Abstract
Background
The underlying mechanism of atopic dermatitis (AD) exacerbated by Staphylococcus aureus has not been established. However, we demonstrated recently that the majority of S. aureus strains colonized in the skin of Korean AD patients carried genes encoding staphylococcal enterotoxin A (SEA) and/or toxic shock syndrome toxin-1 (TSST-1).
Objective
To clarify the role of staphylococcal superantigen, SEA in AD.
Methods
With the lesional skin of 9 AD patients and normal looking skin of one healthy adult, we examined first the expression of SEA, staphylococcal enterotoxin B (SEB), and TSST-1 using immunohistochemical analysis. In addition, we investigated the effects of SEA on the expression of inflammation-related adhesion molecules and cytokines in human HaCaT keratinocytes and Human Umbilical Vein Endothelial Cells (HUVECs) by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and enzyme-linked immunosorbent assay.
Results
Staphylococcal protein A (SPA) and SEA were detected with increased immunoreactivity in AD patients. However, TSST-1 showed mild-to-moderate immunoreactivity in AD patients, whereas SEB was minimally detected. In the double immunofluorescence investigation, SEA and SPA were well co-localized. SEA induced upregulation of adhesion molecules and elicited inflammatory responses in HaCaT keratinocytes and HUVECs.
Conclusion
This study demonstrates the importance of SEA as an immunoinflammatory triggering factor of AD in Koreans.
Keywords: Atopic dermatitis, Staphylococcal enterotoxin A, Staphylococcus aureus
INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease partly evoked by cutaneous infection with microbes, such as Staphylococcus aureus1. The skin of AD patients exhibits a susceptibility to S. aureus and this has been established as a contributing factor in the exacerbation of AD2-4. However, the underlying mechanism has not been well established.
Superantigenic exotoxins produced by S. aureus have been recognized as one of the contributing factors in the exacerbation of AD5. Recently, we noticed a positive prevalence rate of superantigen producing S. aureus in children with AD, which was significantly higher than in the normal control group6,7. Contrary to the previous studies, we demonstrated that the majority of S. aureus strains colonized on the skin of Korean AD patients carried genes encoding staphylococcal enterotoxin A (SEA) and/or toxic shock syndrome toxin-1 (TSST-1)6,7.
Therefore, this study was focused on SEA to clarify the role of staphylococcal superantigens in AD. In detail, we first examined the expression of SEA, staphylococcal enterotoxin B (SEB), and TSST-1 using immunohistochemical analysis in the skin of AD patients. In addition, we examined the correlation between clinical severity and the degree of immunoreactivity of SEA. Second, we investigated the effects of SEA on the expression of inflammation-related adhesion molecules and cytokines in human HaCaT keratinocytes and Human Umbilical Vein Endothelial Cells (HUVECs) by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and ELISA.
MATERIALS AND METHODS
The distribution of SEA, SEB and TSST-1 in the lesional skin of patients with AD (Table 1)
Table 1.
Clinical severity, histopathological stage of dermatitis, and degree of immunoreactivity of the AD cases
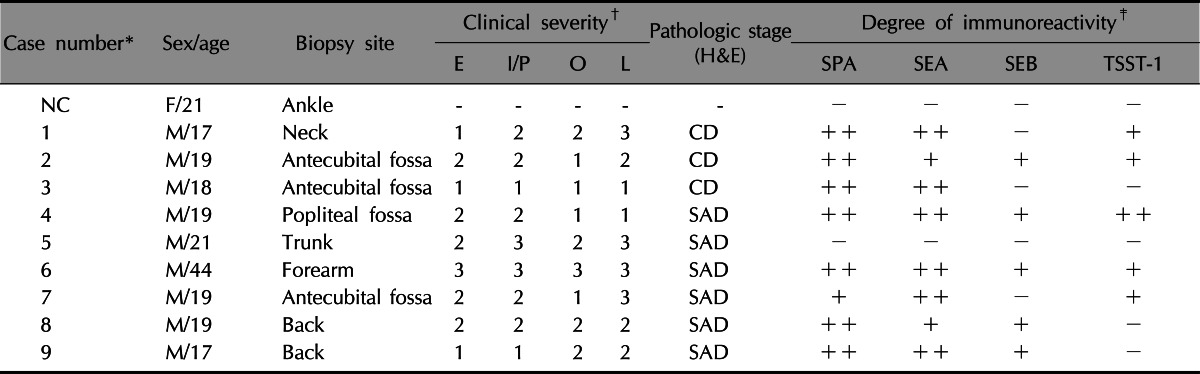
Score: 0 (no clinical manifestation), 1 (mild), 2 (moderate), 3 (severe). AD: atopic dermatitis, E: erythema, I/P: induration/papulation, O: oozing, L: lichenification, SPA: Staphylococcal protein A, SEA: staphylococcal enterotoxin A, SEB: staphylococcal enterotoxin B, TSST-1: toxic shock syndrome toxin-1, NC: normal control, F: female, M: male, CD: chronic dermatitis, SAD: subacute dermatitis.
*Case number of 9 atopic dermatitis, †E-erythema, ‡(-): 0%, (+): <25%, (++): ≥25%.
1) Patients
Nine adolescent or adult AD patients ages 17~44 years, who visited the dermatologic clinic in Kyungpook National University Hospital, were included. The lesional skin from 9 AD patients and normal looking skin from one healthy adult were used in this study. The Kyungpook National University Hospital institutional review board approved the study protocol, prepared in accordance with the Declaration of Helsinki Principles; all participants fully consented to participate in the study.
2) H&E stain and immunohistochemical analysis
For general histopathology, the 5 µm sections of biopsy samples were stained with H&E. The sections were incubated with primary antibodies; polyclonal rabbit anti-SEA, polyclonal rabbit anti-SEB, polyclonal rabbit anti-TSST-1 (Toxin Technology Inc., Sarasota, FL, USA), polyclonal mouse anti-staphylococcal protein A (SPA) (Chemicon Inc., Temecula, CA, USA), and then they were incubated for 16~18 hours at 4℃. Then, the sections were incubated with secondary antibodies; biotin conjugated goat anti-rabbit immunoglobulin G (IgG), and donkey anti-mouse IgG. Then, the sections were incubated for 60 minutes at room temperature with an ABC reagent (Vectastain Elite Kit; Vector Laboratories Inc., Burlingame, CA, USA) and the sections were developed in 0.025% 3,3-diaminobenzidine and 0.003% H2O2 medium at room temperature to visualize peroxidase activity. The sections were counterstained with Mayer's hematoxylin, and mounted in a xylene-based mounting medium, Entellan (Merck & Co., Inc., Darmstadt, Germany). A semi-quantitative analysis for the distribution and degree of several markers was performed according to the following scoring system; negative (-, absence of staining), weakly positive (+, <25% staining), and strongly positive (++, ≥25% staining).
3) Double immunofluorescent analysis
To see whether the distribution of SEA matched the distribution of S. aureus itself, a 2-colored, double immunofluorescent analysis with SEA and SPA was performed. The sections were incubated with the first primary antibody; polyclonal rabbit anti-SEA, for 16~18 hours at 4℃. Next, the sections were incubated with a donkey anti-rabbit IgG fluorescein isothiocyanate-conjugated antibody (Jackson ImmunoResearch Laboratories Inc., Baltimore, MD, USA), followed by incubation with the secondary primary antibody; polyclonal mouse anti-SPA. Then, the sections were incubated with the second secondary antibody; goat anti-mouse IgG Texas-Red conjugated antibody. Photomicrographs were captured digitally at 1,300×1,030 pixel resolution with a DXM-1200C (NIKON, Kawasaki, Japan) camera on a NiKon TE2000-U (NIKON) microscope equipped with fluorescent epi-illumination. The digital images were processed using an image analysis program, MetaMorph software (Universal Imaging, Downingtown, PA, USA).
4) Evaluation of clinical severity
The clinical severity was evaluated using 4 clinical parameters; erythema (E), induration/papulation (I/P), oozing (O), and lichenification (L). The severity was scored as 0 (no clinical manifestation), 1 (mild), 2 (moderate), 3 (severe) for each parameter by 2 designated dermatologists.
5) Statistical method
Statistical analysis was performed with the SAS version 9.1 program (SAS Institute, Cary, NC, USA). The chi-square test was used and a p-value of less than 0.05 was considered statistically significant.
In vitro study on the influence of adhesion molecules and cytokines expression in response to SEA
1) Preparation of recombinant SEA (γSEA) protein
After construction of the recombinant plasmid pET28a-SEA, the section was transformed into Escherichia coli BL21 (DE3). The colony was inoculated in 100 ml Luria-Bertani liquid medium and cultured up to optical density 0.8. The bacteria were induced by 1 mM isopropyl-β-D-thiogalactopyranoside and the SEA protein was over expressed. The resulting lysates were loaded directly onto sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and 2.7 kDa of exogenous protein was observed on the SDS-PAGE8. The over expressed SEA protein was purified using nickel nitrilotriacetate affinity chromatography, then it was analyzed by Western-blotting analysis.
2) RT-PCR analysis
HaCaT cells and HUVECs were cultured in 6-well plates and incubated for 6 hours, 12 hours, 24 hours and 48 hours after the γSEA protein (100 ng/ml) treatment.
Total RNA was isolated from each sample using a TRIzol® Reagent (Invitrogen Corp., San Diego, CA, USA) following the manufacturer's protocol. A variety of inflammation-related adhesion molecules, such as E-selectin, ICAM-1, VCAM-1 and cytokines, such as MCP-1 (monocyte chemoattract protein-1), interleukin (IL)-4, 5, 6, 8, 10, 12, 13, 18 and tumor necrosis factor (TNF)-α, were analyzed by semi-quantitative RT-PCR analysis. First-strand cDNA was obtained using a reverse transcription system (Fermentas Inc., Hanover, MD, USA) with 2 µg of total RNA. The resulting cDNA was used as a template for PCR using gene-specifications. PCR amplification was comprised of 22 cycles at 94℃ for 30 seconds, 55℃ for 30 seconds, and 72℃ for 30 seconds, after which the amplified products were analyzed by conventional agarose gel electrophoresis. The band intensities were measured using an image analysis program, MetaMorph software (Universal Imaging), with data expressed as ratios of each mRNA normalized to 16S rRNA and amplified from the same cDNA sample.
3) ELISA for IL-6 and IL-8
HaCaT cells (3×105 cells) or HUVECs were cultured in 6-well plates containing 3 ml of culture media for 16hour, and these cells were incubated with a γSEA protein (100 ng/ml) treatment for 6 hours, 12 hours, 24 hours, 48 hours, and 72hours. The supernatant was collected to determine the IL-6 and IL-8 released by using commercially available ELISA kits (Human IL-6, Human IL-8 ELISA, Diaclone Research, Besancon, France).
RESULTS
The distribution of SEA, SEB and TSST-1 in the lesional skin of patients with AD
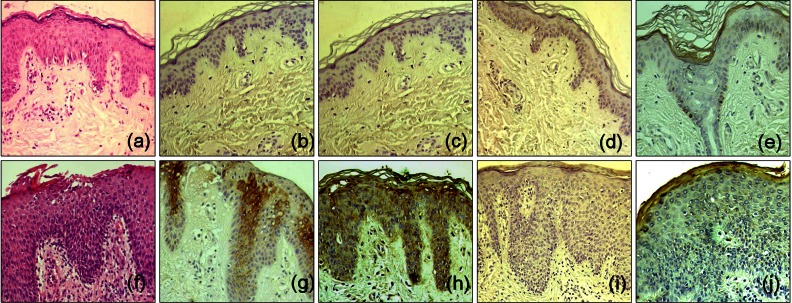
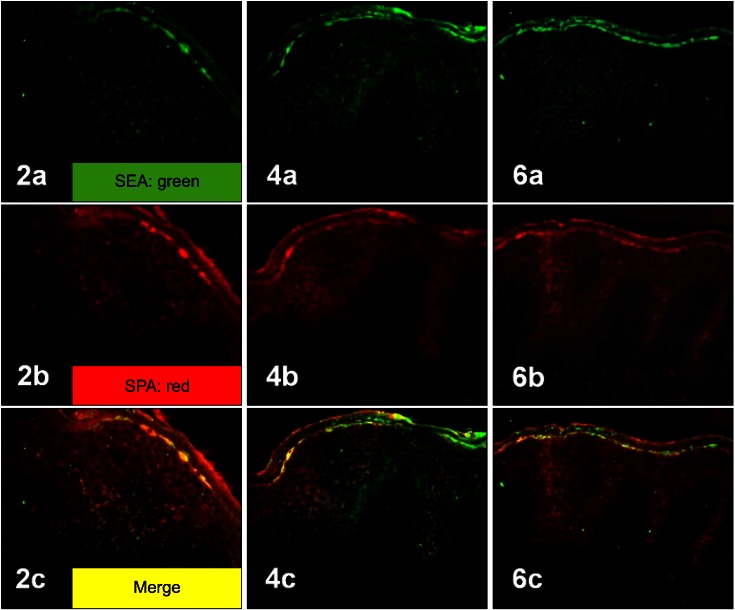
Histopathologically, there was a variable degree of spongiosis and exocytosis in the epidermis and the infiltration of inflammatory cells in the dermis of the lesional skin of the AD patients (Fig. 1F). In the immunohistochemical analysis, SPA and SEA were detected with increased immunoreactivity in AD patients, especially in the upper part of the epidermis (Fig. 1B, C). However, TSST-1 showed mild to moderate immunoreactivity in the upper part of epidermis in the AD patients, whereas SEB was minimally detected (Fig. 1D, E). In the double immunofluorescence, SEA and SPA were well co-localized (Fig. 2). There was no definite correlation between AD severity and amount of production of SEA and/or TSST-1 (p>0.05, Table 1).
Fig. 1.
Histopathological and immunohistochemical findings of biopsy specimens from normal control (a~e) and atopic dermatitis (AD) patients (f~j). H&E stained sections of the healthy skin of the normal control (a) and skin lesion of an AD patient (f). A variable degree of parakeratosis, acanthosis, spongiosis, and/or exocytosis, and sparse-to-moderate inflammatory cells infiltration were observed (×200). Immunohistochemical reactivity of staphylococcal protein A (SPA), recombinant staphylococcal enterotoxin A (SEA) in the skin of a healthy adult (b, c) and AD patients (g, h). Increased intensity of SPA, SEA immunoreactivity was observed in the upper part of the epidermis from all AD patients in comparison with that of a healthy adult (×200). Immunohistochemical reactivity of recombinant staphylococcal enterotoxin B (SEB) in the skin of a healthy adult (d) and AD patients (I). Minimal immunoreactivity of SEB was observed in the lesional skin of almost all AD patients (×200). Finally immunohistochemical reactivity of toxic shock syndrome toxin-1 (TSST-1) in the skin of a healthy adult (e) and AD patients (j). Mild to moderate immunoreactivity of TSST-1 was detected in the lesional skin of AD patients (TSST-1, ×200).
Fig. 2.
Two-colored double-labeled immunofluorescent staining of SEA (a: green), SPA (b: red) and their merged image (c: yellow) in the epidermis of three atopic dermatitis patients (2, 4, 6). Co-localization of SEA and SPA was observed in merged images (yellow), suggesting the coexistence of SEA and Staphylococcus aureus itself in the epidermis of AD patients (2c, 4c, 6c) (2a~6c: ×200). SEA: staphylococcal enterotoxin A, SPA: Staphylococcal protein A, AD: atopic dermatitis.
In vitro study on the influence of adhesion molecules and cytokines in response to SEA
1) Expression of adhesion molecules in HaCaT cells and HUVECs after treatment with γSEA
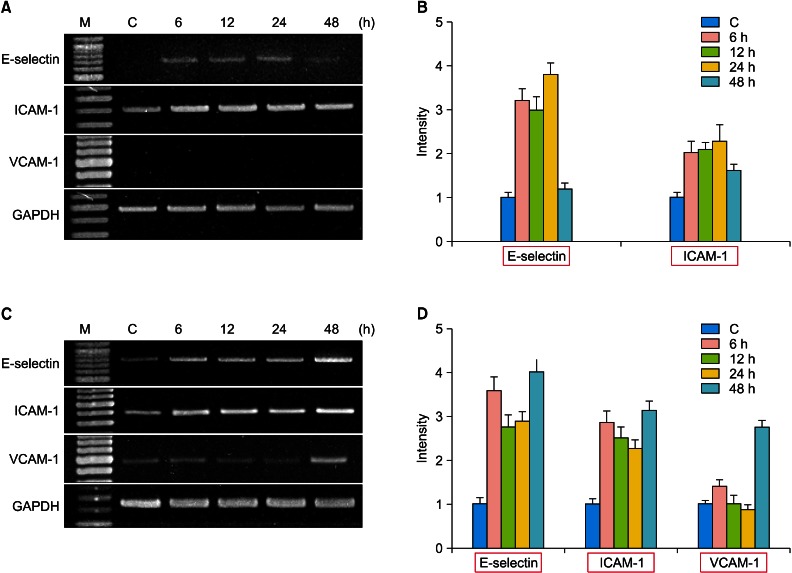
The expression of the E-selectin and ICAM-1 mRNA was upregulated in the HaCaT cells and HUVECs (Fig. 3). The expression of VCAM-1 was significantly increased in HUVECs, but was not detected in the HaCaT cells.
Fig. 3.
RT-PCR analysis for mRNA expression of the inflammation-related adhesion molecules in C, HaCaT cells (A, B) and HUVECs (C, D). The HaCaT cells and HUVECs were incubated for 6, 12, 24 and 48 hours after γSEA protein (100 ng/ml) treatment. (A, C) RT-PCR analysis for mRNA expression of E-selectin, ICAM-1, VCAM-1 and GAPDH was performed. (B, D) The density of each band was measured by a scanning densitometry and then expressed as the mean±standard deviation. The red box denotes significant upregulation of mRNA expression. M: marker, C: control, RT-PCR: reverse transcriptase-polymerase chain reaction, HUVECs: Human Umbilical Vein Endothelial Cells, γSEA: recombinant staphylococcal enterotoxin A.
2) Expression of cytokines in HaCaT cells and HUVECs after treatment with γSEA
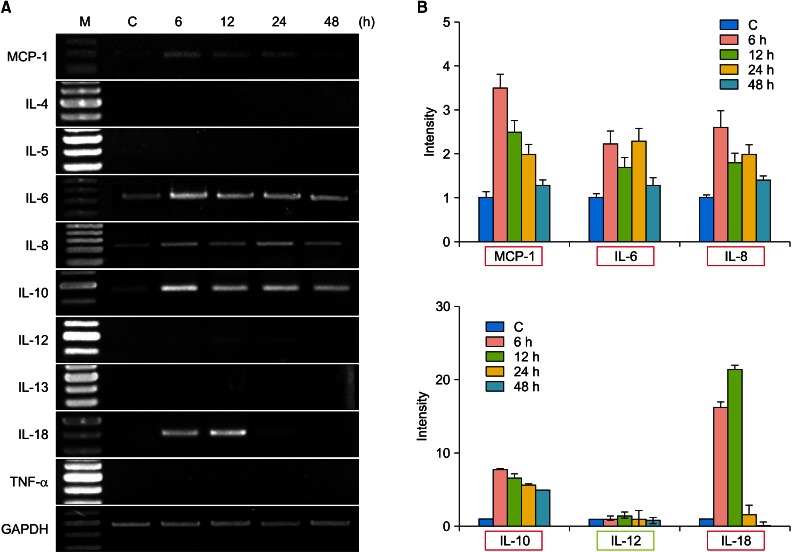
In the HaCaT cells, the expression of MCP-1, IL-6, IL-8, IL-10 and IL-18 mRNAs were significantly upregulated. IL-12 was minimally upregulated and IL-4, IL-5, IL-13 and TNF-α were not detected (Fig. 4). The expression of MCP-1 was significantly increased in the HaCaT cells, but not in the HUVECs. The multiplicity of the cytokines expression was increased in response to the γSEA protein. The results of RT-PCR analysis is summarized in Table 2.
Fig. 4.
RT-PCR analysis for mRNA expression of various cytokines after γSEA protein treatment in HaCaT cells. HaCaT cells were incubated for 6, 12, 24 and 48 hours after γSEA protein (100 ng/ml) treatment. (A) RT-PCR analysis for mRNA expression of various cytokines and GAPDH was performed. (B) The density of each band was measured by a scanning densitometry and then expressed as the mean±standard deviation. The red box denotes significant upregulation and the green box denotes minimal upregulation of mRNA expression. M: marker, C: control, IL: interleukin, TNF: tumor necrosis factor, RT-PCR: reverse transcriptase-polymerase chain reaction, γSEA: recombinant staphylococcal enterotoxin A.
Table 2.
Result of RT-PCR analysis after treatment with γSEA
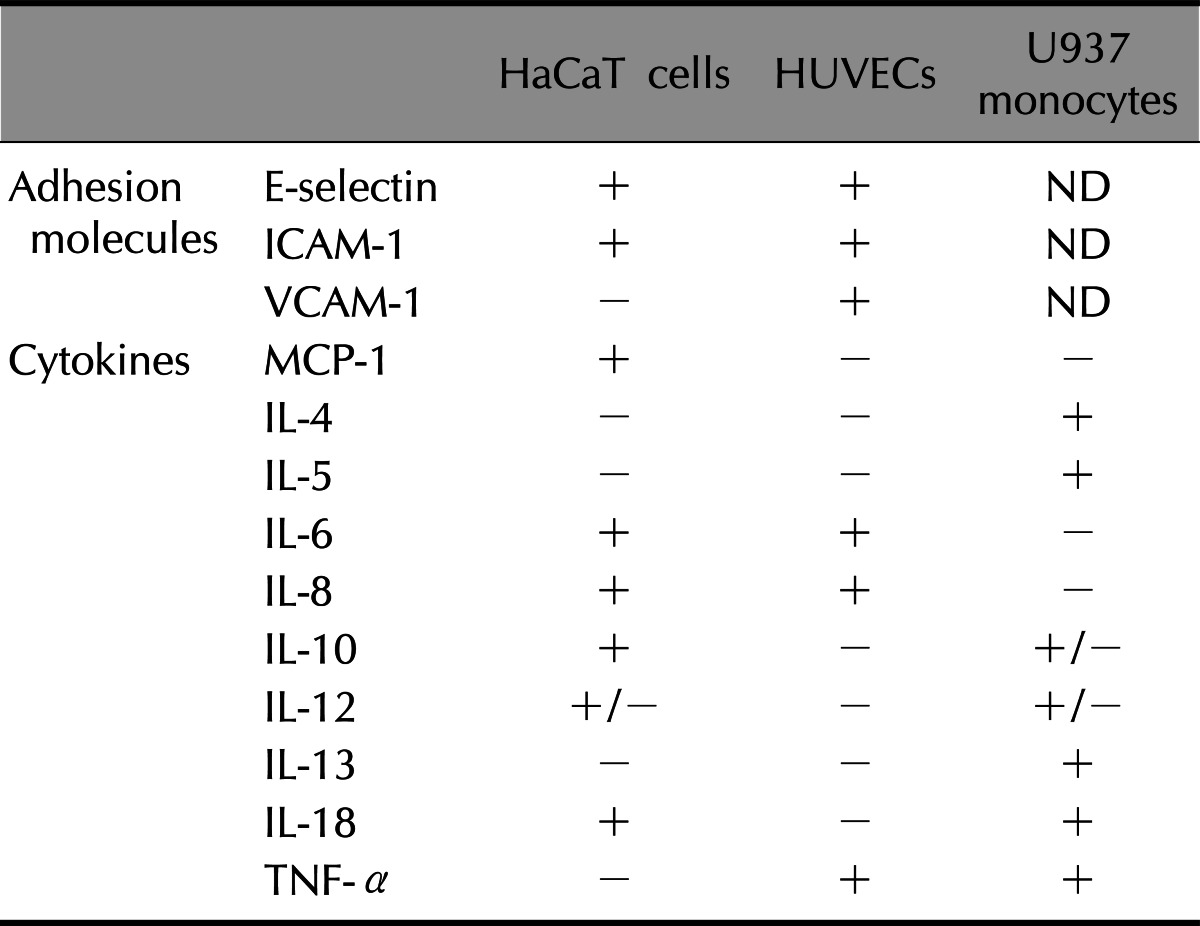
RT-PCR: reverse transcriptase-polymerase chain reaction, γSEA: recombinant staphylococcal enterotoxin A, HUVECs: Human Umbilical Vein Endotherial Cells, IL: interleukin, TNF: tumor necrosis factor, +: significantly upregulated, +/-: minimally upregulated, -: not upregulated, ND: not done.
3) ELISA
ELISA for IL-6 and IL-8 was performed to confirm the results of the RT-PCR (Table 3). Compared with the control group, the levels of IL-6 and IL-8 proteins were higher after treatment with the γSEA protein. The expression of IL-6 and IL-8 after γSEA treatment was similar or higher than the one after LPS treatment, a known potent stimulator of inflammatory cytokines.
Table 3.
Result of ELISA analysis after treatment with γSEA
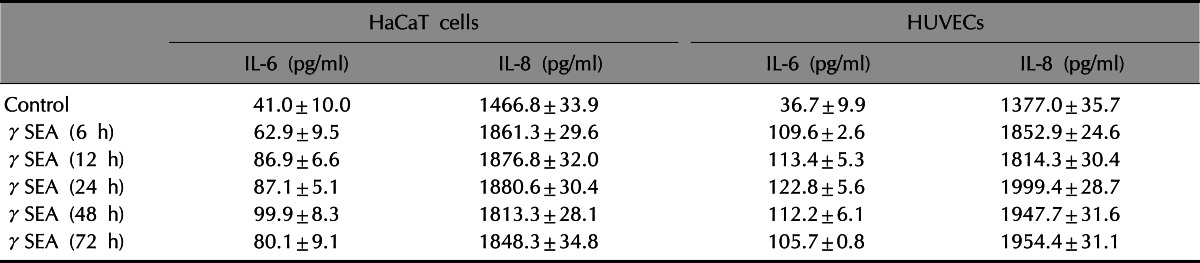
Values are presented as mean±standard deviation. γSEA: recombinant staphylococcal enterotoxin A, IL: interleukin.
DISCUSSION
Our previous data has shown a positive prevalence rate of superantigen producing S. aureus in children with AD and this was significantly higher than in the normal control groups7. However, it was interesting to find that the sea gene was the most prominent superantigenic exotoxin carried by S. aureus in all studied groups6,7. From the current study, SPA was abundantly detected in the skin biopsy specimens obtained from the lesional skin of AD patients, but not in the specimens obtained from a healthy individual. SEA and TSST-1 were also detected in abundantly, with higher levels of SEA than that of TSST-1 in epidermis and upper dermis, whereas only a little SEB detection. However, one subject (Case 5) did not show any immunoreactivity of superantigens (Table 1). Although the prevalence of S. aureus colonization is significantly high in AD patients compared to normal healthy controls, such bacteria is not cultivated from all AD patients. Likewise, though S. aureus may be colonized in AD patients, which is not necessarily to produce superantigens. However, we still can not exclude the possibility of technical error.
Double immunofluorescent analysis with SEA and SPA showed a co-localization of SEA and SPA in the epidermis of AD patients, suggesting that SEA might be produced by S. aureus9. These results match our previous findings well6,7 and strongly imply the importance of SEA as a major staphylococcal exotoxin that is associated with AD. However, there was no definite correlation between AD severity and the amount of SEA produced in this study. Many studies reported that colonization with superantigen-producing S. aureus is associated with increased severity of AD. However, this cannot be validated in our study since the assessment was done only on biopsy-conducted lesional skin and overall AD severity was not determined. Moreover, since AD severity may be influenced by various pathogenic factors, other bacterial products, foods allergens, or environmental factors should be considered as well.
We investigated the effect of SEA in the stimulation of a variety of cells involved in skin inflammatory processes, such as keratinocytes and endothelial cells. Our in vitro study showed that the expression of E-selectin, ICAM-1, MCP-1, IL-6, and IL-8 was significantly upregulated in both HaCaT cells and HUVECs after treatment with SEA. The expression of VCAM-1 was significantly increased in HUVECs, but not detected in HaCaT cells. However, the expression of MCP-1 was increased significantly in HaCaT cells but not in HUVECs. These results demonstrated that bacterial superantigen, especially SEA has been revealed to act as a potent inducer of several pro-inflammatory cytokines and adhesion molecules.
The epidermal keratinocyte has been known to play a key role in the mediating inflammatory and immune responses in the skin of AD. Keratinocytes are not only the targets of inflammation in AD, but also contribute to the initiation and maintenance of inflammation. Keratinocytes act as the source and target of various cytokines, such as IL-1, IL-6, IL-7, IL-8, IL-10, IL-12, and TNF-α10-12. In previous reports, superantigens were able to induce ICAM-1 molecules in cultured human keratinocytes13 and intracutaneous injections of SEB in mice were shown to elicit a strong inflammatory response in the skin, including upregulation of ICAM-1, and induction of VCAM-1 on dermal blood vessels14. Similarly, in this study, upregulation of ICAM-1 was shown in HaCaT cells and upregulation of ICAM-1 and VCAM-1 was shown in HUVEC cells after exposure to SEA. Inflammatory cytokines, such as MCP-1, IL-6, IL-8, IL-10 and IL-18, were also upregulated in HaCaT keratinocytes. Among the upregulated cytokines exposed to SEA, IL-18 was reported to be an important chemokine, which may play an important role in the initiation and amplification of atopic skin inflammation7. According to the Pivarcsi et al.'s study15, abundant expression of IL-18 was observed in the lesional skin of AD patients but not in the normal skin of healthy or non-lesional skin of atopic individuals, indicating a specific association of IL-18 with the AD phenotype. Moreover, IL-18 was significantly induced in the skin of AD patients in vivo and markedly increased in the PBMCs in vitro after topical exposure to SEB37. In this study, a distinctive upregulation of IL-18 was shown in HaCaT cells after exposure to SEA, strongly suggesting that SEA play a role in the initiation and amplification of atopic skin inflammation like SEB.
In further studies, we are planning to make attempts to clarify the role SEA performs as a superantigen in the animal model of AD and in AD patients.
ACKNOWLEDGMENT
This research was supported by grant from Amore-Pacific Grant in 2010.
References
- 1.Ricci G, Patrizi A, Neri I, Bendandi B, Masi M. Frequency and clinical role of Staphylococcus aureus overinfection in atopic dermatitis in children. Pediatr Dermatol. 2003;20:389–392. doi: 10.1046/j.1525-1470.2003.20503.x. [DOI] [PubMed] [Google Scholar]
- 2.Hauser C, Wuethrich B, Matter L, Wilhelm JA, Sonnabend W, Schopfer K. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatologica. 1985;170:35–39. [PubMed] [Google Scholar]
- 3.Hanifin JM, Rogge JL. Staphylococcal infections in patients with atopic dermatitis. Arch Dermatol. 1977;113:1383–1386. [PubMed] [Google Scholar]
- 4.Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113:780–782. [PubMed] [Google Scholar]
- 5.Kim KH, Han JH, Chung JH, Cho KH, Eun HC. Role of staphylococcal superantigen in atopic dermatitis: influence on keratinocytes. J Korean Med Sci. 2006;21:315–323. doi: 10.3346/jkms.2006.21.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DW, Park JY, Park KD, Kim TH, Lee WJ, Lee SJ, et al. Are there predominant strains and toxins of Staphylococcus aureus in atopic dermatitis patients? Genotypic characterization and toxin determination of aureus isolated in adolescent and adult patients with atopic dermatitis. J Dermatol. 2009;36:75–81. doi: 10.1111/j.1346-8138.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim BS, Kim JY, Lim HJ, Lee WJ, Lee SJ, Kim JM, et al. Colonizing features of Staphylococcus aureus in early childhood atopic dermatitis and in mothers: a cross-sectional comparative study done at four kindergartens in Daegu, South Korea. Ann Allergy Asthma Immunol. 2011;106:323–329. doi: 10.1016/j.anai.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Jiang XY, Wang CF, Wang CF, Zhang PJ, He ZY. Cloning and expression of Mycobacterium bovis secreted protein MPB83 in Escherichia coli. J Biochem Mol Biol. 2006;39:22–25. doi: 10.5483/bmbrep.2006.39.1.022. [DOI] [PubMed] [Google Scholar]
- 9.DeDent AC, McAdow M, Schneewind O. Distribution of protein A on the surface of Staphylococcus aureus. J Bacteriol. 2007;189:4473–4484. doi: 10.1128/JB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung DYM, Eichenfield LF, Boguniewicz M. Atopic dermatitis (Atopic eczema) In: Wolff K, Goldsmith LA, Katz Sl, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. Vol. 2. New York: McGraw-Hill Medical Cop.; 2008. pp. 146–158. [Google Scholar]
- 11.Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32:1405–1411. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga T, Katayama I, Yokozeki H, Nishioka K. Superantigen-induced cytokine expression in organ-cultured human skin. J Dermatol Sci. 1996;11:104–110. doi: 10.1016/0923-1811(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 14.Saloga J, Leung DY, Reardon C, Giorno RC, Born W, Gelfand EW. Cutaneous exposure to the superantigen staphylococcal enterotoxin B elicits a T-cell-dependent inflammatory response. J Invest Dermatol. 1996;106:982–988. doi: 10.1111/1523-1747.ep12338479. [DOI] [PubMed] [Google Scholar]
- 15.Pivarcsi A, Gombert M, Dieu-Nosjean MC, Lauerma A, Kubitza R, Meller S, et al. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004;173:5810–5817. doi: 10.4049/jimmunol.173.9.5810. [DOI] [PubMed] [Google Scholar]