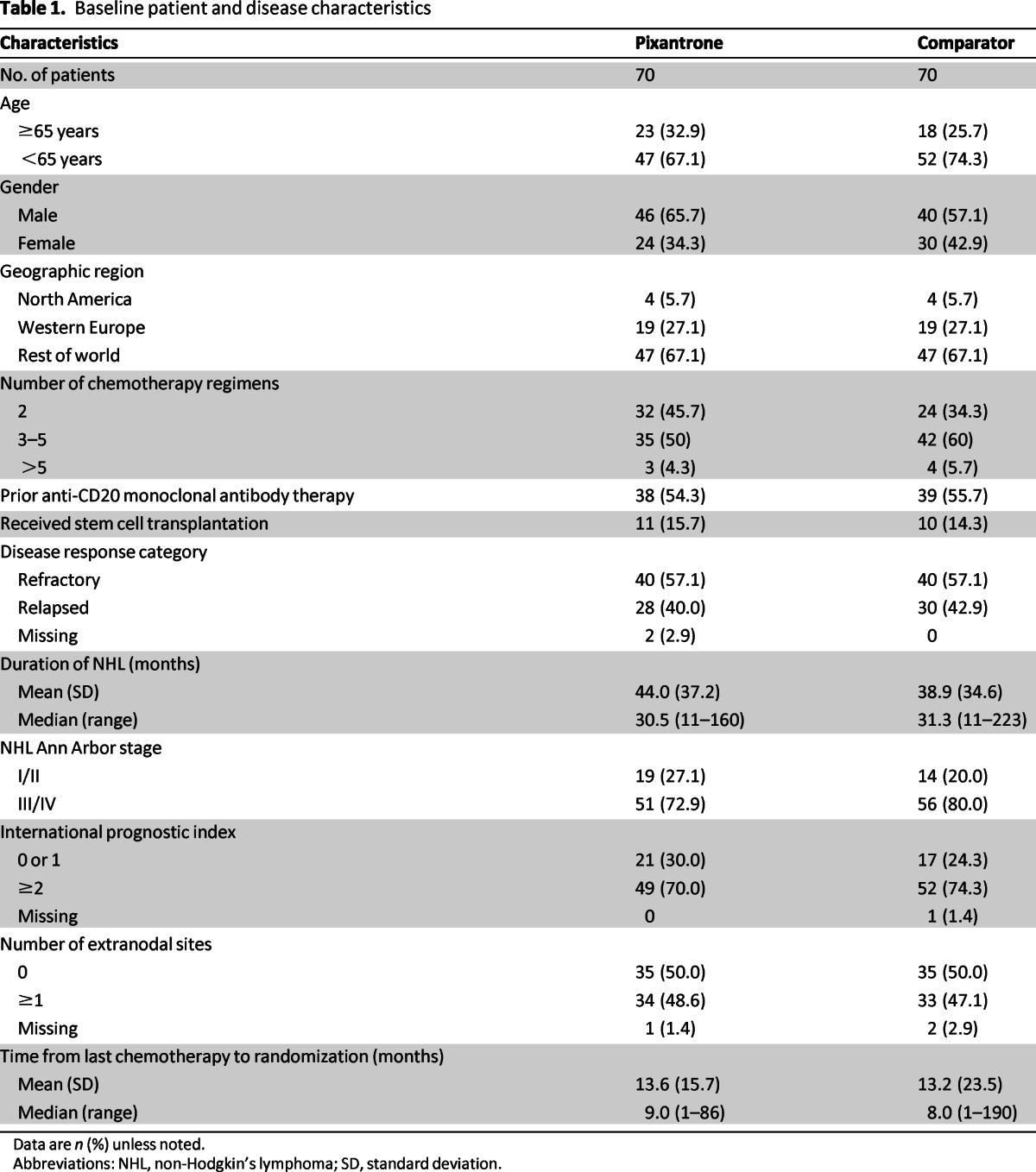
Data are n (%) unless noted.
Abbreviations: NHL, non-Hodgkin's lymphoma; SD, standard deviation.
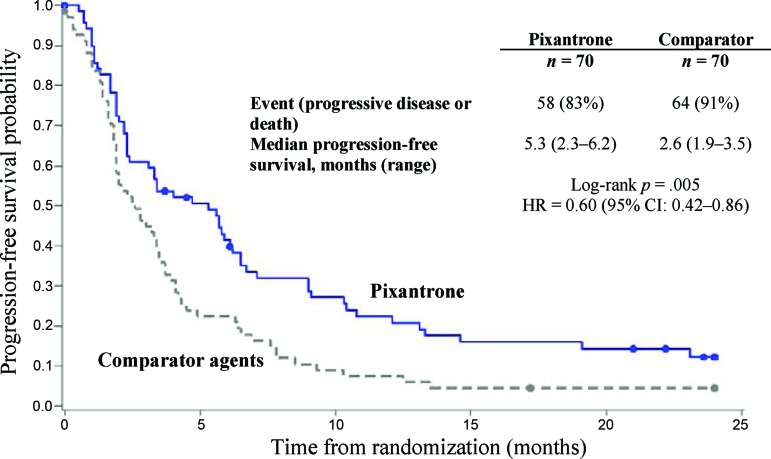
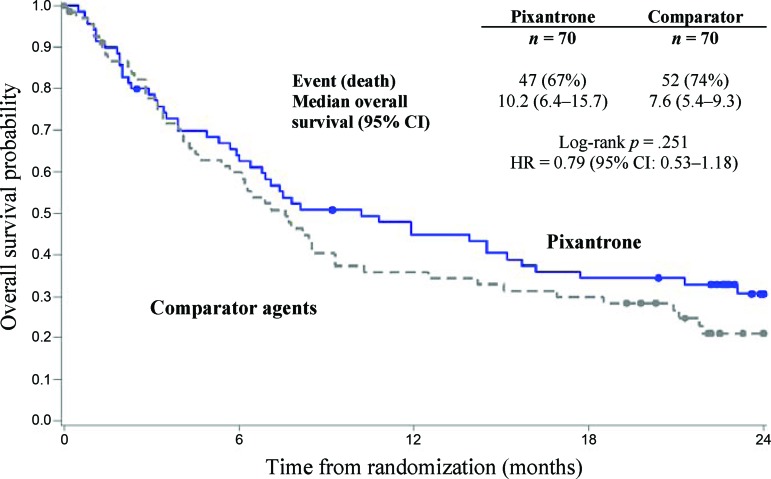
Treatment with pixantrone was associated with a statistically significantly higher rate of CR/CRu and a higher ORR compared with the comparator group (Table 2). Concerning secondary endpoints, pixantrone was associated with a 40% improvement in PFS time (hazard ratio [HR] = 0.60; log-rank p = .005) compared with the control group, with an increase of 2.7 months in median PFS (Fig. 2). The median overall survival for patients treated with pixantrone was 2.6 months longer compared to patients treated with comparator (HR = 0.79; log-rank p = .25; Fig. 3). Duration of complete response (CR/Cru) was 9.6 months in patients treated with pixantrone compared to 4.0 months in patients treated with comparator.
Table 2.
Summary of response per independent assessment panel (intent-to-treat population)
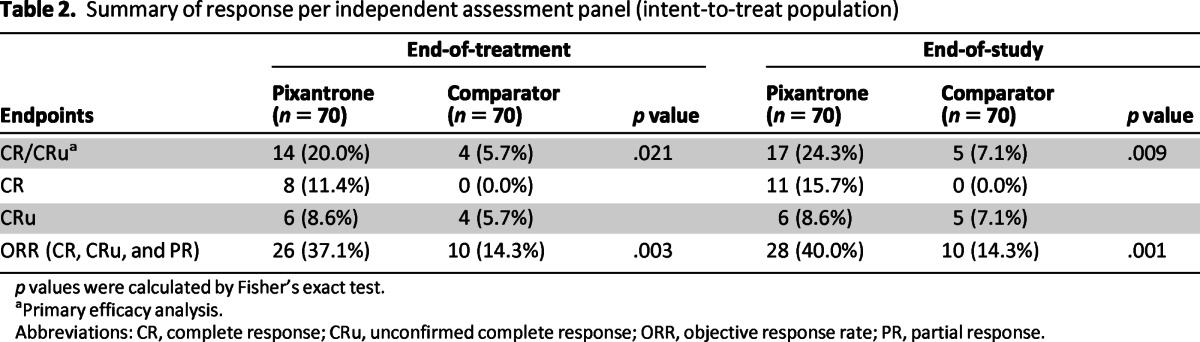
p values were calculated by Fisher's exact test.
aPrimary efficacy analysis.
Abbreviations: CR, complete response; CRu, unconfirmed complete response; ORR, objective response rate; PR, partial response.
Figure 2.
Progression-free survival: end of study.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 3.
Overall survival: end of study.
Abbreviations: CI, confidence interval; HR, hazard ratio.
In exploratory subgroup analyses, factors favoring response to pixantrone included age ≥65 years and women. However, the significance of these differences was difficult to interpret. Other factors associated with more favorable response to pixantrone were “rest of world region” (patients recruited outside North America and Western Europe), absence of prior anti-CD20 treatment or stem cell transplant, and less than three prior chemotherapy regimens (Fig. 4). Concerning the latter, there was a decrease in the response rate to pixantrone with increasing numbers of prior regimens. This was most apparent in patients who had received four or more prior regimens (i.e., use of pixantrone as fifth-line therapy). Nearly all patients (27 of 28) who had received four or more prior regimens had also received prior rituximab. PFS was more favorable in the pixantrone arm versus the comparator arm regardless of prior rituximab use. These data supported the efficacy of pixantrone in patients that have received prior rituximab and up to three prior treatment regimens, although further confirmation is awaited (see Benefit-Risk Assessment).
Figure 4.
Subgroup analysis of complete responses to unconfirmed complete responses by Independent Assessment Panel.
Abbreviations: CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; HR, hazard ratio; IPI, International Prognostic Index; NHL, non-Hodgkin's lymphoma.
There were a total of 38 Western European patients enrolled in PIX 301, 19 per study group. In the pixantrone group, there were three responses (16%; 2 PR and 1 CRu). Most patients from Europe were heavily pretreated with multiple combinations regimens including rituximab and had a short interval from their last regimen; nearly half of the patients had rapidly progressing disease. Compared with other patients enrolled in the study, patients entered in Europe were later-stage patients with highly aggressive disease.
Clinical Safety
Safety data from 12 clinical studies were available and a total of 348 patients received pixantrone, including 68 patients having received any amount of protocol therapy in PIX301 and 129 patients in uncontrolled single-agent trials. The majority of patients in the pivotal trial received at least four cycles of treatment with the recommended dose.
The most common adverse events associated with pixantrone in the pivotal study were neutropenia (50%), leucopenia (25%), anemia (31%), thrombocytopenia (21%), asthenia (23%), pyrexia (23%), cough (22%), decreased ejection fraction (19%), and nausea (18%). One of pixantrone's characteristic is a reversible skin discoloration (Table 3). Neutropenia and leukopenia were the most common grade 3–4 adverse events reported (41% and 23%, respectively, in the pixantrone arm). Most grade 4 neutropenia occurred after cycles 1 and 2 and frequency decreased with subsequent cycles.
Table 3.
Common adverse events in PIX301 (NCT00088530; EudraCT 2004 - 000480-10)
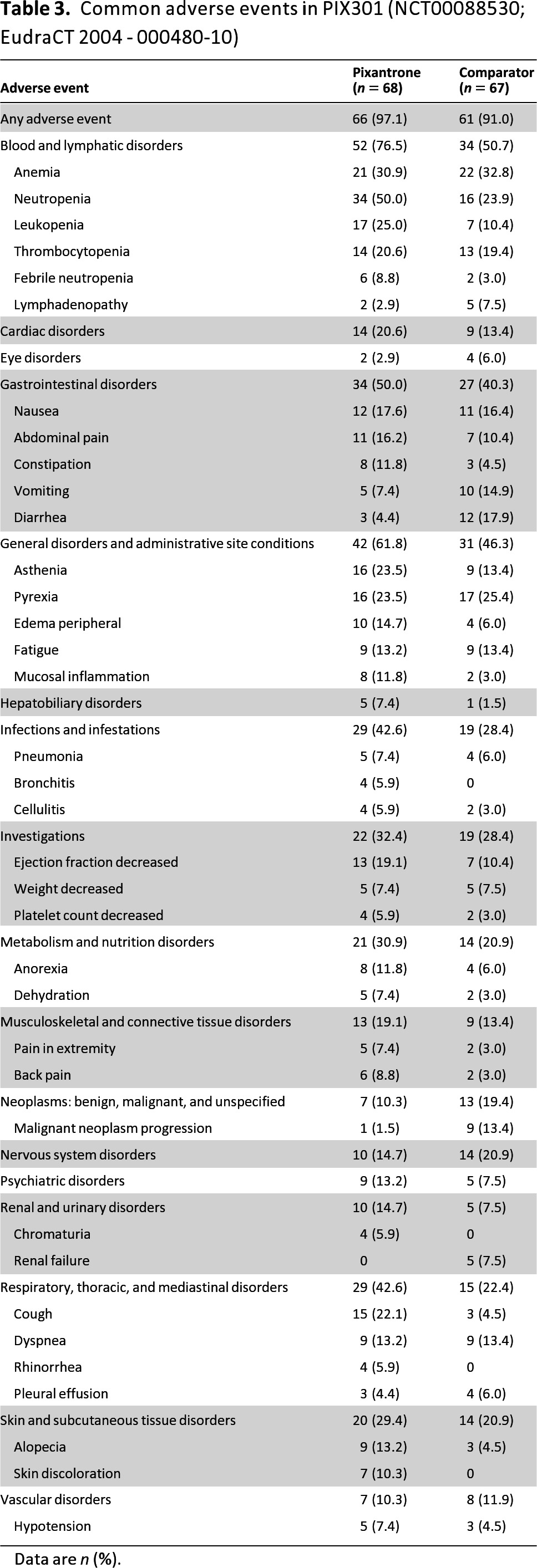
Data are n (%).
Neutropenia was the most common adverse event leading to withdrawal in the pixantrone arm (10%). At the recommended dose and schedule, neutropenia was usually transient, reaching its nadir on days 15–22 following administration on days 1, 8, and 15, with recovery usually occurring by day 28. Thrombocytopenia and anemia were of lower frequency and severity than neutropenia; no difference was observed in blood or platelet transfusions between treatment groups. A careful monitoring of blood counts is required, including leukocyte, red blood cells, platelet, and absolute neutrophil counts. Recombinant hematopoietic growth factors may be used according to institutional or European Society for Medical Oncology guidelines and dose modifications should be considered. The use of pixantrone is contraindicated in the case of profound bone marrow suppression.
Cardiac toxicity was closely monitored in the pivotal study, and a higher incidence of cardiac events was seen in the pixantrone group (35% vs. 21%). Only nine cases of cardiac events were considered related to pixantrone (13%), and all were asymptomatic decreases of ejection fraction. Overall events observed were relatively mild and asymptomatic. There were no clear cases of pixantrone-associated congestive heart failure (CHF) as typically described in the literature for other anthracyclines. However, changes in cardiac function, including decreased left ventricular ejection fraction or fatal CHF, may occur during or after treatment with pixantrone. Cardiac toxicity in general may occur whether or not cardiac risk factors are present. For patients with cardiac disease or risk factors, a careful risk-versus-benefit evaluation should be conducted before considering treatment with pixantrone. Cardiac function should be monitored before initiation of treatment with pixantrone and periodically thereafter. If cardiac toxicity is demonstrated during treatment, the risk versus benefit of continued therapy with pixantrone must be evaluated.
Because infections have been associated with hospitalization, septic shock, and death, pixantrone should not be administered to patients with an active, severe infection or in patients with a history of recurring or chronic infections or with underlying conditions that may further predispose them to serious infection.
Febrile neutropenia, psychiatric disorders, vascular disorders, and asthenia were more common in pixantrone-treated patients who were ≥65 years of age, as was the incidence of cardiac treatment-emergent adverse events (but not of grade 3–4 or ejection fraction decline). Elderly patients suffered less from nausea compared to younger patients. No specific dose adjustment is required in elderly patients (aged ≥65 years).
The majority of deaths within 30 days of last study treatment were stated to be related to the patient's underlying NHL. One death in the pixantrone group was considered to be related to treatment (a 29-year-old woman who died of septic shock on study day 8). None of the deaths within 30 days of last study treatment in the comparator group were considered to be related to treatment. Three deaths that occurred more than 30 days after the last study treatment were considered to be related to treatment; one from acute CHF in the pixantrone arm, one from myelodysplastic syndrome (MDS) in the pixantrone arm, and one from renal failure in the comparator arm.
Pixantrone showed to be more active than the control arm in the group of patients pretreated with up to three regimens, including rituximab. However, additional efficacy data were considered necessary to confirm the benefit of pixantrone in patients who had received prior treatment with rituximab. The applicant company was therefore requested, as a specific obligation for approval, to provide the comprehensive clinical data from the phase III study PIX 306 where pixantrone in combination with rituximab is compared with gemcitabine in combination with rituximab.
Risk Management Plan
A risk management plan identifying relevant important identified/potential risks and important missing information has been agreed upon. Identified risks were cardiac failure, myelotoxicity, serious infections, and tumor lysis syndrome. Potential risks included therapy-related acute myeloid leukemia/MDS, reproductive toxicity, photosensitivity, and potential interactions specifically through CYP1A2 and CYP2C8. Missing information included use in children and safety in people with significant hepatic and renal impairment, severely abnormal cardiac function, elderly patients >75 years of age, nonwhite patients, patients with poor bone marrow reserve, patients with poor performance status, and patients with prior mediastinal radiotherapy. Risk minimization activities (e.g., monitoring, special precautions, contraindications, warnings) to address these risks are detailed in the SmPC [10]. An in vivo phototoxicity study in rodents will be performed using pixantrone dimaleate at relevant clinical doses.
Benefit-Risk Assessment
The CHMP concluded by majority that given the lack of standard of care and the poor prognosis for patients with multiple relapses/refractory aggressive NHL, the improvement seen in CR/CRu supported by the results of secondary endpoints of PFS and OS in the pivotal study were considered clinically relevant. The treatment effect associated with pixantrone was smaller in the subgroup of patients pretreated with rituximab and diminished further with increasing number of prior regimens. Pixantrone showed to be more active than the control arm in the group of patients pretreated with up to three regimens, including rituximab. However, additional efficacy data were considered necessary to confirm the benefit of pixantrone in patients who had received prior treatment with rituximab. The applicant company was therefore requested, as a specific obligation for approval, to provide the comprehensive clinical data from the phase III study PIX 306 where pixantrone in combination with rituximab is compared with gemcitabine in combination with rituximab.
Overall the safety data were considered sufficient to assess the safety profile of pixantrone in the proposed indication. Bone marrow suppression was the most frequent and severe toxicity associated with pixantrone treatment. Neutropenia was the predominant manifestation, whereas thrombocytopenia and anemia occurred at lower frequency and severity. More patients in the pixantrone arm received growth factor support compared to the control group, but blood or platelet transfusions were similar between groups. Infections were common but the incidence of systemic sepsis and opportunistic systemic infections was low.
Cardiac toxicity was closely monitored in the pivotal study and a higher incidence of cardiac events was seen in the pixantrone group, with apparently less severity than that reported with other anthracyclines. There was no demonstrable relationship between cumulative pixantrone dose to symptomatic declines in LVEF or CHF, nor was a relationship seen with prior doxorubicin equivalent cumulative exposure.
The CHMP concluded by majority vote that the benefit-risk balance was considered positive and a conditional approval valid throughout the European Union was granted for pixantrone as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive NHL. The benefit of pixantrone treatment has not been established when used as fifth-line or greater chemotherapy in patients who are refractory to last therapy. A minority of CHMP members disagreed and considered that a positive benefit-risk balance had not been established, noting that the safety profile was unfavorable compared with the control group and that no benefit for pixantrone over the control arm was observed for patients <65 years; men; patients who had previous treatment with anti-CD20, stem cell transplantation, or three or more chemotherapy regimens; and most importantly, patients in North America or Western Europe.
A conditional approval is reserved for medicinal drugs that treat, prevent, or diagnose seriously debilitating diseases or life-threatening diseases or rare diseases (orphan medicinal products) or drugs to be used in emergency situations in response to threats. With this approval, the applicant company is obliged to submit additional data, with a view to confirming that the benefit-risk balance is positive. A conditional approval is only valid for 1 year but can be renewed. The renewal is given on the basis of the confirmation of the benefit-risk balance, taking into account the specific obligations and the timeframe for their fulfillment. Once it is judged that remaining data have been provided or are no longer required, the approval can be converted to a standard approval. If at any time the benefit-risk is considered to be negative, the marketing authorization can be suspended or revoked. The EMA will review new information about pixantrone on an annual basis. Up-to-date information on this medicinal product is available on the website of the EMA (http://www.ema.europa.eu).
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
The scientific assessment as summarized in this report is based on the marketing authorization application submitted by the applicant company and on important contributions from, among others, the rapporteur and co-rapporteur assessment teams, CHMP members, and additional experts. Disclaimer: This publication is a summary of the European Public Assessment Report available in the public domain, together with the summary of product characteristics, and other product information on the EMA website (http://www.ema.europa.eu). Christian Gisselbrecht and Edward Laane are members of the EMA Scientific Advisory Group on Oncology but did not participate in the EMA review of pixantrone. The authors remain solely responsible for the opinions expressed therein.
Author Contributions
Data analysis and interpretation: Beatriz Flores, Ian Hudson, Jan Sjöberg, Kristina Dunder
Manuscript writing: Elias Pean, Francesco Pignatti
Final approval of manuscript: Beatriz Flores, Ian Hudson, Jan Sjöberg, Kristina Dunder, Tomas Salmonson, Christian Gisselbrecht, Edward Laane, Francesco Pignatti
Disclosures
Beatriz Flores: Ipsen. The other authors indicated no financial disclosures.
Section editor: George Canellos: Celgene Business Advisory Board (C/A)
Reviewer “A”: None
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Hazlehurst LA, Krapcho AP, Hacker MP. Correlation of DNA reactivity and cytotoxicity of a new class of anticancer agents: Aza-anthracenediones. Cancer Lett. 1995;91:115–124. doi: 10.1016/0304-3835(95)91035-5. [DOI] [PubMed] [Google Scholar]
- 4.Cavalletti E, Crippa L, Mainardi P, et al. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: Comparative studies against doxorubicin and mitoxantrone. Invest New Drugs. 2007;25:187–195. doi: 10.1007/s10637-007-9037-8. [DOI] [PubMed] [Google Scholar]
- 5.Dawson LK, Jodrell DI, Bowman A, et al. A clinical phase I and pharmacokinetic study of BBR 2778, a novel anthracenedione analogue, administered intravenously, 3 weekly. Eur J Cancer. 2000;36:2353–2359. doi: 10.1016/s0959-8049(00)00342-7. [DOI] [PubMed] [Google Scholar]
- 6.Faivre S, Raymond E, Boige V, et al. A phase I and pharmacokinetic study of the novel aza-anthracenedione compound BBR 2778 in patients with advanced solid malignancies. Clin Cancer Res. 2001;7:43–50. [PubMed] [Google Scholar]
- 7.Borchmann P, Schnell R, Knippertz R, et al. Phase I study of BBR 2778, a new aza-anthracenedione, in advanced or refractory non-Hodgkin's lymphoma. Ann Oncol. 2001;12:661–667. doi: 10.1023/a:1011139016294. [DOI] [PubMed] [Google Scholar]
- 8.Borchmann P, Morschhauser F, Parry A, et al. Phase-II study of the new aza-anthracenedione, BBR 2778, in patients with relapsed aggressive non-Hodgkin's lymphomas. Haematologica. 2003;88:888–894. [PubMed] [Google Scholar]
- 9.Pettengell R, Coiffier B, Narayanan G, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: A phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13:696–706. doi: 10.1016/S1470-2045(12)70212-7. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. European Public Assessment Report for Pixuvri (pixantrone dimaleate) [Accessed March 16, 2013]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002055/human_med_001549.jsp.