Abstract
Background
Tissue inflammation and remodeling have been extensively studied in various tumors in relation with their invasiveness and metastasis.
Objective
The purpose of this study was to investigate the change in tissue inflammation and remodeling markers in cutaneous squamous cell carcinoma (SCC).
Methods
Expression levels of cyclooxygenase-2 (COX-2) as an inflammatory marker and matrix metalloproteinases-2 and -9 (MMPs 2/9) as remodeling markers were studied in mouse and human SCCs. Western blot analysis and RT-PCR for COX-2 and MMPs 2/9 were performed with skin samples from SCC patients and chronic ultraviolet B (UVB)-induced SCC from hairless mice.
Results
mRNA and protein levels of COX-2 and MMPs 2/9 were up-regulated with the higher sensitivity for MMP-9 in mouse SCCs, which were induced by chronic UVB irradiation. Consistently, COX-2 and MMPs 2/9 were up-regulated with the higher sensitivity for MMP-9 in human SCCs.
Conclusion
COX-2 and MMPs 2/9 are up-regulated in well-differentiated cutanous SCC. Our findings indicate that inflammatory and tissue remodeling processes are actively induced during carcinogenesis of cutaneous SCC.
Keywords: Cyclooxygenase 2, MMP-2, MMP-9, SCC
INTRODUCTION
Among different rays within solar light, the ultraviolet B (UVB) component is known to be the major carcinogenic wavelength in inducing non-melanoma skin cancers (NMSCs), such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). UVB-induced cutaneous SCC in mice, which closely mimics human cutaneous SCC in tumorigenesis, can be an ideal in vivo model to test chemopreventive or anticancer agents.
Based on the evidence that inflammatory processes share common pathways with carcinogenic processes, cyclooxygenase-2 (COX-2), a representative inflammatory marker, can be used for a good candidate for tumor marker1. Nuclear factor-κB, a representative transcriptional factor of inflammation, plays a consistent and crucial role in gene regulation for skin cancers2. As a pro-inflammatory cytokine, tumor necrosis factor-α was reported to be closely implicated in carcinogenesis3. On histopathology, tumor mass contains inflammatory foci, demonstrating that lymphocytes are close contact with tumor cells, especially in early SCC4. In previous study, the degree of inflammation was closely related with differentiation status in SCC5.
COXs are key enzymes in the conversion of free arachidonic acid (AA) into a series of pro-inflammatory molecules of eicosanoids, including prostaglandins (PGs). Recent studies clearly demonstrate that AA pathway plays a crucial role in carcinogenesis6-10. As an inducible isoform, COX-2 has been reported to be up-regulated by various motogens including UV lights6. Abnormal PG synthesis is known to be a potential contributing factor in UV-induced skin carcinogenesis7. PG-mediated production of reactive oxygen species and reactive nitrogen species are implicated in the carcinogenesis that included malignant cell transformation8. UVB-induced skin tumors could be inhibited by the oral administration of COX-2 inhibitors, indicating a critical role of COX-2 in skin carcinogenesis10.
The MMP family consists of a group of zinc-dependent enzymes, which can be divided into several subgroups including: collagenases, gelatinases, stromelysins, stromelysin-like matrix metalloproteinases (MMPs), matrilysins, membrane-type MMPs, and other MMPs11. MMPs are actively implicated in inflammation and tissue remodeling by the degradation of a certain subset of protein components of the extracellular matrix12. Abnormal expression of the MMP family might cause the migration of tumor cells or tumor metastasis in SCC, including cutaneous SCC. As examples, knock-out mice targeted for MMP-2 and -7 have reduced tumor progression13,14. Mice lacking MMP-9 have a decreased incidence of invasive tumors in skin carcinogenesis15. Mice deficient in MMP isoforms demonstrate defective angiogenesis for tumor growth16,17. Conversely, over-expression of MMP-1 is related to enhanced tumor formation in a skin cancer model18. MMP-9 promotes tumor growth by enhanced angiogenesis in other mouse tumor models19. Among the MMP family, MMPs 2/9, which are essential enzymes to degrade type IV collagen, a major component of the basement membrane, have been widely studied in SCCs; however, controversy remains among their results20-22. This study was aimed to evaluate whether inflammation and tissue remodeling are actively implicated in cutaneous SCC of the mouse and human by studying COX-2 and MMPs 2/9.
MATERIALS AND METHODS
UVB-induced cutaneous SCC in hairless mice
For animal experiments, official permission to handle mice for our experiments was obtained from the Chonnam National University Institutional Animal Care and Use Committee Animal Committee. Eight-week-old hairless female mice of SKH-1 strain (Charles River Laboratory, Boston, MA, USA) were housed in a radiation cage, in which they moved freely during UVB irradiation with the UV radiator equipped with UVB light bulbs (FL 208.E; Toshiba Electric Co., Tokyo, Japan) emitting a unit dose3 of 7.5×10-4~8×10-4 W/cm2/s2. The UVB dose was measured with the IL 1700 Research Light Meter (International Light, Newburyport, MA, USA). For chronic UV radiation, the mice were irradiated alternately 3 times a week for up to 8 months, for a total dose of approximately 10 J/cm2. The mice were divided into the UVB-irradiated group (n=10) and the UVB-non-irradiated control group (n=6). For histological study, the mid-back of UVB-irradiated or non-irradiated (control) mice was excised by a scalpel, fixed in 10% neutral formalin over night, and processed to prepare paraffin blocks. Tissue sections, 4 mm in thickness, were stained with H&E.
Preparation of skin tissue extracts
Before obtaining human samples from SCC patients, each patient signed an informed consents based on the guidelines determined by local ethics committee and the Declaration of Helsinki Principles (IRB No.: 1-2009-11-136). Excised SCC tumor masses were cut into small pieces with iris scissors, and then, immediately frozen in liquid nitrogen. The frozen samples were pulverized in a mortar containing liquid nitrogen, placed in 0.12 g/ml RIPA lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA [pH 8.0] and protease inhibitor cocktail), and then homogenized with the motor-driven Potter-Elvehjem homogenizer with a teflon pestle (Omni Int., Kennesaw, GA, USA) at 4℃. The homogenates were centrifuged at 25,000×g for 20 min at 4℃, and the resulting supernatant fractions were used for immunoblots. The protein amounts of the tissue extracts were quantified by the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The samples were kept at -70℃ until used.
Western blot analysis
Tissue extracts were mixed with 2×sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris HCl [pH 6.8], 10% glycerol, 2% bromophenol blue, and 5% β-mercaptoethanol), boiled for 5 min, and then cooled on ice for 5 min. The protein components of the skin extracts were separated by electrophoresis in 10% SDS-polyacrylamide gels, and then transferred to a 0.45 µm PVDF membrane (Millipore, Temecula, CA, USA). After identifying blotting conditions with the Ponceau S solution (Sigma, St. Louis, MO, USA), the membrane was reacted with blocking solution (5% skim milk, PBS, 0.1% Tween-20, and PBS-T) for 1 h at room temperature. The following commercially available antibodies were purchased and used as primary antibodies to be incubated with blotting membranes overnight at 4℃; anti-COX-2 (1:1,000; BD Biosciences, San Jose, CA, USA), anti-MMP2 (ab809, 1:1000; Chemicon, Billerica, MA, USA), anti-MMP9 (AF911, 1:1,000; R&D Systems, Minneapolis, MN, USA), and anti-β-actin (ab6276, 1:5000; Abcam, Cambridge, UK). After washing 4 times with TBS-T (Tris-Buffered Saline with 0.1% Tween-20), the sample was reacted with HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:5000; Jackson Imunoresearch, West Grove, PA, USA), and anti-mouse IgG (1:5000; Jackson Immunoresearch, West Grove, PA, USA). The color reaction was performed using an ECL kit (Millipore). A pre-stained protein ladder was used as a standard marker for size verification.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR)
A polymerase chain reaction (PCR) pre-mixture kit (ELPIS, Daejeon, Korea) was used for semi-quantitative PCR. PCR reactions were performed with the following primers for each enzyme: mouse Cox-2 (forward: 5'-agcagatgactgcccaact-'3; reverse: 5'-gaacccaggtcctcgcttat-'3); mouse MMP-2 (forward: 5'-agaaaagattgacgctgtgt-'3; reverse: 5'-ttcacgctcttgagacttt-'3); mouse MMP-9 (forward: 5'-gcatacttgtaccgctatgg-'3; reverse: 5'-tatgatgttatgatggtccc-'3); and β-actin (forward: 5'-tcatgaagtgagacgttgacatccgt-'3; reverse: 5'-cctagaagcacttgcggtgcacgatg-'3). Annealing temperatures were set as 56℃, 58℃ and 60℃. PCR products were analyzed using 1.5% agarose gel electrophoresis, stained with Sybr Safe DNA gel stain buffer (Invitrogen, Carlsbad, CA), and visualized by luminescence (LAS 3000, Fujifilm, Tokyo, Japan).
RESULTS
Chronic UVB irradiation to SKH-1 mice induces well-differentiated cutaneous SCC
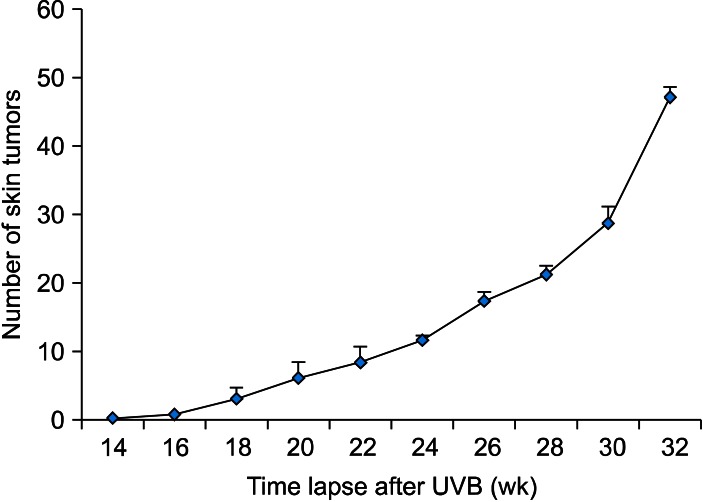
To prepare an animal model for cutaneous SCC, UVB was chronically irradiated to the dorsal skin of SKH-1 mice. When mice were irradiated for approximately 14 weeks, a skin tumor was detected on the dorsal skin of mice, and then the number of tumors was steadily increasing. Further irradiation to 32 weeks, reaching approximately 10 J/cm2 of an accumulated dose of UVB, produced 47.1±17.1 (n=10) skin tumors per mouse (Fig. 1, 2A). In H&E stain, the tumors were shown to be hypergranulosis, hyperkeratosis, atypical keratinocytes with multiple keratin pearls, indicating typical well-differentiated SCCs (Fig. 2B).
Fig. 1.
Induction of UVB-induced skin tumors is time-dependent. To induce skin tumors by chronic UVB irradiation, mice were irradiated 3 times per week for up to 32 weeks. At 14 weeks after starting UVB irradiation, skin tumors could be detected on the back of mice; thereafter, the number of skin tumors was counted by unaided gross visual inspection every week. UVB: ultraviolet B.
Fig. 2.
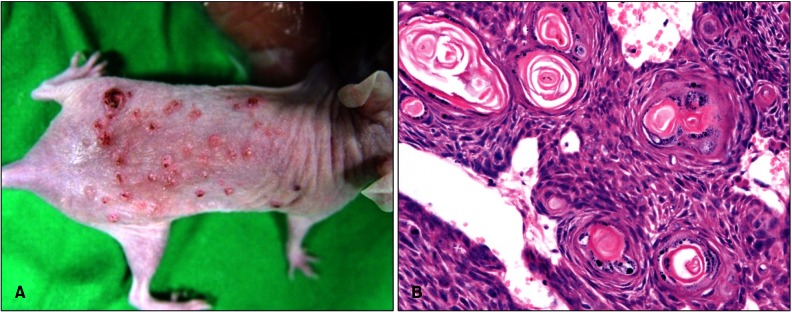
UVB-induced skin tumors are well-differentiated SCCs in mouse. (A) Multiple skin tumors were developed by chronic UVB irradiation on the backs of mice. (B) On a random basis, several tumors were excised with a scalpel, and then paraffin blocks were prepared with H&E staining (×400). UVB: ultraviolet B, SCC: squamous cell carcinoma.
COX-2 and MMPs 2/9 are up-regulated in UVB-induced SCC from mice
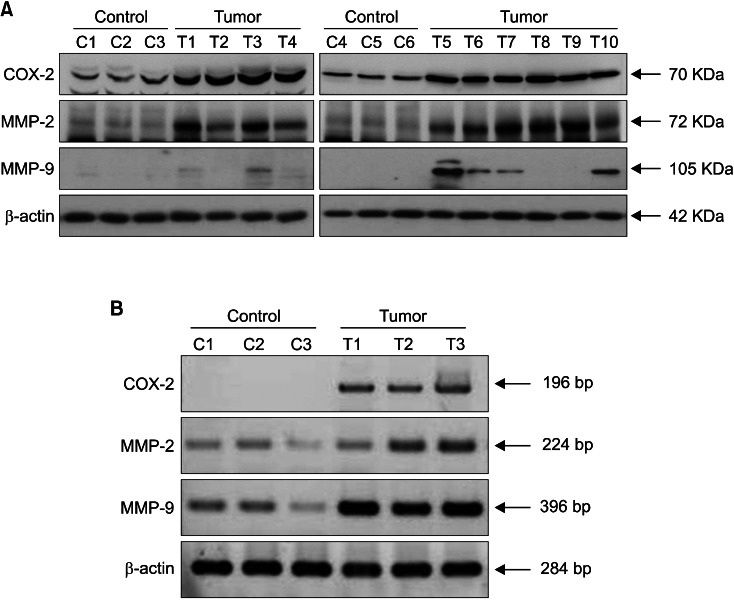
In Western blot analysis, COX-2 protein bands were barely detected in all of normal skin (n=6), but COX-2-positve bands were detected from 9 among 10 SCC samples (n=10) (Fig. 3A). MMP-2 protein bands were detected strongly in both SCC and control normal skin, but the bands became stronger in 7 (T1, T2, T3, T5, T6, T7, T10) among 10 SCCs. MMP-9-positive bands were detected from 7 among 10 SCCs, but the positive band was not detected from all of 6 control skin samples (Fig. 3A). In RT-PCR with 3 sets of samples from SCCs and control skin, which were obtained from separate experiments, mRNA expression levels for COX-2 and MMPs 2/9 were all up-regulated with remarkable differences for COX-2 and MMP-9 in UVB-induced SCCs, indicating the transcriptional regulation of COX-2 and MMPs 2/9 (Fig. 3B). The expression levels of COX-2 and MMPs 2/9 were arbitrarily semi-quantified, and the results were summarized in Table 1.
Fig. 3.
COX-2 and MMPs 2/9 are up-regulated in UVB-induced SCC from mouse. (A) Levels of protein expression for COX-2, MMPs 2/9 were determined by Western immunoblots with tissue extracts from UVB-induced SCCs and normal control skin. Control samples are depicted as C1~C6, and tumor samples are depicted as T1~T10. (B) Total RNAs were prepared from UVB-induced SCCs and control skin, and then RT-PCR for COX-2, MMPs 2/9 were performed. Control samples are depicted as C1~C3, and tumor samples are depicted as T1~T3. COX-2: cyclooxygenase-2, MMPs 2/9: matrix metalloproteinases-2 and -9, UVB: ultraviolet B, SCC: squamous cell carcinoma.
Table 1.
Summary of expression levels of COX-2, MMP-2, and MMP-3 in UVB-induced SCC and normal skin
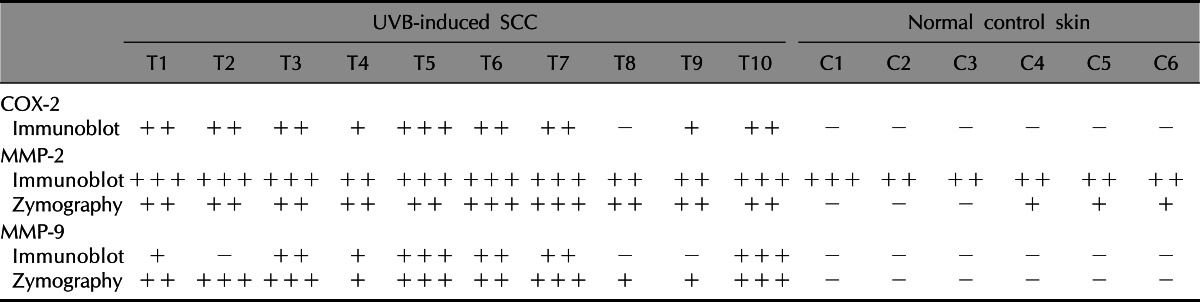
COX-2: cyclooxygenase-2, MMP: matrix metalloproteinase, UVB: ultraviolet B, SCC: squamous cell carcinoma. +: weakly positive, ++: moderately positive, +++: strongly positive, -: negative.
COX-2 and MMPs 2/9 are up-regulated in cutaneous SCC from human
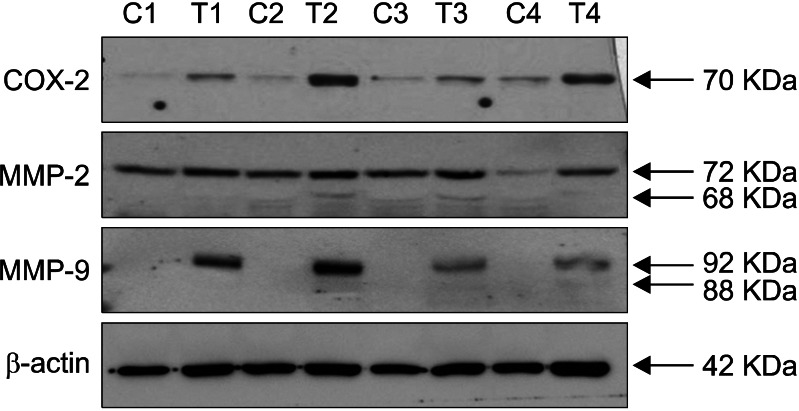
In Western blot analysis, expression levels of COX-2 and MMPs 2/9 were up-regulated in tumor extracts from well-differentiated cutaneous SCCs compared with adjacent normal skin from the same patient (control) in humans (Fig. 4).
Fig. 4.
COX-2 and MMPs 2/9 are up-regulated in cutaneous SCC from human. Expression levels for COX-2 and MMPs 2/9 were determined by Western immunoblots with tissue extracts from cutaneous SCCs (T1~T4) and neighboring normal skin (C1~C4) of the same patient for each sample pair. COX-2: cyclooxygenase-2, MMPs 2/9: matrix metalloproteinases-2 and -9, C: control, T: tumor, SCC: squamous cell carcinoma.
DISCUSSION
Our study shows that cutaneous SCC can be used for a good model for studying the role of inflammation and tissue remodeling in carcinogenesis. Our finding that COX-2 is up-regulated in cutaneous SCC indicates that an active inflammatory reaction is accompanied by skin carcinogenesis. Clinically, active inflammatory tissue reaction can be easily detected in cutaneous SCC patients. A recent study reported that dense inflammatory infiltrate was more associated with well- or moderately-differentiated SCC than poorly-differentiated one, suggesting that inflammatory degree is related with differentiation status in cutaneous SCC5. On a consistent basis, the inflammatory process might be actively induced in well-differentiated SCC, as mouse and human SCC samples in this study were obtained from cutaneous SCCs of the well-differentiated type. COX-2 plays a critical role in the development of UV-induced cancers of NMSCs. Several lines of evidence suggest that PGs play a role in carcinogenesis, and especially COX-2 induction by UV radiation contributes to photocarcinogenesis23. UV-induced carcinogenesis was reduced by down-regulated COX-2 expression in COX-2 knockout mice, while stimulated by the COX-2 over-expression in transgenic mice24. In this study, COX-2 protein bands were detected in 9 of 10 UVB-induced SCCs, while none of normal control skin showed the COX-2-positive band (Table 1). On a consistent basis, COX-2-positive bands were up-regulated in all 4 human SCCs compared with control normal skin from the same patients (Fig. 4). Altogether, our study demonstrates that expression levels of COX-2 are increased in most cutaneous SCCs from mouse and human, indicating that COX-2 can be a good marker for cutaneous SCC.
From this study, the up-regulated expression of MMPs 2/9 in together with COX-2 indicates that tissue remodeling is induced actively during the inflammatory process in cutaneous SCC. MMPs have been widely studied in relation with tumor invasion, metastasis, and prognosis in various tumors13-20. In theory, the activated activities of MMPs 2/9 might be related with the disruption in the integrity of the extracellular matrix, such as type IV collagen, laminin-5, and avb6 integrin, leading to the increased invasive potential of SCC25-27. In a previous study, the latent and active forms of MMPs 2/9 were significantly elevated in oral SCC as compared to adjacent normal tissues22. MMP-2 degrades type IV collagens and fibronectin, which are the major components of the basement membrane, and are also known to be related to angiogenesis, infiltration, and progression of cancer. MMP-9 has a similar function with MMP-2 in related with tissue remodeling and infiltrative growth17. MMP-9 level has been correlated with antigenic markers and poor survival in head and neck SCC patients11. Protein kinase C-mediated MMP-9 was related with the invasiveness of oral SCC28. From this study, MMPs 2/9 were up-regulated in UVB-induced SCCs in mice, which was supported by the results from human SCC. However, our result that MMPs 2/9 are detected at a relatively high rate in well-differentiated SCC suggests to us that there is a limit to the use of MMPs as a prognostic marker for cutaneous SCC. In fact, all of cutaneous SCCs from human and mouse did not show distant metastasis (data not shown). Further studies should be done on the relationship between the levels of COX-2/MMPs and differentiation status of cutaneous SCCs in terms of tumor prognosis. Previous studies suggest that differentiation status, tumor thickness, immunosuppression, localization, and horizontal size are considered as prognostic factors for cutaneous SCCs29,30.
As for sensitivity, MMP-9 was revealed to be a more sensitive marker than MMP-2 for the diagnosis of cutaneous SCC in both mice and humans, as the positive bands for MMP-9 protein expression were detected in cutaneous SCCs from human and mouse, but were not detected in normal control skin (Fig. 3 and 4). However, the sensitivity in the expression of MMP-2 or -9 is supposed to be tumor- and tissue-specific, as other studies suggest that MMP-2 is more sensitive than MMP-926,29. For oral SCC, the activation ratio of MMP-2 discriminated better than that of MMP-9 between patients with and without lymph node metastasis26. Expression levels of MMP 2/9 depending on locations of SCC from the mucosa or skin should be evaluated, as oral SCCs are supposed to be more aggressive than cutaneous SCCs. For malignant melanoma (MM), MMP-2 was closely related with MM-related death, while MMP-9 was not associated with the clinical course of MM31.
Future studies are warranted to evaluate the correlation between MMP level and prognosis or metastatic potential in different types of cutaneous SCC or other types of cutaneous cancers, including BCC and MM.
ACKNOWLEDGMENTS
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A080870).
References
- 1.Mendes RA, Carvalho JF, Waal IV. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. 2009;45:e124–e128. doi: 10.1016/j.oraloncology.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003;15:1–7. doi: 10.1016/s0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 3.Williams GM. Antitumor necrosis factor-alpha therapy and potential cancer inhibition. Eur J Cancer Prev. 2008;17:169–177. doi: 10.1097/CEJ.0b013e3282b6fcff. [DOI] [PubMed] [Google Scholar]
- 4.Boncinelli U, Fornieri C, Muscatello U. Relationship between leukocytes and tumor cells in pre-cancerous and cancerous lesions of the lip: a possible expression of immune reaction. J Invest Dermatol. 1978;71:407–411. doi: 10.1111/1523-1747.ep12558284. [DOI] [PubMed] [Google Scholar]
- 5.Lo Muzio L, Santoro A, Pieramici T, Bufo P, Di Alberti L, Mazzotta P, et al. Immunohistochemical expression of CD3, CD20, CD45, CD68 and bcl-2 in oral squamous cell carcinoma. Anal Quant Cytol Histol. 2010;32:70–77. [PubMed] [Google Scholar]
- 6.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 7.Grewe M, Trefzer U, Ballhorn A, Gyufko K, Henninger H, Krutmann J. Analysis of the mechanism of ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by human epidermoid carcinoma cells. J Invest Dermatol. 1993;101:528–531. doi: 10.1111/1523-1747.ep12365904. [DOI] [PubMed] [Google Scholar]
- 8.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, et al. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280:1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 10.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 11.Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 12.Kolaczkowska E, Arnold B, Opdenakker G. Gelatinase B/MMP-9 as an inflammatory marker enzyme in mouse zymosan peritonitis: comparison of phase-specific and cell-specific production by mast cells, macrophages and neutrophils. Immunobiology. 2008;213:109–124. doi: 10.1016/j.imbio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 14.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii Y, Nakasato Y, Kobayashi S, Yamazaki Y, Aoki T. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res. 2003;22:461–470. [PubMed] [Google Scholar]
- 17.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Armiento J, DiColandrea T, Dalal SS, Okada Y, Huang MT, Conney AH, et al. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995;15:5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993;99:18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- 21.O'Grady A, Dunne C, O'Kelly P, Murphy GM, Leader M, Kay E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007;51:793–804. doi: 10.1111/j.1365-2559.2007.02885.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel BP, Shah PM, Rawal UM, Desai AA, Shah SV, Rawal RM, et al. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol. 2005;90:81–88. doi: 10.1002/jso.20240. [DOI] [PubMed] [Google Scholar]
- 23.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 24.Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002;1:44–47. [PubMed] [Google Scholar]
- 25.Dumas V, Kanitakis J, Charvat S, Euvrard S, Faure M, Claudy A. Expression of basement membrane antigens and matrix metalloproteinases 2 and 9 in cutaneous basal and squamous cell carcinomas. Anticancer Res. 1999;19:2929–2938. [PubMed] [Google Scholar]
- 26.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 27.Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF. alpha v beta 6 Integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol. 2001;116:898–904. doi: 10.1046/j.1523-1747.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- 28.Juarez J, Clayman G, Nakajima M, Tanabe KK, Saya H, Nicolson GL, et al. Role and regulation of expression of 92-kDa type-IV collagenase (MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of the oral cavity. Int J Cancer. 1993;55:10–18. doi: 10.1002/ijc.2910550104. [DOI] [PubMed] [Google Scholar]
- 29.Vinicius de LV, Scapulatempo C, Perpetuo NM, Mohamed F, de Carvalho TS, de Oliveira AT, et al. Prognostic and risk factors in patients with locally advanced cutaneous squamous cell carcinoma of the trunk and extremities. J Skin Cancer. 2011;2011:420796. doi: 10.1155/2011/420796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 31.Väisänen AH, Kallioinen M, Turpeenniemi-Hujanen T. Comparison of the prognostic value of matrix metalloproteinases 2 and 9 in cutaneous melanoma. Hum Pathol. 2008;39:377–385. doi: 10.1016/j.humpath.2007.06.021. [DOI] [PubMed] [Google Scholar]