Dear Editor:
Chronic urticaria may seem a trivial disease but it is a challenge for both the patient and the doctor. For the patient the disease is a tormenting itch that leads to social isolation. The volatile nature of the disease makes diagnosing difficult, and taking a comprehensive history is the most important diagnostic tool1.
The EAACI/GA2LEN/WAO have published two guidelines on the classification and treatment of urticaria2. According to the classification guideline spontaneous urticaria can be divided into chronic and acute urticaria depending on whether the symptoms have lasted for more or less than 6 weeks. The chronic types of urticaria are divided into physical urticaria (cold, delayed pressure, vibratory urticaria, and urticaria factitia), other urticaria types (aquagenic, cholinergic, contact urticaria and exercise induced urticaria/anaphylaxis), and finally, spontaneous urticaria. Chronic spontaneous urticarias may be idiopathic (55%) or autoimmune (45%) as defined by the presence of the immunoglobulin (Ig)G anti-IgE receptor, α subunit antibodies, or IgG anti IgE antibodies. The EAACI/GA2LEN/WAO treatment guideline recommends the use of non-sedating antihistamines up to a fourfold dosage as the first line of treatment, followed by leukotriene antagonist, and lastly systemic immunosuppressants or Omalizumab3.
Omalizumab is an anti-IgE-IgG-antibody, approved for the treatment of allergic asthma, which binds specific IgE molecules, but also reduces the expression of FcεRI on circulating basophils4. The mechanism behind the effect on chronic urticaria is more obscure. FcεRI expression on mast cells in the skin is reduced after Omalizumab therapy, thus reducing mast cell activation and histamine release5.
Urticaria activity is measured by an urticaria activity score of 7 (UAS7), where the number of hives and the itch intensity are each scored on a scale from 0~3 over the preceeding 7 days6. The dermatological quality of life index (DQLI) is a questionnaire that measures the impact of dermatological diseases on the quality of life7. Until now two randomized placebo controlled studies on the effect of Omalizumab on urticaria, and a large number of case reports comprising 1~20 patients each, have been published (thoroughly reviewed by Ivyanskiy et al.8. Taken together, these studies suggest the use of Omlizumab in doses of 150 mg or 300 mg every second or fourth week. The longest treatment period described until now is 15 months.
A total of 15 patients treated with Omalizumab for chronic spontaneous urticaria during 2009~2012 at the Department of Dermatology, Aarhus University Hospital were identified. Previous treatments, concurrent diseases, duration of urticaria, IgE levels (normal<150) before and during treatment, and basophil histamine release assay (BHRA) results (Histamine release [HR] test, Reflab, Copenhagen, Denmark) were registered. UAS7 and DQLI questionnaires were performed before Omalizumab injections. The dosage was either 150 mg/4 week or 300 mg/4 week depending on weight and serum IgE. In case of a lack of medicinal effect, the dosage of Omalizumab was doubled or intervals between injections were reduced to two weeks.
Of the 15 patients (5 males, 10 females) one was excluded due to lack of registration of UAS7 and DQLI regularly, and 1 patient had no effect on his urticaria after 2 months (3 injections) and stopped the treatment (patient characteristics can be found as patient number 14). The average duration of urticaria before Omalizumab treatment was 5.9 years (1~15), and the average observation time in Omalizumab treatment was 15.53 months (2~37). Prior therapies with no effect are listed in Table 1 along with concurrent diseases. Three patients had a positive BHRA (Chronic autoimmune urticaria), 10 were negative (Chronic idiopathic urticaria) and 1 was not determined.
Table 1.
Patient characteristics
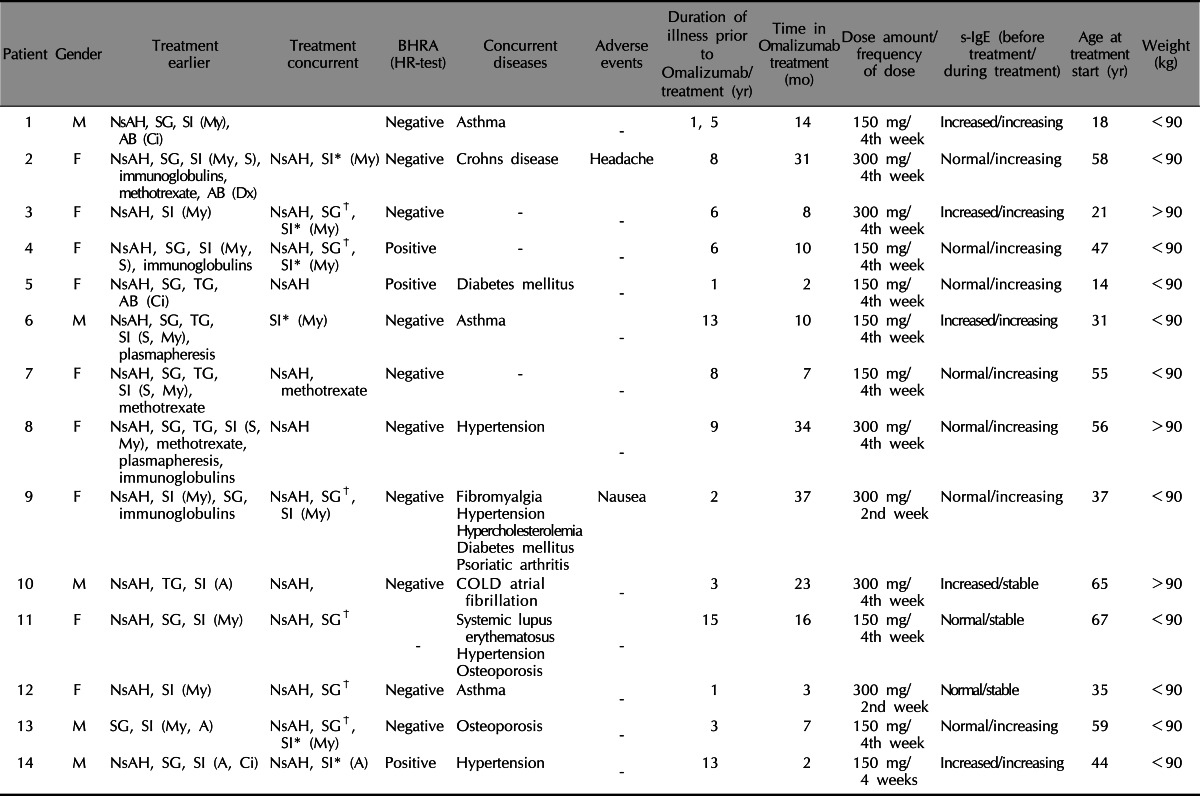
BHRA: basophil histamine release assay, HR-test: histamine release test, s-IgE: serum immunoglubulin E, M: male, F: female, NsAH: non-sedating antihistamines, SG: systemic glucocorticoids, SI: systemic immunesuppressants, My: mycophenolate mophetil, Ci: cyclosporine A, S: cyclosporine A, TG: topical steroid, A: azathioprine, COLD: chronic obstructive lung disease.
*Systemic immunotherapy was tapered down until patients had too many symptoms at the end of the treatment cycle. †In some instances systemic glucocorticoids were given at the beginning of the treatment as a consequence of a severe flare up of urticaria and then tapered off.
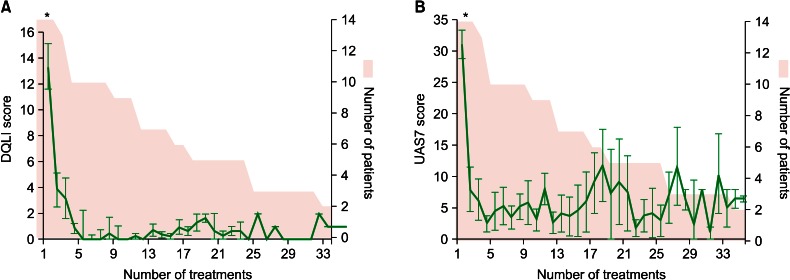
The average DQLI demonstrated a significant decrease after the first month from 13.4 to 3.8 (p<0.001) and also a further significant decrease after the second month (p<0.001), after which it stabilized on a low level (Fig. 1A). UAS7 also showed a significant decrease over the first month from 31.1 to 8.0 (p<0.001) (Fig. 1B) after which it stabilized, but standard deviations were high throughout the whole observation period.
Fig. 1.
(A) Average registered with the number of treatments. The shaded area represents the number of patients at the given number of treatment. (B) UAS7 score registered with the number of treatments. The shaded area again illustrates the number of patients. DQLI: dermatological quality of life index, UAS7: urticaria activity score of 7. *p<0.001 as calculated by one-way ANOVA for values at treatment number 1, 2 and 3.
Of the 14 patients 5 had increased serum immunoglubulin E (s-IgE) (>150 kU/ml) and 9 had normal levels (Table 1). During the treatment s-IgE was increased (doubled or more) in 11 out of 14 patients (p<0.01 using Mann-Whitney-U-test).
The longest documented periods of Omalizumab treatment until now were 15 months. In our material the longest treatment period was 37 months. None of the patients in our study could maintain symptom control without either antihistamines (12 of 14) or another systemic immune suppressant (7 of 14). The significant decrease in DQLI (Fig. 1) demonstrates that Omalizumab is very potent in restoring the patients' quality of life and controlling symptoms. The UAS7 also showed a significant decrease indicating that urticaria activity really is decreased. The high standard deviations reflect that UAS7 was measured in the last week of an injection cycle, where the effect of Omalizumab was wearing off, allowing break through of urticaria symptoms.
An interesting fact is that Omalizumab was efficient independently of s-IgE concentrations, and that s-IgE increased during treatment. The mechanism of this is debated, as is the actual finding. It might be due to the measuring techniques that measure both free and immune-complex bound IgE, thus also Omalizumab bound IgE9. Other studies have not been able to reproduce this finding10. However chronic spontaneous urticaria, whether autoimmune or idiopathic should by definition be independent of IgE, and it may therefore be the down regulation of FcRεI expression on mast cells and basophils that is important.
In conclusion, this study suggests that omalizumab is an excellent treatment choice for severe treatment refractory urticaria. Omalizumab, however, does not seem to be a cure for the disease only a symptomatic treatment, no matter the cause of urticaria. It is a well known phenomena in patients treated with Omalizumab for allergic asthma that s-IgE may increase9,10 and we observed the same phenomenon in our patient cohort suffering from chronic urticaria.
References
- 1.Weller K, Schoepke N, Krause K, Ardelean E, Bräutigam M, Maurer M. Selected urticaria patients benefit from a referral to tertiary care centres--results of an expert survey. J Eur Acad Dermatol Venereol. 2013;27:e8–e16. doi: 10.1111/j.1468-3083.2011.04387.x. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimenez-Arnau A, et al. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–1426. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, et al. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 4.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 5.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 7.Finlay AY, Khan GK. Dermatology Life Quality Index (DQLI)- -a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 8.Ivyanskiy I, Sand C, Thomsen SF. Omalizumab for chronic urticaria: a case series and overview of the literature. Case Rep Dermatol. 2012;4:19–26. doi: 10.1159/000336205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton RG, Marcotte GV, Saini SS. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab (Xolair) therapy. J Immunol Methods. 2005;303:81–91. doi: 10.1016/j.jim.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Steiss JO, Schmidt A, Nährlich L, Zimmer KP, Rudloff S. Immunoglobulin E monitoring and reduction of omalizumab therapy in children and adolescents. Allergy Asthma Proc. 2012;33:77–81. doi: 10.2500/aap.2012.33.3500. [DOI] [PubMed] [Google Scholar]