Abstract
Aseptic abscesses are an emergent entity and have been described in inflammatory bowel disease, especially in Crohn’s disease, and in other diseases. However, aseptic abscesses associated with Behçet’s disease are extremely rare. We report a Japanese male diagnosed with an incomplete type of Behçet’s disease who developed multiple aseptic abscesses of the spleen and liver. In 2002, the spleen abscesses were accompanied by paroxysmal oral aphthous ulcers and erythema nodosum. As the patient’s response to antibiotic treatment was inadequate, a splenectomy was performed. Severe inflammatory cell infiltration, largely of polymorphonuclear neutrophils, was observed without evidence of bacterial or fungal growth. Although the patient had no history of ocular symptoms or genital ulcers, a diagnosis of incomplete Behçet’s disease was made according to the Japanese diagnostic criteria because of the presence of paroxysmal arthritis and epididymitis since 2002. In 2005, multiple liver abscesses developed with right hypochondrial pain and seemed to be attributed to Behçet’s disease because the abscesses yielded negative results during a microbiologic investigation and failed to go into remission under antibiotic therapy. Oral prednisone (15 mg/d) was started in May 2006, and the abscesses dramatically disappeared 4 wk after treatment. Although the patient had a relapse of the liver abscesses in association with the tapering of prednisone, the augmentation of prednisone dosage yielded a response. The abscesses of the liver and spleen were strongly suggested to be attributed to Behçet’s disease. Clinician should be aware of the existence of aseptic abscesses as uncommon manifestations of Behçet’s disease.
Keywords: Behçet’s disease, Aseptic abscess, Spleen, Liver, Prednisone
Core tip: We report a Japanese male diagnosed with an incomplete type of Behçet’s disease who developed multiple aseptic abscesses of the spleen and liver. Spleen abscesses developed with paroxysmal oral aphthous ulcers and erythema nodosum. A splenectomy was performed, and severe neutrophil infiltration was observed without evidence of bacterial or fungal growth. Multiple liver abscesses also developed with right hypochondrial pain and seemed to be attributed to Behçet’s disease because the abscesses yielded negative results during a microbiologic investigation. Oral prednisone (15 mg/d) was started, and the abscesses dramatically disappeared. Clinicians should be aware of the existence of aseptic abscesses as uncommon manifestations of Behçet’s disease.
INTRODUCTION
Behçet’s disease (BD) is a systemic inflammatory disease commonly characterized by oral and genital ulcerations, with involvement of the skin and eye. The manifestations of BD are protean, and all symptoms and signs tend to recur either alone or in combination. Aseptic abscesses (AAs) are characterized by deep, sterile, round lesions consisting of neutrophil infiltration that do not respond to antibiotic therapy but improve with corticosteroid and immunosuppressive drugs. Clinical reports and case series concerning AAs in patients with inflammatory bowel disease, neutrophilic dermatoses, and other diseases have been published[1-5]. Although BD belongs to a group of neutrophilic dermatoses, AAs associated with BD are extremely rare. Here, we describe a patient with BD complicated by AAs of the liver and spleen, which were successfully treated with corticosteroid therapy.
CASE REPORT
In February 2006, a 20-year-old Japanese male suffering from multiple liver abscesses associated with sporadic fever, right hypochondrial pain, and elevated inflammatory markers was admitted to our hospital. In 2002, at the age of 16, he developed oral aphthous ulcers, erythema nodosum, pericardial effusion, and multiple spleen abscesses (Figure 1A). As for the aphthous ulcers and erythema nodosum, resolution occurred spontaneously. Pericardiocentesis was performed. Effusive pericarditis was thought to be the cause of the pericardial fluid; however, it did not reaccumulate after pericardiocentesis. Because empirical antibiotic therapy had the least effect and symptoms such as fever and left hypochondrial pain persisted, an open splenectomy was performed in October 2002. Upon macroscopic examination, sections of the resected spleen showed multiple yellow nodular lesions (10-20 mm in diameter) (Figure 1B). Pathological examination confirmed the presence of severe inflammatory cell infiltration, largely of polymorphonuclear neutrophils, without evidence of bacterial or fungal growth. In addition to oral aphthosis and erythema nodosum, the primary symptoms of BD, paroxysmal arthritis and epididymitis have occurred since 2002. Although the patient had no history of ocular symptoms or genital ulcers, a diagnosis of incomplete BD was made according to the criteria of the BD Research Committee of Japan[6]. Spleen abscesses were suspected to be attributed to BD, but the association was difficult to prove. Paroxysmal oral aphthosis and arthritis persisted after splenectomy, and at the age of 19 in March 2005, multiple abscesses with an aggressive inflammatory response developed in the liver. Antibiotics were unable to demonstrate any benefit, and the liver abscesses seemed to be attributed to BD. Colchicine (with a 1.5 mg maximum daily dose) was started in July 2005. However, the patient had elevated inflammatory markers, fever, and right hypochondrial pain.
Figure 1.
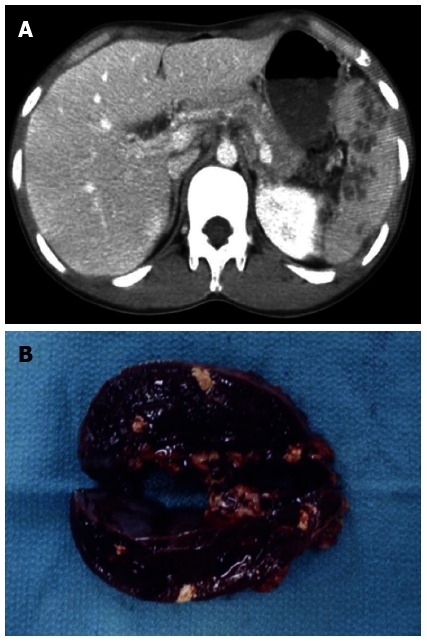
Contrast-enhanced abdominal computed tomography scan showing multiple spleen abscesses (A), and macroscopic findings of the cut surface of the resected spleen (B).
Upon physical examination, the patient did not present with fever, oral aphthosis, erythema nodosum, articular swelling, or abdominal tenderness at the time. The findings from the laboratory examinations are summarized as follows: white blood cell count, 13400/L (normal: 4000-9000/L) with 69% neutrophils; hemoglobin, 14.8 g/dL (normal: 12.0-16.0 g/dL); platelets, 57.1 × 104/L (normal: 14.0-40.0 × 104/L); aspartate aminotransferase, 21 U/L (normal: 10-40 U/L); alanine aminotransferase, 30 U/L (normal: 5-40 U/L); lactate dehydrogenase, 137 U/L (normal: 115-245 U/L); total bilirubin, 0.4 mg/dL (normal: 0.3-1.2 mg/dL); alkaline phosphatase, 583 U/L (normal: 115-359 U/L); gamma-glutamyl transferase, 123 U/L (normal: ≤ 70 U/L); and C-reactive protein (CRP), 11.0 mg/dL (normal: < 0.3 mg/dL). The results of the kidney function tests were within the normal limits. Antinuclear antibodies (speckled staining pattern) showed a 40-fold positive result (normal: < 40-fold). Rheumatoid factor, antineutrophil cytoplasmic antibodies, and HLA-B51 antigen were negative (HLA-A24, B52, and B60 antigens were positive). Colonoscopy showed only a small aphthous ulcer in the terminal ileum; pathological examination showed non-specific chronic inflammation without any evidence of Crohn’s disease (CD), such as noncaseating granuloma. Blood and urine cultures were negative. An abdominal ultrasound scan was performed, which showed multiple round lesions in the liver. Contrast-enhanced abdominal computed tomography scan demonstrated multiple areas of low-attenuation with ring enhancement in the liver (Figure 2A). Despite empirical antibiotic therapy, no clinical improvement was achieved. Ultrasound-guided percutaneous aspiration of the abscess yielded pus containing numerous neutrophils; no microbes were found in the culture. A fine needle biopsy of the liver lesions was performed, and a pathological examination revealed necrotic tissue containing inflammatory cells. Based on this evidence, these lesions were interpreted as aseptic liver abscesses associated with BD, and oral low-dose prednisone (15 mg/d), in addition to colchicine, was initiated in May 2006. A rapid improvement in the patient’s symptoms occurred; the levels of CRP and liver enzymes reached normal range, and the liver abscesses disappeared within 4 wk of the initiation of corticosteroid therapy (Figure 2B). The dose of prednisone was gradually tapered; at 7.5 mg/d prednisone, however, the CRP level increased again, requiring a higher dose of prednisone. Therefore, the patient was maintained on low-dose prednisone therapy (10-12.5 mg/d) to restrain the inflammatory response. Elevated CRP was observed without obvious abnormality in August 2010, and the recurrence of AAs in the liver was detected by abdominal CT without abdominal symptoms in August 2011. After escalating the dose of prednisone to 20 mg/d, the liver abscesses vanished once again. The dose of prednisone was gradually tapered to 15 mg/d, and complete clearance of the liver lesions was achieved in February 2012.
Figure 2.
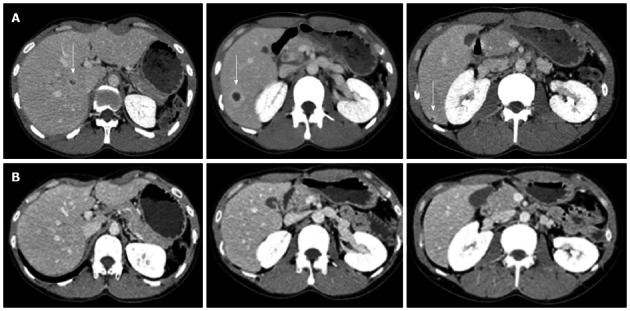
Contrast-enhanced abdominal computed tomography scan showing multiple liver abscesses (arrow) before (A) and 4 wk after corticosteroid therapy (B). Multiple areas of low-attenuation with ring enhancement were seen in the liver as indicated by the arrows (A). However, the areas vanished after treatment (B).
DISCUSSION
We present a patient with BD who developed AAs in the spleen and liver. AAs are an emergent entity. This condition has been described in inflammatory bowel disease (IBD), especially in CD, as well as in other diseases such as Sweet’s syndrome and pyoderma gangrenosum[1-5]. Most patients with AAs have some underlying disease, and it has been proposed that AAs belong to the spectrum of autoinflammatory multifactorial disorders[1]. The diagnosis of AAs is of exclusion on the basis of the following criteria suggested by André et al[1]: (1) deep abscess(es) upon radiologic examination, with neutrophilic features proven by surgical pathology or aspiration when performed; (2) negative blood cultures, negative serologic tests for bacteria, and, when surgical procedure or aspiration are performed, sterile standard, acid-fast bacillus and fungal cultures of pus; (3) failure of antibiotic therapy; and (4) rapid improvement by corticosteroids, sometimes in combination with immunosuppressive drugs. The main clinical manifestations of AAs are fever, abdominal pain, and weight loss. A rare case of BD complicated by multiple intrahepatic abscesses, which was dramatically resolved by antibiotic therapy, was reported[7]. There is also a case report of BD that was suspected to be complicated by sterile cerebral abscesses, although the clinical state of the patient gradually improved without immunosuppressive therapy[8]. In our patient, the liver and spleen abscesses were negative upon microbiologic investigation, and remission was not achieved under antibiotic therapy. Furthermore, the rapid resolution of the abscesses with corticosteroid therapy, even at the time of disease relapse in association with the tapering of prednisone, also favored a diagnosis of BD-associated AAs. Based on a PubMed search of the literature, our patient is the first unfailing case of sterile visceral abscesses associated with BD.
A differential diagnosis of CD vs BD can be difficult. Although ulcerative lesions at the ileocecal area are common in both diseases, we diagnosed the patient as having intestinal BD because of a lack of factors suggestive of CD: there was only one ulcerative lesion, its shape was not longitudinal, and histopathological examination showed no granulomatous lesions. It is also often difficult to differentiate incomplete types of BD from Sweet’s syndrome because some overlapping manifestations exist between BD and Sweet’s disease. Unfortunately, a skin biopsy was not performed. However, we are convinced of the diagnosis of incomplete BD in this case because our patient had a chronic course with remissions and relapses, as well as a history of epididymitis and pericarditis, found in relatively rare manifestations of BD[9].
Neutrophilic dermatoses are well-recognized cutaneous manifestations of systemic diseases such as IBD, and the wide spectrum of the disease includes Sweet’s syndrome and pyoderma gangrenosum. These diseases may share common features such as sterile infiltration of polymorphonuclear leukocytes; BD is analogous to this condition[10]. Extracutaneous neutrophilic infiltrates are observed in all forms of neutrophilic dermatoses, but they predominate in Sweet’s syndrome[11]. Sweet’s syndrome associated with BD has been reported, and there may be similarities in the pathogenesis of BD and Sweet’s disease[12]. Therefore, it seems reasonable that BD was complicated by AAs, although the mechanism remains unclear. Classically, Th1 immune response polarization has been known to be the main characteristic of BD immunopathogenesis[13]. The interleukin 17 (IL-17)-mediated (Th17) immune response may also contribute to immunological aberrations of BD[14]. IL-8-producing T cells were suggested to orchestrate neutrophil-rich pathologies of BD[15]. It was proposed that liver disease occurring in IBD is mediated by an aberrant homing of gut-derived T cells to the liver, with subsequent extensive lymphocyte infiltration of the liver[16]. Therefore, the mechanism by which AAs of the liver and spleen occurred in our case might be explained by an aberrant T cell-mediated immune response.
A patient with CD and associated AAs who underwent successful splenectomy was reported[17]. However, in our study, the patient developed AAs in the liver after splenectomy and finally needed immunosuppressive therapy. The risks of postoperative infection and thrombosis after splenectomy are now widely accepted. Accordingly, splenectomy as a treatment option might not be advisable in cases of AAs associated with BD.
In conclusion, we present a case of aseptic liver and spleen abscesses as the presenting picture of incomplete BD, in which complete remission was only obtained by corticosteroid therapy. The current case illustrates the possibility of the existence of AAs as uncommon manifestations of BD. Thus, physicians should be aware of this possibility to avoid a delay in diagnosis and unnecessarily aggressive therapies such as splenectomy.
Footnotes
P- Reviewers Andre MFJ, Zippi M S- Editor Song XX L- Editor A E- Editor Zhang DN
References
- 1.André MF, Piette JC, Kémény JL, Ninet J, Jego P, Delèvaux I, Wechsler B, Weiller PJ, Francès C, Blétry O, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore) 2007;86:145–161. doi: 10.1097/md.0b013e18064f9f3. [DOI] [PubMed] [Google Scholar]
- 2.Quilichini R, Mazzerbo F, Baume D, Carsuzaa F, Burtey S. [Sweet’s syndrome and aseptic abscess of the spleen] Rev Med Interne. 1996;17:1029–1031. doi: 10.1016/s0248-8663(97)80848-x. [DOI] [PubMed] [Google Scholar]
- 3.Klinger S, Mathis N, Jackson S. Bullous Sweet syndrome associated with an aseptic splenic abscess. Cutis. 2009;84:255–258. [PubMed] [Google Scholar]
- 4.Fukuhara K, Urano Y, Kimura S, Hori K, Arase S. Pyoderma gangrenosum with rheumatoid arthritis and pulmonary aseptic abscess responding to treatment with dapsone. Br J Dermatol. 1998;139:556–558. doi: 10.1046/j.1365-2133.1998.02440.x. [DOI] [PubMed] [Google Scholar]
- 5.Zakout R, Fonseca M, Santos JM, Marques A, Távora I, Oliveira E, Ferreira C, Victorino RM. Multiple aseptic liver abscesses as the initial manifestation of Crohn’s disease: report of a case. Dis Colon Rectum. 2009;52:343–345. doi: 10.1007/DCR.0b013e318199db60. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Kurokawa M, Suzuki N. Behcet’s disease. Clin Exp Med. 2004;4:10–20. doi: 10.1007/s10238-004-0033-4. [DOI] [PubMed] [Google Scholar]
- 7.Gelber AC, Schachna L, Mitchell L, Schwartzman G, Hartnell G, Geschwind JF. Behçet’s disease complicated by pylephlebitis and hepatic abscesses. Clin Exp Rheumatol. 2001;19:S59–S61. [PubMed] [Google Scholar]
- 8.Tokgoz S, Ogmegul A, Mutluer M, Kivrak AS, Ustun ME. Cerebral abscesses in Behcet’s disease: a case report. Turk Neurosurg. 2012;22:116–118. doi: 10.5137/1019-5149.JTN.3297-10.2. [DOI] [PubMed] [Google Scholar]
- 9.Sezen Y, Buyukhatipoglu H, Kucukdurmaz Z, Geyik R. Cardiovascular involvement in Behçet’s disease. Clin Rheumatol. 2010;29:7–12. doi: 10.1007/s10067-009-1302-0. [DOI] [PubMed] [Google Scholar]
- 10.Yim CW, White RH. Behçet’s syndrome in a family with inflammatory bowel disease. Arch Intern Med. 1985;145:1047–1050. [PubMed] [Google Scholar]
- 11.Vignon-Pennamen MD. The extracutaneous involvement in the neutrophilic dermatoses. Clin Dermatol. 2000;18:339–347. doi: 10.1016/s0738-081x(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 12.Wu F, Luo X, Yuan G. Sweet’s syndrome representing a flare of Behçet’s disease. Clin Exp Rheumatol. 2009;27:S88–S90. [PubMed] [Google Scholar]
- 13.Frassanito MA, Dammacco R, Cafforio P, Dammacco F. Th1 polarization of the immune response in Behçet’s disease: a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–1974. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Leng RX, Chen GM, Pan HF, Ye DQ. The role of IL-23/IL-17 axis in the etiopathogenesis of Behçet’s disease. Clin Rheumatol. 2010;29:1209. doi: 10.1007/s10067-010-1531-2. [DOI] [PubMed] [Google Scholar]
- 15.Keller M, Spanou Z, Schaerli P, Britschgi M, Yawalkar N, Seitz M, Villiger PM, Pichler WJ. T cell-regulated neutrophilic inflammation in autoinflammatory diseases. J Immunol. 2005;175:7678–7686. doi: 10.4049/jimmunol.175.11.7678. [DOI] [PubMed] [Google Scholar]
- 16.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 17.Renna S, Mocciaro F, Perricone G, Orlando A, Virdone R, Speciale A, Lima G, Stella M, Cottone M. Is splenectomy a treatment option for aseptic abscesses in patients with Crohn’s disease? Eur J Gastroenterol Hepatol. 2009;21:1314–1316. doi: 10.1097/MEG.0b013e32832bab85. [DOI] [PubMed] [Google Scholar]


