Abstract
OBJECTIVES
To evaluate whether education level is associated with change in cognitive performance.
DESIGN
Prospective cohort study.
SETTING
The Atherosclerosis Risk in Communities (ARIC) Study, a community-based cohort.
PARTICIPANTS
Nine thousand two hundred sixty-eight ARIC participants who underwent cognitive evaluation at least twice over a 15-year period.
MEASUREMENTS
Education was evaluated as a predictor of change in word recall, the Digit Symbol Substitution Test (DSST), and word fluency. A random-effects linear regression model, and a time by educational level interaction was used.
RESULTS
Educational level was highly associated with cognitive performance. The effect on performance of a less than high school education (vs more than high school) was equivalent to the effect of as much as 22 years of cognitive aging, but educational level was not associated with change in cognitive performance in whites or blacks, with the exception of the DSST for whites, in whom those with lower levels of education had less decline in scores.
CONCLUSION
Educational level was not associated with change in cognitive performance, although the higher baseline cognitive performance of individuals with more education might explain lower rates of dementia in more-educated individuals, because more decline would have to take place between baseline higher performance and time at which dementia was diagnosed in more-educated individuals.
Keywords: education, cognition, cognitive reserve
Higher education levels have been strongly associated with lower dementia risk and better cognitive performance in older persons on a variety of standard tests.1–3 There are two possible interpretations; persons with more education must decline further in cognitive performance before it is low enough to merit a dementia diagnosis or are at less risk of cognitive decline.
Recent reviews generally suggest that higher education is indeed associated with less cognitive decline,4,5 despite some conflicting evidence.6 The associations between education and dementia incidence and cognitive change have implications regarding the concept of “cognitive reserve,” which “preserves cognitive function in the face of Alzheimer’s pathology.”7 The observed associations raise the question as to whether education, or even stimulating cognitive activities, improves neural networks or increases synaptic density, enhancing the ability to compensate for neuropathological changes (e.g., from amyloid plaque deposition or cerebrovascular disease) that would otherwise promote cognitive impairment or dementia.
A difficulty in interpreting the literature on this topic is that prior studies have had major biases. Many studies showing that higher education is associated with less cognitive decline adjusted for baseline test scores in their analyses, but this adjustment biases results to favor that finding.8 Other studies used screening measures of global cognitive function, such as the Mini-Mental State Examination (MMSE), which have known “ceiling effects” (are insensitive to differences in cognition at higher levels of performance). Studies limited by these biases generally have shown less cognitive decline in persons with higher education. More-recent studies that have avoided these biases have not demonstrated a similar association.9–12
Previous studies have also generally not adjusted for important medical factors such as diabetes mellitus and hypertension. Unadjusted associations may not be attributable to education itself but to the association between low education levels and more risk factors for vascular disease. Adjusting for vascular risk factors provides an estimate of the direct cognitive effects of education. Direct effects may indicate the important ways in which more education or perhaps the continuing cognitive activities associated with it may preserve cognitive abilities in persons whose brains contain vascular or Alzheimer’s-type neuropathology.
This study analyzed the association between educational attainment and cognitive change in the Atherosclerosis Risk in Communities (ARIC) Study from 1990 to 2006. ARIC provides an ideal setting to examine this association while avoiding some of the biases present in prior studies. ARIC is one of the largest community-based studies with cognitive tests performed at several time points, and the cognitive tests studied are known to have less “ceiling effect” than the MMSE or other screening instruments. Rigorous measurement of vascular risk factors allowed these factors to be adjusted for, and, as recommended, the analyses herein were not adjusted for baseline scores. Three cognitive domains are included in the ARIC cognitive battery, and assessments took place in black and white participants in midlife, whereas other studies of education and cognitive change have been primarily limited to Caucasian populations and cognitive assessments later in life.9 It was hypothesized that higher level of educational attainment would be associated with less decline in scores on the cognitive tests that were repeated throughout ARIC.
METHODS
Study Population
Atherosclerosis Risk in Communities is an ongoing community-based prospective cohort of 15,792 middle-aged adults from four U.S. communities: Washington County, Maryland; Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; and Jackson, Mississippi. A flow diagram of ARIC is shown in Figure 1. Cognitive performance was assessed at Visit 2 (1990–1992), Visit 3 (1993– 1995), Visit 4 (1996–1998), the carotid magnetic resonance imaging (MRI) visit (2004–2006), and the brain MRI visit (2004–2006). All ARIC participants underwent cognitive testing at Visits 2 and 4, whereas only a subsample of the cohort was invited to undergo cognitive testing at Visit 3 and at the brain and carotid MRI visits. The selection for the Visit 3, brain MRI, and carotid MRI visits are described in detail elsewhere.13–15 The Visit 3 brain MRI substudy (n = 1,920) included a random sample of all participants aged 55 and older from Forsyth County and Jackson.14 These participants were invited for another assessment in 2004 to 2006 (the brain MRI visit, n = 1,130).13 The carotid MRI visit (n = 2,066) used a stratified sampling plan, oversampling carotid intimal medial thickness (IMT) to increase the prevalence of larger plaques. 15 The selection factors for the two subsamples (carotid IMT, study center, and age) and the other factors related to follow-up success were adjusted for. Because of the overlap in time for the two MRI visits and the potential for practice effects for participants in both MRI subsamples, the two MRI visits were combined into one visit, using only the first cognitive test scores for participants who were in both MRI visit subsamples. Therefore, we have cognitive assessments at four time points, and Visit 2 was considered baseline. Change in cognition was examined over the 15-year follow-up period.
Figure 1.
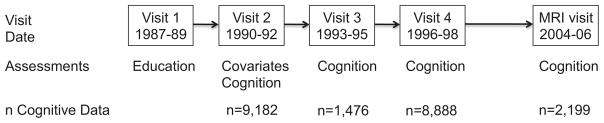
Atherosclerosis Risk in Communities Study flow diagram. MRI = magnetic resonance imaging.
Of the 14,348 participants who attended the baseline visit, 42 who self-identified as other than white or black and 667 with neurological conditions that might affect their cognitive performance at any time during follow-up (stroke, n = 488; dementia, n = 189; multiple sclerosis, n = 1; Parkinson’s disease, n = 1; brain tumor, n = 5; head surgery, n = 10; head radiation, n = 3; and other neurologic disorder, n = 8, with some overlapping) were excluded, leaving 13,639 as the population of interest. The cognitive data from individual visits were excluded if the participant was taking medications at that time that might affect cognitive performance (anxiolytics, hypnotics and sedatives, antipsychotics, anticonvulsants, Parkinson’s disease medications, and dementia medications). Of the 13,639 participants in the population of interest, 3,998 who did not undergo two or more cognitive assessments and 373 who were missing education status or needed covariate data were excluded, leaving a study population of 9,268 (68.0% of the population of interest). The number of these with cognitive assessment at each visit was 9,182 at Visit 2, 1,476 at Visit 3, 8,888 at Visit 4, and 2,199 at MRI visit (Figure 1). Six thousand seven hundred twenty-three participants underwent cognitive testing at two timepoints, 1,878 at three timepoints, and 667 at four timepoints.
Education
Education, assessed at Visit 1 as the highest grade completed in school, was categorized as less than high school completion, high school degree or vocational school, or more than high school (attending or completed college or professional school).
Measures of Cognitive Function
Three standard tests were used to assess cognitive performance: delayed word recall (DWRT), Digit Symbol Substitution Test (DSST) from the Wechsler Adult Intelligence Scale—Revised, and word fluency test (WFT), also known as the Controlled Oral Word Association test, from the Multilingual Aphasia Examination. Identical protocols were used at all visits. Trained examiners administered the tests in a fixed order during one session in a quiet room. Examiner performance was recorded on audiotapes, which were reviewed routinely to ensure consistency.
The DWRT is a test of verbal learning and recent memory. The participant is asked to learn 10 common nouns by using each word in two sentences. After 5 minutes, the participant is asked to recall the nouns. The score is the number of nouns correctly recalled.16
The DSST is a test of executive function and processing speed. The participant translates numbers to symbols using a key. The score is the number of numbers correctly translated within 90 seconds, and the range of possible scores is 0 to 93.17
The WFT is a test of executive function and expressive language. The test includes three 1-minute trials in which the participant is asked to generate words beginning with a particular letter, not including proper names or places. The three letters used were F, A, and S. The score is the total number of correct words generated in 60 seconds.18
Covariates
Covariates included in the regression models were assessed at baseline (1990–1992). Covariates were age, sex, study site, cigarette smoking (never, former, current), diabetes mellitus (defined as fasting glucose ≥126 mg/dL, self-reported history of diabetes mellitus diagnosis, or use of diabetes mellitus medication), hypertension (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of hypertension medication), apolipoprotein (APO) E ε4 genotype (determinant of cognitive performance,19 defined using the single-nucleotide polymorphisms rs429358 and rs7412 and coded as having 0, 1, or 2 ε4 alleles), and carotid IMT.
Statistical Analysis
Baseline means and proportions for participant characteristics were calculated separately according to race and education level. To estimate the association between education level and rate of cognitive decline, race-stratified random-effects (random intercept only) linear regression models for repeated measures were used, which take into account the intraindividual correlation of scores on repeated cognitive tests. The models included educational level, follow-up time, interaction terms between education and follow-up time, age, sex, study site, cigarette smoking status, diabetes mellitus, hypertension, APOE genotype, and carotid IMT.
More than high school degree was the reference education level. The less than high school and high school coefficients in the models reflect the differences in baseline cognitive test score between having less than or equal to a high school education and having more than a high school education, adjusted for other variables in the model. The education group-by-follow-up time interaction terms, the coefficients of interest, test the null hypothesis of no difference in cognitive test score change over time between education groups.
To determine whether the associations differed in older participants, the models were restricted to include only the test scores of the 1,958 participants who were tested on two or more occasions after they had reached age 65. In addition, to reduce the effect of possible floor effects, analyses were repeated after excluding those with the lowest 5% of baseline scores in each race category. Regression models were also used to compare baseline cognitive test scores between individuals seen in 2004 to 2006 and those seen only at the earlier visits.
All P-values are two-sided, and P < .05 was considered statistically significant. Analyses used Stata Version 11 S (StataCorp., College Station, TX).20
RESULTS
Characteristics of Participants
Characteristics of the study population are displayed in Table 1. Women comprised the majority (54%). Approximately 20% were black and 80% were white. Unadjusted cognitive test scores were lowest for individuals with lower educational attainment and highest for individuals with more than a high school education, for all tests and all visits.
Table 1.
Characteristics of Participants Overall and Stratified According to Race and Educational Level
| Characteristic | All Participants (n = 9,268) | Whites
|
Blacks
|
||||
|---|---|---|---|---|---|---|---|
| <High School (n = 1,005) | High School or Equivalent (n = 3,374) | >High School (n = 3,043) | <High School (n = 612) | High School or Equivalent (n = 535) | >High School (n = 699) | ||
| Age, mean ± SD | 56.7 ± 5.6 | 59.1 ± 5.4 | 56.9 ± 5.5 | 56.4 ± 5.6 | 57.6 ± 5.6 | 55.3 ± 5.5 | 54.6 ± 5.3 |
|
| |||||||
| Female, n (%) | 4,968 (53.6) | 470 (46.8) | 1,932 (57.3) | 1,386 (57.3) | 402 (65.7) | 351 (65.6) | 427 (61.1) |
|
| |||||||
| Hypertension, n (%) | 2,903 (31.4) | 323 (32.2) | 954 (28.3) | 723 (23.8) | 336 (55.5) | 256 (48.0) | 311 (44.8) |
|
| |||||||
| Diabetes mellitus, n (%) | 1,135 (12.3) | 161 (16.1) | 348 (10.3) | 246 (8.1) | 150 (24.6) | 114 (21.4) | 116 (16.7) |
|
| |||||||
| Cigarette smoking status | |||||||
|
| |||||||
| Current, n (%) | 1,798 (19.4) | 246 (24.5) | 657 (19.5) | 481 (15.8) | 169 (27.6) | 119 (22.2) | 126 (18.0) |
|
| |||||||
| Former, n (%) | 3,590 (38.7) | 399 (39.7) | 1,346 (39.9) | 1,304 (42.9) | 191 (31.2) | 136 (25.4) | 214 (30.6) |
|
| |||||||
| Never, n (%) | 3,880 (41.9) | 360 (35.8) | 1,371 (40.6) | 1,258 (41.3) | 252 (41.2) | 280 (52.3) | 359 (51.4) |
|
| |||||||
| Number of apolipoprotein E ε4 alleles, n (%) | |||||||
|
| |||||||
| 0 | 6,164 (69.3) | 725 (74.7) | 2,326 (72.1) | 2,054 (70.6) | 331 (55.7) | 310 (59.7) | 418 (61.3) |
|
| |||||||
| 1 | 2,512 (28.2) | 225 (23.2) | 839 (26.0) | 798 (27.4) | 241 (40.6) | 176 (33.9) | 233 (34.2) |
|
| |||||||
| 2 | 224 (2.5) | 20 (2.1) | 62 (1.9) | 56 (1.9) | 22 (3.7) | 33 (6.4) | 31 (4.6) |
|
| |||||||
| Carotid intimal medial thickness, mm, mean ± SD | 0.73 ± 0.18 | 0.77 ± 0.21 | 0.73 ± 0.18 | 0.71 ± 0.17 | 0.77 ± 0.20 | 0.73 ± 0.16 | 0.72 ± 0.14 |
|
| |||||||
| Visit 2 cognitive test scores, mean ± SD | |||||||
|
| |||||||
| Delayed word recall test (n = 9,167) | 6.73 ± 1.47 | 6.26 ± 1.40 | 6.83 ± 1.40 | 7.05 ± 1.39 | 5.76 ± 1.62 | 6.37 ± 1.48 | 6.68 ± 1.47 |
|
| |||||||
| Digit Symbol Substitution Test (n = 9,166) | 46.6 ± 13.4 | 39.8 ± 9.8 | 49.5 ± 10.4 | 53.8 ± 10.1 | 23.7 ± 9.4 | 33.1 ± 10.6 | 41.6 ± 11.9 |
|
| |||||||
| Word fluency test (n = 9,166) | 34.2 ± 12.2 | 27.1 ± 10.5 | 33.5 ± 10.5 | 40.2 ± 11.3 | 20.5 ± 9.9 | 28.5 ± 10.2 | 38.3 ± 11.6 |
|
| |||||||
| Visit 4 cognitive test scores, mean ± SD | |||||||
|
| |||||||
| Delayed word recall test (n = 8,888) | 6.61 ± 1.58 | 6.11 ± 1.54 | 6.71 ± 1.50 | 6.94 ± 1.50 | 5.54 ± 1.73 | 6.14 ± 1.60 | 6.57 ± 1.56 |
|
| |||||||
| Digit Symbol Substitution Test (n = 8,882) | 44.4 ± 13.2 | 37.7 ± 10.1 | 46.9 ± 10.5 | 51.1 ± 10.2 | 22.1 ± 10.5 | 31.4 ± 11.3 | 39.5 ± 11.8 |
|
| |||||||
| Word fluency test (n = 8,884) | 33.9 ± 12.5 | 26.8 ± 10.6 | 33.4 ± 10.9 | 39.6 ± 11.5 | 19.8 ± 10.2 | 27.8 ± 11.0 | 36.9 ± 12.3 |
|
| |||||||
| MRI visit cognitive test scores, mean ± SD | |||||||
|
| |||||||
| Delayed word recall test (n = 2,189) | 6.14 ± 1.69 | 5.93 ± 1.53 | 6.30 ± 1.58 | 6.49 ± 1.68 | 5.11 ± 1.75 | 5.70 ± 1.78 | 6.09 ± 1.63 |
|
| |||||||
| Digit Symbol Substitution Test (n = 2,184) | 38.7 ± 12.5 | 33.4 ± 9.7 | 41.5 ± 9.8 | 45.7 ± 10.3 | 21.1 ± 8.7 | 29.4 ± 10.0 | 37.0 ± 11.1 |
|
| |||||||
| Word fluency test (n = 2,191) | 32.7 ± 12.5 | 26.7 ± 10.2 | 32.6 ± 10.8 | 39.0 ± 11.6 | 18.9 ± 8.1 | 26.2 ± 10.7 | 35.9 ± 11.7 |
SD = standard deviation.
Practice Effects
When performance over sequential visits was compared using the 234 individuals who were tested twice in 2004–2006, only the DWRT demonstrated significant improvement, with a difference of 0.53 words (paired t-test P < .0001). Performance on the DSST declined slightly (mean change = −1.99 points, P < .0001), and WFT performance improved minimally (mean change = 0.28 words, P = .5). Based on this evidence of possible practice effect in individuals tested at both the carotid and brain MRI visits, we chose to use the test results from just the earlier of the two 2004–2006 visits.
Baseline Cognition and Cognitive Change
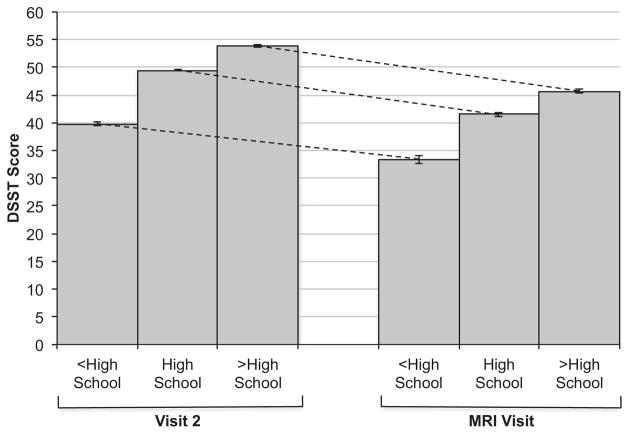
From the fitted model, education level was associated with substantial differences in baseline cognitive scores. For example, on the DSST, white participants with less than a high school education scored 11.84 points lower at baseline, and those with a high school or vocational school education, 4.55 points lower than those with more than a high school education (Table 2). This net difference of 11.84 points is large relative to the mean baseline score of 53.8 points for greater than high school–educated whites (Table 1). That baseline difference in score is large also relative to the statistically significant but small annual decline in score (0.540 per year in whites, Table 2). Education-related differences in baseline score were consistent in direction, significant for all cognitive tests, similar in blacks and whites, and large relative to the modest annual declines in scores for each test. However, neither black nor white participants had significant associations of level of education with change in cognitive performance (Table 2) with one exception: the interaction on the DSST in white participants indicating a slightly smaller decrement in cognitive score per year in individuals with lower educational attainment. Table 2 shows a mean annual change of −0.540 in those with greater than a high school education. The annual change in those with a less than high school education is estimated to be −0.429 (equal to −0.540 +0.111). The small size of the difference in the degree of decline is seen in Figure 2 (using data only from participants who were tested both at visit 2 and the MRI visit) where the dotted lines demonstrate similar slopes across these two visits, despite a significant interaction.
Table 2.
Coefficients for Raw Cognitive Test Scores According to Race, Education Level, and Change over Time
| Factor | Delayed Word Recall Test | Digit Symbol Substitution Test | Word Fluency Test |
|---|---|---|---|
| β (Standard Error) | |||
| Whites, n = 7,422 | |||
| <High school | −0.67 (0.05) b | −11.84 (0.35)b | −12.71 (0.41)b |
| High school or equivalent | −0.28 (0.03)b | −4.55 (0.23)b | −6.92 (0.28)b |
| Time | −0.034 (0.003)b | −0.540 (0.014)b | −0.106 (0.017)b |
| <High school by time interaction | 0.010 (0.007) | 0.111 (0.029)b | 0.013 (0.035) |
| High school or equivalent by time interaction | 0.001 (0.004) | 0.025 (0.019) | 0.044 (0.024) |
| Blacks, n = 1,846 | |||
| <High school | −0.74 (0.08)b | −15.19 (0.56)b | −16.63 (0.61)b |
| High school or equivalent | −0.28 (0.08)a | −7.81 (0.57)b | −9.39 (0.61)b |
| Time | −0.040 (0.006)b | −0.334 (0.031)b | −0.206 (0.030)b |
| <High school by time interaction | −0.014 (0.010) | 0.060 (0.047) | 0.018 (0.045) |
| High school or equivalent by time interaction | −0.014 (0.010) | −0.004 (0.050) | 0.045 (0.047) |
Model is adjusted for age, gender, cigarette smoking, diabetes, hypertension, APOE ε4 genotype, and carotid intimal medial thickness. Reference group is > high school education. Time and interaction terms represent 1-year changes.
P <
001,
.05.
Figure 2.
Mean (95% confidence interval) raw Digit Symbol Substitution Test (DSST) scores for whites at Visit 2 and the magnetic resonance imaging (MRI) visit according to educational level. Figure represents participants who underwent cognitive assessment at Visit 2 and the MRI Visit: <high school (n = 180), high school (n = 714), >high school (n = 650).
Secondary Analyses
A secondary analysis included persons only after they reached age 65. Results were similar to our primary analyses: the only significant education X time interaction term was for the DSST test in whites (P = .0005), indicating, in this smaller population, again slightly reduced decline in individuals with lower levels of education.
An additional secondary analysis excluded individuals with the lowest 5% of baseline test scores within each race category. Changes from our primary analysis were minimal, although the excluded persons appeared to be concentrated largely in the lowest education stratum, and therefore, the decline in that group was steeper (not shown). In addition, blacks with a less than high school education showed a slightly greater decline on the DWRT (β = −0.021, vs −0.014 before the exclusion) than those with greater than a high school education, and this became nominally significant (P = .03).
We saw no evidence that participants followed through 2004–2006 were meaningfully different cognitively than those seen only at the earlier exams. Adjusting for the same covariates as in our final model, baseline scores for those individuals who were seen in 2004–2006 did not differ significantly from the scores of those who were seen only at the earlier visits (participants who were seen in 2004–2006: DWRT whites = 8.34, DWRT blacks = 8.27, DSST whites = 58.9, DSST blacks = 50.6, WFT whites = 24.6, WFT blacks = 22.9; participants who were not seen in 2004–2006: DWRT whites = 8.31, DWRT blacks = 8.12, DSST whites = 59.0, DSST blacks = 49.6, WFT whites = 24.3, WFT blacks = 22.3).We also compared demographic and health-related factors between participants who had two cognitive assessments with those who had three or four assessments (Table S1). Participants with three or four cognitive assessments differed on factors that were used for selection into the Brain MRI or Carotid MRI subsamples (older age, greater carotid IMT) and were more likely to be female and to have less than high school education compared to those with two cognitive assessments, but our analyses adjust for these factors, likely reducing much of the bias associated with them.
DISCUSSION
This analysis of a community-based population demonstrates that level of educational attainment was not associated with decline in cognitive performance over time, in either black or white adults or in adults after attaining the age of 65 years. The only exception was for the DSST test, for which white participants with lower education appeared to have less decline. Our results are consistent with previous studies demonstrating much higher baseline cognitive performance among individuals with higher educational attainment.
The results indicate that the associations between education and baseline cognitive performance are strong, with effect sizes consistently larger than declines associated with the normal aging process. For instance, for whites, on the DSST, the difference in baseline score between individuals with more than a high school education and those with less than a high school education is equivalent to the difference in scores associated with more than a 22-year difference in age (calculated by dividing the coefficient for lower education (−11.84) by the coefficient for 1 year of time (−0.540). It is difficult to imagine any intervention or preventive strategy that could result in a cognitive improvement of this magnitude, although these results must be considered carefully, because it is likely that cultural and many other factors, some of which may not have been adequately adjusted for in the present study, affect baseline score. Nevertheless, less confounding is expected when investigating change than an analysis of baseline score, because many factors that affect cognitive performance (e.g., race, neighborhood, education) are relatively stable over time.
Although not examined here, dementia incidence rates appear to be higher in individuals with less education or with lower cognitive abilities in youth, as reported by many epidemiological studies.1–3 The fact that, in the current study, educational level did not appear to predict cognitive decline suggests that the association between higher education and lower dementia risk may not be due to its association with less cognitive decline. Instead, it may be due to the association between education and “cognitive reserve,” a concept used to explain why individuals with an equivalent degree of Alzheimer’s neuropathology or cerebrovascular injury, for instance, might have different clinical manifestations of disease.21 The results demonstrate that individuals with more education start at a higher cognitive level (substantially higher baseline cognitive scores); they are therefore likely to take much longer to decline enough cognitively to meet a threshold at which point dementia would be diagnosed.
Although the model, which adjusts for smoking, diabetes mellitus, and hypertension, could in theory be adjusting for mediators, it addresses an important question: whether education relates to cognitive change through what might be called “cognitive processes” as opposed to risk factor–mediated vascular processes. In other words, aside from its potential influence on risk factors, is higher education associated with the kind of life that protects persons from cognitive decline because of the intellectual activities they tend to undertake?
If education is not a means by which cognitive decline is actually slowed, techniques and approaches to improve knowledge and provide cognitive stimulation (e.g., an active social life or even performing crossword puzzles) may have no effect on rate of cognitive decline. Although the data do not specifically support or negate any effects of these forms of cognitive stimulation, it could be hypothesized that these activities might improve cognitive performance at the time they are undertaken and therefore increase the difference between an individual’s score and the threshold of scores below which dementia might be diagnosed, thus lengthening the time interval before dementia is found.
These findings support those that others have recently published in which education is not associated with decline in cognition.9–12 The current study benefited from a cognitive battery covering three domains, the availability of data from blacks and whites, and cognitive scores first obtained at midlife before substantial age-related impairments appear. Although other recently published studies have avoided the bias described previously8 (adjustment for baseline cognitive function can induce a spurious statistical association between education and cognitive change) by not adjusting for baseline cognitive status, they have other limitations. In one study of civil servants with repeat cognitive assessment over a 10-year period, including from midlife, education was associated with baseline scores but not cognitive change; there were no black participants in this study and few were female.9 The Cambridge City over 75s Cohort Study found similar associations but without any data on black participants and with a limited cognitive battery (MMSE alone).10 Two studies, with biethnic populations, studied composite global cognitive scores, which are unlikely to be affected by “ceiling effects,” but the Chicago Health and Aging Project8 provided results only for the global score, and the Asset and Health Dynamics among the Oldest Old Study12 provided results for the global and a composite recall score, but no other domains.
The current study was limited by having only one test for each cognitive domain. In addition, only 1,617 individuals had very low levels of education (17.5% of the population with < high school education, with even fewer having only fifth grade or less). If very low levels of education are associated with cognitive decline, this would have been missed in the analyses. In addition, any association between early cognitive abilities and the subsequent acquisition of more education may cloud all studies of the influence of education on late-life cognitive abilities. Another limitation is the potential lack of correspondence between cognitive scores and the latent cognitive abilities they are meant to measure.22
This study suggests that educational level, although of apparent importance in establishing baseline cognitive performance, is not associated with decline in cognitive function over time. These findings were consistent in black and white participants and in a subset of the oldest participants. These findings may help to understand the longdebated idea of “cognitive reserve,” which is expected to protect cognition in the face of brain disease. The findings may be relevant to cognitive interventions or preventive strategies designed to enhance that protection. Clinical trials may be needed to evaluate interventions such as training in memory or executive function, for which small trials have suggested a beneficial role.23 Can a course of such training substantially boost relevant cognitive abilities, as the baseline effects of long-ago completed education the current study and others have observed may suggest, so that decline to dementia takes longer?
Supplementary Material
Baseline characteristics comparing participants with 2 vs 3 or 4 cognitive assessments.
Acknowledgments
The authors thank the ARIC staff and participants for their important contributions.
ARIC is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute Contracts HHSN268201100005C, HHSN268201100006C, HHSN26 8201100007C, HHSN268201100008C, HHSN26820110 0009C, HHSN268201100010C, HHSN268201100011C, HHSN268201100012C, with the ARIC carotid MRI examination funded by U01HL075572–01 and the ARIC brain MRI examination funded by Grant R01-HL70825.
Sponsor’s Role: The sponsor played no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Author Contributions: All authors approved the manuscript. Ms. Schneider performed the analysis and drafted parts of the manuscript (Methods, parts of the Results section). Dr. Sharrett helped design the study, interpreted the results, and performed critical review of the manuscript. Drs. Patel, Alonso, and Coresh edited and performed critical review of the manuscript. Dr. Mosley supervised the collection of data, interpreted the results, and performed critical review of the manuscript. Dr. Selnes and Dr. Selvin interpreted the results and performed critical review of the manuscript. Dr. Gottesman helped design the study, interpreted the results, and drafted sections of the manuscript (background, part of the Results section, and the Discussion).
References
- 1.Riley KP, Snowdon DA, Desrosiers MF, et al. Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela M, Brayne C, Sachdev P, et al. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011;173:1004–1012. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- 3.Whalley LJ, Starr JM, Athawes R, et al. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychol Med. 2006;36:1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- 5.Plassman BL, Williams JW, Jr, Burke JR, et al. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 6.Christensen H, Hofer SM, Mackinnon AJ, et al. Age is no kinder to the better educated: Absence of an association investigated using latent growth techniques in a community sample. Psychol Med. 2001;31:15–28. doi: 10.1017/s0033291799002834. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3:e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 9.Singh-Manoux A, Marmot MG, Glymour M, et al. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70:296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muniz-Terrera G, Matthews F, Dening T, et al. Education and trajectories of cognitive decline over 9 years in very old people: Methods and risk analysis. Age Ageing. 2009;38:277–282. doi: 10.1093/ageing/afp004. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Hebert LE, Scherr PA, et al. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlamangla AS, Miller-Martinez D, Aneshensel CS, et al. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, Apoe genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 15.Wagenknecht LE, Wasserman B, Chambless L, et al. Correlates of carotid plaque presence and composition as measured by MRI: The Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2009;2:314–322. doi: 10.1161/CIRCIMAGING.108.823922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 18.Benton AL, Eslinger PJ, Damasio AR. Normative observations on neuropsychological test performances in old age. J Clin Neuropsychol. 1981;3:33–42. doi: 10.1080/01688638108403111. [DOI] [PubMed] [Google Scholar]
- 19.Blair CK, Folsom AR, Knopman DS, et al. ApoE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64:268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- 20.Barnes LL, Wilson RS, Hebert LE, et al. Racial differences in the association of education with physical and cognitive function in older blacks and whites. J Gerontol B Psychol Sci Soc Sci. 2011;66B:354–363. doi: 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Wilson RS, et al. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65:953–959. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 22.Proust-Lima C, Dartigues JF, Jacqmin-Gadda H. Misuse of the linear mixed model when evaluating risk factors of cognitive decline. Am J Epidemiol. 2011;174:1077–1088. doi: 10.1093/aje/kwr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson MC, Erickson KI, Kramer AF, et al. Evidence for neurocognitive plasticity in at-risk older adults: The Experience Corps program. J Gerontol A Biol Sci Med Sci. 2009;64A:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics comparing participants with 2 vs 3 or 4 cognitive assessments.