Abstract
In this work, cross-sensitivities and environmental influences on the sensitivity and the functionality of an enzyme-based amperometric sensor system for the direct detection of formaldehyde from the gas phase are studied. The sensor shows a linear response curve for formaldehyde in the tested range (0 - 15 vppm) with a sensitivity of 1.9 μA/ppm and a detection limit of about 130 ppb. Cross-sensitivities by environmental gases like CO2, CO, NO, H2, and vapors of organic solvents like methanol and ethanol are evaluated as well as temperature and humidity influences on the sensor system. The sensor showed neither significant signal to CO, H2, methanol or ethanol nor to variations in the humidity of the test gas. As expected, temperature variations had the biggest influence on the sensor sensitivity with variations in the sensor signal of up to 10 % of the signal for 5 vppm CH2O in the range of 25 - 30 °C.
Keywords: enzyme biosensor, selectivity, amperometry, formaldehyde, formaldehyde dehydrogenase
1. Introduction
As formaldehyde, a base chemical used in the scale of megatons in industrial applications [1] is known to be a human carcinogen [2] with toxic and allergenic potential, the development of a highly selective and sensitive sensor for formaldehyde is desirable.
As an advantage, the sensor described in the following allows the online detection of formaldehyde directly from the gas phase, in contrast to analytical methods [3, 4 and 5] or other previous described sensor systems for formaldehyde [6, 7 and 8], often dealing with the problem of long sampling times and supplementary accumulation steps prior to the measurement.
A first presentation of new enzyme-based biosensors is often based on the demonstration of the operational functionality of the novel sensor set-up or the illustration of the new sensing idea. Moreover, initial characterization is often limited to a report on sensitivity and sometimes on long-term stability of the device. In the last 10 years, especially in the case of enzyme-based sensors for formaldehyde, interference measurements were often completely missing [9, 10, 11 and 12], or only of little interest, and the number of tested interferences was restricted [13]. Against the background that selectivity is the outstanding advantage of biosensors due to their highly specific biorecognition elements, this seems to be justified.
However, as Thevenot et al. depicted in 2001 [14] selectivity of an electrochemical enzyme-based biosensor is not only defined by the biological receptor in use. The biological recognition element as well as the transducer and even the measurement mode might affect the selectivity of the whole sensing device.
In bioorganic matrices or complex media, e.g. industrial or private surroundings, several components similar or even those dissimilar to the analyte can interact with the device [15]. This may not only cause detection errors, but also lead to electrode fouling or poisoning in long-term application. Furthermore interactions between interfering media and the biological receptor can cause significant loss of sensitivity when the enzyme is deactivated or partially complexed by interferences.
As two divisions of the IUPAC stated in 1999 (see [14]), the choice of transducer or electrode material and type notably affects the selectivity. Metal electrodes for example are often sensitive to numerous interfering substances, though oxygen electrodes or pH-electrodes show less sensitivity to interferences [14]. In addition, when an amperometric detection mode is used, the selectivity of metal electrodes depends on the potential used for the electrochemical reduction or oxidation of electroactive species.
Hence, even for highly selective biological components interferences from surrounding media, whether liquid or gaseous media, must be considered within the characterization process.
For formaldehyde non-enzymatic gas sensors (e.g. semiconducting devices), it is quite common to evaluate interferences from inorganic and organic matter like methanol, acetone, toluene, benzene, CO, CO2, SO2 etc. [16, 17 and 18]. As one of the few groups evaluating interferences for formaldehyde biosensors Vastarella et al. [15] studied a large group of organic compounds like glycin, glucose, paracetamol, ascorbic acid etc. in the presence or absence of formaldehyde. However, they only evaluated the influence of components in liquid buffer solution.
A detailed evaluation of common organic and inorganic substances found in the environment of an enzyme-based gas sensor for the detection of formaldehyde is still missing.
The intent of the present study is not to illustrate a new sensor set-up for formaldehyde, since the one mentioned here was described in [19]. Rather, we estimate the influences of common interferences like CO, CO2, NO, H2, ethanol, methanol, relative humidity and temperature on the sensitivity and stability of the slightly revised amperometric enzyme-based gas sensor for formaldehyde presented in [19] and demonstrate that with the choice of a proper electrode material and the adequate measurement potential, influences of interfering substances can be limited.
2. Experimental
2.1. Reagents
Formaldehyde dehydrogenase from Pseudomonas putida (EC 1.2.1.46, lyophilised powder: 3 - 4 U/mg) and phenothiazine (approx 98 %) were purchased from Sigma, NAD+ (free acid, approx. 95 %) from Merck. Methanol (approx 99 %, Merck) and Ethanol (technical grade) sample solutions were prepared by dilution. Formaldehyde sample solutions were prepared in the same way using a 36.5 % stock solution (Riedel-de-Haen), containing 10 % methanol as stabilizer. All other chemicals were of analytical grade. Distilled water was used to prepare standard and buffer solutions.
2.2. Gas sample preparation
Gaseous methanol, ethanol and formaldehyde samples were collected from the head space above aqueous solutions of known concentration.
The concentration of methanol and ethanol in the gas phase above aqueous solutions depends on Henry's law constants of the given analyte in water and was calculated from Henry's law following the equation given by Sander [20] (25°C):
| (1) |
kH0: Henry's law constant for standard conditions; [kH0] = M/atm
ca: aqueous-concentration of the analyte; [ca] = M
pg: partial pressure of the analyte in the gas phase; [pg] = atm
For methanol and ethanol a kH0 value of 2.2·102 M/atm and 1.9·102 M/atm was used, respectively [21].
The CH2O concentration in the gas phase above the formaldehyde sample solution was calculated according to the following equation (20 °C) given by Dong et al.[22]:
| (2) |
In Table 1, 2 and 3 the used equilibrium gas phase concentrations above aqueous methanol, ethanol and formaldehyde solutions are specified.
Table 1.
Equilibrium gas phase concentrations above an aqueous methanol solution at 25 °C according to the Henry's law constant given by Snider et al. [21] and Equation (1) given by Sander [20].
| Concentration of CH3OH in the aqueous phase / mM | Concentration of CH3OH in the gas phase / vppm |
|---|---|
| 3 | 13.6 |
| 30 | 136 |
| 60 | 273 |
Table 2.
Equilibrium gas phase concentrations above an aqueous ethanol solution at 25 °C according to the Henry's law constant given by Snider et al. [21] and Equation (1) given by Sander [20].
| Concentration of C2H5OH in the aqueous phase / mM | Concentration of C2H5OH in the gas phase / vppm |
|---|---|
| 2.8 | 14.7 |
| 28.1 | 148 |
| 56.2 | 296 |
Table 3.
Equilibrium gas phase concentrations above an aqueous formaldehyde solution at 20 °C according to Equation (2) given by Dong et al. [22].
| Concentration of CH2O in the aqueous phase / mM | Concentration of CH2O in the gas phase / vppm |
|---|---|
| 2.62 | 0.5 |
| 11.7 | 2 |
| 31.4 | 5 |
| 66.4 | 10 |
| 103 | 15 |
All other gases like CO, CO2, NO, and H2 were prepared by dilution of pure gas (Riesner Gase, Germany) by nitrogen in a self-made gas sensor test bench.
2.3. Preparation of humidified and tempered gas samples
To apply different relative humidity to the samples, nitrogen was saturated with water in fritted wash-bottles in a temperature controlled water bath. Hereafter, the humidified nitrogen was fed to the main sample stream in the gas sensor test bench via heated tubing to avoid water condensation. Humidity was regulated by the temperature of the water quench or the ration of humidified nitrogen in the main sample stream. Thus, it was possible to vary the humidity of the test gas in the range of 23 -90 %rH (25 °C).
Tempered gas samples in the range of 25 - 30 °C were provided by warming the gas stream before applying it to the sensor device.
2.4. Sensor set-up
The sensor is based on the enzymatic conversion of the analyte formaldehyde by NAD+/NADH-dependent formaldehyde dehydrogenase (FDH) from P. putida and subsequent electrochemical detection of formed NADH with the aid of a mediator (Fig. 1). The flux of electrons was recorded in an amperometric measurement at +200 mV vs. Ag/AgCl reference (3M KCl, +210mV vs. NHE) using a potentiostat (PGSTAT 12, Eco Chemie, The Netherlands).
Figure. 1.
Reaction steps for monitoring formaldehyde using the enzyme formaldehyde dehydrogenase (FDH), and phenothiazine (PT) as electrochemical mediator.
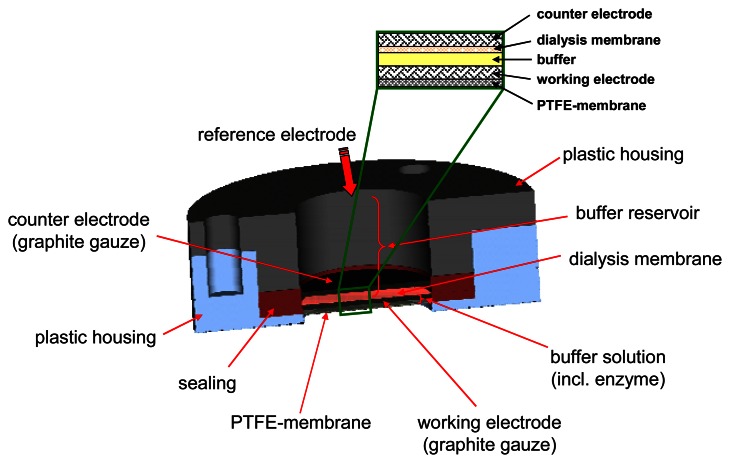
The sensor device consists of a 3-electrode configuration with disks of woven graphite gauze (Alfa Aesar, Ø=15 mm) as working and counter electrode included in a plastic housing (Fig. 2). Both electrodes were contacted with Pt-wire (Ø=0.2 mm). The gas diffuses into the liquid phase via a 15 mm diameter Teflon membrane (FALP02500, Millipore, France). Loss of enzyme is restricted by a dialyses membrane with a MWCO of 12000 - 14000 Daltons (Medicell International, London, Great Britain).
Figure 2.
Sensor set-up: plastic housing with different membranes and electrodes for the detection of the gaseous analyte formaldehyde.
A similar sensor set-up was proposed in our previous publication [19, 23]. For this study, it was optimized for better long-term stability. The stability of the initial sensor [23] was limited to 4 - 6 h. This was mainly attributed to the stability of the mediator 1,2-naphthoquinone-4-sulfonic acid (NQS) as well as to side reactions between the enzyme FDH and NQS. Since NAD+/NADH [23] and the enzyme itself showed high long-term stability (over 96 % for 18 h [19]) in the respective buffer, several new molecules with possible mediating properties were evaluated in cyclovoltametric experiments (results not shown). Phenothiazine (PT) enabling a sensor functionality of up to 20 h was chosen as the most suitable mediator. This improved set-up allowed for the first time to obtain reliable cross-sensitivity data.
In the sensor device presented here, the mediator PT is physically adsorbed on the working electrode by coating the gauze with 50 μl of a 10 mM solution of PT in acetonitrile and consecutive drying at room temperature for 30 min.
The buffer within the sensor contained 0.1 M KCl and 0.1 M KH2PO4 at pH 8.
2.5. Detection of environmental interferences
To evaluate the influence of environmental gaseous components, functionality of the sensing device was first verified in 2 vppm formaldehyde. Subsequently, different gas phase concentrations of the environmental interferences in the absence of formaldehyde were applied to the sensor to study the sensitivity towards those substances. The functionality was checked again at the end of the measurement (2 vppm CH2O). The gas concentrations were verified by an FTIR (6700, Nicolet). Relative humidity was determined by a humidity sensor (HIH 3610, Honeywell).
The concentration range selected for the inorganic gas samples clasp around the concentration or output of the possible source for the interferences and the occupational exposure limits summarized by the International Occupational Safety and Health Information Centre (CIS) [24]. For example, the origin of the interfering CO2 will presumably be exhalation, with up to 4 - 5 % CO2 in the exhaled breath [25]. As another possible source for CO2 engine exhaust has to be mentioned, which is also a source for NO, and CO. The exhaust of diesel engines for example, consists of about 350 - 1000 ppm NO, 300 - 1200 ppm CO and 7 % CO2 among others [26], whereas the occupational exposure limits for those components are much lower. A limit of 25 - 30 ppm for CO [27], 25 ppm for NO [28], and 5000 ppm for CO2[29] was set for an 8-hour working day. Considering this, the concentration ranges listed in Table 4 were chosen for the inorganic species .
Table 4.
Concentration range for the tested environmental inorganic species.
| Environmental interference | Concentration range in the gas phase |
|---|---|
| CO | 0 - 467 vppm |
| NO | 0 - 800 vppm |
| H2 | 0 - 600 vppm |
| CO2 | 0 - 13.7 % (v/v) |
For temperature and humidity influences not only functionality and stability of the sensor were evaluated after the test phase. Different concentrations of the interfering components were added to a formaldehyde sample of 5 vppm and the deviation of the sensor signal for each interfering concentration was investigated.
3. Results and Discussion
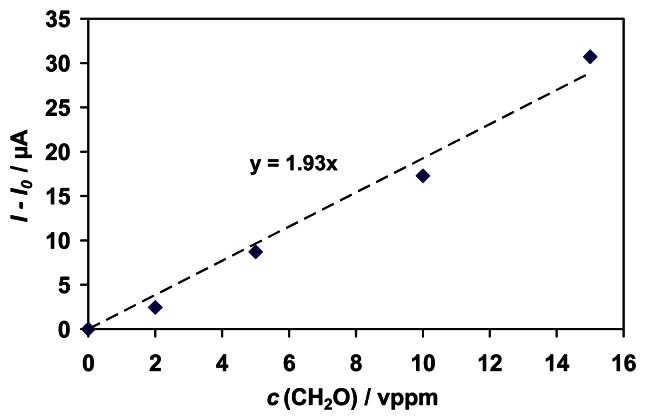
The sensor shows a linear response curve for formaldehyde in the tested range (0 - 15 vppm) with a sensitivity of 1.9 μA/ppm and a detection limit of about 130 ppb (Fig. 3), which meets the threshold limit of 300 ppb set by the American Conference of Governmental Industrial Hygienists (ACGIH) in 2004 [24].
Figure 3.
Response curve to formaldehyde (0 - 15 vppm) for the amperometric enzyme-based biosensor. Potential applied: +200 mV vs. Ag/AgCl (3 M KCl); Buffer solution: 0.1 M KCl/KH2PO4, 5 mM NAD+, pH 8; Enzyme load: 0.5 mg/ 200 μl buffer solution; Mediator: 50 μl of a 10 mM solution of PT in acetonitrile adsorbed on the graphite working electrode.
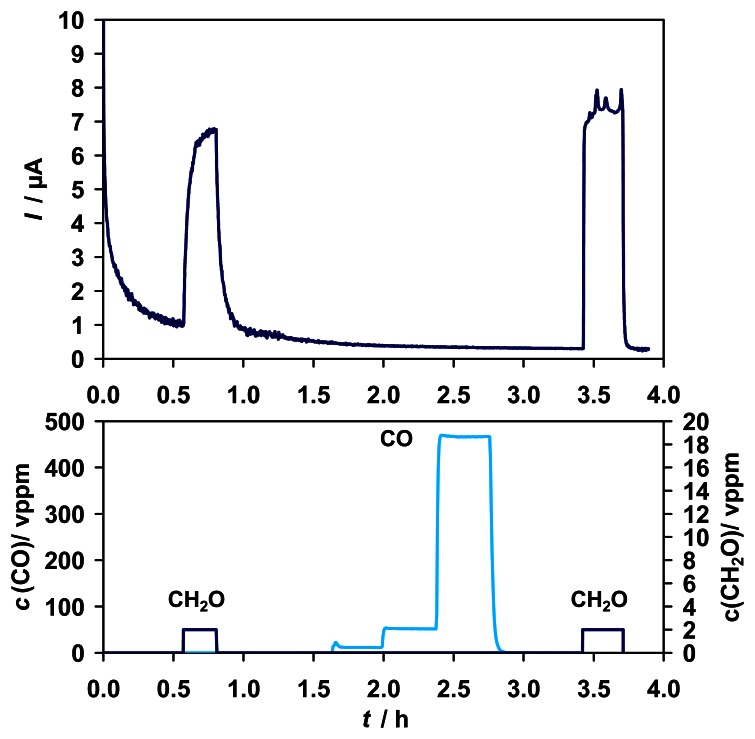
As assumed for an enzymatic biosensor, most tested interferences did not show any influence on the sensor performance. There were no interferences of CO (Fig. 4), methanol (Fig. 6) or H2 (results not shown) in the tested range.
Figure 4.
Top: Amperometric sensor signal for 2 vppm gaseous formaldehyde and CO interferences in the range of 0 - 467 vppm. Bottom: Test cycle concentrations of CO (left y-axis) and formaldehyde (right y-axis).
Figure 6.
Top: Amperometric sensor signal for 2 vppm formaldehyde and interferences from methanol (0 - 276 vppm) and ethanol (0 - 300 vppm). Bottom: Test cycle concentrations of methanol and ethanol (left y-axis) and formaldehyde (right y-axis).
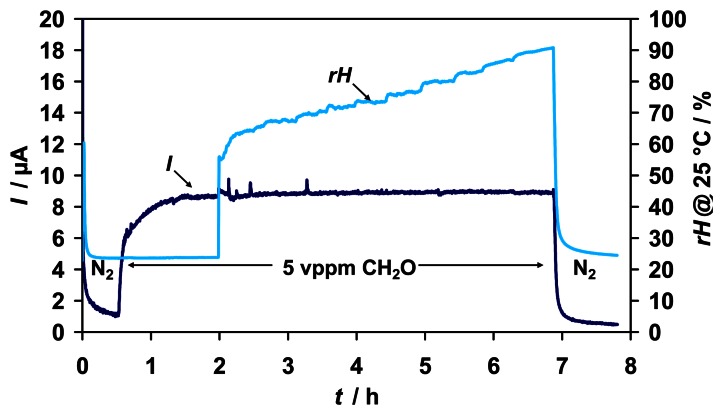
A variation of humidity in the range of 23 - 90 %rH of the test gas did also not affect the sensor signal for 5 vppm formaldehyde (Fig. 5).
Figure 5.
Sensor signal for 5 vppm formaldehyde with varied humidity in the range of 23 - 90 %rH.
As can be seen from Fig. 6, there was a very small response to ethanol. The sensitivity to ethanol was about 0.01 μA/vppm in the range of 0 - 15 vppm but not linearly dependent on the concentration, whereas the sensitivity towards formaldehyde is about 200 times higher, about 1.9 μA/vppm in the same range. Furthermore, a sort of saturation effect for concentrations higher than 150 vppm ethanol was detected. Thus, the sensor functionality was not influenced by ethanol interferences. As Ogushi et al. [30] investigated for FDH from P. putida there is no enzymatic activity towards methanol or ethanol, so the small signal to ethanol is supposed to be an anodic effect where ethanol is directly oxidized to acetaldehyde at the electrode.
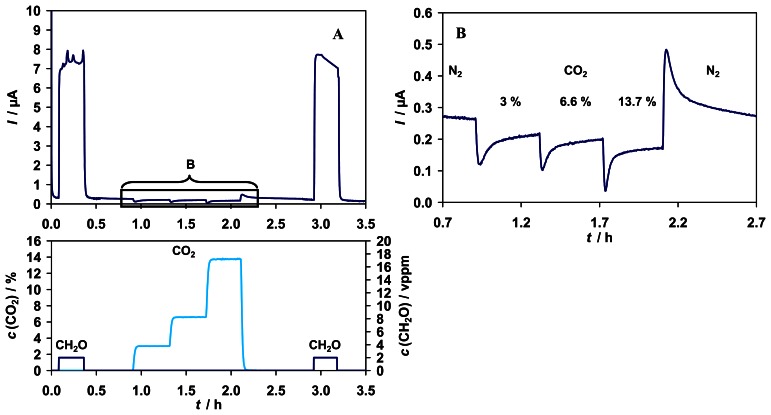
The influence of CO2 in the gas phase was also investigated. Although no cross-reactivity of the enzyme FDH from P. putida with CO2 was observed in an enzyme assay [31], the sensor showed a kind of response to different CO2 concentrations in the range of 0 - 14 % (v/v), see Fig. 7.
Figure 7.
A) Top: Sensor signal for 2 vppm formaldehyde and interferences from CO2 (0 -13.7 %). Bottom: Test cycle concentrations of CO2 (left y-axis) and formaldehyde (right y-axis). B) Signal to CO2 (magnified range).
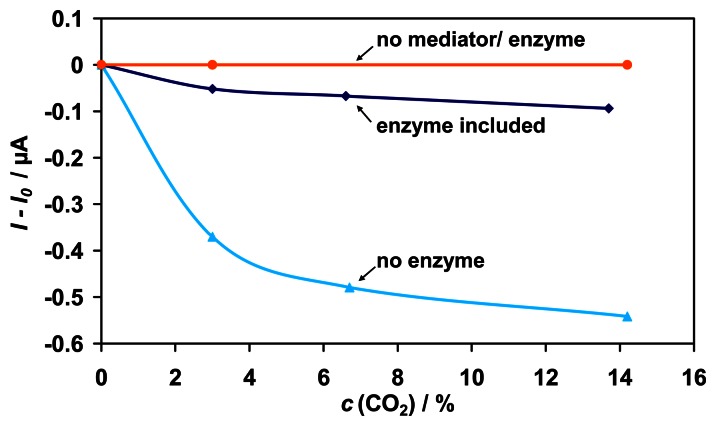
To determine which component is reactive to CO2 in the sensing device, the enzyme, NAD+, and the mediator were successively omitted. As can be seen from Fig. 8, there is even a higher sensitivity to CO2 when no enzyme is present in the sensor device. Therefore, the signal to CO2 is not enzyme-catalyzed. The conversion seems to be catalyzed by the mediator PT, because there is no signal to CO2 in the absence of PT. With the use of another mediator, it should be possible to minimize the sensitivity to CO2.
Figure 8.
Cross-sensitivity of the enzymatic biosensor to CO2 (0 - 14 % (v/v)). Successively, the biological components enzyme, NAD+, and the mediator PT were omitted.
The lower sensitivity to CO2 in the presence of enzyme can probably be ascribed to a kind of occupation of the active electrode surface by the protein molecules. Thus, the reactive surface area for the mediator catalyzed conversion of CO2 is reduced resulting in a lower current signal.
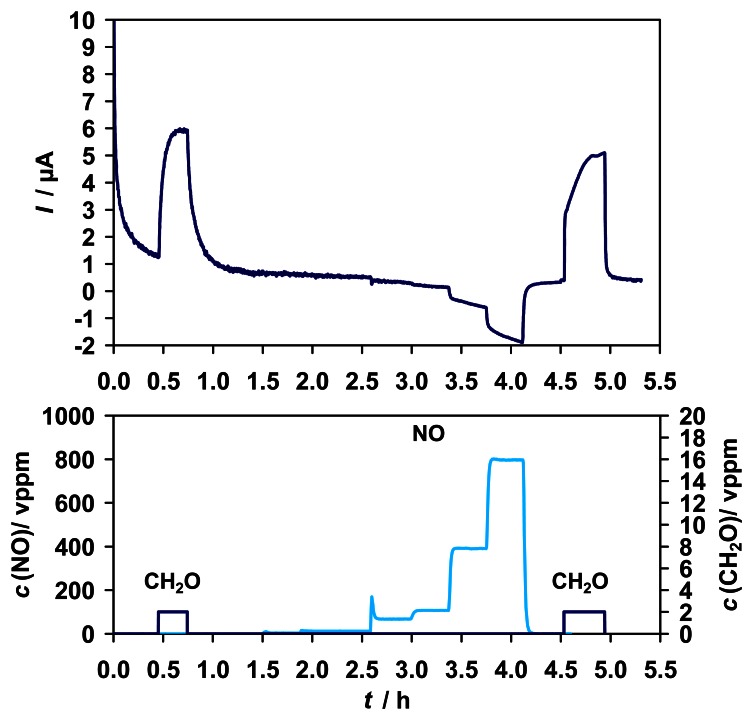
A similar characteristic was determined for NO (0 - 800 vppm). As depicted in Fig. 9, a concentration dependent signal with a sensitivity of about 0.01 μA/ppm in the range of 0 - 15 vppm was detected.
Figure 9.
Top: Amperometric sensor signal for 2 vppm formaldehyde and interferences from NO (0 - 800 vppm) in the gas phase. Bottom: Test cycle concentrations of NO (left y-axis) and formaldehyde (right y-axis).
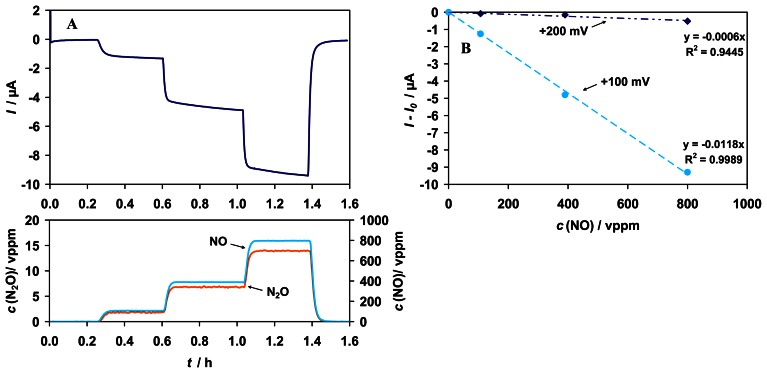
As in the case of CO2 the sensitivity to NO increases in the absence of enzyme and decreases in the absence of the mediator (Fig. 10). Residual sensitivity to NO in the absence of any biological component is attributed to the reduction of NO at the graphite gauze electrode to N2O, evidenced by the detection of 0 - 14 vppm N2O via FTIR (s. Fig. 11 A). The sensitivity to NO can be reduced by the change of the electrode material. For an Au electrode an approximately 3-times smaller sensitivity was achieved than for the graphite gauze (results not shown).
Figure 10.
Cross-sensitivity of the enzymatic biosensor to NO (0 - 800 vppm). Components like the enzyme formaldehyde dehydrogenase, NAD+, and the mediator PT were omitted step by step.
Figure 11.
A) Top: Amperometric sensor signal of the “bio”sensor set-up to NO (0 - 800 vppm) at +100 mV vs. Ag/AgCl at a graphite gauze working electrode. The sensor contained neither enzyme FDH, nor NAD+, nor mediator PT. Bottom: Test cycle concentrations of NO (right y-axis) and N2O concentrations (left y-axis), which were detected downstream the sensor via FTIR. B) Comparison of the response curve characteristic to NO at +100 mV and +200 mV vs. Ag/ACl.
In order to prove that NO reduction at the electrode is responsible for this effect, additional studies without any biological component have been conducted at a lower potential (+100 mV vs. Ag/AgCl). The signal to NO is up to 20 times higher and almost linear. Furthermore, a lower noise level occurs; possibly because of fewer interactions by interfering media from the buffer (Fig. 11 A, B).
In summary, the interference from NO is mainly based on electrochemical reduction to N2O. The interference can be reduced by the right choice of the electrode material and detection potential. The enzyme itself is unaffected by NO, and sensitivity or stability of the sensor to formaldehyde was not affected.
As a condensed summary, the above-mentioned gases that are present in the ambient do neither have an impact of the applicability of this sensor principle to determine the desired analyte formaldehyde nor do they affect the functionality of the sensor device. When signals towards interferences and towards the analyte for the same concentration range are compared, cross-sensitivities are about two decades smaller (s. Fig. 6 or Fig. 9) and barely higher than the noise of the measurement (s. Fig. 6 or Fig. 7).
Finally, temperature variations on the sensor signal were evaluated. The enzymatic reaction as well as the diffusion of the analyte molecules in the gas and the liquid phase depend on temperature. Whereas the dependence of the diffusion rate on the temperature is quite clear, the influence on the enzymatic reaction is not well known in terms of explicit mathematic equations, i.e., the thermal activation energy is unknown. Ando and coworkers [31] specified an activity optimum of the enzyme FDH from P. putida to formaldehyde at about 40 °C in a phosphate buffer, pH 7.5 with an activity and stability decrease at higher temperatures. In this work, the temperature of the test gas was increased stepwise around room temperature in the range of 25 - 30 °C at a constant formaldehyde concentration of 5 vppm.
The signal increases with increasing temperature as assumed (Fig. 12). A quadratic correlation is given as a guide to the eye in the tested temperature range. In this case, a temperature variation of 5 °C leads to a 10 % higher signal based on a signal of 6.2 μA for the concentration of 5 vppm at 25 °C. To obtain more information on the temperature dependence, a modified sensor has to be designed, for example by constructing an actively heated sensor device that allows for expanding the tested temperature range. Thus, the optimum temperature for maximum sensor stability and sensitivity could be determined and a temperature compensation for the sensor could be established.
Figure 12.
Influence of temperature increase on the sensor signal for 5 vppm formaldehyde in the range of 25 - 30 °C. An increase of the sensor signal of about 10 % for a temperature increase of 5 °C is shown based on a signal of 6.2 μA at 25 °C.
4. Conclusion
Various interferences originating from the environment of the sensing device were evaluated in the present work. Via a process of successive elimination of different components it was possible to show that the enzyme FDH itself as sensing element of the amperometric biosensor system showed no cross-sensitivities to almost all tested organic or inorganic gaseous species. Low interferences from CO2 and NO could be attributed to mediator or electrode interactions and were eliminated or minimized by the change of the respective component.
Variations in the humidity of the test gas did not affect the sensor characteristic at all.
As assumed, temperature variations of the test gas affected the sensitivity of the whole sensing device and a more or less quadratic dependence of the sensor signal in the tested temperature range can be approximated. However, exceeding and more detailed data have to be gathered covering a wider temperature range in order to find out the optimum working temperature of the sensing device. Therefore, we are aiming to construct an actively heated sensor device to keep the temperature accurately at the desired value.
The results verify the preliminary observation reported in the introduction. The recognition element itself seems to be quite favorable for a highly selective detection of the analyte formaldehyde. However, selectivity of the whole sensing device does not only depend on the selectivity of the recognition element, but on each participant of the sensor device . It can be engineered to the same content by the right choice of the involved components.
Acknowledgments
The authors like to thank the German research foundation (DFG) for financial support (Ha 4424/ 1-1 and Ha 4424/ 1-3) and Ms. Nadine Losleben, for contributing measurement from her student research project to the presented work.
References and Notes
- 1.Herschkovitz Y., Eshkenazi I., Campbell C. E., Rishpon J. An electrochemical biosensor for formaldehyde. J. Electroanal. Chem. 2000;491:182–187. [Google Scholar]
- 2.International Agency for Research on Cancer Press release N° 153. 2004. http://www.iarc.fr/ENG/Press_Releases/archives/pr153a.html.
- 3.Kawamura K., Kerman K., Fujihara M., Nagatani N., Hashiba T., Tamiya E. Development of a novel hand-held formaldehyde gas sensor for the rapid detection of sick building syndrome. Sens. Actuat. B-Chem. 2005;105:495–501. [Google Scholar]
- 4.Suzuki Y., Nakano N., Suzuki K. Portable sick house syndrome gas monitoring system based on novel colorimetric reagents for the highly selective detection of formaldehyde. Environ. Sci. Technol. 2003;37(24):5695–5700. doi: 10.1021/es0305050. [DOI] [PubMed] [Google Scholar]
- 5.Kim S., Kim H.-J. Comparison of standard methods and gas chromatography method in determination of formaldehyde emission from MDF bonded with formaldehyde-based resins. Bioresour. Technol. 2005;96(13):1457–1464. doi: 10.1016/j.biortech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Kühn M., Rodewyk B., Bryniok D. DE 197 28 663 C1. 1998
- 7.Korpan Y. I., Gonchar M. V., Sibirny A. A., Martelet C., El'skaya A. V., Gibson T. D., Soldatkin A. P. Development of highly selective and stable potentiometric sensors for formaldehyde determination. Biosens. Bioelectron. 2000;15:77–83. doi: 10.1016/s0956-5663(00)00054-3. [DOI] [PubMed] [Google Scholar]
- 8.Kataky R., Bryce M. R., Goldenberg L., Hayes S., Nowak A. A biosensor for monitoring formaldehyde using a new lipophilic tetrathiafulvalene-tetracyanoquinodimethane salt and a polyurethane membrane. Talanta. 2002;56:451–458. doi: 10.1016/s0039-9140(01)00567-7. [DOI] [PubMed] [Google Scholar]
- 9.Vianello F., Stefani A., Paolo M. L., Rigo A., Lui A., Margesin B., Zen M., Scarpa M., Soncini G. Potentiometric detection of formaldehyde in air by an aldehyde dehydrogenase FET. Sensor Actuat. B-Chem. 1996;37:49–54. [Google Scholar]
- 10.Curri M. L., Agostiano A., Leo G., Mallardi A., Cosma P., Della Monica A. Development of a novel enzyme/semiconductor nanoparticles system for biosensor application. Mat. Sci. Eng. C. 2002;22:449–452. [Google Scholar]
- 11.Ali M. B., Korpan Y., Gonchar M., El'skaya A. V., Maaref M. A., Jaffrezic-Renault N., Martelet C. Formaldehyde assay by capacitance versus voltage and impedance measurements using bi-layer bio-recognition membrane. Biosens. Bioelectron. 2006;22(5):575–581. doi: 10.1016/j.bios.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Nikitina O., Shleev S., Gayda G., Demkiv O., Gonchar M., Gorton L., Csoregi E., Nistor M. Bi-enzyme biosensor based on NAD+- and glutathione-dependent recombinant formaldehyde dehydrogenase and diaphorase for formaldehyde assay. Sensor. Actuat. B-Chem. 2007;125(1):1–9. [Google Scholar]
- 13.Hämmerle M., Hall E. A. H. Electrochemical enzyme sensor for formaldehyde operating in the gas phase. Biosens. Bioelectron. 1996;11(3):239–246. [Google Scholar]
- 14.Thevenot D. R., Toth K., Durst R. A., Wilson G. S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001;16(1-2):121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 15.Vastarella W., Nicastri R. Enzyme/semiconductor nanoclusters combined systems for novel amperometric biosensors. Talanta. 2005;66:627–633. doi: 10.1016/j.talanta.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Knake R., Jacquinot P., Hauser P. C. Amperometric detection of gaseous formaldehyde in the ppb Range. Electroanal. 2001;8-9:631–634. [Google Scholar]
- 17.Dirksen J.A., Duval K., Ring T. A. NiO thin-film formaldehyde gas sensor. Sensor. Actuat. B-Chem. 2001;80:106–115. [Google Scholar]
- 18.Zhou K., Ji X., Zhang N., Zhang X. On-line monitoring of formaldehyde in air by cataluminescence-based gas sensor. Sensor. Actuat. B-Chem. 2006;119:392–397. [Google Scholar]
- 19.Achmann S., Hämmerle M., Moos R. Amperometric enzyme-based biosensor for direct detection of formaldehyde in the gas phase: dependence on electrolyte composition. Electroanalysis. 2007;20(4):410–417. [Google Scholar]
- 20.Sander R. Compilation of Henry's law constants for inorganic and organic species of potential importance in environmental chemistry. 1999. p. 3. www.henrys-law.org.
- 21.Snider J. R., Dawson G. A. Tropospheric light alcohols, carbonyls, and acetonitrile: Concentrations in the southwestern United States and Henry's law data. J. Geophys. Res. 1985;90D:3797–3805. [Google Scholar]
- 22.Dong S., Dasgupta P.K. Solubility of gaseous formaldehyde in liquid water and generation of trace standard gaseous formaldehyde. Environ. Sci. Technol. 1986;20:637–640. doi: 10.1021/es00148a016. [DOI] [PubMed] [Google Scholar]
- 23.Achmann S., Hämmerle M., Moos R. In: Dresdner Beiträge zur Sensorik. Gerlach G., Kaden H., editors. Vol. 24. TUDpress; Dresden: 2005. pp. 177–180. article in German. [Google Scholar]
- 24.International Labour Organisation, International Occupational Safety and Health InformationCentre, ICSC 0275. 2004. http://www.ilo.org/public/english/protection/safework/cis/index.htm.
- 25.Hunt D. J. Laser action in human breath and its use for monitoring the carbon dioxide content of air. J. Sci. Instrum. 1967;44:408–410. doi: 10.1088/0950-7671/44/5/430. [DOI] [PubMed] [Google Scholar]
- 26.Kašpar J., Fornasiero P., Hickey N. Automotive catalytic converters: current status and some perspectives. Catal. Today. 2003;77:419–449. [Google Scholar]
- 27.International Labour Organisation, International Occupational Safety and Health InformationCentre, ICSC 0023. 2007. http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/index.htm.
- 28.International Labour Organisation, International Occupational Safety and Health InformationCentre, ICSC 1311. 2004. http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/index.htm.
- 29.International Labour Organisation, International Occupational Safety and Health InformationCentre, ICSC 0021. 2006. http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/index.htm.
- 30.Ogushi S., Ando M., Tsuru D. Substrate specifity of formaldehyde dehydrogenase from Pseudomonas putida. Agric. Biol. Chem. 1984;48(3):597–601. [Google Scholar]
- 31.Ando M., Yoshimoto T., Ogushi S., Rikitake K., Shibata S., Tsuru D. Formaldehyde dehydrogenase from Pseudomonas Putida. J. Biochem. 1979;85(5):1165–1172. [PubMed] [Google Scholar]