Summary
Indoleamine 2,3-dioxygenase (IDO) is an enzyme with known immunosuppressive and tolerogenic effects in cancer. Mounting evidence has associated IDO expression with the induction of regulatory T cells and malignant progression. IDO inhibition may therefore provide a promising therapeutic approach for glioblastoma, where the need for novel treatment is great.
Keywords: Glioblastoma, Immunosuppression, T-Lymphocytes, Regulatory
Commentary
In this issue of Clinical Cancer Research, Wainwright and colleagues present data characterizing the role of IDO-induced immunosuppression in glioma, and specifically address the impact of IDO on the recruitment of regulatory T cells (Tregs) to tumors in the brain (1).
Glioblastoma (GBM) is the most common and aggressive tumor of the central nervous system (CNS). It carries an exceedingly poor prognosis despite aggressive combinations of surgical resection, radiation, and temozolomide chemotherapy, as well as salvage therapy with bevacizumab. These conventional therapies also cause incapacitating, non-specific toxicity to healthy tissues. By contrast, immunotherapy offers an exquisitely precise approach; substantial evidence suggests that T cells have the ability to eliminate large, well-established tumors, even within the “immunologically-privileged” CNS, and without concomitant autoimmunity (reviewed in (2)).
Despite this promise, GBM immunotherapy has been limited to date, in part due to proclivities that the tumor possesses for instigating profound host immunosuppression. While the source of this immune impairment is clearly multifactorial, Tregs are perhaps most frequently implicated as major contributors to cellular immune defects and are present at increased proportions in both the tumors and peripheral blood of patients with GBM (3, 4). In tumors, Tregs curtail the generation and expansion of cellular immune responses, primarily through inhibited production of IL-2 and IFN-γ and the induction of T-cell anergy. Recent studies have suggested that the accumulation of Tregs in brain tumors may be a result of factors associated with the anatomical niche, since intracerebral gliomas in mice were found to contain significantly elevated numbers of Tregs compared to identical tumors implanted subcutaneously (5). While the CCR4/CCL22 axis has certainly been implicated, the data presented here by Wainwright et al. suggest an additional mechanism for Treg-to-GBM recruitment, centered about the enzyme indoleamine 2,3-dioxygenase (IDO).
IDO is an intracellular oxidoreductase that catalyzes the first step of tryptophan degradation. In a variety of tumor models, the expression of IDO by dendritic cells (DCs) in tumor-draining lymph nodes has been shown to potently facilitate antitumor T-cell tolerance, partly via the induction and recruitment of new and existing Tregs (Fig. 1). Currently, it is believed that IDO also inhibits effector T-cell responses through the depletion of essential tryptophan stores. In the absence of tryptophan, effector T cells have been shown to activate the general control nonrepressed-2 (GCN2) stress kinase pathway, which leads to cell cycle arrest and abortive T-cell activation. Interestingly, IDO expression can be induced in DCs through counter-regulatory mechanisms triggered by pro-inflammatory mediators (e.g., IFNγ), or alternatively through interactions with CTLA-4 on the surface of activated Tregs, thereby creating an immunosuppressive feedback “loop.”
Figure 1.
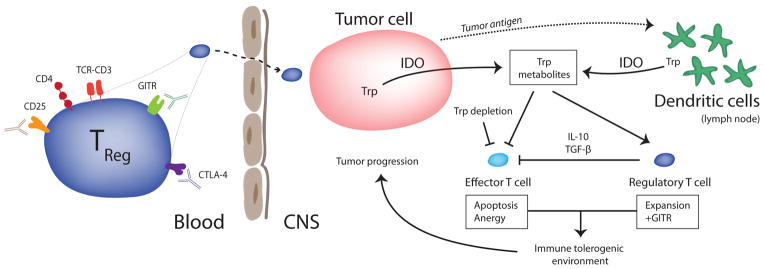
Tregs accumulate in brain tumors and are associated with poor prognosis. Peripheral depletion with anti-CD25 antibodies and CTLA-4 blockade are methods by which antitumor immune responses may be enhanced. IDO-mediated catabolism of tryptophan occurs in both tumor cells and peripherally in DCs. Here, IDO enzymatic activity potentiates an immune tolerogenic environment resulting in the reciprocal induction of Tregs amidst a suppressed effector T-cell response.
Wainwright and colleagues demonstrate that IDO expression by glioma leads to the recruitment of Tregs, to tumors resulting in impaired immune-mediated rejection of brain tumors in both orthotopic and transgenic models of GBM (1). The effects of IDO proved most impactful when expressed by the tumor itself, with peripheral levels being seemingly less relevant. This was clinically correlated insomuch as a retrospectively reviewed database of tumor samples from GBM patients revealed an inverse relationship between IDO expression and survival. Given that an IDO inhibitor, 1-mehtyl-tryptophan (1MT), is now in clinical trials, these data may be leveraged to support the application of IDO inhibition as a novel therapeutic strategy for GBM. The caveat though will be that such inhibition will need to be demonstrated at the level of the tumor, and not systemically. This is especially pertinent given that much of what is known about IDO and its inhibition to date has been limited to its peripheral activity in DCs, which the authors advance as less influential in the setting of GBM.
Of further import, the authors’ data also suggest that it is the constitution of tumor-infiltrating lymphocytes (TILs), and not their absolute number per se, that impacts survival. Previously, a number of efforts have been made to evaluate the prognostic significance of TILs in GBMs; however, these studies have not yielded definitive results to date. In contrast, when considered as a subset of glioma-infiltrating lymphocytes, intratumoral Tregs have been shown to correlate with tumor grade and malignant features. This notion is similarly supported by Wainwright and colleagues, since increased levels of tumor IDO, despite being associated with increased numbers of TILs in general, were simultaneously associated with an increased ratio of Tregs-to-CD8+ T cells, and accordingly poorer survival.
Numerous efforts have been made to selectively modulate Treg activity with hopes of improving immunotherapeutic responses against GBM. Importantly, upon removal of Tregs from the peripheral blood of patients with GBM, normal levels of effector T-cell proliferation and cytokine secretion can be restored in vitro, and in vivo depletion of Tregs in tumor-bearing mice has been shown to significantly prolong survival (3, 6, 7). Given the high levels of CD25 on the surface of Tregs, attempts have been made to selectively eliminate Tregs with denileukin diftitox (a fusion protein of diphtheria toxin and IL-2) and LMB-2 (a fusion protein of an anti-IL-2Rα monoclonal antibody and exotoxin). Both have been utilized to deplete Tregs in humans, but with mixed success. A significant limitation is the collateral depletion of effector T cells that transiently upregulate IL-2Rα upon activation. Moreover, due to its IL-2 targeting moiety, denileukin diftitox has the added drawback of unintentionally eliminating an even broader subset of cells that constitutively express lower affinity IL-2βγ receptors.
Whereas Tregs rely heavily on IL-2 for survival and function, activated effector T cells and tumor-specific memory T cells are not necessarily IL-2 dependent especially when undergoing homeostatic proliferation after lymphodepleting chemotherapy. This has prompted hope that systemic blockade with unarmed anti-IL-2Rα antibodies (e.g., daclizumab, basiliximab) may selectively impair Tregs based on their differential requirement for IL-2. Importantly, in preclinical models and early clinical trials, monoclonal antibodies that block IL-2Rα have been shown to significantly reduce Treg activity without hampering vaccine-induced immunity (8, 9). Although promising, current strategies to deplete Tregs in vivo have not been shown to efficiently eliminate intratumoral Treg populations, a limitation that IDO inhibition may avoid.
Going forward, it will be important to assess whether IDO inhibition may prove more effective or even synergistic with like-minded therapies. For instance, as noted, one component of IDO activity may be interaction with CTLA-4 on Tregs. CTLA-4 has an established role in facilitating Treg activity as well as in limiting activated T-cell responses, and its blockade with ipilimumab has potent therapeutic value in metastatic melanoma, for which it is FDA-approved (10). Likewise, CTLA-4 blockade has proven effective in experimental models of GBM (11) and in compassionate use protocols for patients with brain metastases (12). Given their likely activity along two steps in the same pathway, combining IDO inhibition and CTLA-4 blockade may be one example of a rational therapeutic platform to assess. Clearly, the authors demonstrate IDO inhibition as a promising means of countering Treg recruitment and activity. Clarifying its mechanisms, appropriate immune context, and interaction with other modalities should be emerging priorities.
Acknowledgments
This work was supported in part by the National Institutes of Health 5R01-CA135272–04 (J.H. Sampson), 5P50-NS020023–29 (D.D. Bigner and J.H. Sampson), 3R25-NS065731–03S1 (J.H. Sampson), as well as grants from the Pediatric Brain Tumor Foundation (D.D. Bigner and J.H. Sampson), Ben and Catherine Ivy Foundation (J.H. Sampson), and Cancer Research Institute (B.D. Choi).
Footnotes
The authors do not report conflicts of interest.
References
- 1.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 4.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biollaz G, Bernasconi L, Cretton C, Puntener U, Frei K, Fontana A, et al. Site-specific anti-tumor immunity: differences in DC function, TGF-beta production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol. 2009;39:1323–33. doi: 10.1002/eji.200838921. [DOI] [PubMed] [Google Scholar]
- 6.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 7.Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118:3003–12. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS One. 2012;7:e31046. doi: 10.1371/journal.pone.0031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557–61. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]


