Abstract
BACKGROUND
In observational studies, the relationship between blood pressure and end-stage renal disease (ESRD) is direct and progressive. The burden of hypertension-related chronic kidney disease and ESRD is especially high among black patients. Yet few trials have tested whether intensive blood-pressure control retards the progression of chronic kidney disease among black patients.
METHODS
We randomly assigned 1094 black patients with hypertensive chronic kidney disease to receive either intensive or standard blood-pressure control. After completing the trial phase, patients were invited to enroll in a cohort phase in which the blood-pressure target was less than 130/80 mm Hg. The primary clinical outcome in the cohort phase was the progression of chronic kidney disease, which was defined as a doubling of the serum creatinine level, a diagnosis of ESRD, or death. Follow-up ranged from 8.8 to 12.2 years.
RESULTS
During the trial phase, the mean blood pressure was 130/78 mm Hg in the intensive-control group and 141/86 mm Hg in the standard-control group. During the cohort phase, corresponding mean blood pressures were 131/78 mm Hg and 134/78 mm Hg. In both phases, there was no significant between-group difference in the risk of the primary outcome (hazard ratio in the intensive-control group, 0.91; P = 0.27). However, the effects differed according to the baseline level of proteinuria (P = 0.02 for interaction), with a potential benefit in patients with a protein-to-creatinine ratio of more than 0.22 (hazard ratio, 0.73; P = 0.01).
CONCLUSIONS
In overall analyses, intensive blood-pressure control had no effect on kidney disease progression. However, there may be differential effects of intensive blood-pressure control in patients with and those without baseline proteinuria. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center on Minority Health and Health Disparities, and others.)
Chronic kidney disease is a major public health problem. In national surveys, the prevalence of chronic kidney disease (stages 1 through 4) among adults in the United States increased from 10% during the period from 1988 through 1994 to 13% during the period from 1999 through 2004.1 In 2006, the cost to the federal government for the treatment of end-stage renal disease (ESRD) was $23 billion, and the corresponding treatment cost for chronic kidney disease was $49 billion.2 In the United States, approximately 30% of incident ESRD cases are attributed to hypertension.2 The burden of hypertension-related chronic kidney disease and ESRD is especially high a mong black patients.3
In observational studies, the relationship between blood pressure and the progression of chronic kidney disease or incident ESRD is direct and progressive.3 Yet few trials have tested the effects of intensive blood-pressure control, as compared with traditional control, on the progression of chronic kidney disease, and the findings from such trials have been inconsistent.4–7 Despite a lack of compelling evidence,8 numerous guidelines recommend a reduced blood-pressure target in patients with chronic kidney disease.9–12
Trials in which the outcome variable is ESRD are difficult to conduct, because even high-risk patients typically have a relatively slow rate of decline in kidney function. The average decline in the glomerular filtration rate (GFR) among black patients with hypertensive chronic kidney disease is approximately 2 ml per minute per 1.73 m2 of body-surface area per year,5 which is twice the usual age-associated decline in the general population.13 For a patient with a GFR of 40 ml per minute and with an average decline in GFR of 2 ml per minute per year, it would take 15 years to reach ESRD, which typically occurs at a GFR of approximately 10 ml per minute. However, trials studying the progression of chronic kidney disease rarely exceed 5 years.
In this study, called the African-American Study of Kidney Disease and Hypertension (AASK), we evaluated the effects of an intensive blood-pressure target, as compared with a traditional blood-pressure target, on the progression of chronic kidney disease among black patients with hypertensive chronic kidney disease. On completion of the trial phase, patients were invited to enroll in a cohort phase in which they received recommended therapy with a blood-pressure target of less than 130/80 mm Hg. Using data from both phases of the trial, we now report the longterm effects of a lower blood-pressure target on the progression of chronic kidney disease.
METHODS
PATIENTS
Descriptions of the study methods have been reported previously.5,14–16 All the patients in our study were black and between the ages of 18 and 70 years, and all had hypertensive chronic kidney disease, which was defined as a diastolic blood pressure of more than 95 mm Hg and a GFR of 20 to 65 ml per minute, as measured by 125I-iothala-mate clearance. Principal exclusion criteria were diabetes, which was defined as a fasting glucose level of more than 140 mg per deciliter (7.8 mmol per liter), a random glucose level of more than 200 mg per deciliter (11.1 mmol per liter), or the need for drug therapy for diabetes; a urinary protein-to-creatinine ratio of more than 2.5; malignant hypertension (as defined by each center) during the previous 6 months; secondary hypertension, serious systemic disease, or heart failure; or a specific indication for or contraindication to a study drug.
STUDY DESIGN
The study had two phases, an initial trial phase, followed by a cohort phase. The trial phase had a 3-by-2 factorial design. From February 1995 through September 1998, we randomly assigned 1094 patients to receive either intensive blood-pressure control or standard control. The blood-pressure target was a mean arterial pressure of 92 mm Hg or less in the intensive-control group and 102 to 107 mm Hg in the standard-control group. A mean arterial pressure of 92 is lower than the traditional blood-pressure target of 130/80 mm Hg, which is recommended for patients with chronic kidney disease, and a mean arterial pressure of 107 mm Hg corresponds to the traditional blood-pressure target of 140/90 mm Hg.9 We also randomly assigned patients to one of three initial drug therapies: ramipril, an angiotensin-converting–enzyme (ACE) inhibitor; metoprolol, a sustained-release beta-blocker; or amlodipine, a dihydropyridine calcium-channel blocker. If the blood-pressure target could not be achieved with the highest tolerated dose of the randomly assigned drug, other antihypertensive drugs (furosemide, doxazosin, clonidine, and hydralazine or minoxidil) were sequentially added.
The cohort phase was initiated in April 2002. Between the end of the trial phase on September 30, 2001, and the start of the cohort phase, there was a brief transition period during which the cohort phase was designed and patients were switched from randomized therapy to ramipril. Patients in whom ESRD had not been diagnosed were invited to enroll in the cohort phase, in which they received protocol-driven blood-pressure management on the basis of the results of the primary trial. If patients could not tolerate ramipril therapy, they were switched to an angiotensin-receptor blocker (ARB) that was selected by the clinical site investigator. If the blood-pressure target was not achieved with the highest tolerated dose of ramipril, additional drugs were added, including furosemide, beta-blockers, calcium-channel blockers, centrally acting alphaadrenergic blockers, and direct vasodilators. At the start of the cohort phase, the blood-pressure target was less than 140/90 mm Hg. The target was reduced to less than 130/80 mm Hg in 2004, just after national guidelines recommended this target.9
OUTCOMES
The primary outcome was the progression of chronic kidney disease, which was defined as a doubling of the serum creatinine level (roughly equivalent to a halving of the GFR), a diagnosis of ESRD, or death. Other outcomes were directly related to chronic kidney disease (a doubling of the serum creatinine level or a diagnosis of ESRD) or clinical outcomes (ESRD or death). Serum creatinine was assessed twice at baseline and then every 6 months. ESRD was defined by the initiation of dialysis or receipt of a kidney transplant.
STUDY MEASUREMENTS
Baseline characteristics were summarized for all patients and according to the urinary protein-to-creatinine ratio (>0.22 or ≤0.22, as measured from 24-hour urine collections, with both protein and creatinine measured in milligrams per day). Soon after the start of recruitment, on the basis of emerging evidence from other studies,17 the investigators requested that the data and safety monitoring board test for interactions of trial interventions with proteinuria and review results stratified according to the presence or absence of baseline proteinuria. The cutoff point for proteinuria (a protein-to-creatinine ratio of >0.22) was selected by the investigative team in conjunction with the data and safety monitoring board. The specific threshold was chosen post hoc but before the outcome analyses were performed. This level of proteinuria roughly corresponds to an absolute urinary protein excretion of 300 mg per day, a commonly used threshold for defining proteinuria. All published proteinuria subgroup analyses of the AASK trial have used this threshold.17
Blood-pressure levels and hypertension control were summarized at baseline and then every 2 years in patients who had not yet had the primary outcome. For the trial phase, follow-up time started at the date of randomization. For the cohort phase, follow-up time started at the end of the trial phase and included the transition period. The maximum duration of follow-up was 12.2 years, which corresponded to the interval between the start of enrollment in the trial phase (April 7, 1995) and the end of outcome ascertainment (June 30, 2007).
STUDY OVERSIGHT
The institutional review board at each study center and the studywide scientific advisory committee approved the protocols for the trial and cohort phases. The trial was conducted in accordance with the protocol. (The protocol is available with the full text of this article at NEJM.org.) In each phase of the study, patients provided written informed consent. King Pharmaceuticals provided financial support and donated antihypertensive medications to each clinical center. Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn also donated antihypertensive medications. None of these companies had any role in the design of the study, the accrual or analysis of data, or the preparation of the manuscript.
STATISTICAL ANALYSIS
We calculated the cumulative probability of study outcomes using Kaplan–Meier curves.18 The effects of blood-pressure targets on trial outcomes were evaluated with the use of Cox proportionalhazards regression with adjustment for five prespecified baseline factors (log-transformed urinary protein excretion, age, sex, presence or absence of a history of heart disease, and baseline mean arterial pressure) and randomized drug assignment. An interaction term of follow-up time with the log-transformed baseline protein-to-creatinine ratio was included as a covariate to account for a change in the hazard ratio for baseline urinary protein excretion over time. A timedependent indicator variable for study phase (trial phase vs. cohort phase) was also included to allow the baseline hazard to change at the completion of the trial.
We investigated the relationship between the effects of the blood-pressure target and the level of baseline protein excretion by adding interaction terms between the randomized study groups and the log-transformed baseline protein-to-creatinine ratio and by fitting the basic Cox regression analyses separately for the two strata of the protein-to-creatinine ratio. There was no significant interaction between the randomized drug assignments and assignments to a blood-pressure target. A two-sided P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
PATIENTS
Of the 2802 patients who underwent screening, 1094 were enrolled in the trial phase (see Fig. 1 in the Supplementary Appendix, available at NEJM .org). The baseline characteristics of the patients are shown according to the randomized blood-pressure target and the baseline protein-to-creatinine ratio (≤0.22 vs. >0.22) for the trial phase (Table 1) and the cohort phase (Table 1 in the Supplementary Appendix). At baseline in the trial phase, approximately one third of the patients had proteinuria. Among all patients and within the two strata for the protein-to-creatinine ratio, baseline characteristics were similar in the two blood-pressure groups, with the exception of current smoking, which was more prevalent in the intensive-control group. Among patients with proteinuria, the median protein-to-creatinine ratio was slightly higher in the standard-control group than in the intensive-control group. The primary outcome (a doubling of the serum creatinine level, ESRD, or death) occurred in 328 patients during the trial phase and in 239 patients during the cohort phase.
Table 1.
Baseline Characteristics of 1094 Patients Assigned to Receive Intensive or Standard Blood-Pressure Control, According to the Level of Urinary Protein Excretion.*
| Variable | All Patients | Urinary Protein-to-Creatinine Ratio, ≤0.22 |
Urinary Protein-to-Creatinine Ratio, >0.22 |
|||
|---|---|---|---|---|---|---|
| Intensive Control (N = 540) |
Standard Control (N = 554) |
Intensive Control (N = 357) |
Standard Control (N = 376) |
Intensive Control (N = 181) |
Standard Control (N = 176) |
|
| Age — yr | 54.5±10.9 | 54.7±10.4 | 56.6±10.1 | 55.8±9.67 | 50.4±11.3 | 52.1±11.4 |
| Female sex — no. (%) | 206 (38.1) | 219 (39.5) | 147 (41.2) | 149 (39.6) | 59 (32.6) | 70 (39.8) |
| Current smoker — no. (%) | 182 (33.7) | 139 (25.1) | 117 (32.8) | 87 (23.1) | 64 (35.4) | 51 (29.0) |
| Did not complete high school — no. (%)† | 218 (40.4) | 226 (40.9) | 158 (44.3) | 168 (44.8) | 60 (33.3) | 57 (32.4) |
| Weight — kg | 89.5±20.9 | 89.4±20.5 | 87.4±19.7 | 88.0±20.1 | 93.7±22.6 | 92.4±21.1 |
| Body-mass index‡ | 30.5±6.71 | 30.6±6.47 | 30.0±6.22 | 30.3±6.44 | 31.7±7.47 | 31.3±6.52 |
| Estimated GFR (ml/min/1.73 m2) | 48.1±13.9 | 46.8±14.0 | 51.5±13.2 | 50.7±12.3 | 41.4±12.7 | 38.5±13.6 |
| Serum creatinine — mg/dl | 1.98±0.70 | 2.02±0.70 | 1.79±0.58 | 1.80±0.50 | 2.35±0.77 | 2.48±0.83 |
| Median urinary protein — g/day (interquartile range) | 0.12 (0.04–0.55) | 0.11 (0.04–0.59) | 0.06 (0.03–0.12) | 0.06 (0.04–0.12) | 0.96 (0.54–1.99) | 1.06 (0.63–2.08) |
| Median urinary protein-to-creatinine ratio (interquartile range) | 0.08 (0.03–0.36) | 0.08 (0.03–0.37) | 0.04 (0.02–0.08) | 0.04 (0.02–0.09) | 0.58 (0.35–1.08) | 0.73 (0.42–1.37) |
Plus–minus values are means ±SD. Four patients did not undergo baseline measurement of the urinary protein-to-creatinine ratio. To convert the values for creatinine to micromoles per liter, multiply by 88.4. GFR denotes glomerular filtration rate.
Data were missing for one patient in the intensive-control group and one in the standard-control group.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
BLOOD-PRESSURE LEVELS AND HYPERTENSION CONTROL
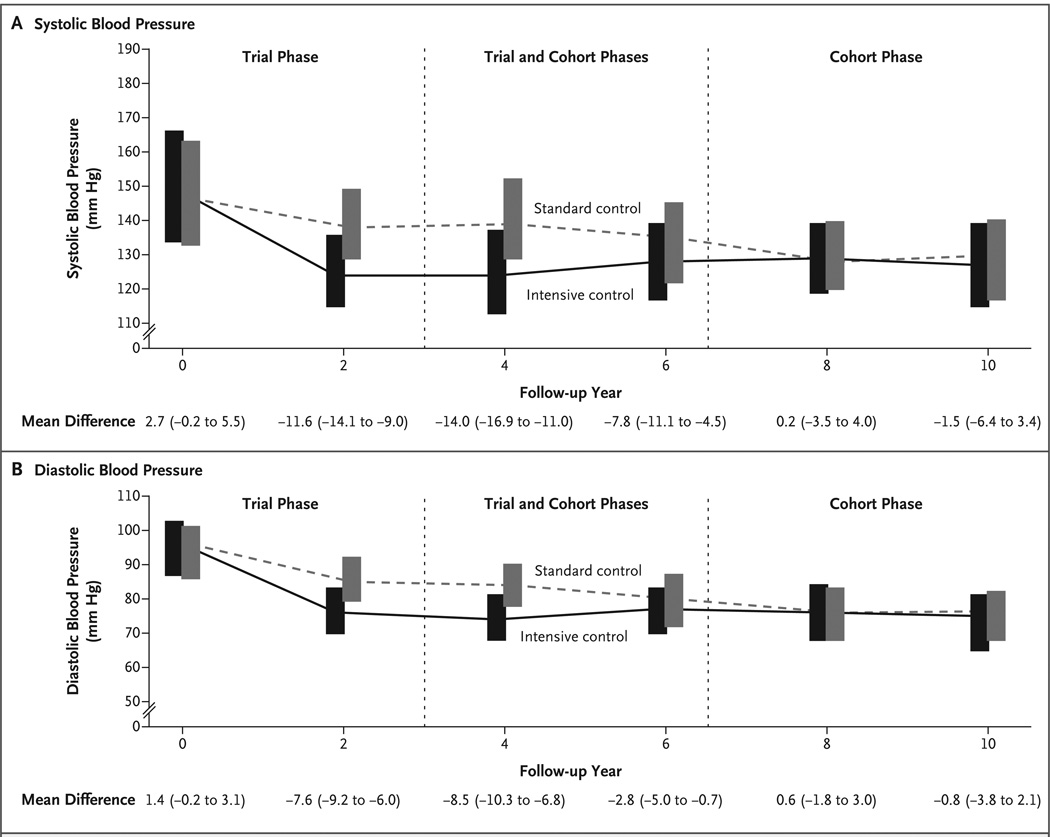
Figure 1 and Table 2 show blood-pressure levels, rates of hypertension control, and medication use according to blood-pressure target among patients who remained at risk for the primary outcome. At baseline, the mean blood pressure was 152/96 mm Hg in the intensive-control group and 149/95 mm Hg in the standard-control group. Throughout the trial phase, the mean blood pressure was significantly lower in the intensive-control group than in the standard-control group (130/78 mm Hg vs. 141/86 mm Hg). During the cohort phase, differences in blood pressure were smaller in magnitude, because all patients had a common blood-pressure target; the mean blood pressure was 131/78 in the intensive-control group and 134/78 mm Hg in the standard-control group. Few patients had poorly controlled blood pressure, which was defined as a blood pressure of 160/100 mm Hg or more (Table 2). Blood-pressure levels that were stratified according to the baseline protein-to-creatinine ratio paralleled the results among all patients (Tables 2 and 3 and Fig. 2 and Fig. 3 in the Supplementary Appendix). Throughout the study, use of ACE inhibitors and ARBs was similar in the two randomized blood-pressure groups.
Figure 1. Blood-Pressure Levels in Patients with Chronic Kidney Disease.
Shown are median systolic (Panel A) and diastolic (Panel B) blood-pressure measurements for patients who received intensive blood-pressure control or standard control over time in the trial and cohort phases. All values are for patients who did not have progression of chronic kidney disease, defined as a doubling of the serum creatinine level, end-stage renal disease, or death (composite primary outcome). The upper edge of each bar corresponds to the 75th percentile, and the bottom edge to the 25th percentile. Patients had at least 3 years of follow-up in the trial phase. The period between 3 and 6.5 years is a mixed period that encompasses the trial phase for early enrollees and the cohort phase for late enrollees. After 6.5 years, all data are for the cohort phase. The values at the bottom of the graphs are the mean difference in blood pressure between the intensive-control group and the standard-control group at various time points; numbers in parentheses are 95% confidence intervals.
Table 2.
Blood-Pressure Levels and Medication Use.*
| Variable | Yr after Randomization | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| No. of patients | ||||||
| Trial phase | ||||||
| Standard control | 554 | 448 | 272 | 46 | 0 | 0 |
| Intensive control | 540 | 456 | 292 | 56 | 0 | 0 |
| Cohort phase | ||||||
| Standard control | 0 | 0 | 39 | 183 | 208 | 116 |
| Intensive control | 0 | 0 | 37 | 189 | 219 | 133 |
| Blood pressure | ||||||
| Systolic pressure (mm Hg) | ||||||
| Standard control | 149±23 | 140±18 | 141±18 | 136±20 | 131±19 | 130±19 |
| Intensive control | 152±25 | 128±21 | 127±20 | 128±16 | 131±20 | 128±20 |
| Diastolic pressure (mm Hg) | ||||||
| Standard control | 95±14 | 86±11 | 84±10 | 80±13 | 76±12 | 75±11 |
| Intensive control | 96±15 | 78±14 | 76±12 | 77±11 | 77±13 | 74±12 |
| Blood pressure <130/80 mm Hg (%) | ||||||
| Standard control | 8.5 | 12.3 | 14.1 | 24.9 | 37.5 | 41.4 |
| Intensive control | 6.7 | 50.9 | 55.0 | 43.7 | 39.3 | 48.1 |
| Blood pressure <140/90 mm Hg (%) | ||||||
| Standard control | 20.2 | 39.7 | 40.2 | 59.8 | 72.1 | 72.4 |
| Intensive control | 19.1 | 75.4 | 75.1 | 71.8 | 73.5 | 73.7 |
| Blood pressure ≥160/100 mm Hg (%) | ||||||
| Standard control | 18.2 | 4.0 | 3.2 | 3.9 | 2.9 | 0.9 |
| Intensive control | 22.4 | 3.9 | 3.3 | 1.6 | 2.7 | 1.5 |
| Medication | ||||||
| ACE inhibitor or ARB (%) | ||||||
| Standard control | 39.7 | 40.0 | 48.9 | 82.1 | 90.9 | 83.6 |
| Intensive control | 39.5 | 41.0 | 48.6 | 79.1 | 88.1 | 91.6 |
| No. of classes of hypertension drugs | ||||||
| Standard control | 2.4±1.1 | 2.7±1.2 | 3.0±1.4 | 3.6±1.4 | 3.9±1.6 | 3.9±1.5 |
| Intensive control | 2.4±1.2 | 3.5±1.1 | 3.6±1.1 | 3.7±1.4 | 3.8±1.3 | 4.2±1.3 |
Plus–minus values are means ±SD. ACE denotes angiotensin-converting enzyme, and ARB angiotensin-receptor blocker.
Table 3.
Event Rates for Primary and Secondary Outcomes, According to Study Phase and Proteinuria Status at Baseline.*
| Variable | Intensive Control | Standard Control | Hazard Ratio (95% CI) |
P Value | ||
|---|---|---|---|---|---|---|
| no./total no. |
rate per 100 person-yr |
no./total no. |
rate per 100 person-yr |
|||
| All patients | ||||||
| Doubling of serum creatinine level, ESRD, or death | ||||||
| Trial phase | 159/540 | 7.0 | 169/554 | 7.3 | 0.88 (0.71–1.09) | 0.24 |
| Cohort phase | 123/377 | 7.9 | 116/382 | 7.7 | 0.95 (0.74–1.23) | 0.70 |
| Both phases | 282/540 | 7.3 | 285/554 | 7.5 | 0.91 (0.77–1.08) | 0.27 |
| Doubling of serum creatinine level or ESRD | ||||||
| Trial phase | 121/540 | 5.3 | 125/554 | 5.4 | 0.91 (0.71–1.18) | 0.49 |
| Cohort phase | 92/377 | 5.9 | 84/382 | 5.5 | 0.99 (0.73–1.33) | 0.95 |
| Both phases | 213/540 | 5.5 | 209/554 | 5.5 | 0.95 (0.78–1.15) | 0.59 |
| ESRD or death | ||||||
| Trial phase | 124/540 | 5.3 | 140/554 | 5.9 | 0.84 (0.66–1.07) | 0.16 |
| Cohort phase | 114/412 | 6.4 | 116/411 | 6.9 | 0.86 (0.67–1.12) | 0.27 |
| Both phases | 238/540 | 5.8 | 256/554 | 6.3 | 0.85 (0.71–1.02) | 0.08 |
| Patients with baseline urinary protein-to-creatinine ratio ≤0.22 | ||||||
| Doubling of serum creatinine level, ESRD, or death | ||||||
| Trial phase | 64/357 | 4.0 | 61/376 | 3.6 | 1.14 (0.80–1.63) | 0.46 |
| Cohort phase | 81/290 | 6.5 | 74/312 | 5.6 | 1.21 (0.88–1.66) | 0.24 |
| Both phases | 145/357 | 5.1 | 135/376 | 4.5 | 1.18 (0.93–1.50) | 0.16 |
| Doubling of serum creatinine level or ESRD | ||||||
| Trial phase | 42/357 | 2.6 | 34/376 | 2.0 | 1.44 (0.91–2.26) | 0.12 |
| Cohort phase | 56/290 | 4.5 | 49/312 | 3.7 | 1.36 (0.92–2.00) | 0.12 |
| Both phases | 98/357 | 3.4 | 83/376 | 2.7 | 1.39 (1.04–1.87) | 0.03 |
| ESRD or death | ||||||
| Trial phase | 47/357 | 2.9 | 50/376 | 2.9 | 0.98 (0.66–1.47) | 0.94 |
| Cohort phase | 72/307 | 5.2 | 62/323 | 4.3 | 1.22 (0.87–1.72) | 0.25 |
| Both phases | 119/357 | 3.9 | 112/376 | 3.6 | 1.12 (0.87–1.45) | 0.39 |
| Patients with baseline urinary protein-to-creatinine ratio >0.22 | ||||||
| Doubling of serum creatinine level, ESRD, or death | ||||||
| Trial phase | 94/181 | 13.9 | 108/176 | 18.3 | 0.74 (0.56–0.99) | 0.04 |
| Cohort phase | 42/86 | 13.7 | 41/68 | 21.1 | 0.66 (0.43–1.03) | 0.07 |
| Both phases | 136/181 | 13.8 | 149/176 | 19.0 | 0.73 (0.58–0.93) | 0.01 |
| Doubling of serum creatinine level or ESRD | ||||||
| Trial phase | 78/181 | 11.5 | 91/176 | 15.4 | 0.76 (0.55–1.04) | 0.08 |
| Cohort phase | 36/86 | 11.8 | 35/68 | 18.0 | 0.68 (0.43–1.09) | 0.11 |
| Both phases | 114/181 | 11.6 | 126/176 | 16.1 | 0.76 (0.58–0.99) | 0.04 |
| ESRD or death | ||||||
| Trial phase | 76/181 | 10.6 | 90/176 | 14.3 | 0.76 (0.56–1.04) | 0.09 |
| Cohort phase | 42/104 | 11.0 | 53/86 | 20.3 | 0.55 (0.37–0.84) | 0.005 |
| Both phases | 118/181 | 10.8 | 143/176 | 16.1 | 0.67 (0.52–0.87) | 0.002 |
Hazard ratios and P values are for the comparison between the intensive-control group and the standard-control group. Hazard ratios have been adjusted for five prespecified baseline factors (log-transformed urinary protein excretion, age, sex, presence or absence of a history of heart disease, and baseline mean arterial pressure), along with a linear interaction term between the log-transformed baseline urinary protein-to-creatinine ratio and follow-up time. ESRD denotes end-stage renal disease.
KIDNEY DISEASE PROGRESSION
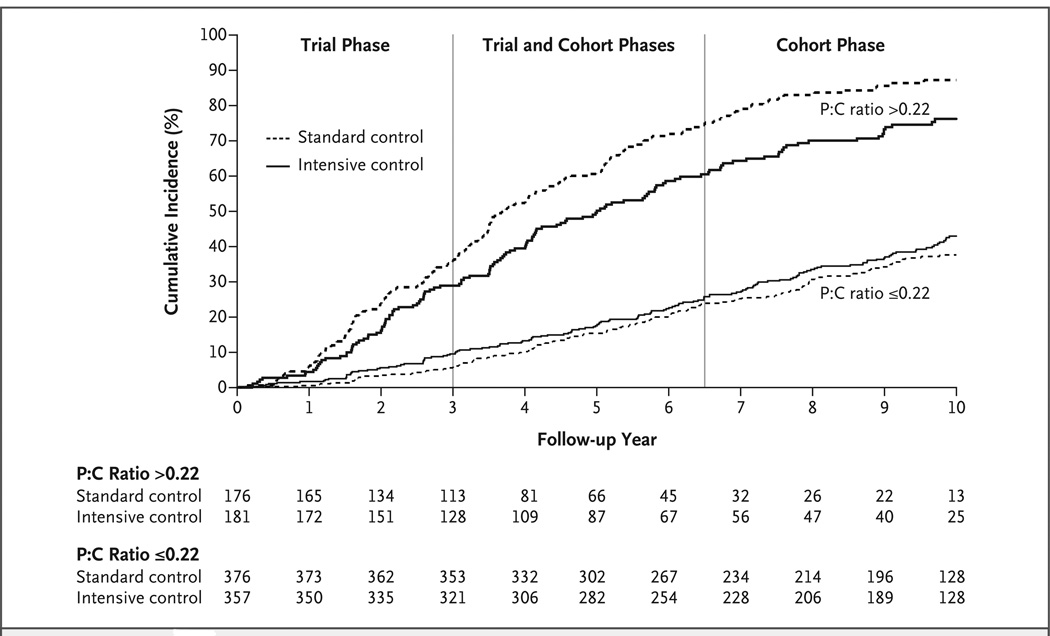
Among all patients and across both phases of the study, there was no significant difference between the intensive-control group and the standard-control group in the primary outcome (hazard ratio in the intensive- control group, 0.91; 95% confidence interval [CI], 0.77 to 1.08; P = 0.27) or secondary outcomes (Table 3). However, the effects of the randomized blood-pressure target differed according to the baseline protein-to-creatinine ratio. Across the entire study, there was a significant interaction between the randomized blood-pressure group and the log-transformed protein-to-creatinine ratio for the primary outcome (P = 0.02 for interaction), the outcome of a doubling of the serum creatinine level or ESRD (P = 0.007 for interaction), and the outcome of ESRD or death (P = 0.02 for interaction). Among patients with a baseline protein-to-creatinine ratio of 0.22 or less, there was no significant between-group difference in the primary outcome (hazard ratio, 1.18; 95% CI, 0.93 to 1.50; P = 0.16), and there was an inconsistent pattern for the outcome of a doubling of the serum creatinine level or ESRD (hazard ratio, 1.39; 95% CI, 1.04 to 1.87; P = 0.03) and the outcome of ESRD or death (hazard ratio, 1.1 2; 95% CI, 0. 87 to 1.45; P = 0.39) (Table 3 and Fig. 2).
Figure 2. Cumulative Incidence of the Composite Primary Outcome, According to Baseline Proteinuria Status.
Among patients with baseline proteinuria, which was defined as a urinary protein-to-creatinine ratio (P:C) of more than 0.22, those who received intensive blood-pressure control had a significantly lower cumulative incidence of the composite primary outcome (a doubling of the serum creatinine level, end-stage renal disease, or death) than those who received standard blood-pressure control (hazard ratio in the intensive-control group, 0.73; 95% confidence interval [CI], 0.58 to 0.93; P = 0.01). However, the between-group difference was not significant among patients with a P:C of 0.22 or less (hazard ratio, 1.18; 95% CI, 0.93 to 1.50; P = 0.16). The values at the bottom of the graph are numbers of patients.
Among patients with a protein-to-creatinine ratio of more than 0.22, those in the intensive-control group had a significant reduction in the risk of the primary outcome (hazard ratio, 0.73; 95% CI, 0.58 to 0.93; P = 0.01) and for the two secondary outcomes: a doubling of the serum creatinine level or ESRD (hazard ratio, 0.76; 95% CI, 0.58 to 0.99; P = 0.04) and ESRD or death (hazard ratio, 0.67; 95% CI, 0.52 to 0.87; P = 0.002). Among patients with a protein-to-creatinine ratio of more than 0.22, the hazard ratio for the primary outcome was 0.74 during the trial phase and 0.66 during the cohort phase. (For additional outcomes, see Tables 4, 5, and 6 in the Supplementary Appendix.)
DISCUSSION
With extended follow-up of patients who were randomly assigned to two different blood-pressure targets, we found that the rate of progression of chronic kidney disease did not differ significantly between the two groups. However, results differed according to the level of the baseline protein-to-creatinine ratio. In patients with a protein-to-creatinine ratio of more than 0.22, intensive blood-pressure control significantly retarded disease progression, according to the primary outcome (a doubling of the serum creatinine level, ESRD, or death) as well as outcomes directly related to chronic kidney disease (a doubling of the serum creatinine level or ESRD) and clinical outcomes (ESRD or death). Intensive blood-pressure control had no significant or consistent effect among patients with a protein-to-creatinine ratio of 0.22 or less. Throughout the trial and cohort phases, the use of medications that block the renin–angiotensin system was similar in the two blood-pressure groups. Hence, it is unlikely that confounding with the use of renoprotective medication accounted for the beneficial effect of intensive blood-pressure control.
Few trials have tested the effects of a reduced blood-pressure target on the progression of Modification of Diet in Renal Disease (MDRD) trial compared the effects of a lower blood-pressure target (mean arterial pressure, <92 mm Hg) to a standard target (mean arterial pressure, <107 mm Hg) in adults with nondiabetic kidney disease from a variety of conditions. During the initial phase of the MDRD trial, assignment to the lower blood-pressure target had no significant effect on the mean change in the GFR during a 3-year period.4 However, subgroup analyses suggested that assignment to the lower target retarded the progression of chronic kidney disease in patients with an increased severity of proteinuria (>1 g per day, with a mean of 2.8 g per day).19 During extended follow-up, patients in the intensive-control group had a reduced risk of ESRD, as compared with the standard-control group.20 However, because patients in the intensive-control group were more likely to have received ACE inhibitors than those in the standard-control group, the benefit might have resulted from a renoprotective effect of ACE inhibition. A recent trial documented beneficial effects of strict blood-pressure control among children with chronic kidney disease in whom the mean protein-to-creatinine ratio at baseline was relatively high (approximately 1.3).7 Hence, in these two trials in which a benefit of a lower blood-pressure target was documented, levels of proteinuria were considerably higher than the corresponding levels among patients in our trial, including those with a protein-to-creatinine ratio of more than 0.22.
The long-term results of the AASK study should be viewed in the context of the findings in the trial phase alone. During the trial phase among all patients, there was no significant benefit of intensive blood-pressure control, as compared with standard control, on the primary GFR outcomes or the clinical composite outcomes, each based on 125I-iothalamate clearance.5 Still, some analyses suggested that intensive blood-pressure control might be beneficial in patients with baseline proteinuria. Specifically, for the primary composite outcome, there was a significant interaction between baseline proteinuria and blood-pressure target (P = 0.02 for interaction). Nonetheless, because the effects of intensive blood-pressure control did not achieve significance in either proteinuria stratum, subgroup results were deemed inconclusive.5
An important issue is time course. It is difficult to precisely identify the onset of a beneficial effect from intensive blood-pressure control in patients with baseline proteinuria. Nonetheless, a benefit appears to have emerged during the trial phase and persisted into the cohort phase. This was evident from the Kaplan–Meier plots, in which curves appeared to separate after approximately 1 year, and from the pattern of hazard ratios (0.74 during the trial phase and 0.66 during the cohort phase).
An unexpected finding was the lack of benefit of the lower target in patients with a baseline protein-to-creatinine ratio of 0.22 or less. Insufficient statistical power is an unlikely explanation. In both the trial and cohort phases, the number of outcomes was similar in the two proteinuria strata: 280 events in patients with a protein-to-creatinine ratio of 0.22 or less and 285 in those with a protein-to-creatinine ratio of more than 0.22. Also, there was no suggestion of a benefit among patients with a protein-to-creatinine ratio of 0.22 or less across both phases (hazard ratio, 1.18; 95% CI, 0.93 to 1.50; P = 0.16). Although a physiological basis for the absence of a protective effect in the subgroup without proteinuria is uncertain, empirical data support the notion that renoprotective therapies, such as ACE inhibitors, are most effective in patients with proteinuria and least effective, or possibly ineffective, in patients without diabetes or proteinuria.6,19–22 In some observational studies 23–25 and in observational analyses of trial data,25 low levels of attained blood pressure have been associated with adverse health outcomes. However, the relationship does not appear to be causal, because among patients with chronic kidney disease or ESRD, a low rate of achievement of the blood-pressure target is often confounded by indicators of poor health.23,26 Overall, it is hard to develop a coherent, biologically plausible argument for a qualitative interaction between harm in patients without proteinuria and benefit in those with proteinuria.
Our study has several strengths, including its focus on an understudied population at high risk for the progression of chronic kidney disease,16 the long duration of follow-up, a high reenrollment rate in the cohort phase, a large and sustained difference in blood pressure between the two randomized groups during the trial phase, the similar use of renoprotective antihypertensive drug therapies in the two blood-pressure groups, and the availability of data on potential confounders, including blood-pressure medications. However, our study also has several limitations. The cohort phase was not a randomized trial, since all the patients had the same blood-pressure target. Still, despite a convergence of blood-pressure levels in the intensive-control and standard-control groups during the cohort phase, a benefit of an intensive blood-pressure target emerged in patients with proteinuria. Second, adjustment of therapy was based on blood pressure as assessed by standard office readings, not on ambulatory blood pressure. We recently documented that elevated nocturnal blood pressure was common among patients in the cohort phase.27 Third, the effect modifier that accounted for our observed findings might not be the presence of proteinuria at baseline but instead might be a variable closely related to it.28 However, we tested for interactions between the blood-pressure target group and three variables (smoking, obesity, and GFR), and none of the interactions were significant (data not shown). Finally, significant subgroup results should be interpreted cautiously, given the potential for chance findings even when the subgroup is pre-specified.29 Nevertheless, our finding that a lower blood-pressure target significantly retarded the progression of chronic kidney disease among patients with proteinuria is consistent with the results of other studies.7,19
In conclusion, although guidelines have recommended a more intensive blood-pressure goal in patients with hypertensive chronic kidney disease,9–12 trial evidence in support of such recommendations is sparse. Subgroup analyses from our study suggest that a lower blood-pressure target may retard disease progression in some patients with hypertensive chronic kidney disease, but the evidence for this benefit is limited to patients with a protein-to-creatinine ratio of more than 0.22 at baseline.
Supplementary Material
Acknowledgments
Supported by grants to each clinical center and the coordinating center from the National Institute of Diabetes and Digestive and Kidney Diseases; by the Office of Research in Minority Health (now the National Center on Minority Health and Health Disparities); by institutional grants from the National Institutes of Health (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, and DK 2818-02); by King Pharmaceuticals, which provided monetary support and antihypertensive medications to each clinical center; and by Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn, which donated antihypertensive medications.
APPENDIX
The authors’ affiliations are as follows: Welch Center for Prevention, Epidemiology, and Clinical Research (L.J.A., B.C.A., E.R.M.), and Johns Hopkins ProHealth (J.C.), Johns Hopkins Medical Institutions, Baltimore; the Department of Medicine, Case Western Reserve University (J.T.W.), and the Department of Quantitative Health Sciences, Cleveland Clinic Foundation (J.J.G., X.W.) — both in Cleveland; the Division of Clinical Epidemiology, University of Utah School of Medicine, Salt Lake City (T.G.); the Division of Kidney, Urologic and Hematologic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD (L.Y.A., J.W.K.); the Hypertensive Diseases Unit Department of Medicine, University of Chicago Pritzker School of Medicine (G.L.B.), and the Department of Medicine, University of Illinois at Chicago (J.P. Lash) — both in Chicago; the Multidisciplinary Research Center, Morehouse School of Medicine (W.H.C.), and Emory Center for Hypertension and Renal Disease Research, Emory University School of Medicine (J.P. Lea) — both in Atlanta; the Department of Medicine, University of Miami Miller School of Medicine, Miami (G.C.); Meharry Medical College (M.L.F.) and the Division of Nephrology, Vanderbilt University School of Medicine (J.B.L.) — both in Nashville; the Veterans Affairs San Diego Healthcare System and Department of Medicine, University of California, San Diego (F.R.G.); the Division of Nephrology, Ohio State University Medical Center, Columbus (L.A.H.); the Division of Cardiovascular Medicine, University of Michigan, Ann Arbor (K.A.J.); Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center and the University of California at Los Angeles (J.D.K.), Los Angeles County–University of Southern California Medical Center and the University of Southern California Keck School of Medicine (S.G.M.), the Department of Internal Medicine, Charles Drew University of Medicine and Science (K.N.), and the Division of Nephrology, University of Southern California Keck School of Medicine (M.J.S.) — all in Los Angeles; the Department of Medicine, Mount Sinai School of Medicine (M.S.L.), and the Department of Medicine, Harlem Hospital Center and Columbia University (V.A.P.) — both in New York; the Cardiovascular Research Foundation, University of Massachusetts Medical School, Worcester (R.A.P.); Howard University and Howard University Hospital, Washington, DC (O.R.); the Division of Nephrology, University of Alabama at Birmingham, Birmingham (S.G.R.); and the Department of Medicine, University of Texas Southwestern Medical Center, Dallas (R.D.T.).
The following persons (listed according to center) participated in the AASK study (asterisks indicate principal investigators, and daggers study coordinators): Case Western Reserve University — J.T. Wright, Jr.,* M. Rahman, R. Dancie,† L. Strauss; Emory University — J. Lea,* B. Wilkening,† A. Chapman, D. Watkins; Harbor–UCLA Medical Center — J.D. Kopple,* L. Miladinovich,† J. Choi, P. Oleskie, C. Secules; Harlem Hospital Center — V. Pogue,* D. Dowie,† H. Anderson, L. Herbert, R. Locko, H. Nurse, J.-T. Cheng, F. Darkwa, V. Dowdy, B. Nicholas; Howard University — O. Randall,* T. Retta, S. Xu,† M. Ketete, D. Ordor, C. Tilghman; Johns Hopkins University — E. Miller,* B. Astor, C. Diggs,† J. Charleston, C. Harris, T. Shields Lawrence Appel (steering committee); Charles R. Drew University — K. Norris,* D. Martins, M. Miller,† H. Howell, L. Pitts; Medical University of South Carolina — D. Cheek,* D. Brooks†; Meharry Medical College — M. Faulkner,* O. Adeyele, K. Phillips,† G. Sanford, C. Weaver; Morehouse School of Medicine — W. Cleveland,* K. Chapman, W. Smith,† S. Glover; Mount Sinai School of Medicine and University of Massachusetts — R. Phillips,* M. Lipkowitz, M. Rafey, A. Gabriel,† E. Condren, N. Coke; Ohio State University — L. Hebert,* G. Shidham, L. Hiremath,† S. Justice; University of Chicago, Chicago — G. Bakris,* J. Lash, L. Fondren,† L. Bagnuolo, J. Cohan, A. Frydrych; University of Alabama, Birmingham — S. Rostand,* D. Thornley-Brown, B. Key†; University of California, San Diego — F.B. Gabbai,* D.T. O’Connor, B. Thomas†; University of Florida — C.C. Tisher,* G. Bichier, C. Sarmiento,† A. Diaz, C. Gordon; University of Miami — G. Contreras,* J. Bourgoignie, D. Florence-Green, J. Junco,† J. Vassallo; University of Michigan — K. Jamerson,* A. Ojo, T. Corbin, D. Cornish-Zirker,† T. Graham, W. Bloembergen; University of Southern California — S. Massry,* M. Smogorzewski, A. Richardson,† L. Pitts; University of Texas Southwestern Medical Center, Dallas – R. Toto,* G. Peterson, R. Saxena, T. Lightfoot,† S.-A. Blackstone, C. Loreto; Vanderbilt University — J. Lewis,* G. Schulman, M. Sika,† S. McLeroy; National Institute of Diabetes and Digestive and Kidney Diseases — L.Y. Agodoa, J.P. Briggs, J.W. Kusek; Data Coordinating Center (Cleveland Clinic Foundation) — J. Gassman,* G. Beck, T. Greene, B. Hu, K. Brittain,† S. Sherer, L. Tuason, C. Kendrick, S. Bi, H. Litowitz, X. Liu, X. Wang, K. Wiggins, C.A. Tatum, N. Patterson; Central Biochemistry Laboratory — F. Van Lente, J. Waletzky, C. O’Laughlin, L. Burton; Scientific Advisory Committee — W. McClellan, L. Adams-Campbell, K. Faber-Langendoen, B. Kiberd, E. Lee, T. Meyer, D. Nathan, J. Stokes, H. Taylor, P.W. Wilson; Cardiovascular Research Foundation — T. deBacker, A. Lansky, S. Slack.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System. Atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 3.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. Endstage renal disease in African-American and white men: 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 5.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihy pertensive dr ug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [Erratum, JAMA 2006;295:2726.] [DOI] [PubMed] [Google Scholar]
- 6.Jafar TH, Stark P, Schmid C, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 7.The ESCAPE Trial Group. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 8.Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database Syst Rev. 2009;3:CD004349. doi: 10.1002/14651858.CD004349.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevent ion, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Kidney Diseases Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and anti-hypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(Suppl 1):S1–S290. [PubMed] [Google Scholar]
- 11.Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2007;16:135–232. doi: 10.1080/08037050701461084. [DOI] [PubMed] [Google Scholar]
- 12.Padwal RS, Hemmelgarn BR, Khan NA, et al. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 1 — blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2009;25:279–286. doi: 10.1016/s0828-282x(09)70491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 14.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(Suppl 2):S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appel LJ, Middleton J, Miller ERIII, et al. The rationale and design of the AASK Cohort Study. J Am Soc Nephrol. 2003;14(Suppl 2):S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Wright JT, Jr, Greene T, et al. Long-term effects of renin-angiotensin system-blocking therapy and a low blood Pressure goalon progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168:832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 18.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Berlin: Springer-Verlag; 1997. [Google Scholar]
- 19.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: a meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [Erratum, Ann Intern Med 2002;137:299.] [DOI] [PubMed] [Google Scholar]
- 22.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting- enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 23.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:830–837. doi: 10.2215/CJN.06201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 26.Coresh J, Longenecker JC, Miller ERIII, Young HJ, Klag MJ. Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9(Suppl):S24–S30. [PubMed] [Google Scholar]
- 27.Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- 28.Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, L agakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.