Abstract
Five millimolar KCN reduced water permeability in 1-millimeter thick slices of potato tuber (Solanum tuberosum L.). One-tenth millimolar ATP and CTP prevented or reversed the reduced permeability. UTP and GTP were not effective. Five millimolar ammonium carbonate or 0.1 millimolar 2,4-dinitrophenol also reduced water permeability, but ATP and CTP were only partially effective in reversing the reduced permeability. Oligomycin, 5 micrograms per milliliter, reduced water permeability, and the reduction was reversed by ATP and CTP. ATP and CTP appear to be involved in maintaining the structure of water pathways into the cell.
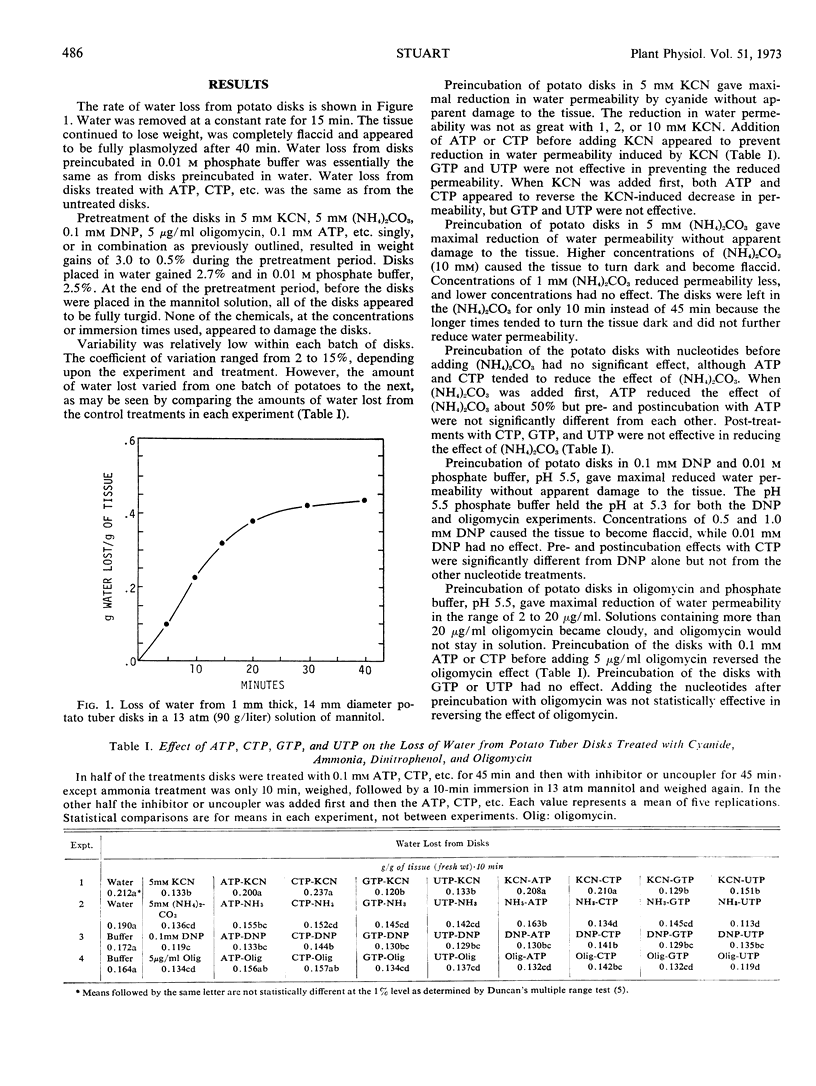
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Reversible Changes in the Hydraulic Permeability of Plant Cell Membranes. Plant Physiol. 1964 Nov;39(6):1043–1050. doi: 10.1104/pp.39.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanebuth W. F., Chasson R. M. The Effect of Light upon Development in Potato Tissue Slices. Plant Physiol. 1972 May;49(5):857–859. doi: 10.1104/pp.49.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Smith B. N., Epstein S., Laties G. G. The prevalence of carbon-13 in respiratory carbon dioxide as an indicator of the types of endogenous substrate. The change from lipid to carbohydrate during the respiratory rise in potato slices. J Gen Physiol. 1970 Jan;55(1):1–17. doi: 10.1085/jgp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopushinsky W. Effect of Water Movement on Ion Movement into the Xylem of Tomato Roots. Plant Physiol. 1964 May;39(3):494–501. doi: 10.1104/pp.39.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEES G. C., WEATHERLEY P. E. The mechanism of water absorption by roots. II. The role of hydrostatic pressure gradients across the cortex. Proc R Soc Lond B Biol Sci. 1957 Dec 3;147(928):381–391. doi: 10.1098/rspb.1957.0057. [DOI] [PubMed] [Google Scholar]
- Nakao T., Tashima Y., Nagano K., Nakao M. Highly specific sodium-potassium-activated adenosine triphosphatase from various tissues of rabbit. Biochem Biophys Res Commun. 1965 Jun 9;19(6):755–758. doi: 10.1016/0006-291x(65)90323-2. [DOI] [PubMed] [Google Scholar]
- Ordin L., Kramer P. J. Permeability of Vicia Faba Root Segments to Water as Measured by Diffusion of Deuterium Hydroxide. Plant Physiol. 1956 Nov;31(6):468–471. doi: 10.1104/pp.31.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENE H. F. The effect of anoxia on water exchange and oxygen consumption of onion root tissues. J Cell Physiol. 1950 Apr;35(2):179–193. doi: 10.1002/jcp.1030350203. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Structural changes in microsomal suspensions. V. Interactions with nucleotides. Arch Biochem Biophys. 1967 Mar 20;118(3):649–658. doi: 10.1016/0003-9861(67)90401-8. [DOI] [PubMed] [Google Scholar]
- Stuart D. M., Haddock J. L. Inhibition of water uptake in sugar beet roots by ammonia. Plant Physiol. 1968 Mar;43(3):345–350. doi: 10.1104/pp.43.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Loos G. M., Samuel E. W. Penetration of Mannitol into Potato Discs. Plant Physiol. 1960 Nov;35(6):848–853. doi: 10.1104/pp.35.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines H. M., Wedding R. T. Some Effects of Ammonia on Plant Metabolism and a Possible Mechanism for Ammonia Toxicity. Plant Physiol. 1960 Nov;35(6):820–825. doi: 10.1104/pp.35.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDDING R. T., VINES H. M. Inhibition of reduced diphosphopyridine nucleotide oxidation by ammonia. Nature. 1959 Oct 17;184(Suppl 16):1226–1227. doi: 10.1038/1841226a0. [DOI] [PubMed] [Google Scholar]
- Woolley J. T. Radial Exchange of Labeled Water in Intact Maize Roots. Plant Physiol. 1965 Jul;40(4):711–717. doi: 10.1104/pp.40.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]



